Abstract
Cyanide (CN = HCN + CN−) is a renowned poison and neurotoxicant that is prevalent throughout the environment. Despite a plethora of studies conducted over the last half century, relatively little is known of its potential to cause adverse health outcomes at sublethal exposures. CN exposure is normally determined from blood, but because CN is rapidly metabolized and cleared from this compartment (t1/2 < 1 h), it is common for several half-lives to have passed before blood samples are drawn for analysis. This variable, coupled with a very narrow toxic index and metabolic diversity within the human population, have rendered accurate assessment of CN exposure, and consequently any predictions of possible adverse health outcomes, highly problematic. Prior studies by us showed the potential of Cys-SCN adducts within human serum albumin (HSA) to act as retrospective surrogates of CN exposure. Here we report the discovery of a stable, SCN adduct at Cys567 formed by reaction of CN with the C-terminal Cys558Cys567 disulfide bond of HSA. Treatment of HSA purified from human serum with base in guanidine hydrochloride releases a readily detectable, uniquely modified, C-terminal-19 mer peptide from Cys567-SCN moieties in all the samples examined thus far. Inclusion of a HSA-Cys567-S13C15N labeled internal standard permits quantitation of the Cys567- SCN adduct by LC-MS/MS in Selective Reaction Monitoring (SRM) of the surrogate peptide with high sensitivity and good precision. Reaction of CN in vitro with the Cys558Cys567 disulfide bond in HSA is specific, rapid, and concentration dependent within a putative, physiologically-relevant range. Data from various human sera demonstrate the potential usefulness of this adduct as a biomarker of CN exposure.
Keywords: Hydrogen cyanide, plasma protein adducts, human serum albumin, biomarkers
Introduction
Recent advances in the capability of mass spectrometry to identify trace quantities of large and small biomolecules have enhanced “adductomics”; the study of reactive xenobiotics that form essentially irreversible chemical bonds with biomolecules. Bioreactive xenobiotics are encountered from numerous sources and can be either inherently reactive or become activated by enzymes within the body. Potentially affected biomolecules include nucleotides within DNA and RNA, low molecular weight compounds such as glutathione and Cys, and proteins. Adducts on protein and DNA normally remain long after the parent compound has cleared the body as judged from conventional analysis of urine, blood and even tissue thereby rendering them potential retrospective biomarkers of exposure.
CN is a poisonous compound that can inflict almost immediate death (1,2). Nonlethal exposures associate with neurological impairments including konzo (3–5), tobacco amblyopia (6,7), and motor neuron disease (8). Parkinsonism-like symptoms also occur with nonlethal CN exposure as do changes in respiratory, cardiovascular, and thyroid function. CN and its sodium and potassium salts are absorbed by inhalation and/or by dermal and oral routes of exposure. Because CN is a very weak acid (pKa = 9.22), once in the body it exists almost entirely as HCN, whose half-life in blood is less than 1 h (9,10). Sources of CN include: cyanogenic glycosides as in cassava root (11,12); industrial processing (13); vehicle exhaust; and cigarette and residential and commercial fire smokes (14,15).
Our studies have focused on developing a retrospective, quantitative assay of CN exposure that can be used to better assess the potential of CN to affect human health. We demonstrated previously that stable Cys-SCN adducts are formed via the reaction of CN with certain disulfides within HSA (16). Digestion of reduced HSA containing Cys-SCN adducts by trypsin at pH 8 caused cyclization of the Cys-SCN adducts to 2-iminothiazoline-4-carboxylyl/2-aminothiazolidine-4-carboxylyl itcCys tautomers (see Table of Contents Figure) with concomitant release from the upstream peptide. Detection of these uniquely modified, itcCys peptides was readily accomplished by nano LC-MS/MS with a linear ion trap mass spectrometer. Because of the long biological half-life of HSA, we postulated that quantitation of HSA Cys-SCN adducts could potentially provide a much more reliable estimate of CN exposure than do current biomarker assays of CN or its metabolites (16). We report here, the discovery of a Cys567-SCN adduct that is formed in a CN concentration-dependent reaction at the most C-terminal Cys558 Cys567 disulfide bond in HSA. A technically straight-forward, quantitative assay for the itcCys567 19-mer peptide surrogate released by GuHCl/NaOH treatment of HSA is described. Serum concentrations of the HSA Cys567-SCN adduct in a small cohort of healthy human volunteers, a CN suicide victim, and a commercial serum containing a higher than normal level of the 567Cys-SCN adduct are presented.
Experimental Procedures
Reagents
Gel code Safe Protein Stain, 4–20% Precise gels and BCA protein assay reagent with BSA as the standard were from Thermo, Rockland, IL. DTNB, KCN, K13C15N, highly purified GuHCl, sodium hydroxide and NH4OH were from Sigma-Aldrich, Saint Louis, MO; DTT, mass-spectral grade HOAC, and NH4HCO3 were from Fluka Biochemica, Milwaukee, WI; Microcon YM-10 filters or Amicon 10K-Ultra filters were from Millipore Corporation, MA; the Bio-Vortexer was from Biospec Products Inc., Bartlesville, OK; lyophilized human plasma and human serum samples were from Sigma-Aldrich or Thermo; human serum (lipid stripped) used for preparation of the Cys567S 13C15N labeled HSA adduct and in many other of the experiments was from Bioresource Technology, Miramar, FL. Whole blood and serum/plasma samples without identifiers were obtained from a healthy cohort of employees of the New York State Dept. of Health, Albany, NY, using an IRB approved protocol. Whole blood and serum/plasma from a suicide victim was obtained within 3 h after death. The HSA homologous-C-terminal peptide H-Ala-Asp-Asp-Lys-Glu-Thr-Cys-Phe-Ala-Glu-Glu-Gly-Lys-Lys-Leu-Val-Ala-Ala-Ser-Gln-Ala-Ala-Leu-Gly-Leu-OH (purity > 90% by HPLC, theoretical mass (M+H+) 2572.9, found (M+H+) 2574.2). was from AnaSpec, San Jose, CA. The Cys corresponds to Cys567 in HSA. The two underlined alanines each contain 3 deuterium atoms in the methyl side chain.
General Methods
Protein concentration
Protein containing solutions were diluted with 0.1% SDS; each was measured in triplicate. Twenty μL of the sample and serial dilutions of the BSA standard supplied in the kit were added to 0.2 mL of the BCA reagent in a 96-well plate, mixed and incubated at room temperature for ~60 min. Color development was read at 570 nm.
SDS PAGE Electrophoresis
Protein solutions were diluted with sample buffer, heated to 80°C for 5 min and separated on the 4–20% gels using the HEPES/SDS running buffer and conditions provided by the manufacturer. The gels were washed extensively with water, and the proteins visualized with the Gel-code dye.
LC-MS/MS
Nano LC-MS/MS was performed with a ThermoFinnigan (San Jose, CA) LTQ ion trap mass spectrometer coupled to a Dionex (Bannockburn, IL) Ultimate 3000 nano-LC system. LTQ XCalibur software (Version 2.0) was used for system operation and data analysis. The LC solvents in MS grade water were: A: 0.1% formic acid; 1% CH3CN and B; 0.1% formic acid: 95% CH3CN. Peptides were separated on a New Objective (Woburn, MA) 75 μm × 10 cm C18 PicoFrit nano-LC column. This nano-LC column is part of a New Objective PicoView nano-Spray Ionization (NSI) source interfaced to the LTQ. Analyses were conducted in the positive ionization mode, at an ESI voltage of 2.0 kV, and heated metal capillary temperature of 150°C. The precolumn is a Dionex Acclaim Pepmap C18100 (300 μm i.d. × 5 mm). Samples (5 μL) are loaded onto the pre-column at 40 μL/min off-line such that the effluents from the sample and wash solutions (100% A) are sent to waste. In-line elution is either by gradient for the samples analyzed by LC-MS/MS (250 nL/min: 5% B to 85% B over 85 min followed by a 5 min wash at 85% B) and isocratic for the samples analyzed by LC-MS/MS (70% solvent B for 25 min at 600 nL/min followed by a 5 min wash at 100% B).
The data-dependent “triple play” method was used to acquire molecular weight, charge state, and sequence information. The “triple play” method started with a molecular weight scan (m/z 440–2000, average of 3 × 50 msec scans), followed by a zoom scan (isolation width m/z 10 window, 10 × 50 msec scans) of a data dependent selected signal, and then an MS/MS (isolation width of m/z 1.5 or 2.5, normalized collision energy 40%, activation Q = 0.25, activation time of 30 msec, 3 × 300 msec scans) of the selected signal. The MS/MS spectra were analyzed using the SEQUEST protein identification algorithm by searching a full human protein database derived from GenBank. Amino-terminal modified Cys peptides were identified by SEQUEST using a dynamic modification to Cys of 26 amu, a peptide mass tolerance of 2.5 amu and a fragment ion tolerance of m/z 1.3. These parameters capture and identify the addition of both CN (26 amu minus 1 H amu = plus 25 amu) and 13C15N (plus 27 amu) in a single MS/MS spectra. The modification of Cys was also confirmed manually in MS/MS spectra, and through observation of the isotope pattern of 13C15N/CN of labeled peptides evident in the corresponding zoom scan spectra.
Once the structure of the itcCys-C-terminal peptide was established, analysis of it was accomplished using the isocratic separation method and employing MS/MS in the SRM mode. The proportion of endogenous (CN) versus labeled (13C15N) itcCys peptides released from HSA was determined from the b12 ion at m/z 1272 peak area for the endogenous peptide and the b12 ion at m/z 1274 peak area for the labeled peptide, 28% (theoretical 31%) of the m/z 1272 peak area was subtracted from the m/z 1274 peak area to correct for isotope overlap. This value was experimentally determined from the peak areas of the m/z 1272 and 1274 fragment ions in the itcCys567 peptide released from HSA to which no exogenous 13C15N had been added. In experiments utilizing the deuterated (D6) peptide standard, peak area for the product ions at m/z 1272 (CN) or m/z 1274 (13C15N; corrected for m/z 1272 contribution) versus m/z 1278 was used.
MS/MS was carried out on (M+2H)2+ at m/z 967.4 using an isolation width of 10 m/z. The scan ranges of the 3 main product ions were m/z 1130–1140, 1200–1210, and 1271–1281. The final peak area of each istopically distinct itcCys peptide component within the m/z 1271.00–1281.00 window was obtained by manual adjustment of the baseline, which was always from the beginning of the peak to a point on the backend after 0.2 min.
Preparation of the deuterium labeled peptide standard
Cyanylation of the peptide Cys was adapted from Vanaman and Stark (17). A 0.3 M DTNB solution was prepared by dissolving 120 mg of DTNB in 7.5 mL 0.1 M HEPES, pH 7.5, adjusting the pH to 7.5 with 0.1 M NaOH, and bringing the final volume to 10 mL with the HEPES buffer. To 2.6 mg of peptide (1 μmol) in an Eppendorf 1.5 mL tube were added 0.1 mL of the DTNB solution (3 μmol) and another 25 μL of the HEPES buffer. The mixture was rocked for 15–30 m and clarified by centrifuging at maximum speed for 3 m. The supernatant was transferred to a clean tube and 50 μL of KCN in water (40 mg/mL = 30 μmol) was added. After 30 m at room temperature, the reaction was stopped by the addition of 50 μL of HOAc.
Purification of the cyanylated peptide was accomplished with a Varian ProStar Model 210 Solvent Delivery Module equipped with a Varian ProStar Model 320 UV/Vis Detector in line with a Pharmacia Superdex 75 10/300 GL column equilibrated in 0.1M NH4OAc, pH 4.5. Two hundred μL of the reaction were injected onto the column. The flow rate was 0.5 mL/min and the detection of eluting compounds was at 230 nm. Fractions (0.25 mL) were collected every 30 sec starting at 25 min and ending at 40 min. Two shouldered peaks eluted between 29 and 31 min, which were subsequently identified by MS/MS as the full length-SCN peptide (~90%) and the shorter itcCys peptide (~10%). Those fractions with an A230 nm absorbance >1 were combined and evaporated to dryness overnight in a speed vacuum apparatus at 40°C. The residue was dissolved in 0.1% HOAc and its absorption at 230 nm determined in triplicate samples. The cyanylated peptide concentration was determined using an extinction co-efficient determined from the absorbance at 230 nm of the parent peptide taken from three carefully weighed samples that were dissolved in 0.1% HOAc (ε230 nm = 3879 mol/L).
Preparation and Quantitation of external standard Cys567S13C15N HSA
To 50 mL of lipid-stripped serum was added 50 μL of K13C15N in water (20 mgs/mL = 0.3 μmol/mL final) and the solution incubated at room temperature overnight. It was then dialyzed overnight against 2 × 2 L of 0.2 M tris, 0.15 M NaCl, pH 7.4, and aliquots frozen at −75°C until use. Several HSA precipitates prepared as described below were dissolved in 0.3 mL of 6 M GuHCl/0.1 M NaOH and pooled. The HSA concentration was determined with the BCA reagent. The pool was again divided into 0.3 mL aliquots, and the cyanylated, deuterated (D6) peptide standard was added in triplicate to final concentrations of 800, 1600, 3200, 6400 and 12800 pmol/ml of serum. The solutions were heated to 50°C for 3 h, and the peptide mixtures isolated and analyzed exactly as described below. The concentration of the itcCys567 labeled peptide and therefore the Cys567S13C15N labeled adduct was 1640 ± 254 pmol/mL of serum (see Results for assumptions used in the calculation).
“Everyday” external standards were prepared by diluting this Cys567S13C15N standard serum with untreated, lipid-stripped serum to achieve Cys567S13C15N adduct concentrations of 62, 125 and 250 pmol/mL of serum. Each of these solutions was processed identically to the test samples (below) except that the starting volume was 0.7 mL since the exogenous, labeled standard was already present. The final solutions containing the endogenous and labeled itcCys567 peptides are stable for several months at 5°C. A 5 μL portion of each is run one or more times during the analysis of several test samples to ensure analysis integrity.
Quantification of CN adducted to Cys567 in test samples
All procedures are performed in Eppendorf tubes and a compatible centrifuge at room temperature. Serum was obtained commercially or by centrifuging coagulated whole blood at 3000 rpm for 10 min. To 645 μL was added 55 μL of the stock Cys567S13C15N serum standard prepared above (final = 125 pmol labeled adduct/mL test serum). An equal volume of saturated (NH4)2SO4 is added, and the mixtures rocked in the mini lab roller (Labnet Int. Edison, NJ) at room temperature for 5–10 min. The precipitated protein is removed by centrifuging at 13,000 rpm for 3 min. Three 0.3 mL aliquots of the (NH4)2SO4 supernatant are added to 0.6 mL of 0.12 M NaOAc, pH 4, and 23 μL of octanoic acid in a clean tube and the mixtures rocked at room temperature for 20 min in a mini lab roller. The precipitated HSA is collected by centrifuging at 10,000 rpm for 10 min and the supernatant carefully decanted. Recovered HSA is thoroughly washed with 1 mL of acetone and dried in a speed vacuum apparatus at 40°C for a minimum of 30 min. Dried HSA may be stored at 5°C for prolonged periods.
The HSA is dissolved in 0.3 mL of 6 M GuHCl/0.1 M NaOH solution (aqueous 6.6 M GuHCl and 1 M NaOH – mixed 9:1 just before use) using a Bio-Vortexer to disperse the aggregated protein. A minimum of 15 min contact with the GuHCl/NaOH solution at room temperature facilitates maximum solubility. The mixture is then incubated at 50°C for 3 h with gentle mixing (500 rpm) in an Eppendorf Thermomixer. At the end of the incubation, the original solution usually remains liquid, but may occasionally form a jelly-like solid. HOAc (2% 0.6 mL) and 23 μL of octanoic acid are added; the suspension is mixed thoroughly and rocked in the mini lab roller for a minimum of 20 min. The precipitated HSA is again separated by centrifuging for 3 min at maximum speed, and a 0.5 mL aliquot of the supernatant is carefully decanted into an Amicon 10K-Ultra filter that had been previously washed with water (0.4 mL) by centrifuging at 10,000 rpm for 10 min. Any remaining water was removed by inverting the spin filter and spinning for 5–10 sec. The solution is spun at 10,000 rpm for 10 min and a 5 μL aliquot of the run through is assayed by LC-MS/MS.
Results
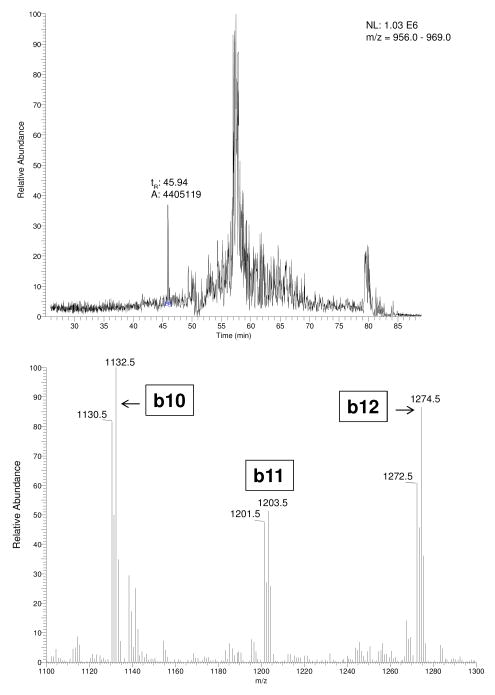
The initial “triple play” full scan spanning m/z 440 to 2000 of the peptides released from purified serum HSA previously treated with a 1:1 mixture of KCN/K13C15N and heated in 6M GuHCl/0.1 M NaOH for 3 h, indicated the presence of the 19 mer-C-terminal peptide containing itcCys567 at its N-terminus. The extracted ion current chromatogram from m/z 956 to 969 encompassing the nominal, doubly-charged molecular species of this CN-modified peptide (m/z 967.4, tR = 45.94 min) is shown in the upper panel of Fig. 1. The partial MS/MS spectrum of the CN-modified peptide spanning m/z 1100 to 1300 revealed the unique (M+2H)2+ isotope pattern of the three product ions and is shown in the lower panel of Fig. 1. The relative abundances of the b-ion doublets at m/z 1130.50/1132.46, 1201.45/1203.50, and 1272.49/1274.51 are consistent with the 1:1 mixture of KCN/K13C15N added and identify the b fragment sequences of itcCys-Phe-Ala-Glu-Glu-Gly-Lys-Lys-Leu-Valb10- Alab11-Alab12. The SEQUEST peptide/protein identification algorithm program confirmed the complete peptide sequence from a sample to which no K13C15N had been added as itcCys-Phe-Ala-Glu-Glu-Gly-Lys-Lys-Leu-Val-Ala-Ala-Ser-Gln-Ala-Ala-Leu-Gly-Leu from a full (M+2H+)2+ scan (Table I).
Figure 1.
LC-MS/MS analysis of peptides released from HSA exposed to 1:1 mixture of KCN/K13C15N, following treatment with base. Upper panel is an extracted ion chromatogram from m/z 956 to 969. The peptide eluting at tR 45.94 min is the doubly charged peptide (m/z 967.4) released by incubation of purified HSA in 6 M GuHCl/0.1 M NaOH at 50°C for 3 hr and separated by LC using the gradient and MS/MS conditions cited under Experimental procedures. The lower panel shows the partial MS/MS product ion spectrum of the doubly charged peptide (m/z 967.4) from m/z 1100–1300. The major product ions observed at m/z 1130.50/1132.46, 1201.45/1203.50, and 1272.49/1274.51 are consistent with the anticipated isotope distribution of any itcCys peptides released from HSA, and the partial HSA sequence of itcCys-Phe-Ala-Glu-Glu-Gly-Lys-Lys-Leu-Valb10-Alab11-Alab12.
TABLE 1.
Sequest AA Sequence Assignment of Peptide Released from HSA by GuHCl/NaOHa
| Sequence | AA Number | b ions | y ions | AA Number |
|---|---|---|---|---|
| itcCys | 1 | 130.0 | 1932.0 | 19 |
| Phe | 2 | 277.1 | 1803.0 | 18 |
| Ala | 3 | 340.1 | 1655.9 | 17 |
| Glu | 4 | 477.2 | 1584.9 | 16 |
| Glu | 5 | 604.2 | 1455.9 | 15 |
| Gly | 6 | 663.2 | 1326.8 | 14 |
| Lys | 7 | 791.3 | 1269.8 | 13 |
| Lys | 8 | 919.4 | 1141.7 | 12 |
| Leu | 9 | 1032.5 | 1013.6 | 11 |
| Val | 10 | 1131.6 | 900.5 | 10 |
| Ala | 11 | 1202.6 | 801.4 | 9 |
| Ala | 12 | 1273.6 | 730.4 | 8 |
| Ser | 13 | 1360.7 | 659.4 | 7 |
| Gln | 14 | 1488.7 | 572.3 | 6 |
| Ala | 15 | 1559.8 | 444.3 | 5 |
| Ala | 16 | 1630.8 | 373.2 | 4 |
| Leu | 17 | 1743.9 | 302.2 | 3 |
| Gly | 18 | 1800.9 | 189.1 | 2 |
| Leu-OH | 19 | 1914.0 | 132.1 | 1 |
HSA not treated with K13C15N was processed and assayed using the conditions described under Experimental Procedures. Amino acid assignments were made from the full (M+2H+)2+ scan 967.4@cid 35.00 (m/z 255.00–1945) of the peptide eluting at 45.82 min. Bolded b and y ions were identified from the scan.
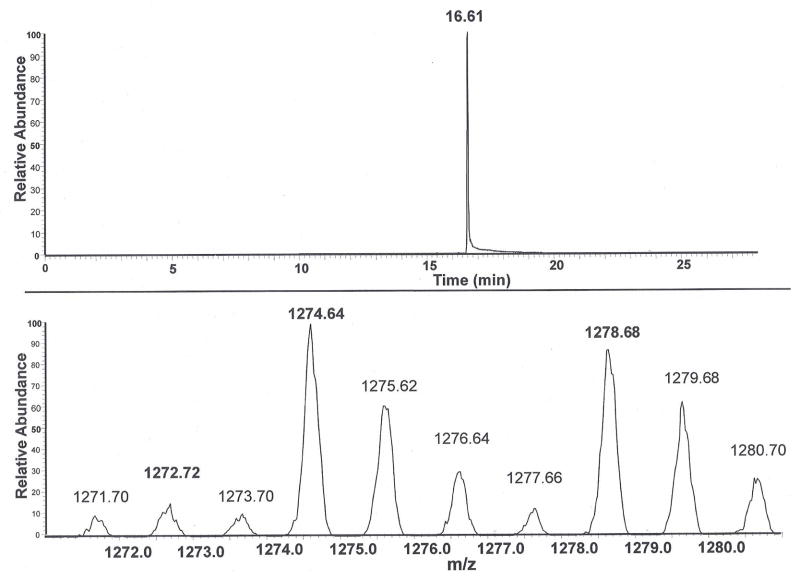
Isocratic elution and MS/MS profile of a mixture containing the peptides released from the Cys567SCN and Cys567S 13C15N adducts in HSA and the deuterium-labeled (D6), cyanylated peptide is shown in Fig. 2. The SRM chromatogram trace in the upper panel shows a single peak eluting at tR 16.61 min, and the mass range from m/z 1271.0 to 1281.0 was employed to capture the two expected isotope variants of the m/z 1272 (b12) product ion fragment from the endogenous Cys567SCN adduct (Fig. 1). The lower panel scan shows the partial product ion spectrum. The unique isotope distribution of the m/z 1272.72, 1274.64 and 1278.68 ions demonstrates the presence of the peptides released from, respectively, the itcCys567SCN and itcCys567S 13C15N adducts in HSA and the deuterium (D6) labeled synthetic peptide. Essentially identical isotope patterns were obtained using m/z windows that permit recognition of the other two major fragmentation ions at m/z 1130.5/1132.5/1136.5 and 1201.5/1203.5/1207.5 described in Fig. 1 (data not shown) thereby unequivocally establishing the sequence of the itcCys567 peptide.
Figure 2.
LC-MS/MS trace of a mixture of itcCys peptides prepared from the Cys567SCN and, Cys567S13C15N adducts in HSA and the full length-deuterium labeled (D6) cyanylated peptide. The deuterium (D6) labeled peptide was added to the HSA solubilized in 6 M GuHCl/0.1M NaOH just prior to heating at 50°C for 3 h. Upper panel is the ion current (m/z 963 to m/z 973) scan obtained by isocratic elution of the mixture and MS/MS analysis. Lower panel is a MS/MS product ion spectrum within in a restricted window of m/z 1271 to m/z 1281, showing the product b12 ion of interest in the peak eluting at 16.61 min. The uniquely high relative abundance of the 1272.72 isotope peaks at m/z 1274.64 and m/z 1278.68 confirm the presence of the peptides produced, respectively, from the itcCys567S13C15N HSA adduct and the deuterium labeled (D6) cyanylated peptide.
Integration of the peak area contribution by each peptide using the XCalibur software permits determination of their relative proportion to one another. The data presented in Figs. 4 through 7 are presented as peak area ratios between two m/z 1272.72, 1274.64 and 1278.68 product ions depending on the nature of the experiment. Since the concentration of the synthetic, deuterium labeled peptide standard is known, the serum concentration of the itcCys567 peptides released from the Cys567SCN and Cys567S 13C15N adducts in HSA can be reasonably determined (see Results pertaining to Fig. 5). The serum concentration of the endogenous Cys567SCN adduct in the human samples (Fig. 8) was determined from the estimated serum concentration of the Cys567S 13C15N HSA adduct added as an internal standard.
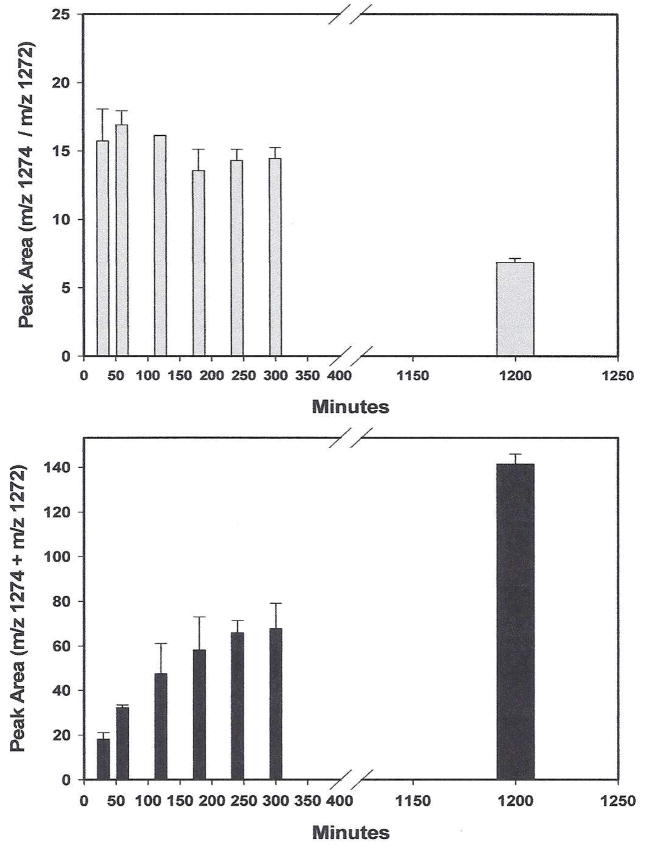
Figure 4.
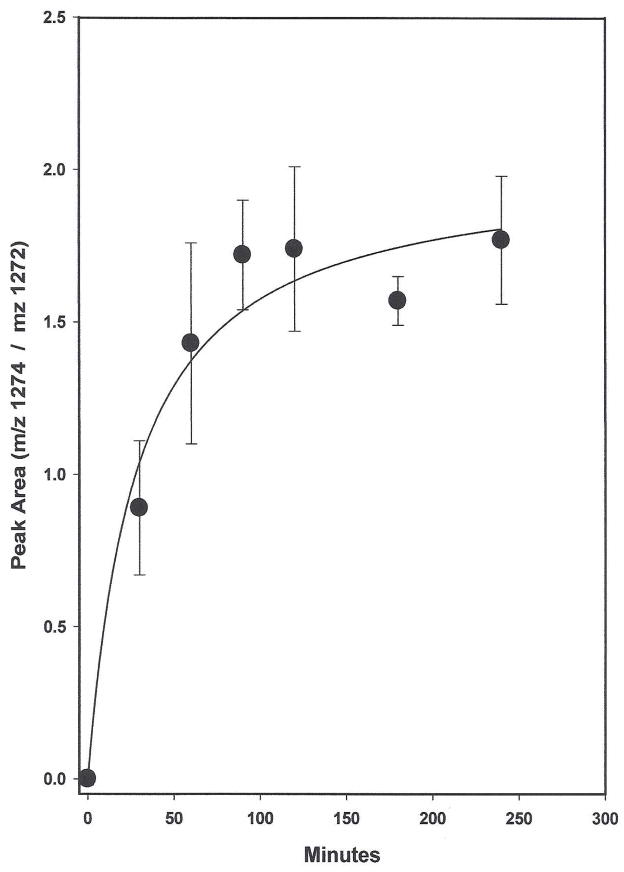
Release of 13C15N and CN itcCys567-C-terminal peptides from HSA by incubation at 50°C in GuHCl/NaOH as a function of time. Human serum was treated with an excess of K13C15N at room temperature overnight.. Triplicate HSA precipitates from it were isolated, dissolved in 0.3 ml of 6 M GuHCl/0.1 NaOH and placed in a Eppendorf Thermomixer at 50°C with gentle shaking. At the times indicated, triplicate samples were removed and the integrated peak areas of the labeled and unlabeled itcCys peptides determined. Data points are the average ± SD of the triplicate samples. Upper panel: m/z 1274/1272 peak area ratios obtained at various times from the itcCys567 13C15N and CN peptides, respectively. Lower panel: data from the same experiment except that the peak areas of the m/z 1274 and 1272 product ions of the itcCys13C15N and itcCysCN peptides, respectively, were summed.
Figure 7.
Concentration-dependent adduction of KCN to HSA Cys567. K13C15N in water was added to 6 × 1 mL aliquots of human serum spanning a concentration range 0 to 8000 pmol/mL. After incubation at 37°C for 1.5 h, the ratio of the itcCys 13C15N and itcCysCN peptides released from HSA was determined from their integrated peak areas. Values are the average ± SD of the triplicate samples. The line is simple regression using all the data points and not forced through zero.
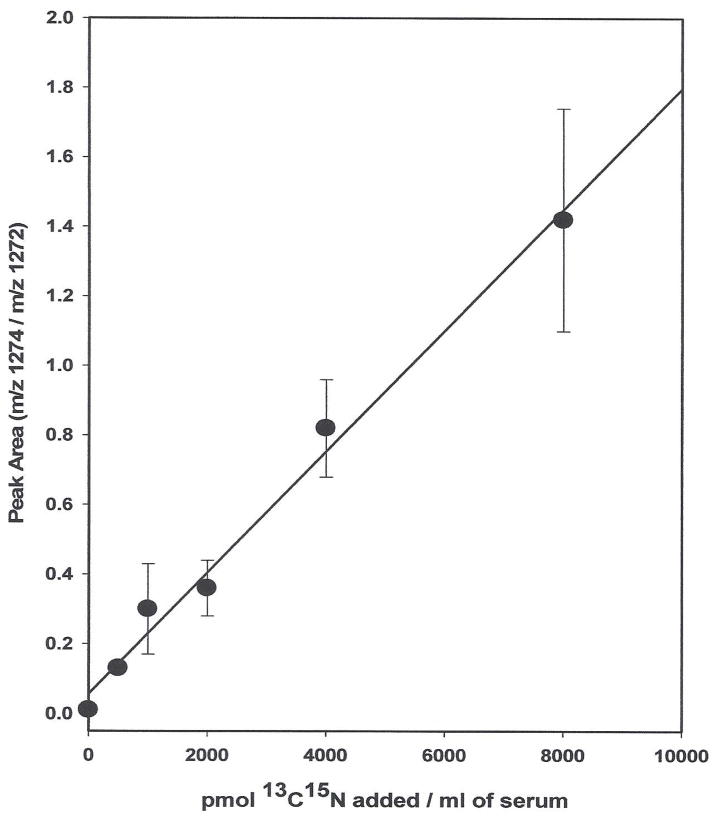
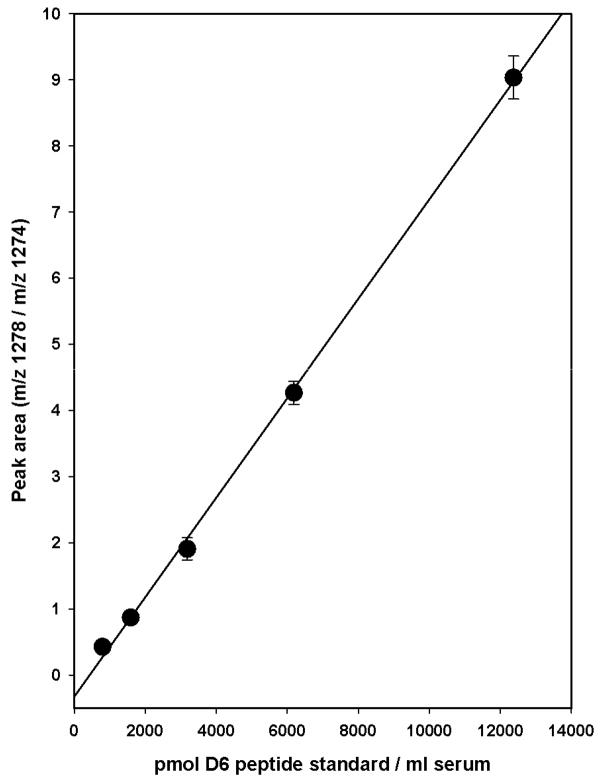
Figure 5.
Quantitation of the 13C15N Cys567 adduct in HSA using the synthetic, deuterated (D6) peptide as the standard. Several HSA precipitates prepared from human serum treated with an excess of of K13C15N were solubilized 0.3 ml of 6M GuHCl/0.1 M NaOH. The deuterated (D6)-cyanylated peptide was added to triplicate samples to mimic a serum concentration range of 800 to 12,500 pmol/mL. The mixtures were incubated at 50°C for 3 h, and the peptides purified and analyzed by LC-MS/MS in SRM. Data are the average ± SD from triplicate samples. The line is a simple regression of the data points and not forced through zero.
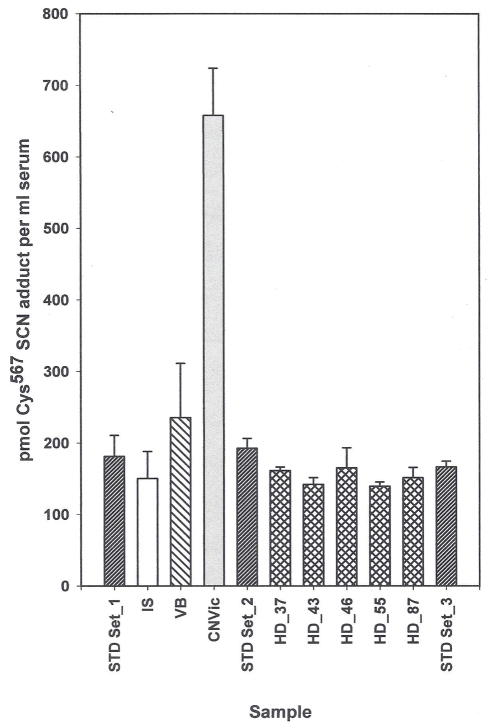
Figure 8.
HSA Cys567-SCN adduct concentration in human serum samples. The internal standard concentration was 125 pmol of itcCys567S13C15N adduct/mL of serum.. STD set 1, 2, 3: “everyday” external 13C15N itcCys567 peptide standards containing 248, 124 and 62 pmol of 13C15N adduct per ml of serum. Sets 1 to 3 are the same samples assayed at different times during the run to ensure the integrity of the assay. Values are the average ± SD from each concentration. Values for the remaining samples are the average ± SD of triplicate samples containing 125 pmol of the labeled adduct per ml of serum added as an internal standard. IS: the untreated serum used to prepare the “everyday” external standards and treated as a test sample. VB: a commercial serum sample from Valley Biomedical that consistently shows an elevated level of adduct relative to other commercial samples. CNVic: a CN overdose- suicide victim whose serum was obtained approximately 3 h after death. HD-37, 43, 46, 55 and 87: male and female sera from nonsmoking healthy NYS Health Department volunteers that were randomly chosen from an 89 member cohort.
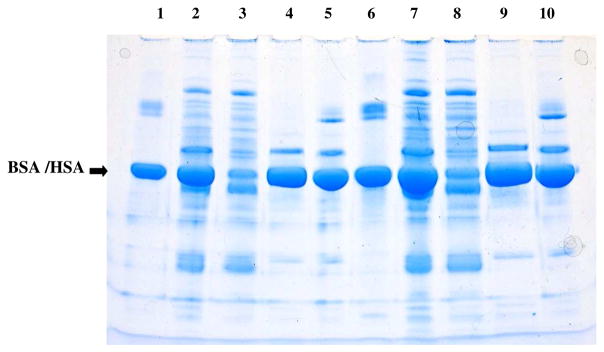
Purification and recovery of the HSA as described under Experimental Procedures is illustrated in the SDS-PAGE gel shown in Fig. 3. For comparison, BSA from the commercially available BCA protein assay kit was used as the standard. The gel was purposely overloaded to visualize contaminating proteins and demonstrate that little if any HSA is lost in the (NH4)2SO4 precipitate or during any other step. Lane protein loads were adjusted for volume differences among the various fractions and assumed a 100% recovery of HSA from the starting serum in every fraction. Visual inspection of the gel shows that the (NH4)2SO4 precipitation removes most of the non-HSA proteins, including the immunoglobulins, and that little HSA is lost in this fraction. Precipitation of the highly purified HSA from the (NH4)2SO4 supernatant at pH 4 with octanoic acid is essentially quantitative. Not shown is that no protein is detectable in the supernatant solutions from which the HSA was precipitated at pH 4 with octanoic acid.
Figure 3.
SDS-Page electrophoresis of proteins present in fractions produced during processing of HSA. Lane 1: 4.4 μg BSA from BCA protein quantitation kit; Lane 2: starting serum; Lane 3: ammonium sulfate precipitate; Lane 4: ammonium sulfate supernatant; Lane 5: octanoic acid precipitated HSA used in the peptide assay. Lanes 6–10 contain double the concentrations of lanes 1–5. Conditions were as described under Experimental Procedures.
Initial time, temperature and condition experiments designed to optimize the release of the HSA C-terminal peptide from the Cys567−SCN adduct indicated that sufficient CN could be formed to react with the HSA Cys558Cys567 disulfide, thereby artificially increasing the amount of the Cys567SCN adduct present. To ensure that this reaction did not confound the results obtained under the peptide release conditions finally adopted, HSA containing a mixture of Cys567-S13C15N and Cys567-SCN adducts was prepared. The level of Cys567-SCN adduct present was endogenous. The proportion of the peptides released was determined as a function of incubation time at 50° in 6 M GuHCl/0.1 M NaOH by integration of the peak areas attributed to the b12 ions at m/z 1274 and 1272. The results of this experiment are presented in Fig. 4. As shown in the upper panel, no detectable change occurred in the ratio of the labeled/unlabeled itcCys567 peptides released during the first 5 h, demonstrating that any CN formed in the reaction during this time is insufficient to react with the Cys558Cys567 disulfide bond in HSA and measurably increase the amount of the Cys567-SCN adduct from that present at 30 min. After incubation for 20 h, however, the peptide ratio produced from the Cys567-S13C15N and Cys567-SCN adducts was clearly lower, consistent with an increase in the Cys567-SCN adduct concentration. The integrated peak areas at m/z 1274 and 1272 are the sums of, respectively, the labeled and unlabeled peptides released in this experiment are shown in the bottom panel of Fig. 4. During the initial 3 h, a progressive increase in the peak area sum occurred, which essentially leveled between 3 and 5 h; indicative of maximum release. At 20 h incubation, however, the peak area sum was much larger that would have predicted from extrapolation of the 3 to 5 h results; again consistent with formation of the Cys567-SCN adduct during prolonged incubation. Based on these results, a 3 h incubation time in 6 M GuHCl/0.1 M NaOH at 50°C was adopted for all future experiments; essentially maximum release of the Cys567-SCN peptide is achieved under these conditions without any confounding Cys567-SCN adduct formation occurring due to the chemical production of CN during the reaction.
The intent of extending the amino acid sequence beyond the Cys residue in the D6 standard was an attempt to more closely mimic the rate of peptide release that occurs in HSA. We subsequently found that conversion of the intact (D6) cyanylated peptide to the itcCys peptide was much more facile than in the corresponding adduct in HSA. Since the intact SCN (D6) peptide is converted to the itcCys (D6) peptide under the conditions used for peptide release from HSA, the 10% itcCys (D6) peptide formed during preparation of the cyanylated standard does not confound its use (see Preparation of the deuterium labeled peptide standard in Experimental Procedures). The HSA Cys567- S13C15N adduct concentration in the commercial serum that was prepared for use as the standard was determined by adding known concentrations of the deuterium (D6)-labeled peptide standard to the HSA purified from the treated serum, releasing the itcCys peptides by treatment with 6 M GuHCl/0.1 M NaOH, and measuring the peak area proportion of the two peptides by LC-MS/MS (Fig. 5). The serum concentration of the deuterated (D6) peptide standard added was estimated from the protein concentration in the 6 M GuHCl/0.1 M NaOH solution (18 mg/ml); and the assumptions that the HSA is100% recovered from serum, that the molecular weight of HSA is 66,500, and that HSA constitutes 60% of the total serum protein, A linear relationship was obtained over the entire concentration range of the deuterium (D6) labeled peptide standard used. Where the peak areas of the two peptides equal 1, the concentration of the Cys567−S 13C15N adduct is 1640 ± 254 pmol/mL of serum.
The time dependent adduction of 13C15N to Cys567 in serum HSA and the 13C15N concentration dependence on the reaction are shown in Figs. 6 and 7, respectively. Literature estimates of the red blood cell to plasma partition vary (18–20). Here, a value of 20 was assumed in determining the concentrations of K13C15N added to serum in both experiments. The 13C15N dose of 6000 pmol/mL serum used in the time dependent experiment approximates a normally lethal dose of 120 nmol CN/mL in whole blood. As illustrated in Fig. 6, incorporation of approximately half of the 13C15N finally adducted to Cys567 occurred within the first 30 min and by 60 min the adduction had essentially subsided, demonstrating the rapidity of the reaction. In the concentration-dependent-incorporation experiment shown in Fig. 7, the 13C15N doses span putative, physiologically-relevant-whole blood concentrations ranging from near normal to above lethal. Reaction was stopped after incubation at 37°C for 90 min. The linear relationship obtained demonstrates that over this concentration range, the adduction of 13C15N to Cys567 is directly proportional to the CN serum concentration. In other experiments not included here, concentration-dependent- linear relationships existed among all three of the differentially labeled itcCys -C-terminal peptides used in these studies; thereby allowing the employment of various strategies to characterize the reaction of CN with the Cys558Cys567 disulfide and to quantitate the Cys567-SCN and Cys567-S13C15N adducts present in HSA.
Figure 6.
Time course of 13C15N adduction to Cys567 in HSA. K13C15N in water (20 mg/mL) was added to 10 ml of untreated serum to a final concentration of 6000 pmol/mL. Aliquots (18 × 0.3 mL) were immediately added to Eppendorf tubes equilibrated at 37°C in an Eppendorf Thermomixer. At 30, 60, 90, 120, 180 and 240 min, 0.3 mL of saturated ammonium sulfate was added to each of 3 tubes and the peptides released from HSA were assayed in the usual manner. Zero time samples were processed immediately after addition of the K13C15N. The ratio of the itcCys13C15N and itcCysCN peptides released was determined from the integrated peak areas of the 1274 and 1272 m/z product ions. Values are the average ± SD of the triplicate samples. The line is hyperbola, single rectangular, 2 parameter using all the data points.
Cys567-SCN adduct concentrations in HSA from various commercial and individual sera are shown in Fig. 8. One commercial lot (VB) consistently showed slightly higher adduct levels than other commercial sera, and the samples obtained from healthy volunteers. Adduct concentrations obtained from 5 samples selected from a nonsmoking, healthy cohort of volunteers are indistinguishable from one another and from the untreated serum used to prepare the 13C15N labeled-standard solutions. The HSA Cys567-SCN adduct concentration in a CN overdose victim was 4 to 5 times higher than that present in the healthy cohort samples, providing support for the in vitro data showing that CN reacts in a concentration dependent reaction with the Cys558Cys567 disulfide bond of HSA in humans.
Discussion
That CN is a potent neurological toxicant and lethal poison is well established (1). Because of the myriad of environmental and natural exposure sources of CN, all people carry a CN body burden, which in whole blood, is 2 to 8 nmol/mL(2). The difference between this “nontoxic concentration”, and the beginning of the putative lethal concentration range, which is estimated at 120 nmol of CN/mL of whole blood, predicts a narrow toxic index. This, coupled with the very fast clearance rate of CN, which can be as rapid as 20 min in some individuals (1,10), has made measurement of chronic and particularly acute CN exposure, and consequently any potential long-term health consequences, extremely problematic.
Numerous highly sensitive biomarker assays for CN in whole blood have been described (21), but that most used is a GC-MS headspace assay (22,23). Although 98% of the CN added to whole blood can be readily recovered and detected by this method, its usefulness as a measure of CN exposure in individuals is extremely limited because the time of sample collection is a highly-significant-dependent variable. Sensitive biomarker assays for the primary CN metabolites SCN (24) and ATCA (15,25–26), which are chemically less reactive, and persist for longer times within the body, have also been developed as probes of CN exposure. Currently, however, insufficient data exist to determine if useful correlations exist between these metabolites and CN levels.
CN in blood is partitioned between the cellular (primarily red blood cell) and plasma fractions, but estimates of the extent of distribution are highly variable, ranging from 5 to 50 percent residing within the plasma (19,27). In plasma and at physiologically relevant concentrations, CN reacts with susceptible disulfides almost exclusively within HSA (16). Whether CN reacts with tissue proteins is understudied, but given the ease with which very low concentrations of CN react with disulfides within HSA, it is highly probable that disulfide bonds in proteins within at least some cell types also undergo this irreversible reaction with CN. Indeed, in studies not reported here, we have demonstrated that CN reacts in vitro with the homologous disulfide in alpha fetoprotein and that the C-terminal peptide is released by base catalysis in the presence of GuHCl/NaOH. If affected proteins are present in cells that slowly regenerate, then such adduct formation could accumulate and account, at least partially, for some of the adverse health conditions associated with CN exposure.
The Cys567-SCN adduct level present in a small cohort of humans (Fig. 8) predict that its concentration in healthy subjects will be 100 to 200 pmol/mL of serum. One commercial serum lot (VB) consistently showed a slightly higher adduct level than all of the other sera, except that from the CN overdose victim. This commercial serum was included in this experiment to illustrate the ability of the assay to discriminate between differences in exposure. The adduct level in the suicide victim almost certainly represents the “ceiling” for the assay given the extremely high dose of CN consumed. The discovery of this 4 to 5-fold increase over normal is highly significant, however, because: 1) it provides additional support for the ability of the Cys567-SCN adduct to retrospectively reflect changes in plasma CN concentrations; and 2) the “ceiling” to normal differential is sufficiently large to diminish the contribution of errors inherent in the assay such as day to day sample preparation, mechanical variation, etc.
The reaction of CN with small molecule disulfides and susceptible protein disulfides yields R-SCN and R′-SH. Under basic conditions, the R-SCN moiety can undergo either of two secondary and tertiary structure-dependent reactions (28–31). One is the β-elimination of SCN which leaves behind a slightly modified, but otherwise intact sequence. The other is cyclization of the R-SCN moiety yielding itcCys. In peptides and proteins, itcCys formation yields upstream and downstream peptides, the latter containing the modified, N-terminal itcCys (16,28). Recently Youso et al. (32) reported the rapid release of SCN from blood proteins by incubation with sodium carbonate buffer, pH 10, at room temperature. When purified HSA, extensively labeled with 13C15N at various Cys residues throughout the protein, was dissolved in pH 10 carbonate buffer and incubated the solution for 1 h at room temperature or at 50°C for 3 h, neither the HSA itcCys567 peptide described here nor SCN or S13C15N, assayed as the pentafluorobenzyl bromide derivative and using similar GC-MS conditions to those described by Youso et al. (32), were detectable (data not shown). Additionally, when solutions containing different proportions of the labeled and unlabeled adducts were subjected to the much more vigorous conditions used by us for peptide release, no SCN or S13C15N was detectable whereas the itcCys567 peptides from the two isotopic variants were readily detectable, and in proportion to their predicted initial concentrations. Similar treatments of the cyanylated, full length, deuterated (D6) peptide standard did release barely detectable levels of SCN, but under every condition examined, the itcCys peptide was by far the major conversion product. Collectively, these results demonstrate that any SCN release from HSA or the cyanylated peptide standard is insufficient to confound the results obtained by assay of the itcCys567C-terminal HSA peptide as a measure of the Cys567-SCN adduct present in HSA.
The discovery that CN reacts with Cys567 of the C-terminal-Cys558Cys567 disulfide in HSA was highly fortuitous for several reasons. First, endogenous levels of the Cys567-SCN adduct are readily detectable as the itcCys peptide released in all the samples examined from healthy volunteers thus far, thereby eliminating concern about reaching the lower limit of detection. Second, K13C15N and the deuterated (D6) synthetic itcCys peptide could be used in studies in vitro to determine various reaction parameters and adduct concentrations at Cys567 independently of the amount of endogenous adduct present. Conversely, in certain experiments, the endogenous adduct also served as an internal standard to measure the amount of isotopic CN adduct present. Third, quantitation of endogenous Cys567 SCN adduct is straight-forward and reproducible using the itcCys567peptide as a surrogate and adding a known amount of HSA Cys567-S13C15N labeled serum as an internal standard. The deuterated (D6) SCN synthetic peptide can also be used as the internal standard, but it must be added just before incubation of the purified HSA with GuHCl/NaOH at 50°C since it would be lost during the initial protein precipitation steps. Additionally, because the HSA concentration in the GuHCl/NaOH varies somewhat from sample to sample, the HSA in solution should be quantitated to ensure the most accurate results. Use of the serum labeled HSA as the internal standard eliminates the HSA quantitation step in test samples since the original endogenous to labeled adduct mixture established at the beginning of the assay is retained throughout. Last, because of the location of the Cys567-SCN adduct in HSA, detection of the peptide released does not require enzyme digestion of HSA, which would seriously complicate the simplicity and reproducibility of the assay. The amount of HSA containing the endogenous Cys567SCN adduct is very low and detection of it requires reconstituted HSA solutions containing upwards of 12 mg/mL. Reduction and alkylation of the HSA at these concentrations, followed by a lengthy incubation with a protease would not only significantly increase the time of analysis, but would yield extremely complex peptide mixtures caused by sample to sample variation in the extent of digestion. The chemical method used yields selective and near quantitative release of the peptide target. Additionally, the HSA from which it is released and any HSA not containing the adduct are readily removed by the precipitation and spin filter treatments. The resultant peptide target solutions are “clean” enough for at least 100 injections on a single precolumn and many more on the separation column.
In summary, the in vitro and in vivo data presented suggest that the reaction of CN with the Cys558Cys567 disulfide in HSA exhibits the properties of a prototypical biomarker. Reaction at this disulfide coupled with itcCys peptide conversion from the Cys567−SCN adduct in HSA is unique to CN. Formation of the adduct occurs rapidly, a necessary requirement for a toxicant that is rapidly cleared from the body, being essentially complete within one to two hours, even at the very low CN concentrations believed to exist in plasma (Fig. 6). The reaction is concentration dependent and linear within estimated nontoxic to lethal levels (Fig. 7), thereby permitting detection of exposures above the “normal” level present within the general population, and it occurs on an abundant plasma protein with a t1/2 of approximately 23 days. Consequently, deviation from the “normal population” level for a given person, particularly one at risk for acute exposure as, for example, a firefighter, other first responder or soldier, should be identifiable in HSA obtained from blood drawn even after a few days post exposure. Last, the reaction of CN with HSA occurs in plasma, where the CN concentration ultimately determines it toxic consequences.
Acknowledgments
Funding Support
Portions of this publication was partially supported by Cooperative Agreement Number U90/CCU216998 to the Wadsworth Center, NYSDOH, from the Chemical Terrorism Laboratory Network program of the U.S. Centers for Disease Control and Prevention (CDC), Atlanta and by American Recovery and Reinvestment Act of 2009 NIEHS grant 1R21ES01685801A1, Retrospective Cyanide Assay from a Unique HSA-CN Adduct. Contents are solely the responsibility of the authors and do not necessarily represent the official views of the CDC.
The use of trade names is for identification only and does not constitute endorsement by the Wadsworth Center. The authors acknowledge the Biological Mass Spectroscopy and Biochemistry Cores for the use of, respectively, the LC-LTQ ion trap mass spectrometer and chromatography/spectroscopy equipment, and Dr. Robert Turesky of the Wadsworth Center for his helpful suggestions in preparing this manuscript.
Abbreviations
- HSA
human serum albumin
- BSA
bovine serum albumin
- SDS
sodium dodecyl sulfate
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- GuHCl
guanidine hydrochloride
- DTNB
dithionitrobenzoic acid
- itcCys
2-iminothiazoline-4-carboxylyl/2-aminothiazolidine-4-carboxylyl-tautomers
- ATCA
2-aminothiazoline-4-carboxylic acid
- PBS
phosphate buffered saline, pH 7.4
- CN
12C14N cyanide gas and/or anion
- KCN
potassium cyanide
- SCN
thiocyanate
- CH3CN
acetonitrile
- LC-MS/MS
liquid chromatography– mass spectrometry/mass spectrometry
- LC-MS/MS
liquid chromatography mass spectrometry/mass spectrometry
- SRM
selective reaction monitoring
References
- 1.Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profile for cyanide. U.S. Department of Health and Human Services, Centers for Disease Control; 2006. http://www.atsdr.cdc.gov/toxprofiles/TP.asp?id=72&tid=19. [PubMed] [Google Scholar]
- 2.Baskin SI, Brewer TG. Cyanide Poisoning. In: Zajtchuk R, Bellamy RF, Sidell FR, Takefugi ET, Franz DR, editors. Medical Aspects of Chemical and Biological Warfare. Office of the Surgeon General, Department of the Army; United States: 1997. pp. 271–286. [Google Scholar]
- 3.Boivin MJ. An ecological paradigm for a health behavior analysis of “konzo”, a paralytic disease of Zaire from toxic cassava. Soc Sci Med. 1997;45:1853–1862. doi: 10.1016/s0277-9536(97)00123-8. [DOI] [PubMed] [Google Scholar]
- 4.Tylleskar T, Banea M, Bikangi N, Cooke RD, Poulter NH, Rosling H. Cassava cyanogens and konzo, an upper motoneuron disease found in Africa. Lancet. 1992;339:208–211. doi: 10.1016/0140-6736(92)90006-o. [DOI] [PubMed] [Google Scholar]
- 5.Tylleskar T, Banea M, Bikangi N, Fresco L, Persson LA, Rosling H. Epidemiological evidence from Zaire for a dietary etiology of konzo, an upper motor neuron disease. Bull World Health Organ. 1991;69:581–589. [PMC free article] [PubMed] [Google Scholar]
- 6.Oku H, Fukushima K, Miyata M, Wakakura M, Ishikawa S. Cyanide with vitamin B12 deficiency as the cause of experimental tobacco amblyopia. Nippon Ganka Gakkai Zasshi. 1991;95:158–164. [PubMed] [Google Scholar]
- 7.Jestico JV, O’Brien MD, Teoh R, Toseland PA, Wong HC. Whole blood cyanide levels in patients with tobacco amblyopia. J Neurol Neurosurg Psychiatry. 1984;47:573–578. doi: 10.1136/jnnp.47.6.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kato T, Kameyama M, Nakamura S, Inada M, Sugiyama H. Cyanide metabolism in motor neuron disease. Acta Neurol Scand. 1985;72:151–156. doi: 10.1111/j.1600-0404.1985.tb00857.x. [DOI] [PubMed] [Google Scholar]
- 9.Ballantyne B. In: Clinical and Experimental Toxicology of Cyanides. Ballantyne B, Marrs T, editors. Wright: Bristol; 1987. pp. 41–127. [Google Scholar]
- 10.Hartung R. Cyanides and nitriles. In: Clayton GD, Clayton FE, editors. Patty’s Industrial Hygiene and Toxicology. 1. John Wiley & Sons; New York, New York: 1982. pp. 4845–4900. [Google Scholar]
- 11.Banea-Mayambu JP, Tylleskar T, Tylleskar K, Gebre-Medhin M, Rosling H. Dietary cyanide from insufficiently processed cassava and growth retardation in children in the Democratic Republic of Congo (formerly Zaire) Ann Trop Paediatr. 2000;20:34–40. doi: 10.1080/02724930092048. [DOI] [PubMed] [Google Scholar]
- 12.Eshiett NO, Ademosun AA, Omole TA. Effect of feeding cassava root meal on reproduction and growth of rabbits. J Nutr. 1980;110:697–702. doi: 10.1093/jn/110.4.697. [DOI] [PubMed] [Google Scholar]
- 13.Young MA. Health Hazards of Electroplating. J Occup Med. 1965;7:348–352. doi: 10.1097/00043764-196507000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Alarie Y. Toxicity of fire smoke. Crit Rev Toxicol. 2002;32:259–289. doi: 10.1080/20024091064246. [DOI] [PubMed] [Google Scholar]
- 15.Logue BA, Maserek WK, Rockwood GA, Keebaugh MW, Baskin SI. The analysis of 2-amino-2-thiazoline-4-carboxylic acid in the plasma of smokers and non-smokers. Toxicol Mech Methods. 2009;19:202–208. doi: 10.1080/15376510802488165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fasco MJ, Hauer CR, III, Stack RF, O’Hehir C, Barr JR, Eadon GA. Cyanide adducts with human plasma proteins: albumin as a potential exposure surrogate. Chem Res Toxicol. 2007;20:677–684. doi: 10.1021/tx6003425. [DOI] [PubMed] [Google Scholar]
- 17.Vanaman TC, Stark GR. A study of the sulfhydryl groups of the catalytic subunit of Escherichia coli aspartate transcarbamylase. The use of enzyme--5-thio-2-nitrobenzoate mixed disulfides as intermediates in modifying enzyme sulfhydryl groups. J Biol Chem. 1970;245:3565–3573. [PubMed] [Google Scholar]
- 18.Vesey CJ, Cole P, Simpson P. Proceedings: Changes in cyanide concentrations induced by sodium nitroprusside (SNP) Br J Anaesth. 1976;48:268. [PubMed] [Google Scholar]
- 19.Ballantyne B. The influence of exposure route and species on the acute lethal toxicity and tissue concentrations of cyanide. Dev Toxicol Environ Sci. 1983;11:583–586. [PubMed] [Google Scholar]
- 20.Lindsay AE, Greenbaum AR, O’Hare D. Analytical techniques for cyanide in blood and published cyanide concentrations from healthy subjects and fire victims. Anal Chim Acta. 2004;511:185–195. [Google Scholar]
- 21.Ma J, Dasgupta PK. Recent developments in cyanide detection: a review. Anal Chim Acta. 2010;673:117–125. doi: 10.1016/j.aca.2010.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dumas P, Gingras G, LeBlanc A. Isotope dilution-mass spectrometry determination of blood cyanide by headspace gas chromatography. J Anal Toxicol. 2005;29:71–75. doi: 10.1093/jat/29.1.71. [DOI] [PubMed] [Google Scholar]
- 23.Calafat AM, Stanfill SB. Rapid quantitation of cyanide in whole blood by automated headspace gas chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;772:131–137. doi: 10.1016/s1570-0232(02)00067-3. [DOI] [PubMed] [Google Scholar]
- 24.Vesey CJ, McAllister H, Langford RM. A safer method for the measurement of plasma thiocyanate. J Anal Toxicol. 1999;23:134–136. doi: 10.1093/jat/23.2.134. [DOI] [PubMed] [Google Scholar]
- 25.Baskin SI, Petrikovics I, Platoff GE, Rockwood GA, Logue BA. Spectrophotometric Analysis of the Cyanide Metabolite 2-Aminothiazoline-4-Carboxylic Acid (ATCA) Toxicol Mech Methods. 2006;16:339–345. doi: 10.1080/15376520600616933. [DOI] [PubMed] [Google Scholar]
- 26.Logue BA, Kirschten NP, Petrikovics I, Moser MA, Rockwood GA, Baskin SI. Determination of the cyanide metabolite 2-aminothiazoline-4-carboxylic acid in urine and plasma by gas chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;819:237–244. doi: 10.1016/j.jchromb.2005.01.045. [DOI] [PubMed] [Google Scholar]
- 27.Vesey CJ, Cole PV, Simpson PJ. Cyanide and thiocyanate concentrations following sodium nitroprusside infusion in man. Br J Anaesth. 1976;48:651–660. doi: 10.1093/bja/48.7.651. [DOI] [PubMed] [Google Scholar]
- 28.Jacobson GR, Schaffer MH, Stark GR, Vanaman TC. Specific chemical cleavage in high yield at the amino peptide bonds of cysteine and cystine residues. J Biol Chem. 1973;248:6583–6591. [PubMed] [Google Scholar]
- 29.Degani Y, Patchornik A. Cyanylation of sulfhydryl groups by 2-nitro-5-thiocyanobenzoic acid. High-yield modification and cleavage of peptides at cysteine residues. Biochemistry. 1974;13:1–11. doi: 10.1021/bi00698a001. [DOI] [PubMed] [Google Scholar]
- 30.Degani Y, Patchornik A, Maclaren JA. Specific cleavage of peptides at cysteinyl residues. J Am Chem Soc. 1966;88:3460–3461. doi: 10.1021/ja00966a069. [DOI] [PubMed] [Google Scholar]
- 31.Degani Y, Neumann H, Patchornik A. Selective cyanylation of sulfhydryl groups. J Am Chem Soc. 1970;92:6969–6971. doi: 10.1021/ja00726a043. [DOI] [PubMed] [Google Scholar]
- 32.Youso SL, Rockwood GA, Lee JP, Logue BA. Determination of cyanide exposure by gas chromatography-mass spectrometry analysis of cyanide-exposed plasma proteins. Anal Chim Acta. 2010;677:24–28. doi: 10.1016/j.aca.2010.01.028. [DOI] [PubMed] [Google Scholar]