Abstract
Context
Antiretroviral therapy has been associated with hypercholesterolemia in HIV-infected children. Few longitudinal studies have been conducted to examine this association, however.
Objective
To evaluate the incidence of and risk factors for development of hypercholesterolemia in a large pediatric study.
Design
Prospective cohort study (Pediatric AIDS Clinical Trials Group 219C).
Participants
A total of 2122 perinatally HIV-infected children free of hypercholesterolemia at entry.
Outcome
Development of hypercholesterolemia (total cholesterol ≥220 mg/dL at 2 consecutive visits). Cox proportional hazards models were used to evaluate risk factors.
Results
Thirteen percent of children had hypercholesterolemia at entry, and an additional 13% developed hypercholesterolemia during follow-up for an incidence rate of 3.4 cases per 100 person-years (95% confidence interval [CI]: 3.0 to 3.9). After adjustment for age, boosted protease inhibitor (PI) use (hazard ratio [HR] = 13.9, 95% CI: 6.73 to 28.6), nonboosted PI use (HR = 8.65, 95% CI: 4.19 to 17.9), and nonnucleoside reverse transcriptase inhibitor use (HR = 1.33, 95% CI: 1.04 to 1.71) were associated with increased risk of hypercholesterolemia, and higher viral load was protective (>50,000 vs. ≤400 copies/mL; HR = 0.59, 95% CI: 0.39 to 0.90). Self-reported adherent subjects had higher risk.
Conclusions
PIs were significant risk factors for hypercholesterolemia. Higher viral load was protective and may reflect non-adherence. Further follow-up is critical to evaluate long-term consequences of chronic PI exposure and hypercholesterolemia.
Keywords: HIV, cholesterol, pediatric, protease inhibitors, dyslipidemia
Treatment of HIV-infected children with highly active antiretroviral therapy (HAART) has led to vastly improved outcomes, including reduced mortality,1 decreased incidence of opportunistic infections,2 and improved quality of life.3,4 Despite these advances, metabolic complications related to antiretroviral treatment (ART) have been noted, including delayed puberty, fat redistribution, insulin resistance. and dyslipidemia.5–8
Several cross-sectional studies have found the use of protease inhibitors (PIs) to be associated with elevated cholesterol in children.9–12 Other studies that examined the incidence of hypercholesterolemia among children on PI therapy observed an increase in cholesterol levels over time.13–15
A few longitudinal studies have also been conducted to examine the association between antiretroviral therapy and incidence of high cholesterol and other metabolic disturbances in HIV-infected children. Beregszaszi et al16 identified PI use as an independent predictor of metabolic disturbance (defined as abnormally high levels of insulin, cholesterol, or triglycerides). Rhoads et al17 compared nonfasting lipid levels in children on HAART with PI, HAART without PI, or no HAART. Both types of HAART regimens were associated with an increase in total cholesterol, low-density lipoprotein (LDL), and high-density lipoprotein (HDL). PI use was independently associated with incidence of high total cholesterol among children participating in the Perinatal AIDS Collaborative Transmission Study–HIV Follow-up After Perinatal Exposure (PACTS-HOPE) study.18 All these studies were relatively small, however, with fewer than 200 subjects.
For this analysis, we examined the incidence of hypercholesterolemia in a large prospectively followed cohort of perinatally HIV-infected children, while adjusting for potentially confounding factors. In addition, we evaluated whether ART, particularly PIs, was associated with increased incidence. This study adds to the earlier cross-sectional evaluation of hypercholesterolemia in this cohort,11 because the ART exposure in our study preceded the development of hypercholesterolemia.
PARTICIPANTS AND METHODS
Study Population
The Pediatric AIDS Clinical Trials Group (PACTG) 219C is a prospective cohort study designed to examine long-term effects of exposure to HIV and antiretroviral therapy among HIV-infected children and uninfected children born to HIV-infected women, as described previously.2,4,11 Enrollment began in September 2000 and included children from 89 sites in the United States and Puerto Rico. Accrual to the study was completed in March 2006, and the study closed to follow-up in May 2007.
This data analysis focused on perinatally HIV-infected children and adolescents. At study entry, a lifetime history of ART and clinical diagnoses was obtained. Children were examined every 3 months; at each study visit, lipid measurements were taken and other clinical and laboratory information was collected, including Centers for Disease Control and Prevention (CDC) HIV clinical classification, CD4 T-lymphocyte percentages, and HIV viral loads. Changes in use of antiretroviral agents, including specific regimens and their start and stop dates, were obtained at each study visit. The study protocol for PACTG 219C was reviewed and approved by the institutional review board at each of the participating sites, and written informed consent was obtained from each child’s parent or guardian or from older participants who could self-consent. Written assent was obtained from children 12 years of age and older when appropriate. The study population for this analysis was perinatally HIV-infected subjects in PACTG 219C who were initially free of hypercholesterolemia at study entry, were not on lipid-lowering agents at any time before development of hypercholesterolemia, and who had at least 2 follow-up cholesterol measurements. We used data submitted to our data management center as of May 2007.
Statistical Analysis
An incident case of hypercholesterolemia was defined as the occurrence of 2 consecutive total cholesterol values greater than or equal to 220 mg/dL among children who had a total cholesterol level <220 mg/dL at baseline. Cholesterol measurements were not required to be fasting; we therefore chose a threshold of 220 mg/dL rather than 200 mg/dL so as to reduce misclassification of our outcome. Children were followed from the time of their first cholesterol measurement up until the time of a second consecutive high cholesterol value, loss to follow-up, or end of the follow-up period.
Incidence rates (IRs) for hypercholesterolemia were calculated as the number of events divided by person-time at risk. Exact 95% confidence intervals (CIs) were estimated under a Poisson distribution. For rate estimation of baseline exposures, children were assumed to continue their baseline exposure level throughout the follow-up period. For time-dependent HAART use, HAART exposure categories were updated throughout follow-up.
Cox proportional hazards models and extended Cox models, including time-dependent covariates, were used to calculate crude hazard ratios (HRs) and HRs adjusted for potential confounders. Our primary exposure of interest was ART. We evaluated the effects of PIs, nonnucleoside reverse transcriptase inhibitors (NNRTIs), and nucleoside reverse transcriptase inhibitors (NRTIs) by first considering baseline use and then use as a time-dependent indicator in an extended Cox model. Ritonavir-boosted PIs (boosted) and PIs not boosted with ritonavir (nonboosted) were considered separately. If children were using a specific ART at baseline or had stopped this use no more than 90 days before baseline, they were classified as exposed to this ART at baseline.We also considered baseline HAARTuse, with HAART defined as combination therapy with at least 3 medications from 2 or more classes of medications. Our HAART categories were defined as HAART with PI, HAART without PI (NNRTI + NRTI), ART without HAART (which could include PI use), and no ART.
We did not consider duration of ART exposure directly. Instead, we considered whether subjects had initiated a specific ART (or HAART regimen) at each point in time. Children who were using a specific ART at baseline were classified as exposed to that ART throughout the follow-up period unless they switched from a nonboosted PI to a boosted PI; at that point, they were reclassified as exposed to a boosted PI. Children who started a specific ART during follow-up were classified as exposed as of the date of initiation of that ART until they switched from a nonboosted PI to a boosted PI.
We also evaluated the hypothesis that use of efavirenz, as compared with other NNRTIs, might particularly increase the incidence of hypercholesterolemia. (This comparison was primarily between efavirenz and nevirapine, because fewer than 2% of children taking NNRTIs during the follow-up period were specifically taking delavirdine.) In addition, we examined stavudine separately from other NRTIs and boosted PIs that included lopinavir separately from boosted PIs that did not include lopinavir. Baseline categories of specific ART use were utilized for descriptive statistics, and time-dependent use of specific ARTs was utilized for analysis of risk factors.
We also examined baseline demographic and health-related characteristics as potential predictors of hypercholesterolemia. Adherence to antiretroviral medications was based on self-reported use (by the child or the parent or guardian) of prescribed regimens during the past 3 days and categorized as 100% or <100%. Viral load was categorized as undetectable (400 or fewer copies/mL), 401 to 5000 copies/mL, 5001 to 50,000 copies/mL, and >50,000 copies/mL. CD4 percentage (CD%) was categorized as less than 15%, 15% to 25%, and higher than 25%.We also considered whether a subject had an AIDS-defining condition at baseline (CDC classification C), age, race/ethnicity, gender, and body mass index (BMI).
Model building was preceded by first calculating unadjusted HRs for each covariate. All ARTs (boosted PI, nonboosted PI, NNRTI, and NRTI) were included together, such that the reference group was no ART use. All univariate predictors of hypercholesterolemia (P value ≤0.10, from the likelihood ratio test) were then entered together into a multivariate model. Covariates that remained significant predictors (P value ≤0.10, from the likelihood ratio test) after multivariate adjustment were retained in the model. Covariates initially identified to be univariate predictors were entered at this point, on an individual basis, into the model and assessed as confounders. Any covariates that changed effect estimates by 15% or more were retained in the model. All analyses were conducted using Unix SAS version 9.1 (SAS Institute Inc., Cary, NC).
RESULTS
There were 2576 perinatally infected children in PACTG 219C enrolled between September 15, 2000 and February 23, 2006. Eleven children had no cholesterol measurements, and another 90 had fewer than 2 follow-up measurements; these 101 children were excluded. Of the 2475 children who had baseline and at least 2 follow-up cholesterol measurements, 324 (13%) had high cholesterol values at baseline and were excluded. Twenty-nine children were on lipid-lowering agents at baseline or during the follow-up period and were excluded. (An additional 15 children initiated lipid-lowering therapy after development of hypercholesterolemia and were included in these analyses.) The remaining 2122 children were included in descriptive analyses as well as in IR estimation and Kaplan-Meier survival estimates. In models evaluating predictors of hypercholesterolemia, subjects missing information on baseline viral load or adherence (n = 186 [9%]) were excluded. (Forty-three children were missing information on CD4% at baseline, but because CD4% was not a predictor, we did not exclude these children from our analyses.)
Table 1 summarizes the baseline characteristics of our study population. Approximately half of the children were female, with an average age of 9 years. Fifty-nine percent were black, non-Hispanic. Most children did not have an AIDS-defining condition at baseline; approximately one third had an undetectable viral load. At baseline, most children (78%) were prescribed HAART therapy.
TABLE 1.
Baseline Characteristics of Perinatally Infected Children Initially Free of Hypercholesterolemia (N = 2122)
| Characteristic | Number (%) |
|---|---|
| Gender | |
| Male | 1027 (48) |
| Female | 1095 (52) |
| Mean age in years (SD), range | 9.21 (4.30), 0.02 to 21.93 |
| <6 | 480 (23) |
| 6 to 9 | 693 (33) |
| 10 to 12 | 535 (25) |
| ≥13 | 414 (19) |
| Race | |
| White/other | 294 (14) |
| Black, non-Hispanic | 1262 (59) |
| Hispanic | 566 (27) |
| AIDS-defining condition (CDC classification C) at baseline | |
| No | 1546 (73) |
| Yes | 568 (27) |
| Missing | 8 (<1) |
| CD4% at baseline | |
| <15 | 218 (10) |
| 15 to 25 | 469 (22) |
| >25 | 1392 (66) |
| Missing | 43 (2) |
| Viral load at baseline | |
| ≤400 | 767 (36) |
| 401 to 5000 | 503 (24) |
| 5001 to 50,000 | 515 (24) |
| >50,000 | 272 (13) |
| Missing | 65 (3) |
| PI use at baseline | |
| No | 680 (32) |
| Yes, nonboosted | 1027 (48) |
| Yes, boosted | 415 (20) |
| NNRTIs at baseline | |
| No | 1404 (66) |
| Yes | 718 (34) |
| NRTIs at baseline | |
| None | 93 (4) |
| 1 | 343 (16) |
| ≥2 | 1686 (80) |
| Baseline HAART use | |
| HAART with PI | 1412 (66) |
| HAART without PI | 250 (12) |
| ART without HAART | 378 (18) |
| No ART | 82 (4) |
| Adherence at baseline | |
| <100% | 318 (15) |
| 100% | 1672 (79) |
| Missing | 132 (6) |
| BMI | |
| <20.0 | 1471 (69) |
| 20.0 to 26.0 | 377 (18) |
| >26.0 | 101 (5) |
| Missing* | 173 (8) |
| BMI z-score | |
| <−2.0 | 1259 (59) |
| −2.0 to −1.0 | 40 (2) |
| >−1.0 to +1.0 | 160 (8) |
| >+1.0 to +2.0 | 388 (18) |
| >2.0 | 102 (5) |
| Missing* | 173 (8) |
BMI not calculated for 132 children who were younger than 2 years old; an additional 41 were missing BMI.
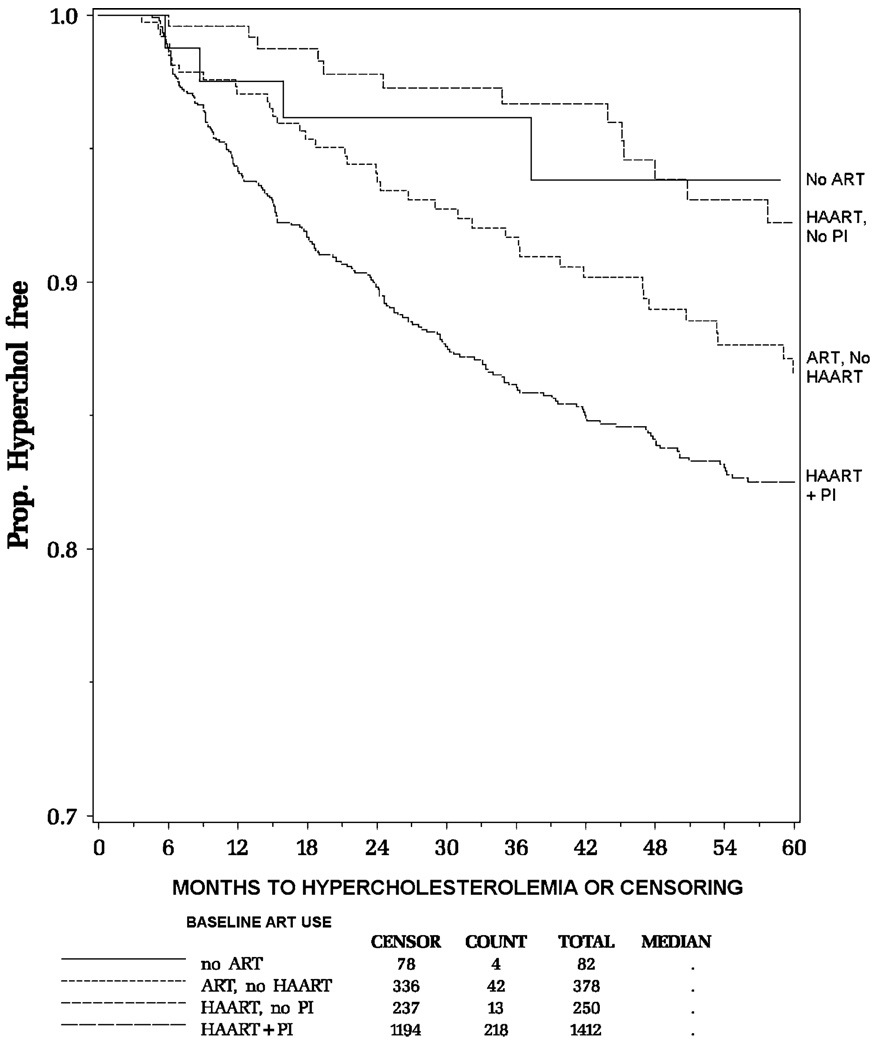
A total of 277 (13%) of the 2122 children developed hypercholesterolemia during a median follow-up of 50.4 months, for an overall IR of 3.4 cases per 100 person-years (95% CI: 3.0 to 3.9). The minimum follow-up time for the cohort was 3.7 months; the maximum follow-up time was 85.1 months. Table 2 summarizes IRs by various baseline and time-dependent factors. Younger children had higher rates (4.2 cases per 100 person-years for children younger than 6 years of age vs. 2.3 cases per 100 person-years for children 13 years of age and older). Children receiving HAART with PI at baseline had a higher rate than those receiving no ART. Figure 1 presents the Kaplan-Meier estimates for the probability of remaining free of hypercholesterolemia by baseline HAART regimen, indicating that children on HAART with PI at baseline had the lowest probability of remaining event-free (ie, the highest cumulative incidence).
TABLE 2.
IRs of Hypercholesterolemia by Selected Characteristics (N = 2122)
| Characteristic | Number | Events | Person-Years | IR per 100 Person-Years (95% CI) |
|---|---|---|---|---|
| Overall rate | 2122 | 277 | 8058.7 | 3.4 (3.0 to 3.9) |
| Baseline age (y) | ||||
| <6 | 480 | 76 | 1792.6 | 4.2 (3.3 to 5.3) |
| 6 to 9 | 693 | 102 | 2746.6 | 3.7 (3.0 to 4.5) |
| 10 to 12 | 535 | 65 | 2048.5 | 3.2 (2.5 to 4.0) |
| ≥13 | 414 | 34 | 1471.0 | 2.3 (1.6 to 3.2) |
| Baseline viral load* | ||||
| ≤400 | 767 | 119 | 2903.0 | 4.1 (3.4 to 4.9) |
| 401 to 5000 | 503 | 61 | 1924.3 | 3.2 (2.4 to 4.1) |
| 5001 to 50,000 | 515 | 57 | 2022.3 | 2.8 (2.1 to 3.6) |
| >50,000 | 272 | 34 | 940.2 | 3.6 (2.5 to 5.0) |
| PI use at baseline | ||||
| No | 680 | 49 | 2657.8 | 1.8 (1.4 to 2.4) |
| Yes, nonboosted | 1027 | 174 | 3957.4 | 4.4 (3.8 to 5.1) |
| Yes, boosted | 415 | 54 | 1443.5 | 3.7 (2.8 to 4.9) |
| NNRTIs at baseline | ||||
| No | 1404 | 180 | 5275.6 | 3.4 (2.9 to 3.9) |
| Yes | 718 | 97 | 2783.1 | 3.5 (2.8 to 4.2) |
| NRTIs at baseline | ||||
| None | 93 | 6 | 323.5 | 1.8 (0.7 to 4.0) |
| 1 | 343 | 64 | 1299.6 | 4.9 (3.8 to 6.3) |
| ≥2 | 1686 | 207 | 6435.6 | 3.2 (2.8 to 3.7) |
| Baseline HAART use | ||||
| HAART with PI | 1412 | 218 | 5307.2 | 4.1 (3.6 to 4.7) |
| HAART without PI | 250 | 13 | 966.5 | 1.3 (0.7 to 2.3) |
| ART without HAART | 378 | 42 | 1505.0 | 2.8 (2.0 to 3.8) |
| No ART | 82 | 4 | 280.0 | 1.4 (0.4 to 3.7) |
| Time-dependent HAART use† | ||||
| HAART with PI | 1662 | 248 | 5182.9 | 4.8 (4.2 to 5.4) |
| HAART without PI | 459 | 7 | 949.5 | 0.7 (0.3 to 1.5) |
| ART without HAART | 640 | 19 | 1330.8 | 1.4 (0.9 to 2.2) |
| No ART | 594 | 3 | 595.5 | 0.5 (0.1 to 1.5) |
| Blinded | 1 | 0 | 0.008 | 0.0 (0.0 to 0.005)‡ |
Sixty-five children were missing a baseline viral load.
Numbers do not add up to 2122, because children can contribute time to more than 1 exposure group during follow-up.
One-sided, 97.5% CI.
FIGURE 1.
Estimated probability of remaining free of hypercholesterolemia, by baseline HAART regimen.
Most children used HAART with PI at some point during follow-up (1662 [78%] of 2122 children). The IR of hypercholesterolemia during HAART with PI use was 4.8 cases per 100 person-years (95% CI: 4.2 to 5.4). In contrast, the IR during use of HAART without PI was significantly lower, with 0.7 cases per 100 person-years (95% CI: 0.3 to 1.5), similar to the rate during periods of no ARTuse (0.5 cases per 100 person-years; 95% CI: 0.1 to 1.5). When children were using ART that was not a HAART regimen (which could include regimens with or without PIs), the IR was 1.4 cases per 100 person-years (95% CI: 0.9 to 2.2). Among children who were on a regimen of ART without HAART at some point during the study period, 11% of the person-time included PI use. The intermediate risk of hypercholesterolemia in this group is likely explained by the use of PIs by some children.
Boosted PI use, nonboosted PI use, and NNRTI use as well as younger age, lower viral load, and self-reported perfect adherence were significant risk factors for development of incident hypercholesterolemia (Table 3). The effects of boosted and nonboosted PIs were particularly strong and similar in magnitude. The adjusted HR for use of lopinavir-boosted PI was 14.0 (95% CI: 6.75 to 29.2), similar to that for boosted PI use that did not include lopinavir (13.5; 95% CI: 6.22 to 29.2). In addition, although we found that NNRTIs generally increased risk, there was no difference in the risk conferred by the specific use of efavirenz relative to that of other NNRTIs (primarily nevirapine) (for efavirenz use, HR = 1.41, 95% CI: 1.05 to 1.90; for other NNRTI use, HR = 1.25, 95% CI: 0.91 to 1.71). The use of NRTIs was not associated with the risk of developing hypercholesterolemia (including the specific use of stavudine). Children who, at a baseline interview of the person primarily responsible for medication dosing (the parent or guardian or the child), reported perfect adherence to ART use during the previous 3 days and those children with a nondetectable or low viral load were also more likely to develop hypercholesterolemia than were children with imperfect adherence or those with a higher viral load. Gender, race, an AIDS-defining condition at baseline, BMI, and CD4% at baseline showed no significant association for risk of hypercholesterolemia.
TABLE 3.
Predictors of Hypercholesterolemia Based on Univariate and Multivariate Cox Models (N = 1936)
| Characteristic | Unadjusted* HR (95% CI) |
P | Adjusted† HR (95% CI) |
P |
|---|---|---|---|---|
| ART use during follow-up period | ||||
| No ART use | 1.00 | 1.00 | ||
| Nonboosted PI use | 8.56 (4.14 to 17.7) | 8.65 (4.19 to 17.9) | ||
| Boosted PI use | 11.6 (5.66 to 23.9) | <0.001 | 13.9 (6.73 to 28.6) | <0.001 |
| NNRTI use | 1.27 (0.99 to 1.63) | 1.33 (1.04 to 1.71) | ||
| NRTI use | 1.56 (0.21 to 11.4) | 1.53 (0.21 to 11.1) | ||
| Viral load (copies/mL) | ||||
| ≤400 | 1.00 | 1.00 | ||
| 401 to 5000 | 0.77 (0.56 to 1.06) | 0.09 | 0.93 (0.68 to 1.28) | 0.01 |
| 5001 to 50,000 | 0.67 (0.48 to 0.93) | 0.63 (0.45 to 0.89) | ||
| >50,000 | 0.84 (0.56 to 1.25) | 0.59 (0.39 to 0.90) | ||
| Age, y | ||||
| ≥13 | 1.00 | 1.00 | ||
| 10 to 12 | 1.32 (0.87 to 2.01) | 0.08 | 1.35 (0.89 to 2.06) | 0.04 |
| 6 to 9 | 1.55 (1.05 to 2.29) | 1.66 (1.12 to 2.48) | ||
| <6 | 1.64 (1.08 to 2.48) | 1.67 (1.10 to 2.55) | ||
| Adherence at baseline | ||||
| <100% | 1.00 | 1.00 | ||
| 100% | 1.79 (1.19 to 2.69) | 0.002 | 1.62 (1.07 to 2.45) | 0.01 |
Unadjusted ART model contains each time-dependent ART variable; reference group is no ART use.
Model includes all variables listed in the table.
DISCUSSION
This is the first large prospective cohort study to examine the effect of PIs and other antiretroviral medications on the incidence of hypercholesterolemia among an HIV-infected pediatric population. In addition to the 13% of children with hypercholesterolemia at baseline, 13% developed hypercholesterolemia during the course of follow-up. Furthermore, the IR seemed to be constant over the period of follow-up, suggesting that the proportion of children with hypercholesterolemia is likely to continue to increase over time. We found that the use of regimens containing PIs greatly increased this incidence. Mechanisms hypothesized to explain the association between PIs and elevated lipid levels include their potential roles in increasing the synthesis of lipids by liver enzymes19 and inhibiting the proteins involved in lipid metabolism and adipocyte differentiation.20,21 NNRTI use, younger age, optimal viral suppression, and self-reported perfect adherence were also associated with increased incidence.
A concerning clinical implication of our findings is the potential for development of premature atherosclerotic disease in this group. Although longitudinal studies of the consequences of dyslipidemia in HIV-infected children have yet to be reported, it has been noted that HIV-infected children treated with HAART have elevations in their cholesterol similar to those seen in patients heterozygous for familial hypercholesterolemia and may have a similar risk for premature atherosclerotic disease.15 In the Bogalusa Heart Study, autopsies were performed on subjects aged 2 to 39 years. High BMI, elevated blood pressure, elevated serum concentrations of total and LDL cholesterol, and elevated triglycerides in childhood correlated with the extent of fibrous plaques found in the coronary arteries and aorta in children and young adults.22 The association of HIV and its management with other risk factors for cardiovascular disease is unclear. An earlier cross-sectional study in PACTG 219C found a positive association between hypercholesterolemia and elevated systolic blood pressure on univariate but not multivariate analysis and no association with BMI.11 Similar to this earlier study, we found younger age to be associated with increased incidence on multivariate analysis, adjusting for viral load and adherence. Although the explanation for this finding remains unclear, the youngest patients, who will have the longest duration of PI exposure, were the most likely to develop hypercholesterolemia.
Although clinical management guidelines have recently been updated,23,24 they do not specifically address hypercholesterolemia in HIV-infected children and youth. For HIV-infected adults with dyslipidemia, the Adult AIDS Clinical Trials Group (AACTG) recommends following the National Cholesterol Education Program Guidelines, which stress dietary and lifestyle modifications as the initial intervention. 25,26 Switching the HAART regimen of patients with dyslipidemia to a PI-sparing regimen is an attractive option. In at least 2 studies, switching from PIs to efavirenz has been shown to result in improvement of cholesterol and triglyceride levels, while maintaining virologic suppression.27,28 Another option is switching the PI in the regimen to atazanavir, which seems to be associated with a lower risk of dyslipidemia.29 These options may be unavailable for many perinatally infected children at this time, however, because these patients often have a long history of ART treatment before HAART or a history of poor adherence, which has resulted in the accumulation of significant antiretroviral resistance mutations. A high incidence of virologic failure has been reported in a study among children switched to atazanavir.30 In addition to formulation as a capsule only, the use of atazanavir is not yet recommended for extremely young children because of the lack of data on appropriate dosage.31
Pediatricians are beginning to use lipid-lowering medications in HIV-infected children with dyslipidemia. In our study, 15 (5.4%) of the 277 children who developed hypercholesterolemia began lipid-lowering therapy. The American Academy of Pediatrics recommends a management strategy based on risk stratification by disease status, with consideration of cardiovascular risk factors and comorbidities.23 Although “chronic inflammatory disease” is listed as a criterion for placement in tier II or the “moderate risk” tier, HIV infection is not specifically addressed. Goals of therapy are recommended for BMI, blood pressure, fasting glucose, and LDL cholesterol. LDL cholesterol goals range from ≤160 mg/dL for the lowest risk tier to ≤100 mg/dL for the highest risk tier. Therapeutic lifestyle change is recommended as the initial intervention. For patients not achieving the goal of treatment with lifestyle change, disease-specific management, including drug therapy, is recommended. The class of agents known as statins reduces cholesterol biosynthesis through competitive inhibition of 3-hydroxy-3-methylglutaryl-coenzyme A reductase, the enzyme catalyzing the early rate-limiting step in cholesterol biosynthesis. These agents are preferred for the management of patients with isolated hypercholesterolemia.32 Although some statins have been approved for use in children and are recommended in a number of clinical circumstances by the American Academy of Pediatrics for children older than the age of 10 years, most statins are metabolized by the cytochrome P450 pathway and pharmacokinetic interactions with PIs may be significant.33 The International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) is planning a safety and pharmacokinetic study of atorvastatin in children and youth being treated with a PI. This should provide useful information to facilitate the updating of dyslipidemia treatment guidelines for HIV-infected children.
Although our prospective study has significant strengths, including a large sample size facilitating a thorough assessment of risk factors and confounders, there are a number of limitations. Cholesterol measurements were generally obtained in the nonfasting state; thus, we are not able to report on LDL or HDL cholesterol or triglycerides. Nonfasting total cholesterol measurements are considered useful dyslipidemia screening tools for children and adolescents, however.34 Furthermore, there is good agreement between fasting and nonfasting total cholesterol measurements among adults when categories (high vs. normal) of cholesterol are compared.35 A study in HIV-infected children found no significant difference in group means for total cholesterol obtained in the fasting versus nonfasting state.12 Our study focuses on elevated total cholesterol as the sole metabolic complication. Adult and pediatric studies suggest that the overall prevalence of metabolic complications may be higher when one considers other disorders such as fat maldistribution, insulin resistance, bone disease, avascular necrosis, or mitochondrial toxicity.32,36
We did not collect information on factors such as diet, smoking, or family history of heart disease. Although these are important risk factors, most are unlikely to be associated with ART use; thus, they would not confound its association with hypercholesterolemia. Most children had a BMI z-score less than 2, and BMI was not associated with hypercholesterolemia risk.
Another potential limitation is that we did not consider the duration of ART exposure before the period of observation, and it is thus possible that our effect sizes were somewhat underestimated. Because inclusion of exposure duration would have likely increased the HRs for PI use, which were already large and statistically significant, the interpretation of our results is not affected.
The results of this study indicate that the use of PIs leads to a marked increase in total cholesterol levels among HIV-infected children and adolescents. Despite the potential negative consequences of treating HIV-infected children with PIs, the benefits associated with PI-based treatment outweigh the toxicities. Important questions remain, such as the incidence of other metabolic complications and their association with dyslipidemia, whether dyslipidemia in this group of children is associated with an increased risk of cardiovascular disease, and optimal management strategies for dyslipidemia in this group. Continued follow-up studies similar to PACTG 219C are needed to evaluate the long-term effects of ART on lipodystrophy, abnormal glucose metabolism, hypertension, abnormal bone mineralization, and lipid abnormalities.
ACKNOWLEDGMENTS
The authors thank the children and families for their participation in PACTG/IMPAACT 219C and the individuals and institutions involved in the conduct of PACTG/IMPAACT 219C.
Funded by the United States National Institute of Allergy and Infectious Diseases and the National Institute of Child Health and Human Development. These institutions were involved in the design, data collection, and conduct of protocol 219C but were not involved in the present analysis, the interpretation of the data, the writing of the manuscript, or the decision to submit for publication.
Supported by the Statistical and Data Analysis Center at Harvard School of Public Health, under National Institute of Allergy and Infectious Diseases cooperative agreement 5 U01 AI41110 with the Pediatric AIDS Clinical Trials Group (PACTG) and cooperative agreement 1 U01 AI068616 with the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Group.
APPENDIX
The following institutions and individuals participated in PACTG protocol 219C, by order of enrollment: Baylor Texas Children’s Hospital, Baylor, TX: Mary E. Paul, MD, Chivon D. Jackson, RN, BSN, ADN, Faith Minglana, RN, BSN, and Heidi Schwarzwald, MD; University of Florida, Jacksonville, FL: Mobeen H. Rathore, MD, Ayesha Mirza, MD, Kristy Champion, and Almer Mendoza; Chicago Children’s Memorial Hospital, Chicago, IL: R. Yogev, and E. Chadwick; University of Puerto Rico, University Children’s Hospital AIDS Program, San Juan, PR: Irma L. Febo, MD, Licette Lugo,MD, Ruth Santos, RN, and Ibet Heyer, RN; Bronx Lebanon Hospital Center, Bronx, NY; M. Purswani, S. Baksi, E. Stuard, and M. Dummit; San Juan Hospital, San Juan, PR: M. Acevedo, M. Gonzalez, L. Fabregas, and M.E. Texidor; University of Miami, Miami, FL: Gwendolyn B. Scott, MD, Charles D. Mitchell, MD, Claudia Florez, MD, and Joan Gamber; University of Medicine and Dentistry of New Jersey, Newark, NJ: Arlene Bardeguez, MD, Arry Dieudonne, MD, Linda Bettica, and Juliette Johnson; Charity Hospital of New Orleans and Earl K. Long Early Intervention Clinic, New Orleans, LA: M. Silio, T. Alchediak, C. Boe, M. Cowie, and R. Van Dyke; University of California, San Diego Mother, Child, and Adolescent HIV Program, San Diego, CA: Stephen A. Spector, MD, Rolando M. Viani, MD, MTP, Mary Caffery, RN, MSN, and Kimberly Norris, RN; Howard University Hospital, Department of Pediatrics, Washington, DC: Sohail Rana, MD, Helga Finke, MD, Patricia Yu, MS, and Jhoanna Roa, MD; Jacobi Medical Center, Bronx, NY: M. Donovan, R. Serrano, M. Burey, and R. Auguste; St. Christopher’s Hospital for Children, Philadelphia, PA: J. Chen, and J. Foster; Baystate Medical Center Children’s Hospital, Springfield, MA: B. W. Stechenberg, D. J. Fisher, A. M. Johnston, and M. Toye; Los Angeles County Medical Center/University of Southern California, Los Angeles, CA: J. Homans, M. Neely, L. S. Spencer, and A. Kovacs; Children’s Hospital Boston, Boston, MA: S. Burchett, and N. Karthas; Children’s Hospital of Michigan, Detroit, MI: E. Moore, and C. Cromer; St. Jude Children’s Research Hospital, Memphis, TN: Aditya Gaur, MD, Katherine Knapp, MD, Nehali Patel, MD, and Marion Donohoe, RN, MSN, PNP; New York University School of Medicine/Bellevue Hospital, New York, NY: Maryam Minter, RN, Thomas Hastings, Seham Akleh, and William Borkowsky, MD; The Children’s Hospital at Downstate, Brooklyn, NY: E. Handelsman, H. J. Moallem, D. M. Swindell, and J. M. Kaye; The Columbia-Presbyterian Medical Center and Cornell University–New York Presbyterian Hospital, New York, NY: A. Higgins, M. Foca, P. LaRussa, and A. Gershon; The Children’s Hospital of Philadelphia, Philadelphia, PA: Steven D. Douglas, MD, Richard M. Rutstein, MD, Carol A. Vincent, CRNP, MSN, and Patricia C. Coburn, RN, BSN; Children’s Hospital of Oakland, Oakland, CA: Ann Petru, MD, Teresa Courville, RN, MN, Katherine Eng, RN, PNP, and Karen Gold, RN, MA; University of California, San Francisco, Moffitt Hospital, San Francisco, CA: Diane W. Wara, MD, Nicole Tilton, PNP, and Mica Muscat, PNP; Children’s Hospital, University of Colorado, Denver, Aurora, CO: E. McFarland, and C. Salbenblatt; Johns Hopkins University, Pediatrics, Baltimore, MD: N. Hutton, B. Griffith, M. Joyner, and C. Kiefner; Children’s Hospital and Regional Medical Center, University of Washington, Seattle, WA: Michele Acker, MN, ARNP, Ann J. Melvin, MD, MPH, Kathleen M. Mohan, ARNP, and Suzanne Phelps, MS; Metropolitan Hospital Center, New York, NY: Mahrukh Bamji, MD, Indu Pathak, MD, Savita Manwani, MD, and Ekta Patel, MD; Children’s National Medical Center, Washington, DC: Diana Dobbins, RN, MSN, Deidre Wimbley, RN, Tracy Perron, RN, and Hans Spiegel, MD, PhD; University of Massachusetts Medical School, Worcester, MA: K. Luzuriaga, and R. Moriarty; University of Alabama at Birmingham, Birmingham, AL: R. Pass, and M. Crain; University of Maryland School of Medicine, Baltimore, MD: D. Watson, J. Farley, K. Klipner, and C. Hilyard; Schneider Children’s Hospital, New Hyde Park, NY: V. R. Bonagura, S. J. Schuval, C. Colter, and L. Campbell; Boston Medical Center, Boston, MA: Stephen I. Pelton, MD, E. R. Cooper, MD, Lauren Kay, RN, and Ann Marie Regan, PNP, MEd; University of Illinois, Chicago, IL: K. C. Rich, K. Hayani, M. Bicchinella, and J. Camacho; State University of New York, Stony Brook, NY: Sharon Nachman, MD, Denise Ferraro, RN, Jane Perillo, PNP, and Michele Kelly, PNP; North Broward Hospital District, Ft. Lauderdale, FL: Ana M. Puga, MD, Guillermo Talero, MD, James Blood, MSW, and Stefanie Juliano, MSW; Duke University, Pediatrics, Durham, NC: Carole Mathison, Kareema Whitfield, Felicia Wiley, RN, and Margaret Donnelly, PA; Harlem Hospital, New York, NY: S. Champion, M. Frere, M. DiGrado, and E. J. Abrams; Cook County Hospital, Chicago, IL: James B. McAuley, MD, Kenneth M. Boyer, MD, Maureen Haak, RN, MSN, and Jamie Martinez, MD; University of South Alabama, Birmingham, AL: Mary Mancao, MD, and Benjamin Estrada, MD; Connecticut Children’s Medical Center, Hartford, CT: Juan C. Salazar, MD, MPH, and Gail Karas, RN; University of North Carolina at Chapel Hill, Chapel Hill, NC: Tom Belhorn, MD, PhD, Jean Eddleman, ACSW, CCSW, and Betsy Pitkin, RN; Ruiz Arnau University Hospital, Bayamon, PR: W. Figueroa, and E. Reyes; State University of New York Upstate Medical University, Syracuse, NY: L. B. Weiner, K. A. Contello, W. A. Holz, and M. J. Famiglietti; Children’s Medical Center of Dallas, Dallas, TX: A. Winborn, G. Wendel; University of Florida at Gainesville, Gainesville, FL: R. Lawrence, J. Lew, C. Delany, and C. Duff; Children’s Hospital at Albany Medical Center, Albany, NY: A. D. Fernandez, P. A. Hughes, N. Wade, and M. E. Adams; Lincoln Medical and Mental Health Center, Bronx, NY: D. Gomez, J. Sinanan; Phoenix Children’s Hospital, Phoenix, AZ: J. P. Piatt, J. Foti, and L. Clarke-Steffen; Public Health Unit of Palm Beach County, West Palm Beach, FL: J. Sleasman, and C. Delaney; Medical College of Georgia, Augusta, GA: Stuart Foshee, MD, Chitra S. Mani, MD, Dennis L. Murray, MD, and Christopher White, MD; Yale University School of Medicine, New Haven, CT:Warren A. Andiman, MD, Leslie Hurst, MS, Janette de Jesus, MD, and Donna Schroeder, BS; Vanderbilt University Medical Center, Nashville, TN: G. Wilson; University of Rochester Medical Center, Rochester, NY: Geoffrey A. Weinberg, MD, Francis Gigliotti, MD, Barbra Murante, MS, RN, PNP, and Susan Laverty, RN; St. Joseph’s Hospital and Medical Center, Paterson, NJ: N. Hutchcon, and A. Townley; Emory University Hospital, Atlanta, GA: S. Nesheim, and R. Dennis; University of South Florida, Tampa, FL: P. Emmanuel, J. Lujan-Zilberman, C. Graisberry, and S. Moore; Children’s Hospital of the King’s Daughters, Norfolk, VA: R. G. Fisher, K. M. Cunnion, T. T. Rubio, and D. Sandifer; Medical University of South Carolina, Columbia, SC: G. M. Johnson; University of Mississippi Medical Center, Jackson, MS: H. Gay, and S. Sadler; Harbor-UCLA Medical Center, Torrance, CA: Margaret A. Keller, MD, Nasser Redjal, MD, Spring Wettgen, RN, PNP, and Sheryl Sullivan, LVN; Mount Sinai Medical Center, New York, NY: D. Johnson; Children’s Hospital of Los Angeles, Los Angeles, CA: J. Church, T. Dunaway, and C. Salata; Long Beach Memorial Hospital, Long Beach, CA: Susan Marks, RN, Karen Elkins, PNP, Jagmohan Batra, MD, and Audra Deveikis, MD; Robert Wood Johnson Medical School, New Brunswick, NJ: S. Gaur, P. Whitley-Williams, A. Malhotra, and L. Cerracchio; Sinai Children’s Hospital, Chicago, IL: M. Dolan, J. D’Agostino, and R. Posada; The Medical Center, Pediatric, Columbus, GA: C. Mani, and S. Cobb; Medical College of Virginia, Richmond, VA: S. R. Lavoie, and T. Y. Smith; Cooper Hospital–University Medical Center, Camden, NJ: A. Feingold, and S. Burrows-Clark; University of Cincinnati, Cincinnati, OH: J. Mrus, and R. Beiting; Columbus Children’s Hospital, Columbus, OH: M. Brady, J. Hunkler, and K. Koranyi; Sacred Heart Children’s Medical Services of Florida, Pensacola, FL: W. Albritton; St. Luke’s/Roosevelt Hospital Center, New York, NY: R. Warford, and S. Arpadi; Incarnation Children’s Center, New York, NY: A. Gershon, and P. Miller; Montefiore Medical-Albert Einstein College of Medicine, Bronx, NY: A. Rubinstein, and G. Krienik; Children’s Hospital of Los Angeles, Los Angeles, CA: A. Kovacs and E. Operskalski; San Francisco General Hospital, San Francisco, CA: D. Wara, A. Kamrin, S. Farrales, N. Tilton, and M. Muscat; Cornell University–New York Presbyterian Hospital, New York, NY: R. Johan-Liang, and K. O’Keefe; St. Louis Children’s Hospital, St. Louis, MO: K. A. McGann, L. Pickering, and G. A. Storch; North Shore University Hospital, Manhasset, NY: S. Pahwa, and L. Rodriquez; and Oregon Health and Science University, Portland, OR: P. Lewis, and R. Croteau.
REFERENCES
- 1.Gortmaker S, Hughes M, Cervia J, et al. for the Pediatric AIDS Clinical Trials Group Protocol 219 Team. Effect of combination therapy including protease inhibitors on mortality among children and adolescents infected with HIV-1. N Engl J Med. 2001;345:1522–1528. doi: 10.1056/NEJMoa011157. [DOI] [PubMed] [Google Scholar]
- 2.Gona P, Van Dyke R, Williams P, et al. Incidence of opportunistic and other infections in HIV-infected children in the HAARTera. JAMA. 2006;296:292–300. doi: 10.1001/jama.296.3.292. [DOI] [PubMed] [Google Scholar]
- 3.Storm D, Boland M, Gortmaker S, et al. for the Pediatric AIDS Clinical Trials Group Protocol 219 Study Team. Protease inhibitor combination therapy, severity of illness, and quality of life among children with perinatally acquired HIV-1 infection. Pediatrics. 2005;115:e173–e182. doi: 10.1542/peds.2004-1693. [DOI] [PubMed] [Google Scholar]
- 4.Lee G, Gortmaker S, McIntosh K, et al. for the Pediatric AIDS Clinical Trials Group Protocol 219C Team. Quality of life for children and adolescents: impact of HIV infection and antiretroviral treatment. Pediatrics. 2006;117:273–283. doi: 10.1542/peds.2005-0323. [DOI] [PubMed] [Google Scholar]
- 5.Buchacz K, Rogol A, Lindsey J, et al. for the Pediatric AIDS Clinical Trials Group 219 Study Team. Delayed onset of pubertal development in children and adolescents with perinatally acquired HIV infection. J Acquir Immune Defic Syndr. 2003;33:56–65. doi: 10.1097/00126334-200305010-00009. [DOI] [PubMed] [Google Scholar]
- 6.Vigano A, Mora S, Testolin C, et al. Increased lipodystrophy is associated with increased exposure to highly active antiretroviral therapy in HIV-infected children. J Acquir Immune Defic Syndr. 2003;32:482–489. doi: 10.1097/00126334-200304150-00003. [DOI] [PubMed] [Google Scholar]
- 7.Bitnun A, Sochett E, Dick P, et al. Insulin sensitivity and beta-cell function in protease inhibitor-treated and -naive human immunodeficiency virus-infected children. J Clin Endocrinol Metab. 2005;90:168–174. doi: 10.1210/jc.2004-0125. [DOI] [PubMed] [Google Scholar]
- 8.Sanchez Torres A, Munoz Muniz R, Madero R, et al. Prevalence of fat redistribution and metabolic disorders in human immunodeficiency virus-infected children. Eur J Pediatr. 2005;164:271–276. doi: 10.1007/s00431-004-1610-y. [DOI] [PubMed] [Google Scholar]
- 9.Bitnun A, Sochett E, Babyn P, et al. Serum lipids, glucose homeostasis and abdominal adipose tissue distribution in protease inhibitor-treated and naïve HIV-infected children. AIDS. 2003;17:1319–1327. doi: 10.1097/00002030-200306130-00006. [DOI] [PubMed] [Google Scholar]
- 10.Melvin A, Lennon S, Mohan K, et al. Metabolic abnormalities in HIV type 1-infected children treated and not treated with protease inhibitors. AIDS Res Hum Retroviruses. 2001;17:1117–1123. doi: 10.1089/088922201316912727. [DOI] [PubMed] [Google Scholar]
- 11.Farley J, Gona P, Crain M, et al. for the Pediatric AIDS Clinical Trials Group Study 219C Team. Prevalence of elevated cholesterol and associated risk factors among perinatally HIV-infected children (4–19 years old) in Pediatric AIDS Clinical Trials Group 219C. J Acquir Immune Defic Syndr. 2005;38:480–487. doi: 10.1097/01.qai.0000139397.30612.96. [DOI] [PubMed] [Google Scholar]
- 12.Lainka E, Oezbek S, Falck M, et al. Marked dyslipidemia in human immunodeficiency virus-infected children on protease inhibitor-containing antiretroviral therapy. Pediatrics. 2002;110:56–62. doi: 10.1542/peds.110.5.e56. [DOI] [PubMed] [Google Scholar]
- 13.Temple M, Koranyi K, Nahata M. Lipodystrophy in HIV-infected pediatric patients receiving protease inhibitors. Ann Pharmacother. 2003;37:1214–1218. doi: 10.1345/aph.1A444. [DOI] [PubMed] [Google Scholar]
- 14.Solorzano Santos F, Gochicoa Rangel L, Palacios Saucedo G, et al. Hypertriglyceridemia and hypercholesterolemia in human immunodeficiency virus-1-infected children treated with protease inhibitors. Arch Med Res. 2006;37:129–132. doi: 10.1016/j.arcmed.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 15.Cheseaux J, Jotterand V, Aebi C, et al. Hyperlipidemia in HIV-infected children treated with protease inhibitors: relevance for cardiovascular diseases. J Acquir Immune Defic Syndr. 2002;30:288–293. doi: 10.1097/00126334-200207010-00004. [DOI] [PubMed] [Google Scholar]
- 16.Beregszaszi M, Dollfus C, Levine M, et al. Longitudinal evaluation and risk factors of lipodystrophy and associated metabolic changes in HIV-infected children. J Acquir Immune Defic Syndr. 2005;40:161–168. doi: 10.1097/01.qai.0000178930.93033.f2. [DOI] [PubMed] [Google Scholar]
- 17.Rhoads M, Smith C, Tudor-Williams G, et al. Effects of highly active antiretroviral therapy on paediatric metabolite levels. HIV Med. 2006;7:16–24. doi: 10.1111/j.1468-1293.2005.00337.x. [DOI] [PubMed] [Google Scholar]
- 18.Carter R, Wiener J, Abrams E, et al. for the Perinatal AIDS Collaborative Transmission Study-HIV Follow-up After Perinatal Exposure (PACTS-HOPE) Group. Dyslipidemia among perinatally HIV-infected children enrolled in the PACTS-HOPE Cohort, 1999–2004: a longitudinal analysis. J Acquir Immune Defic Syndr. 2006;41:453–460. doi: 10.1097/01.qai.0000218344.88304.db. [DOI] [PubMed] [Google Scholar]
- 19.Lenhard J, Croom D, Weiel J, et al. HIV protease inhibitors stimulate hepatic triglyceride synthesis. Arterioscler Thromb Vasc Biol. 2000;20:2625–2629. doi: 10.1161/01.atv.20.12.2625. [DOI] [PubMed] [Google Scholar]
- 20.Zhang G, MacNaul K, Szalkowski D, et al. Inhibition of adipocyte differentiation by HIV protease inhibitors. J Clin Endocrinol Metab. 1999;84:4274–4277. doi: 10.1210/jcem.84.11.6234. [DOI] [PubMed] [Google Scholar]
- 21.Mooser V, Carr A. Antiretroviral therapy-associated hyperlipidemia in HIV disease. Curr Opin Lipidol. 2001;12:313–319. doi: 10.1097/00041433-200106000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Newman W, Freedman D, Voors A, et al. Relation of serum lipoprotein levels and systolic blood pressure to early atherosclerosis: the Bogalusa Heart Study. N Engl J Med. 1986;314:138–144. doi: 10.1056/NEJM198601163140302. [DOI] [PubMed] [Google Scholar]
- 23.American Academy of Pediatrics. Cardiovascular risk reduction in high risk populations. Pediatrics. 2007;119:618–621. doi: 10.1542/peds.2006-3557. [DOI] [PubMed] [Google Scholar]
- 24.McCrindle B, Urbina E, Dennison B, et al. Drug therapy of high-risk lipid abnormalities in children and adolescents: a scientific statement from the American Heart Association, Atherosclerosis, Hypertension, and Obesity in Youth Committee, Council of Cardiovascular Disease in the Young, with the Council on Cardiovascular Nursing. Circulation. 2007;115:1948–1967. doi: 10.1161/CIRCULATIONAHA.107.181946. [DOI] [PubMed] [Google Scholar]
- 25.Dubé M, Stein J, Aberg J, et al. Guidelines for the evaluation and management of dyslipidemia in human immunodeficiency virus (HIV)-infected adults receiving antiretroviral therapy: recommendations of the HIV Medical Association of the Infectious Disease Society of America and the Adult AIDS Clinical Trials Group. Clin Infect Dis. 2003;37:613–627. doi: 10.1086/378131. [DOI] [PubMed] [Google Scholar]
- 26.The Expert Panel of the National Cholesterol Education Program. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 27.McComsey G, Bhumbra N, Ma J, et al. Impact of protease inhibitor substitution with efavirenz in HIV-infected children: results of the First Pediatric Switch Study. Pediatrics. 2003;111:e275–e281. doi: 10.1542/peds.111.3.e275. [DOI] [PubMed] [Google Scholar]
- 28.Vigano A, Aldrovandi G, Giacomet V, et al. Improvement in dyslipidaemia after switching stavudine to tenofovir and replacing protease inhibitors with efavirenz in HIV-infected children. Antivir Ther. 2005;10:917–924. [PubMed] [Google Scholar]
- 29.Johnson M, Grinsztejn B, Rodriguez C, et al. 96-Week comparison of once-daily atazanavir/ritonavir and twice-daily lopinavir/ritonavir in patients with multiple virologic failures. AIDS. 2006;20:711–718. doi: 10.1097/01.aids.0000216371.76689.63. [DOI] [PubMed] [Google Scholar]
- 30.Macassa E, Delaugerre C, Teglas J, et al. Change to a once-daily combination including boosted atazanavir in HIV-1-infected children. Pediatr Infect Dis J. 2006;25:809–814. doi: 10.1097/01.inf.0000234069.37972.94. [DOI] [PubMed] [Google Scholar]
- 31.Working Group on Antiretroviral Therapy and Medical Management of HIV-Infected Children. Guidelines for the use of antiretroviral agents in pediatric HIV infection. [Accessed June 29, 2007];2006 :1–126. Available at: http://aidsinfo.nih.gov/ContentFiles/PediatricGuidelines.pdf.
- 32.Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. 2005;352:48–62. doi: 10.1056/NEJMra041811. [DOI] [PubMed] [Google Scholar]
- 33.Fichtenbaum C, Gerber J, Rosenkranz S, et al. the NIAID AIDS Clinical Trials Group. Pharmacokinetic interactions between protease inhibitors and statins in HIV seronegative volunteers: ACTG Study A5047. AIDS. 2002;16:569–577. doi: 10.1097/00002030-200203080-00008. [DOI] [PubMed] [Google Scholar]
- 34.US Preventive Services Task Force. Screening for lipid disorders in children: US Preventive Services Task Force recommendations statement. Pediatrics. 2007;120:e215–e219. doi: 10.1542/peds.2006-1812. [DOI] [PubMed] [Google Scholar]
- 35.Craig S, Amin R, Russell D, et al. Blood cholesterol screening: influence of fasting state on cholesterol results and management decisions. J Gen Intern Med. 2000;15:395–399. doi: 10.1046/j.1525-1497.2000.03509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McComsey G, Leonard E. Metabolic complications of HIV therapy in children. AIDS. 2004;18:1753–1768. doi: 10.1097/00002030-200409030-00004. [DOI] [PubMed] [Google Scholar]