Abstract
Objectives
Lynch Syndrome is an autosomal dominant condition characterized by early onset colorectal cancer (CRC) and is associated with cancers of the gastrointestinal and reproductive tracts. Germline mutations in DNA mismatch repair (MMR) genes have been causally associated with cancers of Lynch Syndrome. We investigated the occurrence of prostate cancer (PCa) in families with a history of colorectal cancer to assess prostate cancer as a feature of the Lynch Syndrome spectrum.
Methods
Family pedigrees containing at least one CRC case as well as those meeting guidelines for Lynch Syndrome were identified and tumors were requested from participants who underwent radical prostatectomy (RP). Selected families were analyzed for association with type of PCa and clinical characteristics of aggressive disease. Microsatellite Instability (MSI) analysis was preformed on available tumors and correlated to loss of expression in MMR genes by immunohistochemical (IHC) staining.
Results
95 individuals were identified as members of potential Lynch Syndrome families who underwent RP and 35 tumors from 31 families were received for MSI analysis. Two tumors from two unrelated families with known MMR mutations were MSI-high and one additional case from a third family was MSI-low. The remainder of the prostate cancer cases demonstrated no evidence of MSI.
Conclusions
PCa incidence in families enriched for hereditary PCa with a history of Lynch Syndrome cancers is not strongly suggestive of the presence of an MMR mutation. However prostate tumors in known MMR mutation carriers did display MSI and loss of gene expression suggesting that PCa may arise in Lynch Syndrome due to defective DNA mismatch repair.
Keywords: Lynch Syndrome, Prostate Cancer, MMR mutations, Microsatellite instability
Introduction
Prostate cancer (PCa) is the most common cancer diagnosed among American men and the second leading cause of cancer deaths with an estimated 217,730 new cases and 32,050 deaths expected in the United States in 2010 [1]. Aside from increasing age and African American race, family history is the only other confirmed risk factor for PCa suggesting that germline variation may play a role in PCa risk. PCa has shown marked clinical heterogeneity and to date no clear distinctions have been made between the causes of hereditary prostate cancer and those of sporadic disease. Identifying genetic susceptibility to PCa using linkage strategies has been a difficult endeavor for research groups across the globe and is a task that has been further complicated by the high incidence of sporadic disease in the general population [2]. An alternate way to examine the role of inherited genes is to consider PCa as part of known clinical cancer syndromes.
Lynch Syndrome is the most common hereditary colorectal cancer (CRC) predisposing syndrome accounting for 2–5% of all CRC. Individuals with Lynch syndrome have a probability of developing CRC that approaches 70% by the age of 70 [3]. Lynch-associated CRC occurs at a median age of 44 years which is about 20 years earlier than the median age of onset in sporadic CRC [4]. In addition to CRC, affected individuals are at an increased risk of other extra-colonic Lynch-associated tumors including malignancies of the endometrium, stomach, small bowel, ovary, pancreas, ureter or renal pelvis, biliary tract, brain and pancreas [5, 6]. The occurrence of prostate cancer among families with Lynch syndrome has been reported [7] however it has not been conclusively investigated as a feature of the Lynch cancer spectrum.
Lynch syndrome arises as a consequence of hereditary mutations in one of the DNA mismatch repair (MMR) genes and follows an autosomal dominant mode of inheritance. Mutations in MLH1, MSH2, and MSH6 are most common and account for 50%, 39%, and 7% of the mutations found in Lynch families, respectively. Mutations in PMS2 are less commonly involved in Lynch syndrome cancers [8].
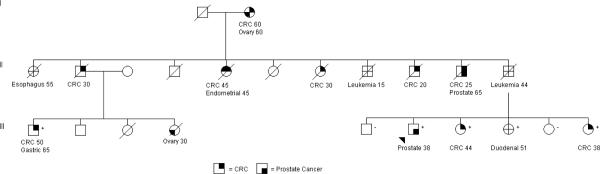
We decided to investigate PCa as a feature of Lynch syndrome after a patient (Patient 29) with a known MMR mutation (MSH2) sought prostate cancer screening at the age of 38 and was subsequently diagnosed with Gleason 7 adenocarcinoma. He was then seen by physicians at the University of Michigan Comprehensive Cancer Center (UMCCC) for treatment of his PCa and enrolled in the Prostate Cancer Genetics Project (PCGP) (Figure 1). The proband and three of his siblings were enrolled in the PCGP, including a sister diagnosed with colon cancer at 44, a second sister diagnosed with duodenal cancer at age 51, and their unaffected brother. Genetic testing of the MMR genes identified a 1704–1705 delAG in MSH2. The proband and his two sisters with cancer were each carriers of the mutation and their unaffected brother did not have the mutation. To further evaluate PCa as a potential feature of the Lynch Syndrome spectrum, we examined the presence of Microsatellite Instability (MSI) and MMR mutations in prostate tumors obtained from families with CRC incidence enrolled in the PCGP.
Figure I.
Pedigree of Patient 29 (indicated by the arrow). Ages at onset have been rounded to the closest 5-year interval, and generations I and II and other family history was excluded to protect the anonymity of the subjects.
Materials and methods
Participants
The PCGP of the UMCCC is an ongoing project to characterize the molecular basis for inherited forms of prostate cancer. To enrich for heritable forms of the disease, enrollment into the PCGP was restricted to (1) men diagnosed with prostate cancer with at least one living first- or second-degree relative also diagnosed with prostate cancer or (2) men diagnosed with prostate cancer at ≤55 years of age without a family history of the disease. All participants were asked to provide a blood sample, extended family history information, access to medical records, and signed written informed consent. All PCGP pedigrees were reviewed for the presence of CRC as well as concordance with established clinical guidelines for the identification of probable Lynch syndrome including Amsterdam I [9] and Amsterdam II [10] criteria and Bethesda guidelines [11]. Ten percent of the pedigrees were viewed independently by a second reader to confirm their correct assignment to each of the clinical guidelines, and all pedigrees were reviewed by a certified genetic counselor. Prostate specimens were requested for microsatellite analysis from individuals belonging to pedigrees most enriched for CRC. Tumor blocks were requested from patients who underwent radical prostatectomy both internally within the University of Michigan Health System (UMHS) and from external institutions. Ninety-five tumors were requested and 35 tumors from 31 unique families were received for an overall response rate of 36.8%. For families with more than one PCa case, only the data from the individual who is a first degree relative of a CRC case or the individual with the youngest age at onset is presented.
Microsatellite Analysis
Microdissection techniques followed with xylene and Proteinase K treatment were used to isolate DNA from paraffin embedded tissues. PCR reactions were prepared for each of 9 microsatellite markers: BAT26, BAT40, D10S197, D17S250, BAT34c4, BAT25, D18S55, D5S346, and ACTC (primer sequences available upon request). PRC products were then analyzed using ABI 3100 Genetic Analyzer with GeneMapper 4.0 software. MSI was defined as the presence of alleles in tumor DNA that were not in normal tissue DNA for a given marker. Tumors showing instability at >30% of markers were considered MSI-high, tumors with instability at <30% of markers were considered MSI-low, and tumors without MSI at any markers were considered microsatellite stable (MSS).
Immunohistochemical Staining
IHC staining for MMR genes was conducted using the FE11 antibody from Oncogene (Cambridge, MA) for MSH2 and the G168-728 antibody from PharMingen (San Diego, CA) for MLH1.
Results
Within 1127 PCGP families, 327 (29.0%) reported CRC in at least one person in their family history (Table 1). As expected, families enrolled with familial clustering of PCa were more ikely to contain a case of CRC in their family: 173 (34.1%) families with an affected relative pair and 80 (29.9%) families who met criteria for hereditary prostate cancer (HPC) reported CRC in their family as compared to 69 (21.3%) families enrolled for early onset PCa without a family history of disease although the average age of relatives was younger in the early onset PCa group. A total of 26 (2.3%) pedigrees met one of the clinical guidelines for identifying probable Lynch Syndrome (Amsterdam I or II). HPC families were most likely to meet some form of Lynch Criteria (3.4%), followed by affected relative pairs (2.4%), and early onset families (1.2%), respectively. Similarly, HPC families were most likely to meet less stringent Bethesda guidelines (8.6%) that indicate a family should undergo a clinical workup for Lynch syndrome.
Table 1.
PCGP Colon Cancer Families by Modified Reason for Enrollment*
| Lynch Syndrome Criteria | ||||||
|---|---|---|---|---|---|---|
| Reason for Enrollment | All Families | Families with CRC | Bethesda | Amsterdam I | Amsterdam II | Lynch All |
| Affected relative paira | 507 | 173 (34.1) | 39 (5.7) | 2 (0.4) | 10 (2.0) | 12 (2.4) |
| Age at diagnosis ≤55b | 324 | 69 (21.3) | 11 (3.4) | 2 (0.6) | 2 (0.6) | 4 (1.2) |
| Hereditary PCac | 268 | 80 (29.9) | 23 (8.6) | 0 (0.0) | 9 (3.4) | 9 (3.4) |
| Other/blankd | 28 | 5 (17.9) | 0 (0.0) | 1 (3.6) | 0 (0.0) | 1 (3.6) |
| Total | 1127 | 327 (29.0) | 73 (6.5) | 5 (0.4) | 21 (1.9) | 26 (2.3) |
Data shown is number (percent)
Affected relative pair: two living first- or second- degree relatives with PCa
Age at diagnosis ≤ 55: men diagnosed with PCa without family history of disease
Hereditary prostate Cancer: ≥3 affected men in a nuclear family, or affected men in 3 vertical generations of a family, or two men diagnosed in a family before age 55
Other: did not meet PCGP inclusion criteria, referred to study by physician
Clinical characteristics of 31 unrelated patients (data on 4 related individuals not shown) screened for MSI are shown in Table 2. The individuals had a median age at prostate cancer diagnosis of 55 years of age, median pre-diagnosis PSA of 6 ng/dL, and a median Gleason score of 7. The majority of patients (74.2%) had localized disease. Fourteen families met HPC criteria, 12 were affected relative pairs, 4 were early onset families, and 1 family was enrolled in the PCGP via referral and had no additional prostate cancer cases within his family. Most families (19) met the less stringent Bethesda guidelines, 5 met Amsterdam II criteria, 2 met Amsterdam I criteria and 5 families had CRC but were not suggestive of Lynch Syndrome. Three of the patients were also diagnosed with CRC.
Table 2.
Characteristics of Individuals screened for MSI
| Individual | Reason for Enrollment† | Age at Dx | Pre-Dx PSA | Gleason | Stage‡ | CRC | Criteria^ | MSI Status^^ |
|---|---|---|---|---|---|---|---|---|
| 1 | HPC | 52 | 15.60 | 7 | Met. | N | Am-II | MSS |
| 2 | HPC | 63 | 4.50 | 6 | Localized | N | Beth. | MSS |
| 3 | HPC | 51 | 4.50 | 6 | Localized | N | None | MSS |
| 4 | HPC | 63 | 53.20 | 7 | Localized | Y, 71 | Beth. | MSS |
| 5 | Early Onset | 55 | 3.80 | 6 | Localized | N | Beth. | MSS |
| 6 | Early Onset | 53 | 9.20 | 6 | Localized | N | None | MSS |
| 7 | HPC | 54 | 4.00 | 6 | Localized | N | Beth. | MSS |
| 8 | ARP | 51 | 6.50 | 7 | Loc. Adv. | N | Beth. | MSS |
| 9 | ARP | 59 | 6.20 | 6 | Localized | Y, 55 | None | MSS |
| 10 | ARP | 64 | 3.27 | 6 | Localized | N | Beth. | MSS |
| 11 | HPC | 60 | 5.30 | 7 | Localized | N | Beth. | MSS |
| 12 | ARP | 52 | 7.40 | 7 | Localized | N | Beth. | MSS |
| 13 | HPC | 57 | 7.40 | 6 | Localized | N | Beth. | MSS |
| 14 | ARP | 47 | 6.70 | 7 | Localized | N | Beth. | MSS |
| 15 | ARP | 56 | N/A | 5 | Localized | N | Am-II | MSS |
| 16 | ARP | 56 | 3.70 | 7 | Localized | N | Beth. | MSS |
| 17 | ARP | 55 | 4.00 | 7 | Localized | N | Beth. | MSS |
| 18 | HPC | 54 | 3.50 | 7 | Localized | N | Beth. | MSI-L |
| 19 | HPC | 70 | 4.40 | 6 | Localized | N | None | MSS |
| 20 | ARP | 41 | 7.00 | 7 | Localized | N | None | MSS |
| 21 | HPC | 58 | 8.30 | 6 | Localized | N | Am-II | MSS |
| 22 | ARP | 62 | 2.70 | 9 | Loc. Adv. | N | Beth. | MSS |
| 23 | HPC | 66 | 5.80 | 8 | Loc. Adv. | N | Beth. | MSS |
| 24 | Early Onset | 50 | 8.60 | 6 | Localized | N | Beth. | MSS |
| 25 | ARP | 68 | 60.00 | N/A | Loc. Adv. | N | Beth. | MSS |
| 26 | HPC | 54 | 56.70 | 7 | Localized | N | Am-II | MSS |
| 27 | HPC | 56 | 3.40 | 7 | Loc. Adv. | N | Am-II | MSS |
| 28 | HPC | 48 | 4.70 | 8 | Loc. Adv. | N | Beth. | MSS |
| *29 | Early Onset | 38 | 175.00 | 7 | Loc. Adv. | N | Am-I | MSI-H |
| 30 | ARP | 54 | 5.50 | 7 | Localized | N | Beth. | MSS |
| *31 | Other | 69 | 9.50 | 8 | Localized | Y, 57 | Am-I | MSI-H |
Denotes individuals with known mismatch repair gene mutations.
HPC= Hereditary Prostate Cancer, ARP= Affected Relative Pair
Localized = T1 or T2, N0 and M0, Locally Advanced = T3 or T4, N0 and M0, Metastatic = N1 or M1
Am-I= Amsterdam I criteria, Am-II= Amsterdam II criteria, Beth.= Bethesda Guidelines
MSS = Microsatellite stable, MSI-L= MSI-low, MSI-H= MSI-high
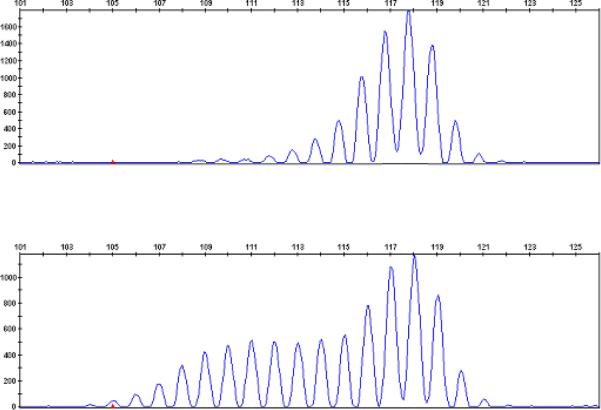
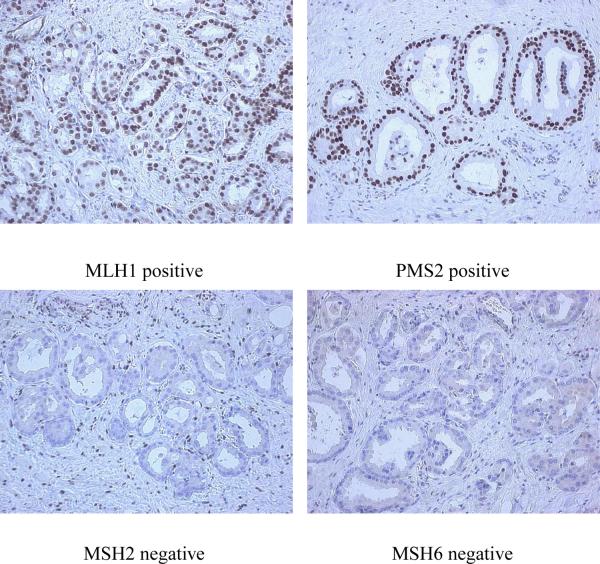
Tumor specimens from all 35 cases of prostate cancer were assessed for MSI and two prostate tumors from families with known MMR mutations, both meeting Amsterdam I criteria, were shown to be MSI-high. One tumor from a family meeting Bethesda guidelines was MSI-low. The additional 4 tumors from relatives of men shown in Table 2 were screened for MSI and also demonstrated to be MSI. Figure 2 depicts an example of MSI at marker BAT40 in Patient 29. The MSI phenotype is evident from the presence of additional repetitive DNA sequences of varying sizes in DNA isolated from tumor tissue. These repeats are not seen in the corresponding germline DNA isolated from normal tissue. IHC staining of his prostate tumor (Figure 3) confirmed complete loss of MSH2 and MSH6 expression as a result of the patient's MMR mutation in MSH2. The 28 remaining tumors were assessed to be MSS.
Figure 2.
MSI from Patient 29 for marker BAT40. DNA from normal tissue (top) and DNA from tumor tissue (bottom) are shown.
Figure 3.
Tumor tissue IHC staining from Patient 29 confirms his known mutation in MSH2. The patient shows a loss of expression of MSH2 and loss of MSH6. Expression of MLH1 and PMS2 were normal.
Discussion
This investigation of the occurrence of prostate cancer in families enriched for CRC suggests that prostate cancer is an uncommon feature of Lynch Syndrome, as typically characterized by the hallmark molecular signatures of microsatellite instability and lack of expression of mismatch repair proteins. These data also suggest that prostate cancer is unlikely to serve as a sentinel cancer suggestive of Lynch Syndrome. Of the 31 tumors received from unrelated individuals, 28 were MSS indicating that their prostate cancer did not arise as a consequence of an MMR mutation. Previous studies of PCa cell lines and primary tumors have identified MSI [12, 13] and defects of MMR genes [14] in human PCa. However, MSI has been rarely detected in hereditary prostate cancer at and when detected, was in patients from families with both familial CRC and HPC [15]. Such evidence suggests that widespread MSI is a rare event in hereditary forms of prostate cancer and that mutations in MMR genes do not serve an important role in the initiation of most common forms of prostate cancer.
The individuals in this study were selected from a prostate cancer research study enriched for hereditary forms of prostate cancer. Therefore, MSI in prostate tumors from these patients may be less likely. Similarly, these families may be more likely to harbor mutations that contribute to prostate cancer and less likely to have MMR mutations, which explain the majority of MSS tumors seen. In order to identify MSI in prostate tumors, it may be important to investigate prostate cancer incidence within the context of families enriched for hereditary forms of CRC as opposed to PCa. Recently Grindedal, et al. reported that MMR mutations were responsible for prostate cancers of MMR mutation carriers identified from the Cancer Registry of Norway, suggesting that PCa is a component of the Lynch syndrome spectrum. The study reported that MMR mutation carriers were 5.9 times more likely to be diagnosed with prostate cancer than normal controls and used immunohistochemistry to confirm that the prostate tumors could be attributed to loss of MMR gene product [16].
It is also possible that some of the individuals identified were from families with Lynch Syndrome but that the men who developed prostate cancer did not inherit the MMR mutation. Thus, their prostate cancer could not be attributed to Lynch Syndrome and would not display the hallmark MSI phenotype. Without sequencing several members of the same families for MMR gene mutations we are unable to conclude whether the affected men were from families with mutation.
A third explanation for our result could be that the individuals selected were from families with Lynch Syndrome and inherited the mutation but that other PCa mutations were more penetrant and their MMR mutation did not contribute to the molecular phenotype of their prostatic tumor. This may be possible in families with Lynch-associated tumors derived from one lineage and an aggregation of PCa cases from the other lineage. However, this explanation is unlikely as the two tumors from patients with known mutations were both MSI-high, suggesting that MMR mutations are highly penetrant and directly contribute to the pathogenesis of prostate cancer in these men.
In conclusion, PCa is an uncommon feature of Lynch Syndrome, but it is rare among individuals with familial prostate cancer. PCa alone is not strongly suggestive of Lynch Syndrome or the presence of an MMR mutation. Further studies of families with Lynch Syndrome are recommended to quantify the frequency, absolute risk, and relative risk of prostate cancer among mutation carriers.
Acknowledgements
Supported by a grant from the Donald and Jo Anne Petersen Endowed Research Fund of the University of Michigan Comprehensive Cancer Center. The University of Michigan Prostate Cancer Genetics Project (PCGP) is supported by the National Cancer Institute R01 CA79596 (K.A. Cooney). Funds were also provided from University of Michigan School of Public Health and the Public Health Genetics Interdepartmental Concentration Internship program. We thank all PCGP men and their families who generously volunteered their time to participate in our study. We also gratefully acknowledge Dr. Stephen N. Thibodeauau for his assistance with tumor staining and genetic testing of Patient 29 and Dr. Kirk Wojno for his assistance with pathology specimens.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J Clin. 2010 doi: 10.3322/caac.20073. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 2.Schaid DJ. The complex genetic epidemiology of prostate cancer. Hum Mol Genet. 2004;13(Spec No 1):R103–R121. doi: 10.1093/hmg/ddh072. [DOI] [PubMed] [Google Scholar]
- 3.Stoffel E, Mukherjee B, Raymond VM, Tayob N, Kastrinos F, Sparr J, et al. Calculation of risk of colorectal and endometrial cancer among patients with Lynch syndrome. Gastroenterology. 2009;137(5):1621–1627. doi: 10.1053/j.gastro.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lynch HT, Lynch PM, Lanspa SJ, Snyder CL, Lynch JF, Boland CR. Review of the Lynch syndrome: history, molecular genetics, screening, differential diagnosis, and medicolegal ramifications. Clin Genet. 2009;76(1):1–18. doi: 10.1111/j.1399-0004.2009.01230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watson P, Riley B. The tumor spectrum in the Lynch syndrome. Fam Cancer. 2005;4(3):245–248. doi: 10.1007/s10689-004-7994-z. [DOI] [PubMed] [Google Scholar]
- 6.Kastrinos F, Mukherjee B, Tayob N, Wang F, Sparr J, Raymond VM, et al. Risk of pancreatic cancer in families with Lynch syndrome. JAMA. 2009;302(16):1790–1795. doi: 10.1001/jama.2009.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soravia C, van der KH, Brundler MA, Blouin JL, Wijnen J, Hutter P, et al. Prostate cancer is part of the hereditary non-polyposis colorectal cancer (HNPCC) tumor spectrum. Am J Med Genet. 2003;121A(2):159–162. doi: 10.1002/ajmg.a.20106. [DOI] [PubMed] [Google Scholar]
- 8.Peltomaki P, Vasen H. Mutations associated with HNPCC predisposition -- Update of ICG-HNPCC/INSiGHT mutation database. Dis Markers. 2004;20(4–5):269–276. doi: 10.1155/2004/305058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vasen HF, Mecklin JP, Khan PM, Lynch HT. The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (ICG-HNPCC) Dis Colon Rectum. 1991;34(5):424–425. doi: 10.1007/BF02053699. [DOI] [PubMed] [Google Scholar]
- 10.Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology. 1999;116(6):1453–1456. doi: 10.1016/s0016-5085(99)70510-x. [DOI] [PubMed] [Google Scholar]
- 11.Umar A, Boland CR, Terdiman JP, Syngal S, de la CA, Ruschoff J, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96(4):261–268. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cunningham JM, Shan A, Wick MJ, McDonnell SK, Schaid DJ, Tester DJ, et al. Allelic imbalance and microsatellite instability in prostatic adenocarcinoma. Cancer Res. 1996;56:4475–4482. [PubMed] [Google Scholar]
- 13.Uchida T, Wada C, Wang C, Ishida H, Egawa S, Yokoyama E, et al. Microsatellite instability in prostate cancer. Oncogene. 1995;10(5):1019–1022. [PubMed] [Google Scholar]
- 14.Chen Y, Wang J, Fraig MM, Metcalf J, Turner WR, Bissada NK, et al. Defects of DNA mismatch repair in human prostate cancer. Cancer Res. 2001;61(10):4112–4121. [PubMed] [Google Scholar]
- 15.Ahman AK, Jonsson BA, Damber JE, Bergh A, Gronberg H. Low frequency of microsatellite instability in hereditary prostate cancer. BJU Int. 2001;87(4):334–338. doi: 10.1046/j.1464-410x.2001.00104.x. [DOI] [PubMed] [Google Scholar]
- 16.Grindedal EM, Moller P, Eeles R, Stormorken AT, Bowitz-Lothe IM, Landro SM, et al. Germ-line mutations in mismatch repair genes associated with prostate cancer. Cancer Epidemiol.Biomarkers Prev. 2009;18(9):2460–2467. doi: 10.1158/1055-9965.EPI-09-0058. [DOI] [PubMed] [Google Scholar]