Abstract
Even brief interruption of cardiac compressions significantly reduces critical coronary perfusion pressure during cardiopulmonary resuscitation (CPR). End-tidal CO2 (ETCO2) monitoring may provide a continuous non-invasive method of assessing return of spontaneous circulation (ROSC) without stopping to auscultate for heart rate (HR). However, the ETCO2 value that correlates with an audible HR is unknown. Our objective was to determine the threshold ETCO2 that is associated with ROSC following asphyxia-induced asystole. Neonatal swine (n=46) were progressively asphyxiated until asystole occurred. Resuscitation followed current neonatal guidelines with initial ventilation with 100% O2 followed by cardiac compressions followed by epinephrine for continued asystole. HR was auscultated every 30 sec and ETCO2 was continuously recorded. A receiver operator curve was generated using the calculated sensitivity and specificity for various ETCO2 values where a positive test was defined as the presence of HR >60 bpm by auscultation. An ETCO2 cut off value of 14 mmHg is the most sensitive ETCO2 value with the least false positives. When using ETCO2 to guide uninterrupted CPR in this model of asphyxia-induced asystole, auscultative confirmation of return of an adequate HR should be performed when ETCO2 ≥14 mmHg is achieved. Correlation during human neonatal CPR needs further investigation.
INTRODUCTION
Although the need for neonatal cardiopulmonary resuscitation (CPR) in the delivery room is rare (1, 2), morbidity and mortality rates are extremely high for newborns requiring CPR (3). During CPR it is critical that adequate coronary perfusion be achieved in order to establish return of spontaneous circulation (ROSC) (4-7). Adult clinical data and experimental models of ventricular fibrillation-induced cardiac arrest demonstrate that even brief interruption of compressions significantly reduces blood flow and coronary perfusion pressure and reduces ROSC and survival (8-10). No information is available from neonatal models of asphyxia-induced cardiac arrest.
Current adult CPR guidelines recommend a ratio of 30 compressions to 2 breaths for medical providers in order to limit interruptions of compressions (11) and even uninterrupted compression-only CPR for lay-providers in the field (12). Pediatric life support guidelines for medical providers emphasize a chest compression/ventilation ratio of 15:2 for all children except neonates (11). American Academy of Pediatrics/American Heart Association Neonatal Resuscitation Program (NRP) guidelines recommend a ratio of 3 compressions to 1 breath and pausing every 30 seconds to auscultate for return of a heart rate (HR) (13). Such frequent interruptions of compressions may limit the ability to achieve an adequate coronary perfusion pressure for ROSC.
End-tidal CO2 (ETCO2) monitoring is a non-invasive tool that has been shown to predict and demonstrate ROSC during experimental and human adult cardiac arrest (14-18). Carbon dioxide is produced by cellular metabolism and is subsequently transported by the venous system to the right heart where it is pumped into the lungs and diffuses into the exhaled air where it can be measured as ETCO2 (19). Thus, CO2 production, alveolar ventilation, and pulmonary perfusion interact to determine ETCO2. When CO2 production and cardiac output are stable, ETCO2 changes are typically due to changes in alveolar ventilation. During CPR, if ventilation is held constant and CO2 production is assumed to be very low and thus constant, excretion of CO2 through the lungs depends on pulmonary perfusion and therefore relates to cardiac output (14, 20-22).
We hypothesized that ETCO2 monitoring might provide a continuous non-invasive method of assessing ROSC without interrupting compressions during neonatal CPR. As a first step, we needed to determine if there is a consistent threshold ETCO2 that is associated with return of an audible HR > 60 bpm following asphyxia-induced asystole. The infrequent and unexpected need for neonatal CPR coupled with the difficulty of obtaining informed consent for such studies has impeded the design and completion of rigorous delivery room CPR studies. Thus, we designed an animal study to determine the threshold ETCO2 that is associated with ROSC during CPR in asphyxiated, asystolic neonatal piglets whose cardiopulmonary compromise parallels that of asphyxiated infants who require CPR at birth.
METHODS
This investigation was approved by the Institutional Review Board for Animal Research at The University of Texas Southwestern Medical Center at Dallas.
Surgical preparation
A convenience sample of 46 American domestic swine (mean ±standard deviation; weight: 2.2 ± 0.6 kg, postnatal age: 8 ± 4 days) were studied. All piglets were administered ketamine (20 mg/kg intramuscularly) as premedication and then were instrumented under pentobarbital anesthesia of 20 mg/kg intravenous bolus followed by 10 mg/kg/hr with additional boluses as needed to prevent spontaneous breathing. All surgical sites were infiltrated with 1% Xylocaine (Steris Laboratories Inc., Phoenix, AZ). A tracheotomy was performed with placement of a 3.5 mm endotracheal tube. Piglets were ventilated (Harvard Apparatus Rodent Respirator, model 680, Millis, MA) with 70% nitrous oxide and 30% O2 using rates of 60 breaths/min and tidal volume adjusted to achieve arterial partial pressure of CO2 (PaCO2) in the mid 40s mmHg range during the stabilization period. Catheters were positioned by aseptic technique in the right and left external jugular vein, the left internal jugular vein and the left common carotid artery and left femoral artery. Following catheter placement, the inspired gas was changed to 70% nitrogen and 30% O2. The piglet’s body temperature was maintained between 38 and 39°C using a thermal blanket wrapped around the body and circulating warm water (40-45°C) through the blanket.
Experimental protocol (Figure 1)
Figure 1. Experimental protocol.
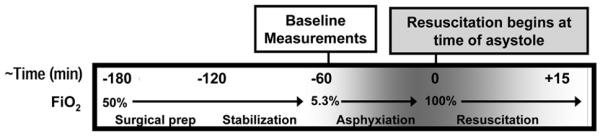
This model gradually induces asphyxia to create a biochemical profile similar to asphyxiated infants who require CPR in the delivery room. FiO2, fraction of inspired O2.
Following instrumentation, animals were allowed to stabilize for 60 minutes prior to acquisition of baseline measurements. Asphyxia was induced by changing ventilatory gases to 7.5% CO2 and 5.3% O2, and the ventilator rate was reduced by 10 breaths/min every 15 minutes until asystole occurred. Asystole was defined as mean arterial pressure = 0 mmHg on the continuous blood pressure tracing and confirmed by auscultation of an absent HR. Resuscitation was implemented by a 4 member NRP-trained resuscitation team. One team member was assigned to each of the following roles: 1) positive pressure ventilation with a self-inflating bag, 2) manual chest compressions, 3) blood sampling and administration of intravenous medications, and 4) code supervisor, who coordinated the timing and sequence of resuscitation interventions as defined in the protocol (Table S1, http://links.lww.com/PDR/XXX). Once asystole occurred, the initial steps of neonatal resuscitation (positioning, suctioning, and stimulation) were simulated for 30 seconds. Asphyxia was reversed by initiating resuscitation using positive pressure ventilation via a self-inflating anesthesia bag with 100% inspired O2. Resuscitation continued with the initiation of manual cardiac compressions. Depth of compressions and compressor fatigue were assessed by real-time evaluation of the aortic compression pressures as displayed on the monitor. Our goals were to achieve an aortic compression pressure around 50-60 mmHg. Compressions were followed by 0.01-0.03 mg/kg intravenous epinephrine doses in 3 minute intervals in accordance with NRP guidelines until ROSC (defined as a HR ≥60 beats per minute) occurred. If there was no return of spontaneous circulation after 15 minutes of resuscitation, resuscitation efforts ceased. If ROSC was achieved, the piglet was maintained on the ventilator for 2 hours without additional medications or interventions prior to euthanasia (using 200 mg/kg pentobarbital) unless death occurred early.
Measurements
HR and arterial blood pressure were recorded on either a 2 channel recorder (220, Gould, Oxnard, CA) or a Power Lab data acquisition system (model 16/30, AD Instruments, Colorado Springs, CO). Other measures, including O2 saturation, HR, ETCO2 and minute ventilation were recorded with a CO2SMO Plus Respiratory Profile Monitor (Novametrix, Wallingford, CT). These vital signs and respiratory parameters were continuously monitored. Blood chemistries (arterial blood gas, hematocrit, lactate, glucose) were obtained at control and subsequently every 15 minutes during asphyxia, every 3 minutes during the resuscitation phase, and every 30 minutes during the post-resuscitation phase. The arterial pH, PCO2 and PO2 were analyzed by an Instrumentation Laboratory Micro gas analyzer (15 μL of blood). The microhematocrit method was used to determine hematocrit. Serum glucose concentrations were measured using an Accu-Chek Advantage glucometer (Roche Diagnostics, Indianapolis, IN). The plasma concentration of lactic acid was determined by a quantitative enzymatic determination assay (Sigma, St. Louis, MO).
Statistical Analysis
Data analysis was completed using Sigma Stat 11.0 (SPSS, Chicago, IL). The results are reported as the mean ± standard deviation. Non-parametric analyses were employed when indicated. A p-value ≤ 0.05 was considered statistically significant. A receiver-operator characteristic (ROC) curve (a plot of the true positives against the false positives for different test cut-off points) was generated for various ETCO2 values where a positive test was defined as an ETCO2 value that was associated with return of a HR > 60 bpm. The optimal ETCO2 cut-off value on the curve was selected based on a combination of a-priori criteria including: 1) significant area under the curve compared to a 45° line of equality, 2) optimum sensitivity and specificity values, 3) maximum perpendicular distance (d) above the 45° line of equality, and 4) the highest proportion of correct predictions (accuracy).
RESULTS
At baseline prior to initiation of asphyxia, piglets had HR, pH, PaCO2, ETCO2, lactate, and glucose levels similar to healthy term neonates (Table 1). The mean time from initiation of asphyxia to asystole was 48 ± 13 min. As expected with asphyxiation, by the time asystole occurred the piglets developed a severe mixed respiratory and metabolic acidosis similar to infants who require CPR in the delivery room (3). ETCO2 levels had climbed concordant with the respiratory acidemia.
Table 1.
Piglet characteristics at baseline and asystole (n=46)
| Baseline | Asystole * | |
|---|---|---|
| Heart rate | 163 ± 26 | 0 ± 0 |
| Mean Arterial Pressure (mmHg) | 78 ± 12 | 0 ± 0 |
| Arterial pH | 7.38 ± 0.05 | 6.84 ± 0.17 |
| Arterial PCO2 (mmHg) | 43 ± 5 | 96 ± 25 |
| Arterial PO2 (mmHg) | 192 ± 33 | 29 ± 12 |
| ETCO2 (mmHg) | 40 ± 4 | 70 ± 23 |
| Glucose (mg%) | 80 ± 23 | 114 ± 102 |
| Hematocrit (%) | 30 ± 5 | 30 ± 4 |
Measurements were obtained at the time of asystole prior to initiation of resuscitation
The actual mean (±SD) aortic compression (systolic) and relaxation (diastolic) pressures attained with this experimental neonatal CPR model were the following: 1) 1 min after initiation of CPR the mean systolic compression pressure was 56±8 mmHg, with a diastolic pressure of 4± 2 mmHg; 2) Immediately following the first dose of epinephrine the mean systolic compression pressure remained 60±11 mmHg, with a diastolic pressure of 5±3 mmHg; 3) Immediately prior to return of spontaneous circulation, mean systolic compression pressure increased to 95±9 mmHg, with increased diastolic pressures of 19±3 mmHg.
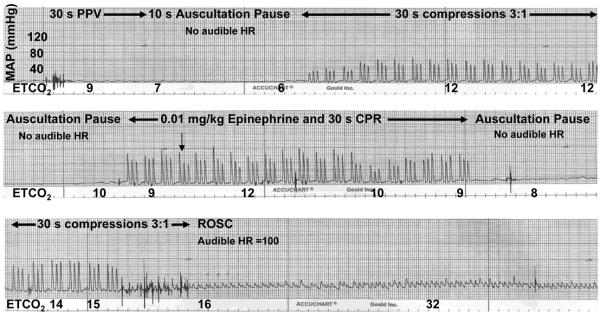
A representative blood pressure/HR/ETCO2 tracing is shown in Figure 2. Immediately prior to cardiac arrest, the high ETCO2 values (due to asphyxia) drifted down as pulmonary blood flow became increasingly limited. Following 30s of positive pressure ventilation, the remaining CO2 was ventilated off of the lung, and ETCO2 fell to near zero. When a small amount of pulmonary blood flow was reestablished by initiation of cardiac compressions, a slight increase in ETCO2 was detected. A sudden increase in ETCO2 was observed in all animals following ROSC (usually following epinephrine administration) as pulmonary blood flow quickly improved with the resumption of the pumping action of the heart. ROSC was achieved in 42 of 46 piglets..
Figure 2. Representative tracing of ETCO2 and mean arterial pressure (MAP) during CPR.
Following initial positive pressure ventilation (PPV), ETCO2 values fell from asphyxia values to 6 mmHg and then gradually increased as blood pressure increased with initiation of CPR. ETCO2 values declined during each 10 second pause to auscultate for HR, reflecting loss of blood flow with interruption of cardiac compressions. After epinephrine administration, blood pressure improved and ETCO2 increased to 15 mmHg, at which point ROSC occurred and an audible HR was detected. The hatch marks along the bottom of each frame represent 1 sec.
The sensitivity, specificity, false positive, false negative, positive likelihood ratio, and % correct prediction for return of an audible heart rate greater than 60 bpm for a range of ETCO2 values are seen in Table 2. The receiver-operator curve is seen in Figure 3 and area under the curve statistics was significant at 0.94 (95% confidence interval 0.88-1.00). An ETCO2 cut off higher than 14 mmHg had a sensitivity of 93%, specificity of 81%, positive likelihood ratio of 5 and 88% accuracy at predicting return of HR above 60. The distance d calculated from the ROC analysis for the ETCO2 critical value of 14 mmHg or higher was 0.524.
Table 2.
ROC analysis for ETCO2 corresponding to return of audible heart rate > 60 bpm
| ETCO2 cut off value (mmHg) | 12 | 13 | 14 | 15 |
|---|---|---|---|---|
| Sensitivity (%) | 100% | 95% | 92% | 73% |
| Specificity (%) | 68% | 78% | 81% | 96% |
| False Negative(%) | 0% | 4% | 6% | 24% |
| False Positive (%) | 22% | 15% | 13% | 2% |
| Likelihood Ratio | 3.20 | 4.35 | 4.95 | 23.62 |
| Distance (d) | 0.486 | 0.519 | 0.524 | 0.499 |
| Correct predictions (%) | 86% | 87% | 88% | 84% |
Figure 3. ROC curve for ETCO2 detection of an audible heart rate following asystole.
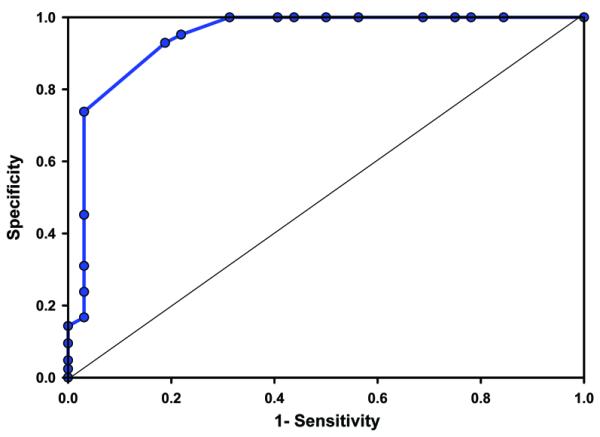
The sensitivity (true positive rate) was plotted against 1 – specificity (false positive rate) for each ETCO2 value. Area 0.94, 95% confidence interval (0.88-1.00).
DISCUSSION
In this neonatal model of asphyxia-induced asystole, increasing ETCO2 values appear to correlate with ROSC and an audible HR greater than 60 bpm. Given the consistent threshold above 14 mmHg at which ETCO2 correlates with return of an adequate HR, it may be useful to guide uninterrupted neonatal CPR. An ETCO2 cut off higher than 14 mmHg appears most useful for determining when to interrupt CPR to auscultate for a HR by providing the lowest combination of false positives and false negatives and the best fit with optimal calculated distance to an ideal curve using ROC analysis with a sensitivity of 93%, 81% specificity, and a positive likelihood ratio of 5.
The current NRP recommendation to interrupt cardiac compressions every 30 seconds for a 6 second auscultation pause to check for ROSC (13) may not be optimal. “In an adult animal model, such breaks in cardiac compressions” further delay reestablishment of adequate coronary perfusion pressure which is very dependent on ongoing uninterrupted compressions (23). NRP also recommends checking for the presence or absence of a palpable pulse during the resuscitative effort to assess the adequacy of artificial perfusion during cardiac compressions (13); however, coronary perfusion pressure (which is calculated as the aortic diastolic blood pressure – the right atrial diastolic blood pressure) is not impacted by the difference in systolic and diastolic pressures represented by a palpable pulse but rather by the aortic diastolic pressure itself (24). Thus, monitoring ETCO2 trends during CPR would allow uninterrupted cardiac compressions and might provide a better indicator of the effectiveness of perfusion during compression administration.
ETCO2 is a measure of the partial pressure of carbon dioxide at the end of an exhaled breath and is mainly determined by alveolar ventilation, pulmonary perfusion (right cardiac output) and CO2 production due to metabolism. During acutely low cardiac output states as in cardiac arrest, decreased pulmonary blood flow becomes the primary determinant of ETCO2 resulting in low values (25-26). The concept of change in ETCO2 reflecting the changes in pulmonary blood flow in the presence of constant cardiac compressions and ventilation has been utilized to assess circulatory status during cardiac arrest and resuscitation in adults (17). In experimental models of ventricular fibrillation induced cardiac arrest, ETCO2 concentration during ongoing CPR correlates with cardiac output, coronary perfusion pressure, and successful resuscitation from cardiac arrest (27-28).
Experimental animal adult studies of atraumatic cardiac arrest have reported increasing ETCO2 to be also associated with ROSC (21). In such models an ETCO2 threshold of 15 mmHg predicted ROSC with a positive predictive value 91% and negative predictive value of 91% (25). In contrast to adult models of ventricular fibrillation, animal models of brief asphyxial pediatric cardiac arrest reported initially elevated ETCO2 levels reflective of the high alveolar PCO2 present at the time of asphyxial arrest. This was followed by a subsequent decrease in PCO2 once ventilation was initiated (22, 29) as the PCO2 present in the lung at the time of arrest was ventilated off and no further CO2 was brought to the lung due to arrested perfusion. In our neonatal model of asphyxia-induced asystole we observed similar findings with high initial ETCO2 at the time of asystole that decreased following 30 seconds of adequate PPV. This pattern of ETCO2 changes is different from that observed in ventricular fibrillation arrest because PCO2 levels are typically normal at the time of cardiac arrest from ventricular fibrillation as opposed to very elevated at the time of asphyxial cardiac arrest. No prior study of asphyxial cardiac arrest has determined ETCO2 values that correlate with return of an audible HR >60 bpm which is the current clinical goal for stopping cardiac compressions following asphyxia-induced asystole in neonates.
A strength of this translational study is the use of a piglet asphyxia model that closely mimics delivery room events with gradual onset of severe asphyxia leading to asystole. The presence of a dedicated focused clinical resuscitation team with current NRP training, along with designated roles during the resuscitation, a supervisor leading the code and a recorder for accuracy of documentation makes this piglet asphyxia model an ideally controlled mega code environment. We attempted to control for depth of compressions and compressor fatigue by real-time assessment and adjustment of the pulse pressures generated by the compressor. This controlled setting allowed us to generate an ROC curve related to the predictive values of ETCO2, with minimum confounding variables.
The following limitations should be considered before general application of ETCO2 guidance in future clinical neonatal resuscitation trials. The current model is one where the animals have already undergone fetal to neonatal transition and in addition are sedated/anesthetized. The findings are still relevant despite this limitation, because the distribution of cardiac output in the fetus and post-transitional neonate during asphyxial episodes are qualitatively similar (30-32). In addition, responsiveness and reactivity of the cerebral circulation to factors that modulate cerebral blood flow such as hypoxia qualitatively remain intact under barbiturate anesthesia (33-34). Another limitation is that manual ventilation and chest compressions could cause ETCO2 to fluctuate with the effort of compression and rate of ventilation (20, 35), so uniform compressions need to be delivered for ETCO2 to be used as a predictor of ROSC. In addition, acute and chronic illness with co-morbidities can result in a ventilation/perfusion mismatch, which can limit the accuracy of ETCO2 (36). This is unlikely to be a problem in the post transitional neonatal piglet model currently utilized in this study. The effect of resuscitation medications such as epinephrine needs to be carefully recorded and should also be taken into consideration. According to adult studies, NaHCO3 can transiently increase ETCO2, while epinephrine can lead to decreased levels (37). Lastly, this model is based on heart rate assessment in term animals and does not include evaluation for pseudo-pulseless electrical activity where there is no clinically palpable pulses but presence of blood flow. The model does not address issues of prematurity or low birth weight.
In conclusion, our study using this piglet model of asystole due to asphyxia demonstrates that capnometry can be used as a predictor of ROSC, and may be a useful substitute for frequent pauses in cardiac compressions in order to auscultate HR during neonatal CPR. Further investigation is needed to determine if uninterrupted ETCO2-guided CPR can improve time to return of spontaneous circulation and short and long-term outcomes following neonatal resuscitation.
Supplementary Material
Acknowledgments
This work was supported by an American Academy of Pediatrics Neonatal Resuscitation Program Research Grant. In addition, L.F.C. and L.H.are supported by Grant Number KL2RR024983, titled, “North and Central Texas Clinical and Translational Science Initiative” from the National Center for Research Resources (NCRR, NIH).
ABBREVIATIONS
- CPR
cardiopulmonary resuscitation
- d
distance
- ETCO2
end-tidal CO2
- HR
heart rate
- NRP
neonatal resuscitation program
- PPV
positive pressure ventilation
- ROC
receiver operator characteristic
- ROSC
return of spontaneous circulation
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.pedresearch.org).
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Perlman JM, Risser R. Cardiopulmonary resuscitation in the delivery room. Arch Pediatr Adolesc Med. 1995;149:20–25. doi: 10.1001/archpedi.1995.02170130022005. [DOI] [PubMed] [Google Scholar]
- 2.Wyckoff MH, Perlman JM, Laptook AR. Use of volume expansion during delivery room resuscitation in near-term and term infants. Pediatrics. 2005;115:950–955. doi: 10.1542/peds.2004-0913. [DOI] [PubMed] [Google Scholar]
- 3.Barber CA, Wyckoff MH. Endotracheal versus intravenous epinephrine during neonatal cardiopulmonary resuscitation in the delivery room. Pediatrics. 2006;118:1028–1034. doi: 10.1542/peds.2006-0416. [DOI] [PubMed] [Google Scholar]
- 4.Ralston SH, Voorhees WD, Babbs CF. Intrapulmonary epinephrine during prolonged cardiopulmonary resuscitation: improved regional flow and resuscitation in dogs. Ann Emerg Med. 1984;13:79–86. doi: 10.1016/s0196-0644(84)80566-1. [DOI] [PubMed] [Google Scholar]
- 5.Sanders AB, Ewy GA, Taft TV. Prognostic and therapeutic importance of the aortic diastolic pressure in resuscitation from cardiac arrest. Crit Care Med. 1984;12:871–873. doi: 10.1097/00003246-198410000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Niemann JT, Criley JM, Rosborough JP, Niskanen RA, Alferness C. Predictive indices of successful cardiac resuscitation. Ann Emerg Med. 1985;14:521–528. doi: 10.1016/s0196-0644(85)80774-5. [DOI] [PubMed] [Google Scholar]
- 7.Halperin HR, Tsitlik JE, Guerci AD, Mellits ED, Levin HR, Shi A-Y, Chandra N, Weisfeldt ML. Determinants of blood flow to vital organs during cardiopulmonary resuscitation in dogs. Circulation. 1986;73:539–550. doi: 10.1161/01.cir.73.3.539. [DOI] [PubMed] [Google Scholar]
- 8.Kern KB, Hilwig RW, Berg RA, Ewy GA. Efficacy of chest compression-only BLS CPR in the presence of an occluded airway. Resuscitation. 1998;39:179–188. doi: 10.1016/s0300-9572(98)00141-5. [DOI] [PubMed] [Google Scholar]
- 9.Berg RA, Sanders AB, Kern KB, Hilwig RW, Heidenreich JW, Porter ME, Ewy GA. Adverse effects of interrupting chest compressions for rescue breathing during cardiopulmonary resuscitation for ventricular fibrillation cardiac arrest. Circulation. 2001;104:2465–2470. doi: 10.1161/hc4501.098926. [DOI] [PubMed] [Google Scholar]
- 10.Kern KB, Hilwig RW, Berg RA, Sanders AB, Ewy GA. Importance of continuous chest compressions during cardiopulmonary resuscitation: improved outcome during a simulated single lay-rescuer scenario. Circulation. 2002;105:645–649. doi: 10.1161/hc0502.102963. [DOI] [PubMed] [Google Scholar]
- 11.ECC Committee, Subcommittees and Task Forces of the American Heart Association 2005 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Part 3. Overview of CPR. Circulation. 2005;112:IV-12–IV-18. doi: 10.1161/CIRCULATIONAHA.105.166550. [DOI] [PubMed] [Google Scholar]
- 12.Sayre MR, Berg RA, Cave DM, Page RL, Potts J, White RD. Hands-Only (Compression-Only) Cardiopulmonary Resuscitation: A Call to Action for Bystander Response to Adults Who Experience Out-of-Hospital Sudden Cardiac Arrest: A Science Advisory for the Public From the American Heart Association Emergency Cardiovascular Care Committee. Circulation. 2008;117:2162–2167. doi: 10.1161/CIRCULATIONAHA.107.189380. [DOI] [PubMed] [Google Scholar]
- 13.Kattwinkel J. Textbook of Neonatal Resuscitation. 5th Ed American Academy of Pediatrics/American Heart Association; Elk Grove IL: 2006. pp. 10–20. [Google Scholar]
- 14.Weil MH, Bisera J, Trevino RP, Rackow EC. Cardiac output and end-tidal carbon dioxide. Crit Care Med. 1985;13:907–909. doi: 10.1097/00003246-198511000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Trevino RP, Bisera J, Weil MH, Rackow EC, Grundler WG. End-tidal CO2 as a guide to successful cardiopulmonary resuscitation: a preliminary report. Crit Care Med. 1985;13:910–911. doi: 10.1097/00003246-198511000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Garnett AR, Ornato JP, Gonzalez ER, Johnson EB. End-tidal carbon dioxide monitoring during cardiopulmonary resuscitation. JAMA. 1987;257:512–515. [PubMed] [Google Scholar]
- 17.Falk JL, Rackow EC, Weil MH. End-tidal carbon dioxide concentration during cardiopulmonary resuscitation. N Engl J Med. 1988;318:607–611. doi: 10.1056/NEJM198803103181005. [DOI] [PubMed] [Google Scholar]
- 18.Kern KB, Sanders AB, Voorhees WD, Babbs CF, Tacker WA, Ewy GA. Changes in expired end-tidal carbon dioxide during cardiopulmonary resuscitation in dogs: a prognostic guide for resuscitation efforts. J Am Coll Cardiol. 1989;13:1184–1189. doi: 10.1016/0735-1097(89)90282-9. [DOI] [PubMed] [Google Scholar]
- 19.Bhende MS. End-tidal carbon dioxide monitoring in pediatrics – concepts and technology. J Postgrad Med. 2001;47:153–156. [PubMed] [Google Scholar]
- 20.Sanders AB, Kern KB, Otto CW, Milander MM, Ewy GA. End-tidal carbon dioxide monitoring during cardiopulmonary resuscitation. JAMA. 1989;262:1347–1351. [PubMed] [Google Scholar]
- 21.Idris AH, Staples ED, O’Brien DJ, Melker RJ, Rush WJ, Duca KD Del, Falk JL. End-tidal carbon dioxide during extremely low cardiac output. Ann Emerg Med. 1994;23:568–572. doi: 10.1016/s0196-0644(94)70080-x. [DOI] [PubMed] [Google Scholar]
- 22.Bhende MS, Karasic DG, Karasic RB. End-tidal carbon dioxide changes during cardiopulmonary resuscitation after experimental asphyxial cardiac arrest. Am J Emerg Med. 1996;14:349–350. doi: 10.1016/S0735-6757(96)90046-7. [DOI] [PubMed] [Google Scholar]
- 23.Ewy GA, Zuercher M, Hilwig RW, Sanders AB, Berg RA, Otto CW, Hayes MM, Kern KB. Improved neurological outcome with continuous chest compressions compared with 30:2 compressions-to-ventilations cardiopulmonary resuscitation in a realistic swine model of out-of-hospital cardiac arrest. Circulation. 2007;116:2525–2530. doi: 10.1161/CIRCULATIONAHA.107.711820. [DOI] [PubMed] [Google Scholar]
- 24.Wyckoff MH, Berg RA. Optimizing chest compressions during delivery room resuscitation. Semin Fetal Neonatal Med. 2008;13:410–415. doi: 10.1016/j.siny.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 25.Callaham M, Barton C. Prediction of outcome of cardiopulmonary resuscitation from end-tidal carbon dioxide concentration. Crit Care Med. 1990;18:358–362. doi: 10.1097/00003246-199004000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Domsky M, Wilson RF, Heins J. Intraoperative end-tidal carbon dioxide values and derived calculations correlated with outcome: prognosis and capnography. Crit Care Med. 1995;23:1497–1503. doi: 10.1097/00003246-199509000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Sanders AB, Ewy GA, Bragg S, Atlas M, Kern KB. Expired PCO2 as a prognostic indicator of successful resuscitation from cardiac arrest. Ann Emerg Med. 1985;14:948–952. doi: 10.1016/s0196-0644(85)80235-3. [DOI] [PubMed] [Google Scholar]
- 28.Gudipati CV, Weil MH, Bisera J, Deshmukh HG, Rackow EC. Expired carbon dioxide: a noninvasive monitor of cardiopulmonary resuscitation. Circulation. 1988;77:234–239. doi: 10.1161/01.cir.77.1.234. [DOI] [PubMed] [Google Scholar]
- 29.Berg RA, Henry C, Otto CW, Sanders AB, Kern KB, Hilwig RW, Ewy GA. Initial end-tidal CO2 is markedly elevated during cardiopulmonary resuscitation after asphyxial cardiac arrest. Pediatr Emerg Care. 1996;12:245–248. doi: 10.1097/00006565-199608000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Behrman RE, Lees MH, Peterson EN, De Lannoy CW, Seeds AE. Distribution of the circulation in the normal and asphyxiated fetal primate. Am J Obstet Gynecol. 1970;108:956–969. doi: 10.1016/0002-9378(70)90341-8. [DOI] [PubMed] [Google Scholar]
- 31.Cohn HE, Sacks EJ, Heymann MA, Rudolph AM. Cardiovascular responses to hypoxemia and acidemia in fetal lambs. Am J Obstet Gynecol. 1974;120:817–824. doi: 10.1016/0002-9378(74)90587-0. [DOI] [PubMed] [Google Scholar]
- 32.Leffler CW, Busija DW, Beasley DG, Fletcher AM, Green RS. Effects of Indomethacin on cardiac output distribution in normal and asphyxiated piglets. Prostaglandins. 1986;31:183–190. doi: 10.1016/0090-6980(86)90045-6. [DOI] [PubMed] [Google Scholar]
- 33.Hohimer AR, Bissonnette JM. Effects of cephalic hypotension, hypertension, and barbiturates on fetal cerebral flood flow and metabolism. Am J Obstet Gynecol. 1989;161:1344–1351. doi: 10.1016/0002-9378(89)90695-9. [DOI] [PubMed] [Google Scholar]
- 34.Donegan JH, Traystman RJ, Koehler RC, Jones MD, Jr, Rogers MC. Cerebrovascular hypoxic and autoregulatory responses during reduced brain metabolism. Am J Physiol. 1985;249:H421–H429. doi: 10.1152/ajpheart.1985.249.2.H421. [DOI] [PubMed] [Google Scholar]
- 35.Steedman DJ, Robertson CE. Measurement of end-tidal carbon dioxide concentration during cardiopulmonary resuscitation. Arch Emerg Med. 1990;7:129–134. doi: 10.1136/emj.7.3.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LaValle TL, Perry AG. Capnography: assessing end-tidal CO2 levels. Dimens Crit Care Nurs. 1995;14:70–77. [PubMed] [Google Scholar]
- 37.Callaham M, Barton C, Matthay M. Effect of epinephrine on the ability of end-tidal carbon dioxide readings to predict initial resuscitation from cardiac arrest. Crit Care Med. 1992;20:337–343. doi: 10.1097/00003246-199203000-00008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.