Abstract
Dengue virus (DENV) NS1 is a versatile non-structural glycoprotein that is secreted as a hexamer, binds to the cell surface of infected and uninfected cells, and has immune evasive functions. DENV NS1 displays two conserved N-linked glycans at N130 and N207. In this study, we examined the role of these two N-linked glycans on NS1 secretion, stability, and function. Because some groups have reported reduced yields of infectious DENV when N130 and N207 are changed, we analyzed glycosylation-deficient NS1 phenotypes using a transgenic expression system. We show that the N-linked glycan at position 130 is required for stabilization of the secreted hexamer whereas the N-linked glycan at residue 207 facilitates secretion and extracellular protein stability. Moreover, NS1 mutants lacking an N-linked glycan at N130 did not interact efficiently with complement components C1s and C4. In summary, our results elucidate the contribution of N-linked glycosylation to the function of DENV NS1.
Keywords: Dengue virus, Flavivirus, non-structural protein NS1, N-linked glycosylation, complement
Introduction
Dengue virus (DENV) is a mosquito-transmitted Flavivirus of global medical concern. Annually, more than 50 million human infections are reported with approximately 25,000 deaths due primarily to dengue hemorrhagic fever and shock syndrome (DHF/DSS) (Gubler, 1998; Halstead, 2007). Four serotypes of DENV circulate and severe disease is preferentially associated with secondary infection with a different serotype. Although antibody-dependent enhancement of DENV infection in Fc-γ receptor-bearing cells has been proposed to initiate pathogenesis (Halstead, 1988), the precise pathologic mechanism for DHF/DSS remains uncertain. Effects of virulent strains, a pro-inflammatory cytokine storm secondary to exuberant activation of poorly lytic cross-reactive T cells, and excessive complement activation also have been proposed to cause the capillary leakage syndrome associated with DHF/DSS (reviewed in (Clyde, Kyle, and Harris, 2006)).
The ∼11 kb genomic RNA encodes a polyprotein that is cleaved by viral and host proteases to generate three structural (capsid (C), pre-membrane/membrane (prM/M) and envelope (E)) and seven non-structural (NS1, NS2A, NS2B, NS3, NS4A, NS4B and NS5) proteins. DENV NS1 is a 48 kDa non-structural glycoprotein that contains twelve invariant cysteine residues, six intramolecular disulfide bonds, and two conserved N-linked glycans (Chambers et al., 1990). Within infected cells, NS1 expression is required for efficient viral RNA replication through an uncertain mechanism (Khromykh et al., 1999; Lindenbach and Rice, 1997; Mackenzie, Jones, and Young, 1996). DENV NS1 is translocated into the endoplasmic reticulum through a signal sequence localized near the C-terminus of E protein. Within the endoplasmic reticulum, DENV NS1 is modified such that N-linked glycans are added at positions N130 and N207 (Falgout and Markoff, 1995). Further modification occurs during transit through the Golgi complex where a complex-type sugar is added to the N130 glycan but the N207 glycan is retained in the high mannose form (Pryor and Wright, 1994). NS1 is secreted at high levels into the extracellular environment during DENV infection, predominantly as a hexamer (Flamand et al., 1999), with significant accumulation (up to 50 μg/ml) in the sera of infected patients (Alcon et al., 2002; Avirutnan et al., 2006; Libraty et al., 2002; Young et al., 2000). Soluble NS1 also can bind back to the plasma membrane of cells through an interaction with specific sulfated glycosaminoglycans (GAGs) (Avirutnan et al., 2007). Finally, NS1 is expressed directly on the surface of infected cells, possibly via glycosylphosphatidyl inositol (GPI) linkage (Jacobs et al., 2000), lipid raft association (Noisakran et al., 2008) or through an as yet undefined mechanism.
NS1 has been implicated in DENV pathogenesis through mechanisms that are enigmatic. High plasma levels of NS1 and terminal complement complex C5b-9 detected in DENV-infected patients correlate with severe disease outcome (Avirutnan et al., 2006; Libraty et al., 2002). NS1 has been proposed to enhance infection in hepatocytes in cell culture (Alcon-LePoder et al., 2005), facilitate immune complex formation (Avirutnan et al., 2006), elicit auto-antibodies that cross-react with platelet and extracellular matrix proteins, and promote endothelial cell damage via antibody-dependent complement-mediated cytolysis (Chang et al., 2002; Lin et al., 2003; Sun et al., 2007). NS1 also has immune evasion functions as it can directly antagonize complement activation. West Nile virus (WNV) NS1 binding and recruitment of factor H, a negative regulator of the alternative pathway, decreases complement activation and C5b-9 membrane attack complex deposition on the cell surface (Chung et al., 2006a). DENV NS1 also exhibits complement antagonistic activity by binding C4 and C1s, leading to C4 degradation and depressed complement activation (Avirutnan et al., 2010). Finally, soluble DENV NS1 may interact with clusterin, a complement regulator that inhibits terminal complement complex formation (Kurosu et al., 2007). Attenuation of complement activation by NS1 protects DENV from direct complement-dependent neutralization by mannose binding lectins (Avirutnan et al., 2010; Fuchs et al., 2010).
Several studies with different Flaviviruses have suggested that the N-linked glycans on NS1 are essential for its function. A yellow fever virus (YFV) NS1 mutant lacking the first N-linked glycosylation site (N130) exhibited a decrease in viral growth, reduced plaque size, delayed cytopathic effect, and impaired NS1 secretion (Muylaert et al., 1996). Mutation of the first or both DENV NS1 N-linked glycosylation sites also caused reduced virus growth (Crabtree, Kinney, and Miller, 2005; Pryor et al., 1998; Tajima, Takasaki, and Kurane, 2008). In vivo experiments have shown that loss of N-linked glycans on NS1 results in attenuated YFV, DENV, and WNV infection in mice (Muylaert et al., 1996; Pryor et al., 1998; Whiteman et al., 2010).
Because of the potentially confounding impact on viral replication, we studied the effects of mutant forms of NS1 lacking either the first (N130), second (N207) or both N-linked glycans on the biology of NS1 in a heterologous viral system. We generated recombinant double subgenomic Sindbis viruses (SINV) that ectopically express wild type or mutant NS1 lacking either the first (N130), second (N207) or both N-linked glycosylation sites to compare the phenotypes of cell surface expression and binding, secretion, stability, and interaction with complement proteins. Mutation of N207 altered secretion and cell surface levels of NS1. In comparison, mutation of N130 affected the stability of hexameric NS1 and bound less well to human C4, C4b, C1s proenzyme, or C1s. In summary, N-linked glycosylation on NS1 modulates cell surface expression, secretion, stability, and function.
Results
Generation of SINV encoding DENV-2 NS1
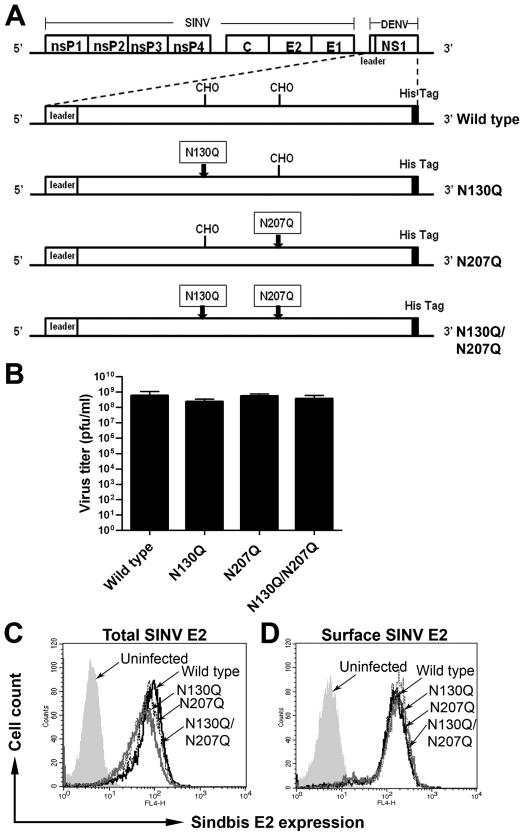
According to prior reports, DENV mutants lacking N-linked glycans on NS1 are attenuated and fail to replicate efficiently in mammalian cells (Crabtree, Kinney, and Miller, 2005; Pryor et al., 1998; Tajima, Takasaki, and Kurane, 2008). To study the effects of N-linked glycosylation on NS1 in a system that is not affected by changes in infectivity, we generated recombinant double subgenomic Sindbis viruses (SINV) that express ectopically wild type or mutant NS1 lacking either the first (N130), second (N207) or both N-linked glycosylation sites (Fig. 1A). As expected, recombinant SINV encoding wild type and mutant DENV-2 NS1 replicated equivalently (Fig. 1B), confirming that expression of the variant DENV-2 NS1 did not differentially affect SINV replication. This was corroborated by analyzing levels of total and surface expression of SINV envelope protein 2 (SINV E2) in infected cells by flow cytometry (Fig. 1C and D).
Figure 1. Expression of wild type and N-linked glycan mutant DENV NS1 does not differentially affect SINV replication.
(A) Schematic diagram of SINV vector encoding DENV NS1 constructs. Wild type or mutants with a glutamine substitution at the first (N130Q), second (N207Q), or both glycosylation sites (N130Q/N207Q) of DENV-2 NS1 were inserted into SINV 39HK vector backbone after the nonstructural nsP1-4 and structural proteins, capsid, envelope 1 and 2. The leader sequence was derived from the last 24 amino acids of DENV-2 E protein. A hexa-histidine tag (His6 Tag) was added onto the carboxy-terminus of each construct. The boxes indicate glutamine for asparagine substitutions within the glycosylation sites and arrows indicate the sites of mutations. CHO indicates the position of N-linked glycans. (B-D) Transgenic expression of variant DENV NS1 does not affect SINV replication. BHK21 cells were infected with SINV expressing wild type and NS1 mutants at an MOI of 1.0. The supernatants were collected 18 h post-infection and subjected to plaque assay (B). Level of SINV infection was also assessed by total (C) and surface (D) SINV-E2 staining with mouse hyper-immune serum against SINV.
The second N-linked glycosylation site (N207) influences surface expression and secretion of DENV NS1
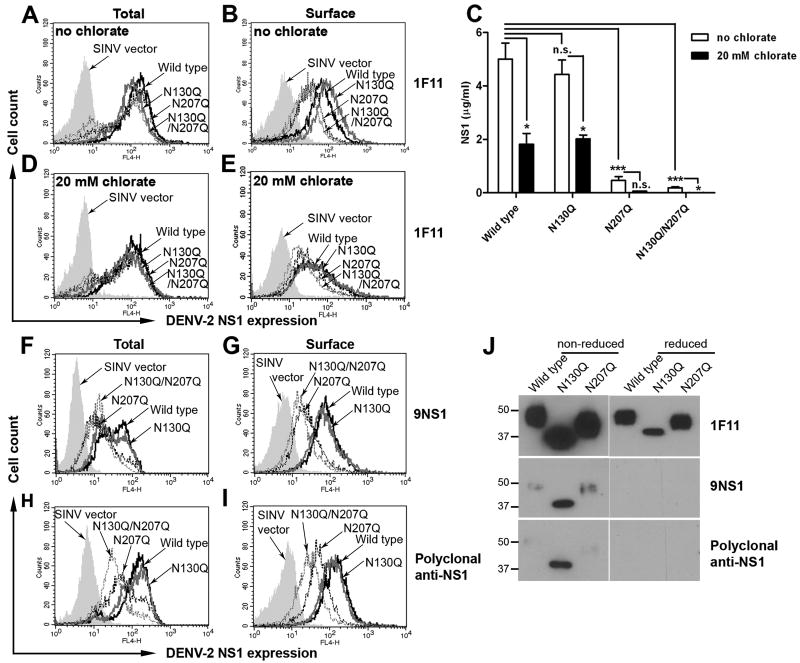
Next, we determined the level of DENV-2 NS1 expression in infected cells using the 1F11 anti-DENV-2 NS1 MAb, which recognizes a linear determinant on NS1 (Puttikhunt et al., 2003). Importantly, consistent with the data of SINV E2 expression (Fig. 1C and D), the total (intracellular + surface) levels of NS1 expression were comparable in cells infected with SINV expressing wild type and all glycosylation mutants of NS1 (Fig. 2A). However, N207Q and N130Q/N207Q showed reduced surface expression of NS1 (2.6-fold, P < 0.05), suggesting that the N-linked glycan at N207 is important for accumulation of DENV NS1 on the plasma membrane (Fig. 2B).
Figure 2. N-linked glycan (N207) is important for surface localization and secretion of DENV NS1.
(A-E) Decreased surface localization and secretion was observed in N207Q and N130Q/N207Q mutant NS1. Infected cells were harvested at 18 h post-infection and total (A) and surface (B) DENV NS1 expression was determined by immunofluorescence using 1F11 anti-DENV-2 NS1 MAb, which recognizes a linear epitope on NS1. Levels of secreted NS1 were quantified by ELISA of supernatants harvested at 18 h post-infection (C, open bars). To deplete cellular pools of sulfated GAGs, cells were treated with 20 mM sodium chlorate at 6 h after infection and the cells and supernatants were harvested 12 h later for analysis of total (D) and surface NS1 (E) by immunofluorescence using NS1-specific MAb 1F11 and secreted NS1 levels by ELISA (C, filled bars) respectively. (F-I) Conformation-dependent anti-NS1 antibodies did not efficiently recognize N207 mutants. Infected cells were harvested at 18 h post-infection and total (F and H) and surface (G and I) DENV NS1 expression was determined using the conformational-dependent cross-reactive 9NS1 MAb (F and G) and polyclonal anti-DENV-2 NS1 (H and I). J. Immunoreactivity of purified wild type and NS1 mutants. Equal amounts (100 ng) of wild type and mutant NS1 were boiled prior to 4-12% SDS-PAGE in the presence (reducing) or absence (non-reducing) of β-mercaptoethanol. Western blot analysis was performed subsequently with 1F11, 9NS1 or polyclonal anti-NS1. Error bars indicate standard error of the mean (SEM) from five independent experiments. Asterisks indicate statistical significant of NS1 secretion compared to wild type DENV2 NS1 (n.s., not significant; *, P < 0.05; ***, P < 0.0001). Histograms represent 3 to 5 independent experiments.
Flavivirus NS1 displayed on the surface of cells can arise from two sources: a dimeric form that directly associates with the plasma membrane (Winkler et al., 1989) through an unidentified mechanism that requires charged amino acids near the NH2-terminus of NS1 (Youn et al., 2010), or a secreted hexamer that binds back to sulfated GAGs on the cell surface (Avirutnan et al., 2007). To begin to address how mutation of the N-linked glycan at the N207 position altered surface levels, we assessed whether loss of N-linked glycans modulated secretion of NS1. Supernatants from BHK21 cells infected with wild type or glycosylation mutants of SINV-DENV-2-NS1 were harvested at 18 h after infection and analyzed with an NS1 capture ELISA. Notably, we observed no significant difference in the levels of NS1 secretion between wild type (5.00 μg/ml) and N130Q (4.43 μg/ml) NS1 (P > 0.5), whereas the levels of N207Q and N130Q/N207Q were 10-25-fold reduced (0.46 μg/ml and 0.19 μg/ml, respectively; P < 0.0001) (Fig. 2C). Thus, the N-linked glycan at position N207 is crucial for accumulation of DENV NS1 in the supernatant.
To assess whether the difference in surface expression levels of wild type and the N207Q mutant of NS1 was due to differential binding back to the plasma membrane of soluble secreted NS1, we treated BHK21 cells with 20 mM sodium chlorate at 6 h after SINV infection to deplete the surface GAGs (Baeuerle and Huttner, 1986). Treatment of the cells with sodium chlorate did not appreciably alter total (intracellular + surface) NS1 expression (Fig. 2D). In comparison, reduction of surface GAGs resulted in similar surface levels of NS1 regardless of whether cells were infected with SINV-DENV-2-NS1 wild type or mutant proteins (Fig. 2E); interestingly, with sodium chlorate treatment, the surface levels of wild type NS1 were equivalent to that seen with N207Q or N130Q/N207Q in untreated conditions (Fig. 2B). These findings were not explained by a marked reduction of NS1 in the supernatant as a consequence of sodium chlorate treatment, as only a two- to three-fold decrease in NS1 levels was detected in the supernatant of cells infected with SINV expressing wild type and N130Q NS1, and both wild type and N130Q still retained several-fold higher NS1 levels than the N207Q mutants (Fig. 2C). Collectively, these data suggest that the increased cell surface expression of wild type and N130Q NS1 is due to the higher levels of secreted NS1 that accumulate and bind back to the plasma membrane.
N-linked glycans have been shown to facilitate the interaction of a glycoprotein with cellular chaperones to obtain its final correct conformation and processing through the secretory pathway (Trombetta and Helenius, 1998). To determine whether the lower level of secretion of N207Q and N130Q/N207Q was due to protein misfolding that could impair transport through the cellular secretory pathway, we analyzed total and cell-surface NS1 in SINV-DENV-2-NS1 infected cells using conformation-dependent monoclonal (9NS1) (Fig. 2F and G) or polyclonal antibodies (Fig. 2H and I), and compared this to 1F11, which recognizes a linear determinant on NS1 (Fig. 2B). The reactivity of these antibodies for wild type and mutant NS1 was established after boiling in the presence or absence of β-mercaptoethanol, with subsequent SDS-PAGE and Western blot analysis (Fig. 2J); only 1F11 detected wild type and mutant NS1 under both non-reducing and reducing conditions, as 9NS1 and polyclonal antibodies failed to recognize any form of NS1 under reducing condition. Consistent with the data observed with 1F11 (Fig. 2A and B), N207Q and N130Q/N207Q displayed lower levels of surface NS1 expression compared to that of wild type and N130Q (Fig. 2G and I). However, in contrast to the results with 1F11, the total (intracellular + surface) levels of N207Q and N130Q/N207Q detected by both conformation-dependent NS1 specific Abs were decreased (Fig. 2F and H). These results suggest that the conformation of N207Q and N130Q/N207Q is different, and potentially misfolded, from that of wild type and N130Q NS1.
N-linked glycosylation does not affect NS1 binding to the cell surface
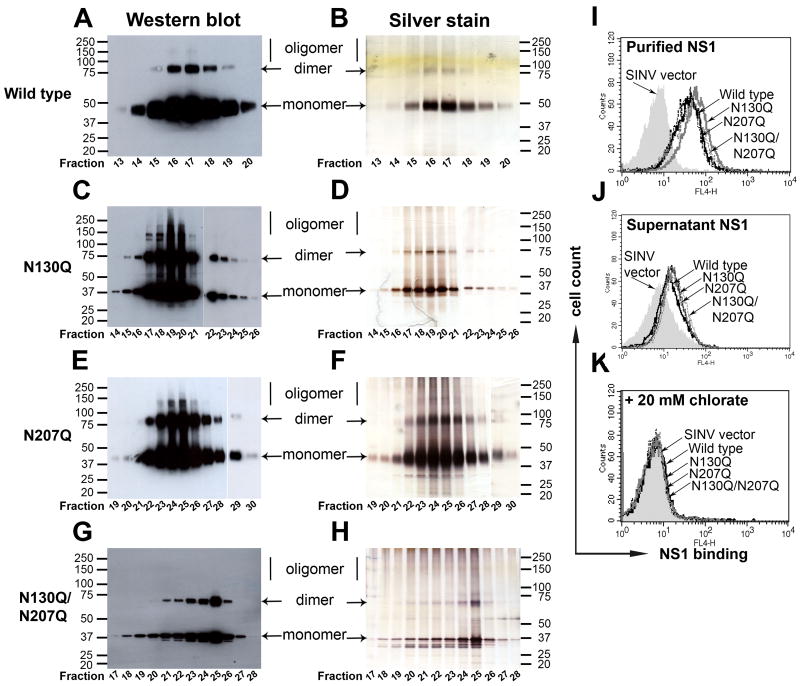
Soluble DENV-2 NS1 binds directly to different types of cells including epithelial and mesenchymal cells in a GAG-dependent manner (Avirutnan et al., 2007). As alteration of N-linked glycosylation on proteins can interfere with GAG interactions (Hershkovitz et al., 2008), we assessed the binding efficiency to the cell membrane of NS1 lacking N-linked glycans. We purified the NS1 variants from supernatants of SINV-DENV-2-NS1 infected BHK21 cells by immunoaffinity chromatography. We obtained relatively large amounts of purified wild type and N130Q NS1 from small starting volumes. However, because of the low yield for N207Q and N130Q/N207Q, larger volumes were required. Western blot of wild type NS1 demonstrated bands that corresponded to those obtained with silver staining (Fig. 3A and B), and were primarily in a monomeric form (as expected), after boiling and solubilization in SDS. N130Q (Fig. 3C and D), N207Q (Fig. 3E and F) and N130Q/N207Q (Fig. 3G and H) all migrated at lower molecular weights secondary to their loss of N-linked glycan(s). Consistent with previous findings under these solubilization conditions, NS1 also migrated as a dimer in all samples. This confirms that glycosylation did not affect NS1 dimerization (Pryor et al., 1998; Pryor and Wright, 1994), although we did observe degradation products in the N207Q NS1 variants (Fig. 3F and H). Notably, NS1 without N130 glycosylation (N130Q and N130Q/N207Q) appeared as a sharper band when compared to that of wild type or N207Q NS1, which still had intact complex oligosaccharides at N130 (Fig. 3D and H). Purified wild type and N-linked glycan mutant NS1, used at equivalent concentrations, demonstrated no differences in NS1 binding to the cell surface (Fig. 3I). Similar results were obtained if cells were incubated with unpurified bulk concentrated supernatants containing equivalent concentrations of wild type and mutant NS1 (Fig. 3J). Cell binding of wild type, N130Q, N207Q and N130Q/N207Q required surface GAGs as treatment with 20 mM sodium chlorate abolished binding completely (Fig. 3K). Overall, these data suggest that N-linked glycosylation of NS1 does not directly influence GAG interactions on the cell membrane.
Figure 3. N-linked glycosylation does not affect secreted DENV NS1 from binding back to the cell surface.
Wild type and mutant NS1 were purified by immunoaffinity chromatography. Serum-free supernatants from SINV-DENV-2-NS1 infected BHK21 cells at an MOI of 1.0 were collected at 18 to 20 h post-infection and purified over an anti-NS1 MAb 2G6 column. Samples of eluted fractions were boiled prior to 4-12% SDS-PAGE under non-reducing condition and analyzed by Western blot with 1F11 anti-DENV-2 NS1 MAb (wild type (A), N130Q (C), N207Q (E) and N130Q/N207Q (G)) and silver staining (wild type (B), N130Q (D), N207Q (F) and N130Q/N207Q (H)); the numbers below each gel correspond to the eluted fractions. (I-K) Binding of wild type and mutant NS1 to the cell surface depends on GAGs. Immunoaffinity purified NS1 (10 μg/ml) (I) or serum-free supernatants of SINV-DENV-2-NS1-infected cells containing 2 μg/ml of NS1 (J) were incubated with BHK21 cells and NS1 binding was detected by 2G6 anti-DENV-2 NS1 MAb. Binding was also performed after BHK21 cells were pre-treated with 20 mM sodium chlorate overnight and incubated with purified NS1 (K). Histograms are representative of 3 independent experiments.
Stability of secreted DENV NS1 depends on N-linked glycans
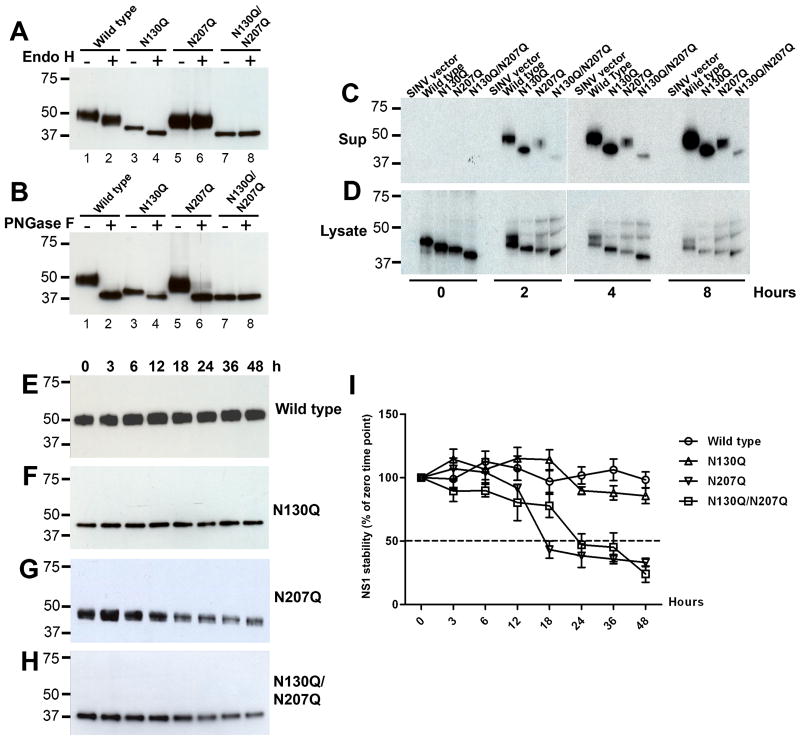
Addition of N-linked glycans to viral proteins can influence folding, stability and solubility (Doms et al., 1993; Malvoisin and Wild, 1994; Ng, Hiebert, and Lamb, 1990; Rademacher, Parekh, and Dwek, 1988). Because less (10 to 25-fold, respectively) N207Q and N130Q/N207Q NS1 are present in the supernatant of SINV-DENV-2-NS1 infected cells compared to the wild type or N130Q mutant (Fig. 2C), we tested whether the N-linked glycosylation at N207 affected NS1 stability. To begin, we first confirmed that the appropriate type of N-linked glycan was present on NS1 in the context of the SINV expression system. Supernatants from SINV-DENV-2-NS1 infected cells containing N207Q and N130Q/N207Q NS1 were adjusted to the same concentration (2.0 μg/ml) as wild type or N130Q NS1. All NS1 were digested with endoglycosidase H (Endo H), which removes high-mannose-content sugars (Tarentino and Maley, 1974), or peptide N-glycosidase F (PNGase F), which cleaves high mannose, hybrid, and complex oligosaccharides from N-linked glycoproteins (Maley et al., 1989; Tarentino, Gomez, and Plummer, 1985). Predicted molecular sizes of undigested wild type, N130Q, N207Q and N130Q/N207Q NS1 are 48, 39, 43, and 37 kDa, respectively (Fig. 4A and B, lanes 1, 3, 5 and 7). After treatment with Endo H, only the wild type and N130Q NS1, but not N207Q nor N130Q/N207Q NS1, were digested to 45 and 37 kDa, respectively (Fig. 4A, lanes 2 and 4). In comparison, all NS1 except N130Q/N207Q showed a shift in migration to 37 kDa after treatment with PNGase F (Fig. 4B, lanes 2, 4, 6 and 8). These results confirm that NS1 synthesized in cells using the SINV expression system retains N-linked glycans similar to that observed in DENV infection (Crabtree, Kinney, and Miller, 2005; Pletnev, Bray, and Lai, 1993): a complex-type sugar at N130 and a high mannose sugar at N207.
Figure 4. N-linked glycan at N207 influences the rate of accumulation and stability of secreted DENV NS1.
Secreted NS1 from SINV-DENV2-NS1 infected BHK cells (2 μg/ml) was treated with Endo H (A) or PNGase F (B) for 3 h at 37°C. Western blot analysis of enzyme digested NS1 was assessed by 4-12% SDS-PAGE under reducing condition using 1F11 anti-DENV2-NS1 MAb. (C and D) BHK21 cells infected at an MOI of one were harvested at 14 h and then were radiolabeled with [35S] and chased at different time points. Supernatants (C) and lysates (D) were collected, pre-cleared with isotype control Sepharose, and immunoprecipitated with 1F11 anti-DENV-2 NS1 MAb and protein A-Sepharose. (E-H) Western blot analysis of secreted NS1. Supernatants containing 2 μg/ml of wild type (E), N130Q (F), N207Q (G), and N130Q/N207Q (H) NS1 were incubated at 37°C prior to analysis by 12% SDS-PAGE under reducing conditions followed by western blotting with 1F11 anti-DENV2 NS1 MAb. Results are representative of two or three independent experiments. I. Using quantitative densitometry, the intensity of wild type, N130Q, N207Q, and N130Q/N207Q NS1 at each time point was compared to level of NS1 (100%) at the starting time (0 h). Error bars indicate standard error of the mean corresponding to three independent experiments.
Given that there was no detectable change in carbohydrate modifications, we assessed the role of individual N-linked glycans on the stability of secreted NS1. Pulse-chase labeling followed by immunoprecipitation was performed, and wild type and mutant NS1 were analyzed in the cell-associated and extracellular pools. At the start time of the chase (0 h), the intracellular expression levels of wild type, N130Q, N207Q, and N130Q/N207Q were comparable (Fig. 4D). Over time, as expected, the signal in the cell lysates declined as NS1 was secreted into the supernatant to varying degrees (Fig. 4C). Intracellular NS1 showed a minimal difference in gel migration during the chase period for each mutant, reflecting the addition of high mannose glycans for each available N-linked glycosylation site. In comparison, NS1 in the supernatant showed a pattern consistent with conversion of the N-linked glycan at N130 to a larger complex-type oligosaccharide (Winkler et al., 1988). Consistent with the ELISA results (see Fig. 2C), labeled forms of wild type and N130Q NS1 accumulated to higher levels than N207Q or N130Q/N207Q. As the NS1 levels in the lysates of cells expressing N207Q and N130Q/N207Q were relatively similar compared to wild type and N130Q, the lower amounts of N207Q mutants in the supernatant were not caused by the instability of nascently generated intracellular NS1. To assess the effect of N-linked glycans on the stability of secreted NS1, serum-free supernatants of SINV-DENV-2-NS1 infected cells were harvested at 18 h post-infection, concentrated, adjusted to equal NS1 concentration (2 μg/ml), and incubated at 37°C. At indicated time points, equal amounts of each NS1 were subjected to SDS-PAGE under reducing conditions, and immunoblotted with the 1F11 NS1-specific MAb. Remarkably, little change in the levels of soluble wild type or N130Q NS1 was observed during a 48 h incubation period (Fig. 4E and F). In contrast, N207Q and N130Q/N207Q showed time-dependent degradation after 12 and 18 h, respectively (Fig. 4G and H). Using quantitative densitometry, we calculated the half-life of the wild type and variant NS1 in solution: whereas wild type or N130Q was quite stable (t ½ > 48 h), N207Q and N130Q/N207Q showed more rapid degradation (t ½ of 17.2 and 23.4 h respectively) (Fig. 4I). Overall, these data suggest that the high mannose N-linked glycan at N207 affects both the rate of accumulation and the stability of secreted DENV NS1.
N-linked glycosylation influences stability of hexameric NS1
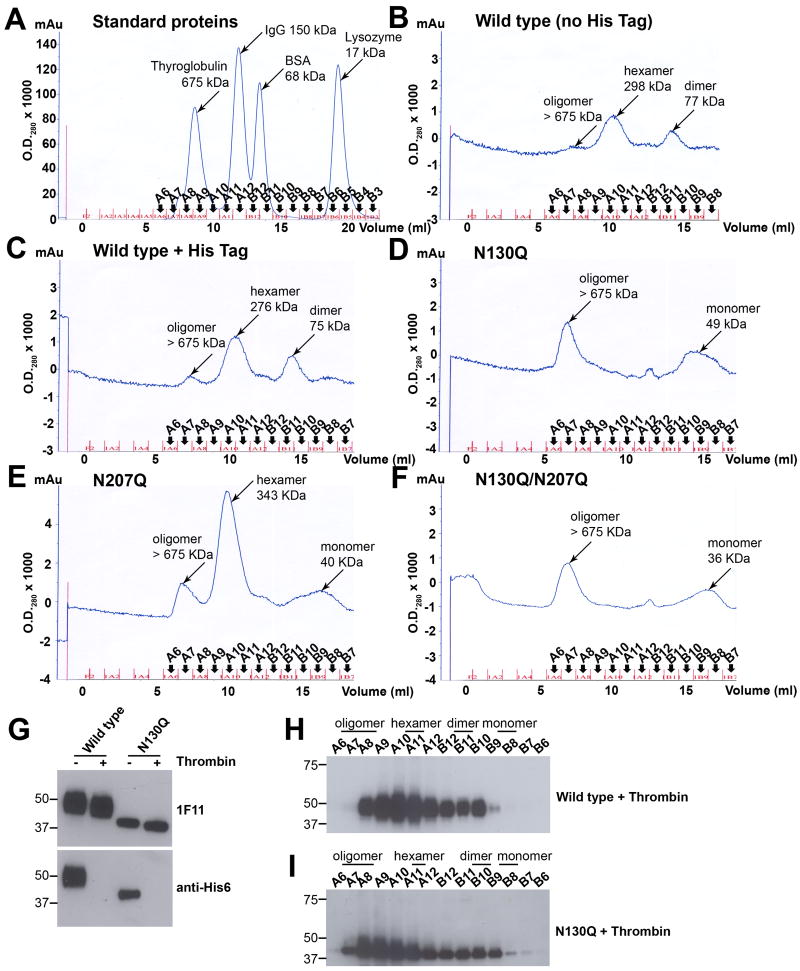
Given that N-linked glycans affected the stability of NS1 in solution, we evaluated their impact on oligomer formation. DENV and WNV NS1 can be isolated as dimers, hexamers, and even larger-order oligomers in solution after chromatography (Avirutnan et al., 2010; Flamand et al., 1999). To evaluate the effect of N-linked glycans on oligomer formation, the peak elution fractions from an antibody (2G6) immunoaffinity column of NS1 variants were subjected to size exclusion chromatography on a Superdex 200 column with standards ranging from 17 to 675 kDa (Fig. 5A). Wild type NS1, with (Fig. 5C) or without (Fig. 5B) a carboxy terminal hexa-histidine tag, that was isolated by immunoaffinity chromatography after alkaline (pH 11.5) elution according to standard NS1 purification protocols (Alcon et al., 2002; Avirutnan et al., 2006; Flamand et al., 1999; Youn et al., 2010), showed similar size exclusion profiles consisting of dimer (∼77 kDa), hexamer (∼285 kDa) and a higher-order oligomer with a predicted size of larger than 675 kDa. In contrast, the major population of N130Q was the higher-order oligomer. Although a ∼49 kDa monomer population was present, the hexameric species was noticeably absent (Fig. 5D). For the N207Q variant, a slightly larger, ∼343 kDa “hexameric” peak (fraction A10) compared to wild type NS1 (fraction A10-A11; see Fig. 5C) was detected along with lesser amounts of monomer and higher-order oligomer (Fig. 5E). The size exclusion profile of N130Q/N207Q NS1 followed the pattern of N130Q, with NS1 species found primarily as monomer and higher-order oligomer (Fig. 5F).
Figure 5. The N-linked glycan at position N130 is required for stabilization of the secreted hexamer.
(A-F) Size exclusion chromatography of immunoaffinity purified DENV-NS1. The peak fractions of NS1 were pooled and adjusted to 30 μg/ml. Size exclusion profiles of affinity-purified NS1 was determined by injecting 1 ml of sample onto a Superdex 200 column. Samples included standard proteins (A), wild type NS1 without hexa-histidine tag (B), wild type NS1 with hexa-histidine tag (C), N130Q (D), N207Q (E) and N130Q/N207Q (F). (G-I) Size exclusion chromatography of NS1 lacking hexa-histidine tag in bulk supernatants. Serum-free supernatants harvested from SINV-DENV-2-wild type or N130Q NS1-infected BHK cells at 18 h post-infection were treated with 0.05 unit of thrombin for 16 h at room temperature and concentrated 50-fold. A portion of thrombin-treated NS1 was subjected to 4-10% SDS-PAGE under reducing condition, followed by Western blotting with 1F11 anti-NS1 MAb or anti-His6 antibody (G). Thrombin-treated wild type or N130Q supernatants (1 ml) were injected onto a Superdex 200 column. Samples of eluted fractions were boiled prior to 4-12% SDS-PAGE under reducing condition and analyzed by Western blot with 1F11 anti-DENV-2 NS1 (Wild type (H), N130Q (I)). Results are representative of three to four independent experiments.
Elution at high pH (11.5) from the immunoaffinity column could differentially affect protein stability and oligomer formation of the variant NS1. To confirm that a change in oligomer formation was not an artifact of the alkaline elution, we independently purified hexa-histidine tagged versions of wild type and N130Q NS1 by nickel-affinity column under neutral pH (7.3) elution conditions. Based on immunoblot analysis of SDS detergent-treated samples, two species of wild type (Supplementary Fig. 1A) and N130Q (Supplementary Fig. 1C) NS1 were detected by electrophoretic analysis, a monomer and dimer, although the ratios were distinct with less of the monomeric form of the mutant NS1. The peak fractions (A2) from the nickel-affinity column of wild type and N130Q NS1 were also subjected to size exclusion chromatography. Similar to the results seen after immunoaffinity chromatography with high pH elution (see Fig. 5C and D), wild type NS1 eluted predominantly as a hexamer (Supplementary Fig. 1B) whereas N130Q NS1 eluted largely as a higher-order oligomer (Supplementary Fig. 1D).
To validate these findings, we analyzed the oligomeric state of NS1 directly in the supernatants without affinity purification. To ensure that the carboxy terminal hexa-histidine tag did not influence the oligomeric stage of NS1, serum-free supernatants from SINV-DENV-2-wild type or N130Q NS1 infected cells were treated with 0.05 unit of thrombin prior to size exclusion chromatography. Cleavage of the hexa-histidine tag from the carboxy terminus of NS1 was confirmed by Western blotting with 1F11 or anti-His6 antibody (Fig. 5G). Thrombin-treated samples showed a slight reduction in molecular weight compared to untreated samples as detected by anti-NS1 MAb 1F11. Importantly, only untreated samples were reactive with anti-His6 antibody. These results indicated that thrombin efficiently removed the hexa-histidine tag from wild type and N130Q NS1. Subsequently, the thrombin treated supernatants were injected onto a Superdex 200 size exclusion column, followed by reducing SDS-PAGE and Western blot with 1F11 anti-NS1 MAb. Supernatants containing both wild type NS1 and N130Q NS1 showed mixed populations including dimer, hexamer, and higher-order oligomers (Fig. 5H and I). Analogous to the sizing profile of affinity-purified NS1, the major population of wild type NS1 in supernatants was hexamer (fractions A10 and A11). In contrast to the size exclusion profiles of affinity-purified NS1 in which the hexamer peak was absent (Fig. 5D and Supplementary Fig. 1D), bulk supernatant containing N130Q also contained hexameric NS1 (Fig. 5I, fraction A11). However, the primary elution peaks of N130Q (fractions A8 and A9) corresponded to forms of NS1 that were larger than hexamer (Fig. 5I). Taken together, these data suggest that N130 influences the oligomeric assembly of secreted NS1 by affecting the efficiency of the generation and/or stabilization of the secreted hexamer.
Loss of N-linked glycan at N130 affects NS1 binding to human complement components
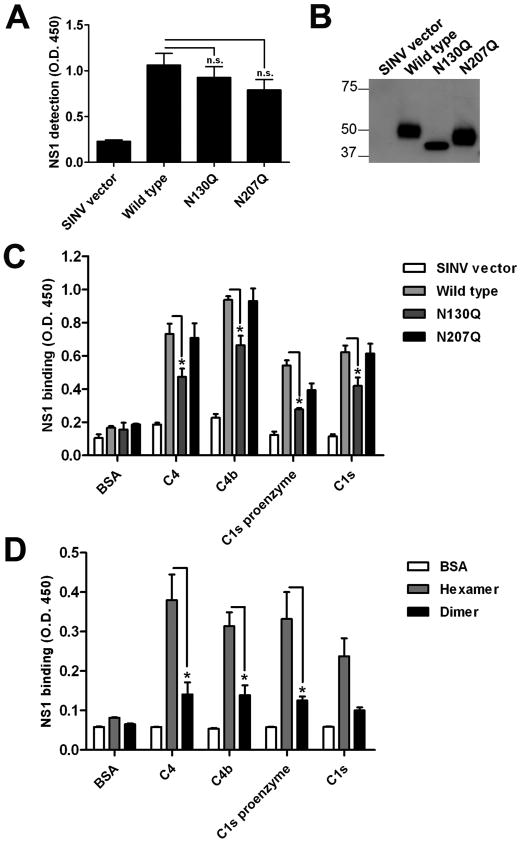
A previous study showed that DENV NS1 binds C4 and C1s to antagonize complement activation (Avirutnan et al., 2010). We therefore investigated the impact of N-linked glycans on these interactions. Complement components were coated on an ELISA plate, and serum-free supernatants from BHK21 cells infected with SINV-DENV-2-NS1 containing wild type and variant NS1 (N130Q or N207Q) at a concentration of 5 μg/ml were added to each well. Bound NS1 was detected by anti-His6 antibody. Importantly, equivalent amounts of wild type and mutant NS1 were applied as judged by ELISA or immunoblot (Fig. 6A and B). While both wild type and N207Q NS1 interacted efficiently with human C4, C4b, C1s proenzyme, and C1s, N130Q NS1 showed reduced binding (Fig. 6C) (P < 0.05), suggesting that the complex-type glycan at N130 is required for efficient NS1 interaction with complement. Because N130 modulates secreted NS1 hexamer generation and/or stability, it is also possible that the low level of NS1 hexamer in N130Q supernatant is responsible for the reduction in complement interaction. To evaluate whether the oligomeric state of DENV NS1 influences the binding to complement components, immunoaffinity purified wild type NS1 was subjected to size exclusion chromatography to separate hexamer and dimer (see Fig. 5C). At equimolar amounts, hexameric NS1 showed enhanced binding to complement proteins compared to dimeric NS1 (Fig. 6D). These data suggest that the altered oligomeric state and decreased amount of hexamer associated with secretion of N130Q NS1 explains, in part the reduced binding to complement proteins.
Figure 6. Loss of N-linked glycan at position N130 affects NS1 binding to human complement components.
Equal amounts (5 μg/ml) of NS1 in culture supernatants were used in complement binding ELISA. Concentration was determined by: (A) direct coating of NS1 to microtiter plates and detection with anti-His6 tag MAb, and (B) Western blot with 1F11 anti-DENV-2 NS1. Binding of wild type and mutant NS1 to human complement components was assessed by ELISA (C). Microtiter plates were coated with BSA, complement proteins C4, C4b, C1s proenzyme or C1s (15 μg/ml). Supernatants with equal concentrations (5 μg/ml) of wild type or mutant NS1 from SINV-DENV-2-NS1-infected cells were added and bound NS1 was detected. (D) Hexameric NS1 interacts most efficiently with complement components. Immunoaffinity purified wild type NS1 hexamer and dimer isolated from a Superdex 200 column at equimolar concentration (40 nM) were added to BSA or complement components and bound NS1 was detected with anti-His6 tag MAb. Error bars indicate standard error of the mean corresponding to 3 or 4 independent experiments. Asterisks indicate binding that is statistically different compared to (C) wild type DENV2 NS1 or (D) hexameric NS1 (n.s., not significant; *, P < 0.05).
Discussion
Although the significance of N-linked glycans on Flavivirus NS1 has been evaluated by several groups over the past two decades, an impediment to its study has been the reduced growth of infectious viruses containing mutations that abolish carbohydrate addition (Crabtree, Kinney, and Miller, 2005; Muylaert et al., 1996; Pryor et al., 1998; Tajima, Takasaki, and Kurane, 2008). To circumvent this, we expressed wild type and N-linked glycan mutated forms of NS1 as a transgene using a double subgenomic SINV. Rather than generate N→A mutations that abolish the N-linked glycan site (Crabtree, Kinney, and Miller, 2005; Muylaert et al., 1996; Pryor et al., 1998; Pryor and Wright, 1994; Tajima, Takasaki, and Kurane, 2008), we engineered, like several other groups, the more minimal N→Q to retain polarity and size of the amino acid side chain (Ito et al., 2007a; Ito et al., 2007b; Kayser et al., 2010; Zhou and Tsai, 2009). Using the double subgenomic expression system, we showed as expected, (a) wild type or mutant NS1 had no differential effect on SINV replication and (b) the total intracellular levels of wild type and mutant NS1 were comparably produced. With this as a starting point, we defined how a loss of individual N-linked glycans modulated surface expression, secretion, stability, oligomer formation, and binding phenotypes.
Our experiments suggest that a loss of N-linked glycans at N207 but not N130 of DENV NS1 resulted in reduced secretion. Although analysis of N-linked glycans on secretion of Flavivirus NS1 has been studied previously, the results have varied (Flamand et al., 1999; Muylaert et al., 1996; Pryor and Wright, 1994). Consistent with our data, transient transfection of DENV NS1 encoded by an expression plasmid in COS-7 cells also showed that mutation of N207 diminished secretion (Pryor and Wright, 1994). In contrast, NS1 secretion from SW13 human adrenal carcinoma cells was impaired in YFV lacking N-linked glycans at N130 (Muylaert et al., 1996). Moreover, treatment of DENV-1 infected Vero cells with N-linked glycosylation inhibitors (i.e. swainsonine and deoxymannojirimicin) that blocked conversion to complex-type sugars at N130 also decreased NS1 secretion (Flamand et al., 1999). The apparent discrepancy in the latter two studies with infectious virus may be explained by the confounding effects on the replication: mutation of YFV N130 and treatment of DENV-infected cells with N-linked glycosylation inhibitors impaired viral infectivity, which indirectly affects the accumulation of NS1 in the supernatant. Additionally, for YFV, both first and second glycosylation sites, in contrast to DENV, are processed to complex-type sugars (Despres, Girard, and Bouloy, 1991; Post, Carvalho, and Galler, 1991).
Based on its reduced accumulation in the supernatant, we hypothesized that the N-linked glycan at residue N207 might regulate NS1 transport through the secretory pathway or affect protein stability. Indeed, a previous study suggested that a loss of the N-linked glycan at N207 decreased intracellular levels of DENV-2 NS1 because of its apparent effects on dimer stability (Pryor and Wright, 1994). However, our pulse-chase experiments showed remarkably similar levels of intracellular NS1. Consistent with this, flow cytometric analysis of SINV-DENV-2 NS1 infected cells also showed equivalent total levels of wild type and variant NS1. These results are more concurrent with a study in which mutation of N207 in the context of DENV-2 infection did not affect the intracellular levels of dimeric NS1, as judged by gel electrophoretic analysis (Pryor et al., 1998). In comparison, pulse-chase analysis of supernatants at early time points revealed that NS1 containing mutations at N207 had a defect in secretion, which could not be accounted for by the change in the stability. This could be due to problems in folding of N207Q NS1, since N-linked glycans facilitate interactions with the molecular chaperones (e.g., calnexin or calreticulin) in the endoplasmic reticulum (Trombetta and Helenius, 1998). In support of this hypothesis, we observed conformation-dependent binding of anti-NS1 MAbs (9NS1) and polyclonal antibodies to intracellular or cell surface NS1 in variants containing N207Q. Thus, the N-linked glycan at N207 likely affects secretion and extracellular NS1 accumulation, in part because of its impact on folding.
NS1 on the cell surface in Flavivirus infection can arise from two independent sources: (a) soluble NS1 binds back to the plasma membrane through interactions with sulfated GAGs (Avirutnan et al., 2007); and (b) NS1 is directly transported to and expressed on the plasma membrane, although it lacks a transmembrane domain or canonical targeting motif (Winkler et al., 1989; Winkler et al., 1988). Our experiments suggested that the N-linked glycan at position N207 but not N130 was required for efficient cell surface attachment of NS1. To distinguish between the “binding back” and “direct transport” mechanisms, we assessed surface levels of wild type and N-linked variants of NS1 in cells treated with sodium chlorate, which inhibits GAG production and NS1 binding back to cell surface (Avirutnan et al., 2007). When GAGs were depleted from the cell surface, the difference in surface levels of NS1 between the wild type, N130Q, N207Q, and N130Q/N207Q disappeared, suggesting that differential N-linked glycosylation did not affect direct transport of NS1 to the cell surface. Consistent with this, a mutant WNV NS1 lacking all three conserved N-linked glycosylation sites did not affect plasma membrane expression (Youn et al., 2010). Taken together, our experiments suggest that the higher cell surface expression levels of wild type and N130Q compared to that of N207Q and N130Q/N207Q NS1 is due to the greater secretion, extracellular accumulation, and binding back to the plasma membrane.
A previous study suggested that the conformation of DENV NS1 might change during the transit through the Golgi system such that complex-type N-linked glycans induced or stabilized a mature form of NS1 hexamer (Flamand et al., 1999). This hypothesis is consistent with our results showing the lack of complex-type oligosaccharides at N130 resulted in a loss of hexameric NS1 and an increase in the higher-order oligomers after purification. This effect, however, was not just associated with highly purified forms of NS1, as size exclusion chromatography of bulk supernatant from SINV-DENV-2 N130Q NS1 infected cells also showed a relative decrease in the hexamer and increase in higher-order oligomer. The phenotype though was less apparent, possibly because secreted cellular proteins may stabilize hexamer formation even for NS1 lacking the N130 N-linked glycan.
Interaction of Flavivirus NS1 with the complement components C4, C1s, and factor H protects virus and infected cells from immune recognition and neutralization (Avirutnan et al., 2010; Chung et al., 2006a). NS1 proteins lacking the N-linked glycan at N130 showed reduced binding to C1s and C4 compared to wild type and N207Q NS1. This could be due to the skewing of oligomeric forms of N130Q as hexameric NS1 bound more efficiently to complement components compared to other NS1 species. Alternatively, N-linked glycan at position N130 may directly participate in the interaction between NS1 and complement components. Interestingly, despite its defect in relative secretion, the N207Q mutant still efficiently bound complement components. We speculate that the DENV-2 variants having an N207Q mutation, which show no apparent growth defect in cell culture but are attenuated in mice (Pryor et al., 1998), are less virulent in part, because the low levels of secreted NS1 generated are not sufficient to protect the virus from complement attack. In summary, the two N-linked glycans on DENV-2 NS1 differentially regulate surface expression, secretion, stability, and complement binding. Our study provides new insight as to how N-linked glycosylation of NS1 is linked to its biological properties.
Materials and Methods
Cell culture
BHK21 (clone 15) cells were grown in Dulbecco's modified Eagle's medium (DMEM, Sigma) supplemented with 10% fetal bovine serum (FBS, Omega Scientific), 100 IU/ml penicillin, 100 μg/ml streptomycin, 10 mM HEPES, pH 7.3, and 10 mM nonessential amino acids (Cellgro) in a 5% humidified CO2 incubator at 37°C.
Generation and purification of wild type and mutant DENV NS1
Recombinant wild-type or N130Q, N207Q, and N130Q/N207Q mutants of DENV-2 NS1 were expressed as a transgene using a double subgenomic Sindbis virus (SINV) expression system with the SINV 39 E2H55K70 infectious cDNA clone (39HK; gift of W. Klimstra, Pittsburgh, PA) (Ryman et al., 2007). Briefly, the DENV-2 NS1 gene of strain 16681, including a 24-amino acid leader sequence from the carboxy terminus of the E gene, was amplified from infected Vero cells by RT-PCR using the following oligonucleotide primers (NS1 sequence underlined): forward, 5′ GCGGCCGCACCATGGTCTCACTGTCTGTGACACTAG 3′; reverse, 5′ TCAAGCTGTGACCAAGGAGTTGAC 3′. The product was subcloned into the 39HK backbone after digestion with NotI and PmeI restriction enzymes. SINV 39HK-DENV-2 NS1 was also introduced with a hexa-histidine tag at the carboxy-terminus by PCR using the following oligonucleotide primers: forward, 5′ GCGGCCGCACCATGGTCTCACTGTCTGTGACACTAG 3′; reverse, 5′ TCAATGATGATGATGATGATGTGATCCACGAGGAACTAGAGCTGTGACCAAGGAGTTGAC 3′. Site-specific mutations were introduced into the wild type DENV-2 NS1 after subcloning into the AT vector (Novagen) using QuickChange II Site-Directed Mutagenesis Kit (Stratagene). The mutations included N130→Q, N207→Q, and N130→Q/N207→Q double mutant using the following oligonucleotide primers: N130Q forward 5′ GCTCTCTACAGAGTCTCATCAACAGACCTTTCTCATTGATGG 3′; N130Q reverse 5′ CCATCAATGAGAAAGGTCTGTTGATGAGACTCTGTAGAGAGC 3′; N207Q forward 5′ GGATAGAAAGTGCACTCCAAGACACATGGAAGATAGAGAAAGC 3′; N207Q reverse 5′ GCTTTCTCTATCTTCCATGTGTCTTGGAGTGCACTTTCTATCC 3′. After NotI restriction enzyme digestion, wide type and mutant NS1 were cloned into 39HK double subgenomic SINV cDNA. The cloning and mutagenesis were verified by sequencing on both strands. Plasmids were subsequently linearized with XhoI and transcribed in vitro using SP6 DNA-dependent RNA polymerase (mMESSAGE mMACHINE kit, Ambion, Austin, TX). RNA was electroporated into BHK21 cells with 3 consecutive electrical pulses of 850 V at 25 μF and ∞ Ω using Gene Pulser Xcell electroporator (BioRad). Recombinant SINV were collected at 24 h post-infection and titrated by plaque assay on BHK21 cells.
To generate purified NS1, SINV encoding wild type, N130Q, N207Q or N130Q/N207Q NS1 were used to infect BHK21 cells at a multiplicity of infection (MOI) of one in serum-free DMEM. Supernatant was harvested at 24 h after infection and infectious SINV was inactivated after addition of 0.05% NP-40. Wild type and mutant NS1 were purified by 2G6 anti-DENV-2 NS1 MAb affinity chromatography (Avirutnan et al., 2006) after elution with diethylamine (Sigma) pH 11.5. In some experiments, NS1 containing a carboxy-terminal hexa-histidine tag was purified by nickel affinity chromatography (GE Healthcare) using an imidazole (EMD Chemicals) elution gradient at pH 7.3. The peak fractions containing NS1 were subjected to Superdex 200 size exclusion chromatography (GE Healthcare) as described previously (Avirutnan et al., 2010).
Analysis of surface and total SINV E2 and DENV-2 NS1 expression
BHK21 cells were infected with SINV-DENV-2 NS1 at an MOI of one and harvested at 18 h post-infection. For total antigen detection, infected cells were fixed with 4% paraformaldehyde, permeabilized with 0.1% (w/v) saponin (Sigma), and incubated with either 1:400 mouse hyper-immune serum against SINV E2 (ATCC) or 1F11 (recognizing a linear epitope) anti-DENV2 NS1 MAb (Puttikhunt et al., 2003) followed by 4 μg/ml Alexa Fluor-647 goat anti-mouse IgG (Invitrogen). For analysis of surface antigens, unfixed cells were stained with antibodies as described above and propidium iodide (0.5 μg/ml, Molecular Probes) was added to exclude dead cells. Alternatively, infected cells were incubated with conformation-dependent 9NS1 MAb (Chung et al., 2006b) or polyclonal anti-DENV-2 NS1. In some experiments, cells were infected with SINV-DENV-2 NS1 viruses for 6 h, washed 5 times with PBS, and SO42- free Joklik Modification Minimum Essential Medium Eagle (Sigma) supplemented with 20 mM sodium chlorate (Sigma) was added to prevent generation of sulfated GAGs (Baeuerle and Huttner, 1986). Levels of surface and total NS1 were assessed by flow cytometry (FACSCalibur, BD Biosciences). Data were processed with CellQuest Pro software (Becton Dickinson).
DENV-2 NS1 ELISA
Maxi-Sorp microtiter plates (Nunc) were coated with 5 μg/ml of 2E11 anti-DENV2 NS1 MAb (Puttikhunt et al., 2003) at 4°C overnight. Non-specific binding sites were blocked with 2% bovine serum albumin (BSA, Sigma) in PBS for 2 h at room temperature. After five washes with 0.05% Tween-20 in PBS (PBST), 50 μl of cell supernatants from SINV-DENV-2- NS1 infected cells or purified NS1 (diluted in PBS containing 0.1% BSA) was added to each well and incubated for 1 h at room temperature. The plates were washed five times with PBST followed by sequential 1 h incubations at room temperature with 50 μl of 2 μg/ml mouse anti-His6 antibody (Quidel), 1μg/ml biotinylated goat anti-mouse IgG (Sigma) and 1μg/ml HRP-conjugated streptavidin (Vector Laboratories). After six final PBS washes, signal was developed by adding 50 μl of TMB substrate (DAKO) and 25 μl of 2N H2SO4 stop solution to each well. The optical density (O.D.) at 450 nm was determined by a 96-well plate reader (Genio Pro; Tecan Instruments).
NS1 binding to BHK21 cells
BHK21 cells were incubated with supernatants containing 2 μg/ml NS1 as determined by NS1 capture ELISA. After a 1 h incubation on ice, the cells were incubated with 2G6 anti-DENV2 NS1 MAb (Puttikhunt et al., 2003) followed by 4 μg/ml Alexa Fluor-647 goat anti-mouse IgG and 0.5 μg/ml propidium iodide to exclude dead cells. Levels of NS1 on the cell surface were determined by flow cytometry. In some experiments, purified wild type or mutant NS1 (10 μg/ml) were used instead of supernatants. To reduce cell-associated GAGs (Baeuerle and Huttner, 1986), BHK21 cells were washed 5 times with PBS and incubated overnight in SO42- free DMEM supplemented with 20 mM sodium chlorate prior to NS1 binding.
SDS-PAGE and Western blot analysis
Purified NS1 or supernatant from SINV-DENV-2-NS1 virus-infected BHK21 cells was collected and boiled at 98°C for 10 min prior to 4-12% SDS-PAGE (NuPAGE, Invitrogen) under non-reducing or reducing condition. Western blots were performed using 1F11 anti-DENV-2 NS1 MAb followed by incubation with 1:5000 HRP-conjugated goat anti-mouse IgG (GE Healthcare). Development was with West Pico Enhanced Chemiluminescence (Thermo Scientific). Silver staining was performed using a ProteoSilver™Silver Stain Kit (Sigma).
DENV-2 NS1 deglycosylation
Supernatants from SINV-DENV-2-NS1 infected BHK21 cells were digested for 3 h with endoglycosidase H (EndoH) or peptide N glycosidase F (PNGaseF) (NEB) according to manufacturer's protocols. Reactants were then subjected to 4-12% SDS-PAGE under reducing condition followed by western blot with 1F11 anti-DENV-2 NS1 MAb.
Pulse-chase experiments
Pulse-chase experiments were performed after infecting BHK21 monolayers (6 × 105 cells/well in a 6-well plate) with SINV-DENV-2-NS1 viruses at an MOI of one for 12 h. Cells were starved by replacing culture media with methionine- and cysteine-free DMEM (Invitrogen) for 15 minutes, incubated with 50 μCi [35S]cysteine-methionine for 20 min, washed with PBS, and cultured for indicated times with complete DMEM supplemented with 10% FBS. Supernatants and cell lysates were collected at 0, 2, 4, and 8 h after labeling, pre-cleared with anti-Flavivirus E (4G2) isotype control MAb-Sepharose and immunoprecipitated at 4°C overnight with anti-DENV NS1 specific (1F11) MAb and protein G-Sepharose (Invitrogen). Immunoprecipitates were washed three times with RIPA buffer (10 mM Tris pH 7.4, 0.1% SDS, 1% sodium deoxycholate, 1% NP-40, 0.15 M NaCl) and bound proteins were eluted in SDS reducing sample buffer, heated at 98°C for 3 min, and subjected to 12% SDS-PAGE under reducing condition. Gels were dried, soaked for 30 min in Amplify (GE Healthcare), and exposed to an intensifying X-ray film (Kodak).
Thrombin cleavage of hexa-histidine tag
Supernatants from SINV-DENV2-NS1 infected BHK21 cells were collected at 18 h post-infection and treated with 0.05 unit of thrombin (Enzyme Research) for 16 h at room temperature. Cleavage of the hexa-histidine tag was confirmed by Western blot analysis with anti-NS1 MAb 1F11 and anti-His6 antibodies.
Complement protein ELISA
Maxi-Sorp microtiter plates were incubated with 15 μg/ml purified C4, C4b, C1s proenzyme or C1s (all from Complement Technologies) at 4°C overnight. Plates were washed 5 times with PBS and non-specific binding sites were blocked with 2% heat inactivated BSA for 2 h at 37°C. After 5 washes with PBS, SINV-DENV-2-NS1 infected BHK supernatants (containing 5 μg/ml of NS1 as determined by capture ELISA) were added to the wells and incubated for 2 h at room temperature. Plates were washed 5 times with PBST followed by sequential 1 h incubations at room temperature with 2 μg/ml anti-His6 MAb, 1 μg/ml biotinylated goat anti-mouse IgG, and 2 μg/ml HRP-conjugated strepavidin. Plates were next washed 6 times with PBS, and then the O.D. at 450 nm was evaluated as described above. In some experiments, wild type NS1 fractions were injected to a Superdex 200 column to separate the NS1 hexamer and dimer populations. Binding of these NS1 species to complement proteins was performed by ELISA as outlined above.
Statistical analysis
Datasets were analyzed by a two-tailed, unpaired t test. Statistical significance was achieved when P values were < 0.05. Results from the plaque formation assay, quantitative NS1 ELISA and complement-NS1 binding ELISA were analyzed with Prism software (GraphPad Software, Inc.). Simple linear regression analysis of the ELISA data was analyzed using StatView software (SAS Institute, Inc.).
Supplementary Material
Serum-free supernatants from SINV-DENV-2-NS1-infected BHK21 cells at an MOI of one were collected at 18 to 20 h post-infection and purified over a nickel affinity column. Samples of eluted fractions were boiled prior to 4-12% SDS-PAGE under non-reducing conditions and analyzed by Western blot using 1F11 anti-DENV-2 NS1 (wild type (A), N130Q (C)). The peak fraction (A2) containing equal amount of nickel affinity-purified wild type (B) or N130Q (D) NS1 as quantitated by O.D. 280 was injected onto a Superdex 200 column.
Acknowledgments
We thank W. Klimstra and D. Lenschow for the SINV expression vectors, S. Youn for DENV-2 NS1 expression constructs, D. Fremont, D. Spitzer, E. Miller, C. Nelson, M. Barrow, and S. Youn for experimental help and advice, and C. Puttikhunt and W. Kasinrerk for providing DENV NS1 specific Abs. This work was supported by the Midwest Regional Centers for Excellence for Biodefense and Emerging Infectious Disease Research (U54-AI057160 to M.S. Diamond and J.P. Atkinson), and the National Center for Genetic Engineering and Biotechnology (BIOTEC), Thailand (P. Avirutnan). P. Somnuke is supported by an M.D.-Ph.D. training fellowship from Medical Scholars Program, Mahidol University. P. Avirutnan has been supported by National Institutes of Health postdoctoral training grants from the Divisions of Dermatology and Rheumatology in the Department of Medicine, Washington University School of Medicine.
Abbreviations
- BSA
bovine serum albumin
- BHK
baby hamster kidney fibroblast
- DENV
dengue virus
- DHF/DSS
dengue hemorrhagic fever/dengue shock syndrome
- GAG
glycosaminoglycan
- GPI
glycosylphosphatidyl inositol
- MAb
monoclonal antibody
- NS1
non-structural protein-1
- SINV
Sindbis virus
- SINV-DENV-2 NS1
Sindbis virus encoding dengue virus serotype 2 non-structural protein-1
- WNV
West Nile virus
- YFV
Yellow Fever virus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alcon-LePoder S, Drouet MT, Roux P, Frenkiel MP, Arborio M, Durand-Schneider AM, Maurice M, Le Blanc I, Gruenberg J, Flamand M. The secreted form of dengue virus nonstructural protein NS1 is endocytosed by hepatocytes and accumulates in late endosomes: implications for viral infectivity. J Virol. 2005;79(17):11403–11. doi: 10.1128/JVI.79.17.11403-11411.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcon S, Talarmin A, Debruyne M, Falconar A, Deubel V, Flamand M. Enzyme-linked immunosorbent assay specific to Dengue virus type 1 nonstructural protein NS1 reveals circulation of the antigen in the blood during the acute phase of disease in patients experiencing primary or secondary infections. J Clin Microbiol. 2002;40(2):376–81. doi: 10.1128/JCM.40.2.376-381.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avirutnan P, Fuchs A, Hauhart RE, Somnuke P, Youn S, Diamond MS, Atkinson JP. Antagonism of the complement component C4 by flavivirus nonstructural protein NS1. J Exp Med. 2010;207(4):793–806. doi: 10.1084/jem.20092545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avirutnan P, Punyadee N, Noisakran S, Komoltri C, Thiemmeca S, Auethavornanan K, Jairungsri A, Kanlaya R, Tangthawornchaikul N, Puttikhunt C, Pattanakitsakul SN, Yenchitsomanus PT, Mongkolsapaya J, Kasinrerk W, Sittisombut N, Husmann M, Blettner M, Vasanawathana S, Bhakdi S, Malasit P. Vascular leakage in severe dengue virus infections: a potential role for the nonstructural viral protein NS1 and complement. J Infect Dis. 2006;193(8):1078–88. doi: 10.1086/500949. [DOI] [PubMed] [Google Scholar]
- Avirutnan P, Zhang L, Punyadee N, Manuyakorn A, Puttikhunt C, Kasinrerk W, Malasit P, Atkinson JP, Diamond MS. Secreted NS1 of dengue virus attaches to the surface of cells via interactions with heparan sulfate and chondroitin sulfate E. PLoS Pathog. 2007;3(11):e183. doi: 10.1371/journal.ppat.0030183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeuerle PA, Huttner WB. Chlorate--a potent inhibitor of protein sulfation in intact cells. Biochem Biophys Res Commun. 1986;141(2):870–7. doi: 10.1016/s0006-291x(86)80253-4. [DOI] [PubMed] [Google Scholar]
- Chambers TJ, Hahn CS, Galler R, Rice CM. Flavivirus genome organization, expression, and replication. Annu Rev Microbiol. 1990;44:649–88. doi: 10.1146/annurev.mi.44.100190.003245. [DOI] [PubMed] [Google Scholar]
- Chang HH, Shyu HF, Wang YM, Sun DS, Shyu RH, Tang SS, Huang YS. Facilitation of cell adhesion by immobilized dengue viral nonstructural protein 1 (NS1): arginine-glycine-aspartic acid structural mimicry within the dengue viral NS1 antigen. J Infect Dis. 2002;186(6):743–51. doi: 10.1086/342600. [DOI] [PubMed] [Google Scholar]
- Chung KM, Liszewski MK, Nybakken G, Davis AE, Townsend RR, Fremont DH, Atkinson JP, Diamond MS. West Nile virus nonstructural protein NS1 inhibits complement activation by binding the regulatory protein factor H. Proc Natl Acad Sci U S A. 2006a;103(50):19111–6. doi: 10.1073/pnas.0605668103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung KM, Nybakken GE, Thompson BS, Engle MJ, Marri A, Fremont DH, Diamond MS. Antibodies against West Nile Virus nonstructural protein NS1 prevent lethal infection through Fc gamma receptor-dependent and -independent mechanisms. J Virol. 2006b;80(3):1340–51. doi: 10.1128/JVI.80.3.1340-1351.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clyde K, Kyle JL, Harris E. Recent advances in deciphering viral and host determinants of dengue virus replication and pathogenesis. J Virol. 2006;80(23):11418–31. doi: 10.1128/JVI.01257-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree MB, Kinney RM, Miller BR. Deglycosylation of the NS1 protein of dengue 2 virus, strain 16681: construction and characterization of mutant viruses. Arch Virol. 2005;150(4):771–86. doi: 10.1007/s00705-004-0430-8. [DOI] [PubMed] [Google Scholar]
- Despres P, Girard M, Bouloy M. Characterization of yellow fever virus proteins E and NS1 expressed in Vero and Spodoptera frugiperda cells. J Gen Virol. 1991;72(Pt 6):1331–42. doi: 10.1099/0022-1317-72-6-1331. [DOI] [PubMed] [Google Scholar]
- Doms RW, Lamb RA, Rose JK, Helenius A. Folding and assembly of viral membrane proteins. Virology. 1993;193(2):545–62. doi: 10.1006/viro.1993.1164. [DOI] [PubMed] [Google Scholar]
- Falgout B, Markoff L. Evidence that flavivirus NS1-NS2A cleavage is mediated by a membrane-bound host protease in the endoplasmic reticulum. J Virol. 1995;69(11):7232–43. doi: 10.1128/jvi.69.11.7232-7243.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamand M, Megret F, Mathieu M, Lepault J, Rey FA, Deubel V. Dengue virus type 1 nonstructural glycoprotein NS1 is secreted from mammalian cells as a soluble hexamer in a glycosylation-dependent fashion. J Virol. 1999;73(7):6104–10. doi: 10.1128/jvi.73.7.6104-6110.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs A, Lin TY, Beasley DW, Stover CM, Schwaeble WJ, Pierson TC, Diamond MS. Direct complement restriction of flavivirus infection requires glycan recognition by mannose-binding lectin. Cell Host Microbe. 2010;8(2):186–95. doi: 10.1016/j.chom.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11(3):480–96. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead SB. Pathogenesis of dengue: challenges to molecular biology. Science. 1988;239(4839):476–81. doi: 10.1126/science.3277268. [DOI] [PubMed] [Google Scholar]
- Halstead SB. Dengue. Lancet. 2007;370(9599):1644–52. doi: 10.1016/S0140-6736(07)61687-0. [DOI] [PubMed] [Google Scholar]
- Hershkovitz O, Jarahian M, Zilka A, Bar-Ilan A, Landau G, Jivov S, Tekoah Y, Glicklis R, Gallagher JT, Hoffmann SC, Zer H, Mandelboim O, Watzl C, Momburg F, Porgador A. Altered glycosylation of recombinant NKp30 hampers binding to heparan sulfate: a lesson for the use of recombinant immunoreceptors as an immunological tool. Glycobiology. 2008;18(1):28–41. doi: 10.1093/glycob/cwm125. [DOI] [PubMed] [Google Scholar]
- Ito K, Ishimaru T, Kimura F, Matsudomi N. Importance of N-glycosylation positioning for secretion and folding of ovalbumin. Biochem Biophys Res Commun. 2007a;361(3):725–31. doi: 10.1016/j.bbrc.2007.07.066. [DOI] [PubMed] [Google Scholar]
- Ito K, Seri A, Kimura F, Matsudomi N. Site-specific glycosylation at Asn-292 in ovalbumin is essential to efficient secretion in yeast. J Biochem. 2007b;141(2):193–9. doi: 10.1093/jb/mvm021. [DOI] [PubMed] [Google Scholar]
- Jacobs MG, Robinson PJ, Bletchly C, Mackenzie JM, Young PR. Dengue virus nonstructural protein 1 is expressed in a glycosyl-phosphatidylinositol-linked form that is capable of signal transduction. Faseb J. 2000;14(11):1603–10. doi: 10.1096/fj.14.11.1603. [DOI] [PubMed] [Google Scholar]
- Kayser V, Chennamsetty N, Voynov V, Forrer K, Helk B, Trout BL. Glycosylation influences on the aggregation propensity of therapeutic monoclonal antibodies. Biotechnol J. 2010 doi: 10.1002/biot.201000091. [DOI] [PubMed] [Google Scholar]
- Khromykh AA, Sedlak PL, Guyatt KJ, Hall RA, Westaway EG. Efficient trans-complementation of the flavivirus kunjin NS5 protein but not of the NS1 protein requires its coexpression with other components of the viral replicase. J Virol. 1999;73(12):10272–80. doi: 10.1128/jvi.73.12.10272-10280.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krych-Goldberg M, Hauhart RE, Subramanian VB, Yurcisin BM, 2nd, Crimmins DL, Hourcade DE, Atkinson JP. Decay accelerating activity of complement receptor type 1 (CD35). Two active sites are required for dissociating C5 convertases. J Biol Chem. 1999;274(44):31160–8. doi: 10.1074/jbc.274.44.31160. [DOI] [PubMed] [Google Scholar]
- Kurosu T, Chaichana P, Yamate M, Anantapreecha S, Ikuta K. Secreted complement regulatory protein clusterin interacts with dengue virus nonstructural protein 1. Biochem Biophys Res Commun. 2007;362(4):1051–6. doi: 10.1016/j.bbrc.2007.08.137. [DOI] [PubMed] [Google Scholar]
- Libraty DH, Young PR, Pickering D, Endy TP, Kalayanarooj S, Green S, Vaughn DW, Nisalak A, Ennis FA, Rothman AL. High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J Infect Dis. 2002;186(8):1165–8. doi: 10.1086/343813. [DOI] [PubMed] [Google Scholar]
- Lin CF, Lei HY, Shiau AL, Liu CC, Liu HS, Yeh TM, Chen SH, Lin YS. Antibodies from dengue patient sera cross-react with endothelial cells and induce damage. J Med Virol. 2003;69(1):82–90. doi: 10.1002/jmv.10261. [DOI] [PubMed] [Google Scholar]
- Lindenbach BD, Rice CM. trans-Complementation of yellow fever virus NS1 reveals a role in early RNA replication. J Virol. 1997;71(12):9608–17. doi: 10.1128/jvi.71.12.9608-9617.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie JM, Jones MK, Young PR. Immunolocalization of the dengue virus nonstructural glycoprotein NS1 suggests a role in viral RNA replication. Virology. 1996;220(1):232–40. doi: 10.1006/viro.1996.0307. [DOI] [PubMed] [Google Scholar]
- Maley F, Trimble RB, Tarentino AL, Plummer TH., Jr Characterization of glycoproteins and their associated oligosaccharides through the use of endoglycosidases. Anal Biochem. 1989;180(2):195–204. doi: 10.1016/0003-2697(89)90115-2. [DOI] [PubMed] [Google Scholar]
- Malvoisin E, Wild F. The role of N-glycosylation in cell fusion induced by a vaccinia recombinant virus expressing both measles virus glycoproteins. Virology. 1994;200(1):11–20. doi: 10.1006/viro.1994.1157. [DOI] [PubMed] [Google Scholar]
- Muylaert IR, Chambers TJ, Galler R, Rice CM. Mutagenesis of the N-linked glycosylation sites of the yellow fever virus NS1 protein: effects on virus replication and mouse neurovirulence. Virology. 1996;222(1):159–68. doi: 10.1006/viro.1996.0406. [DOI] [PubMed] [Google Scholar]
- Ng DT, Hiebert SW, Lamb RA. Different roles of individual N-linked oligosaccharide chains in folding, assembly, and transport of the simian virus 5 hemagglutinin-neuraminidase. Mol Cell Biol. 1990;10(5):1989–2001. doi: 10.1128/mcb.10.5.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noisakran S, Dechtawewat T, Avirutnan P, Kinoshita T, Siripanyaphinyo U, Puttikhunt C, Kasinrerk W, Malasit P, Sittisombut N. Association of dengue virus NS1 protein with lipid rafts. J Gen Virol. 2008;89(Pt 10):2492–500. doi: 10.1099/vir.0.83620-0. [DOI] [PubMed] [Google Scholar]
- Pletnev AG, Bray M, Lai CJ. Chimeric tick-borne encephalitis and dengue type 4 viruses: effects of mutations on neurovirulence in mice. J Virol. 1993;67(8):4956–63. doi: 10.1128/jvi.67.8.4956-4963.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post PR, Carvalho R, Galler R. Glycosylation and secretion of yellow fever virus nonstructural protein NS1. Virus Res. 1991;18(2-3):291–302. doi: 10.1016/0168-1702(91)90025-q. [DOI] [PubMed] [Google Scholar]
- Pryor MJ, Gualano RC, Lin B, Davidson AD, Wright PJ. Growth restriction of dengue virus type 2 by site-specific mutagenesis of virus-encoded glycoproteins. J Gen Virol. 1998;79(Pt 11):2631–9. doi: 10.1099/0022-1317-79-11-2631. [DOI] [PubMed] [Google Scholar]
- Pryor MJ, Wright PJ. Glycosylation mutants of dengue virus NS1 protein. J Gen Virol. 1994;75(Pt 5):1183–7. doi: 10.1099/0022-1317-75-5-1183. [DOI] [PubMed] [Google Scholar]
- Puttikhunt C, Kasinrerk W, Srisa-ad S, Duangchinda T, Silakate W, Moonsom S, Sittisombut N, Malasit P. Production of anti-dengue NS1 monoclonal antibodies by DNA immunization. J Virol Methods. 2003;109(1):55–61. doi: 10.1016/s0166-0934(03)00045-4. [DOI] [PubMed] [Google Scholar]
- Rademacher TW, Parekh RB, Dwek RA. Glycobiology. Annu Rev Biochem. 1988;57:785–838. doi: 10.1146/annurev.bi.57.070188.004033. [DOI] [PubMed] [Google Scholar]
- Ryman KD, Gardner CL, Burke CW, Meier KC, Thompson JM, Klimstra WB. Heparan sulfate binding can contribute to the neurovirulence of neuroadapted and nonneuroadapted Sindbis viruses. J Virol. 2007;81(7):3563–73. doi: 10.1128/JVI.02494-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun DS, King CC, Huang HS, Shih YL, Lee CC, Tsai WJ, Yu CC, Chang HH. Antiplatelet autoantibodies elicited by dengue virus non-structural protein 1 cause thrombocytopenia and mortality in mice. J Thromb Haemost. 2007;5(11):2291–9. doi: 10.1111/j.1538-7836.2007.02754.x. [DOI] [PubMed] [Google Scholar]
- Tajima S, Takasaki T, Kurane I. Characterization of Asn130-to-Ala mutant of dengue type 1 virus NS1 protein. Virus Genes. 2008;36(2):323–9. doi: 10.1007/s11262-008-0211-7. [DOI] [PubMed] [Google Scholar]
- Tarentino AL, Gomez CM, Plummer TH., Jr Deglycosylation of asparagine-linked glycans by peptide:N-glycosidase F. Biochemistry. 1985;24(17):4665–71. doi: 10.1021/bi00338a028. [DOI] [PubMed] [Google Scholar]
- Tarentino AL, Maley F. Purification and properties of an endo-beta-N-acetylglucosaminidase from Streptomyces griseus. J Biol Chem. 1974;249(3):811–7. [PubMed] [Google Scholar]
- Trombetta ES, Helenius A. Lectins as chaperones in glycoprotein folding. Curr Opin Struct Biol. 1998;8(5):587–92. doi: 10.1016/s0959-440x(98)80148-6. [DOI] [PubMed] [Google Scholar]
- Whiteman MC, Li L, Wicker JA, Kinney RM, Huang C, Beasley DW, Chung KM, Diamond MS, Solomon T, Barrett AD. Development and characterization of non-glycosylated E and NS1 mutant viruses as a potential candidate vaccine for West Nile virus. Vaccine. 2010;28(4):1075–83. doi: 10.1016/j.vaccine.2009.10.112. [DOI] [PubMed] [Google Scholar]
- Winkler G, Maxwell SE, Ruemmler C, Stollar V. Newly synthesized dengue-2 virus nonstructural protein NS1 is a soluble protein but becomes partially hydrophobic and membrane-associated after dimerization. Virology. 1989;171(1):302–5. doi: 10.1016/0042-6822(89)90544-8. [DOI] [PubMed] [Google Scholar]
- Winkler G, Randolph VB, Cleaves GR, Ryan TE, Stollar V. Evidence that the mature form of the flavivirus nonstructural protein NS1 is a dimer. Virology. 1988;162(1):187–96. doi: 10.1016/0042-6822(88)90408-4. [DOI] [PubMed] [Google Scholar]
- Youn S, Cho H, Fremont DH, Diamond MS. A short N-terminal peptide motif on flavivirus nonstructural protein NS1 modulates cellular targeting and immune recognition. J Virol. 2010;84(18):9516–32. doi: 10.1128/JVI.00775-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young PR, Hilditch PA, Bletchly C, Halloran W. An antigen capture enzyme-linked immunosorbent assay reveals high levels of the dengue virus protein NS1 in the sera of infected patients. J Clin Microbiol. 2000;38(3):1053–7. doi: 10.1128/jcm.38.3.1053-1057.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Tsai HM. N-Glycans of ADAMTS13 modulate its secretion and von Willebrand factor cleaving activity. Blood. 2009;113(4):929–35. doi: 10.1182/blood-2008-07-167775. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Serum-free supernatants from SINV-DENV-2-NS1-infected BHK21 cells at an MOI of one were collected at 18 to 20 h post-infection and purified over a nickel affinity column. Samples of eluted fractions were boiled prior to 4-12% SDS-PAGE under non-reducing conditions and analyzed by Western blot using 1F11 anti-DENV-2 NS1 (wild type (A), N130Q (C)). The peak fraction (A2) containing equal amount of nickel affinity-purified wild type (B) or N130Q (D) NS1 as quantitated by O.D. 280 was injected onto a Superdex 200 column.