Abstract
Successful vaccines (i.e., tetanus and diphtheria) can induce long-lived Ab levels that are maintained by bone marrow plasma cells and plasma Ab levels do not correlate with numbers of blood memory B cells. Destruction of CD4+ T cells early in HIV-1 acute infection may result in insufficient induction of neutralizing Ab responses; thus, an HIV-1 vaccine should elicit high levels of durable Abs by long-lived plasma cells to be protective. We asked if HIV-1 envelope-specific memory responses were sustained by memory B cells in the settings of HIV-1 gp120 envelope vaccination and chronic HIV-1 infection. Levels of anti-HIV-1 envelope plasma Abs and memory B cells were found to correlate in both settings. Moreover, whereas the expected half-life of plasma Ab levels to protein vaccines was >10 years when maintained by long-lived plasma cells, anti-envelope Ab level half-lives were ~33– 81 wk in plasma from antiretroviral drug-treated HIV-1+ subjects. In contrast, anti-p55 Gag Ab level half-life was 648 wk, and Ab titers against influenza did not decay in-between yearly or biennial influenza vaccine boosts in the same patients. These data demonstrated that HIV-1 envelope induces predominantly short-lived memory B cell-dependent plasma Abs in the settings of envelope vaccination and HIV-1 infection. The inability to generate high titers of long-lived anti-envelope Abs is a major hurdle to overcome for the development of a successful HIV-1 vaccine.
Despite an improvement in effectiveness and utilization of antiretroviral therapies worldwide and improved prevention efforts, HIV-1 infection continues to spread, with an estimated 2.7 million new infections worldwide in 2007 and 56,300 new HIV cases in the United States in 2006 (1, 2). Thus, the development of a safe and effective HIV-1 vaccine remains a global priority (1, 3). The recent lack of efficacy of T cell-based and gp120 envelope-based vaccine phase III clinical trials has dramatically emphasized that two major and central goals of HIV-1 vaccine design are the induction of both broadly neutralizing Abs and long-lived protective Ab responses (1, 3). Therefore, understanding B cell responses to HIV-1 in the settings of infection and vaccination has become a key aim of research toward design of a preventive HIV-1 vaccine (1, 3– 6).
In many viral infections or vaccinations, the initial burst of Ab after reexposure is dependent on the presence of Ag and is mediated by differentiation of memory B cells into short-lived plasma cells (5, 7, 8). In contrast, long-lived Ab responses generated by natural infections or vaccines are not dependent on the continuous presence of memory B cells but are rather produced by long-lived plasma cells that reside in the bone marrow and do not require Ag for continued production of Ab (5, 7, 9, 10).
Amanna et al. have recently described that the Ab responses to tetanus toxoid and diphtheria vaccines have half-lives of 11 and 19 years, respectively, and that the serum Ab levels present long-term in these successful vaccinations did not correlate with blood memory B cell levels (11). This lack of correlation of memory B cell levels with serum Ab implied that, with these vaccines, serum Ab was maintained by long-lived plasma cells in the bone marrow and not by memory B cells (11, 12).
In this paper, we have studied the plasma Ab and memory B cell responses to HIV-1 envelope in the context of chronic HIV-1 infection and in the setting of immunization of HIV-1-negative subjects with recombinant gp120 (rgp120).3 We found a significant correlation between blood anti-Env memory B cell levels and plasma anti-Env Ab titers in both chronic HIV-1 infection and after vaccination with rgp120, suggesting that in these settings plasma Ab was maintained predominantly by short-lived memory B cells. Ab response duration was measured over the course of ~380 wk of antiretroviral therapy (ART) in HIV-1-infected subjects in whom plasma viremia was completely suppressed. We found an apparent half-life of anti-Env Ab plasma levels between 33 (gp41) and 81 (gp120) wk following initiation of ART leading to viral load control, compared with longer (i.e., years) plasma level half-lives for HIV-1 p55 Gag, tetanus toxoid, and influenza Abs. Taken together, our observations demonstrate that anti-Env Ab titers are shorter lived than p55 Gag, influenza, and tetanus toxoid Ab titers, and that the HIV-1 envelope does not elicit long-lived B cell memory to the degree of other immunogens.
Materials and Methods
Memory B cell study population
Twenty-six HIV-1 chronically infected patients were enrolled at the Duke Infectious Diseases Clinic for this study. Of them, 18 were on therapy and 8 were off therapy at the time of enrollment (Table I). Nineteen HIV-negative individuals were enrolled as controls.
Table I.
Clinical data of HIV-1-positive subjects at enrollmenta
| Patient ID | CD4 | VL | Gender | Age (years) | YOD |
|---|---|---|---|---|---|
| Group I: Off ART | |||||
| 8982-026 | 406 | 153,440 | M | 62 | 1988 |
| 8982-029 | 580 | 5,104 | M | 29 | 2001 |
| 8982-037 | 479 | 35,840 | M | 39 | 2006 |
| 8982-127 | n/a | n/a | F | 34 | 2006 |
| 8982-130 | 758 | 1,219 | M | 47 | 1989 |
| 8982-131 | 838 | 8,144 | M | 49 | 2006 |
| 8982-133 | 504 | 79,360 | M | 56 | 2006 |
| 8982-134 | 651 | 71,700 | F | 33 | 2005 |
| Group II: On ART | |||||
| 8982-018 | 109 | 14,704 | M | 51 | 1991 |
| 8982-019 | 729 | 47,040 | M | 47 | 1991 |
| 8982-025 | 14 | 57,700 | F | 48 | 1995 |
| 8982-028 | n/a | 2,960 | M | 38 | 1995 |
| 8982-030 | 205 | 33,440 | M | 31 | 2000 |
| 8982-036 | n/a | n/a | F | 45 | 2000 |
| 8982-038 | n/a | n/a | M | 48 | 1994 |
| 8982-039 | 255 | 726 | M | 54 | 1996 |
| 8982-040 | 255 | 15,362 | M | 48 | 2002 |
| 8982-041 | 318 | 14,688 | F | 42 | 1994 |
| 8982-043 | 18 | 179,200 | M | 50 | 1992 |
| 8982-044 | n/a | n/a | M | 42 | 1999 |
| 8982-126 | 189 | <100 | M | 50 | 2003 |
| 8982-128 | 129 | 379 | M | 46 | 1997 |
| 8982-132 | 510 | <100 | M | 47 | n/a |
| 8982-135 | 127 | <48 | F | 51 | 1997 |
| 8982-136 | 169 | 13,900 | M | 44 | 1993 |
| 8982-137 | 131 | <48 | M | 53 | 1996 |
CD4 indicates CD4 counts (cells/mm3); VL, viral load (copies/ml) determined at the time of enrollment; YOD, year of diagnosis; n/a, data not available; M, male; F, female.
An additional eight HIV-1 chronically infected subjects who were treated with ART for between 125 and 387 wk with suppression of plasma viremia, and for whom only retrospective plasma samples were available, were studied for levels of plasma anti-gp120, -gp41, -p55, -tetanus toxoid, and -influenza (2007 Fluzone vaccine; Sanofi Pasteur) IgG Abs over time of ART using a sensitive Luminex assay as previously described (13) and direct-binding ELISAs. The relative Ab level half-lives were determined over time as described below under statistical methods.
Aliquots of frozen PBMCs from 25 vaccinated volunteers and 5 placebo recipients were obtained from the VaxGen clinical trial (VAX004). Vaccinees were administered HIV MN and GNE8 rgp120 proteins in alum (14). It has been previously reported that in animal models (15) and in humans (16) Ag-specific B cells peak as early as 1 wk after immunization. Therefore, to get as close as possible to this time point, we obtained VAX004 patient and control peripheral blood samples (plasma and cells) 2 wk after the fourth immunization. VAX004 specimens were obtained from the Division of AIDS (DAIDS), a division of the National Institute of Allergy and Infectious Diseases (National Institutes of Health, Department of Health and Human Services), and from Global Solutions for Infectious Diseases. All human studies were approved by the Institutional Review Board of the Duke University Medical Center.
PBMC isolation
PBMCs were isolated from acid citrate dextrose-treated blood using Accuspin tubes (Sigma-Aldrich) according to the manufacturer’s instructions. Briefly, 25 ml of whole blood was loaded onto the frit of each 50-ml tube and centrifuged for 20 min at 800 × g at 22°C. Plasma was removed and the PBMCs were transferred to 50-ml conical tubes, washed twice with HBSS without calcium and magnesium, and resuspended in 10 ml of RPMI 1640 (Invitrogen) plus 10% heat-inactivated FBS (Gemini Bio-Products), 100 U/ml penicillin, and 100 μg/ml streptomycin (Sigma-Aldrich). FBS had been previously tested for lack of mitogenic activity by [3H]thymidine incorporation assay and the same lot has been used throughout the experiments. Viability was checked using the Guava ViaCount kit (Guava Technologies) according to the manufacturer’s instructions.
PBMC thawing
Cryopreserved PBMCs were rapidly thawed, treated with Benzonase (Novagen), washed twice using warm media, resuspended in RPMI 1640 plus 20% FBS, and incubated overnight at 37°C in 5% CO2 humidified atmosphere before proceeding with experiments.
Memory B cell stimulation
Memory B cells were driven into Ab-secreting cells (ASCs) by culturing 2 × 106 PBMCs/ml in RPMI 1640 plus 10% human AB serum (Gemini Bio-Products), 100 U/ml penicillin, 100 μg/ml streptomycin, pokeweed mitogen (1/1000) prepared as previously described (17), 24 μg/ml CpG-oligodeoxynucleotide 10103 (Coley Pharmaceuticals), and 5 ng/ml IL-15 (R&D Systems) for 6 days at 37°C, 5% CO2 in humidified atmosphere on a rocking platform. Optimal culture conditions were previously established. Human AB serum was previously tested for lack of mitogenic activity by [3H]thymidine incorporation assay and the same lot has been used throughout the experiments. Unstimulated cells were cultured simultaneously. Cells were then washed twice and resuspended in RPMI 1640 plus 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin. Viability was determined using the Guava Via-Count kit, and cells were resuspended at proper concentrations before being used in the ELISPOT assay.
Evaluation of survival of HIV+ and HIV− PBMCs upon stimulation
To evaluate the extent of PBMC loss after in vitro stimulation, we compared the recovery ratio, defined as the ratio of viable PBMCs at the end of culture to the number of PBMCs at culture initiation, of stimulated and unstimulated PBMCs from chronically HIV-1-infected and uninfected subjects after a 6-day incubation. The recovery ratio of HIV-1-positive and HIV-1-negative unstimulated PBMCs was comparable (0.35 ± 0.15 and 0.37 ± 0.21, respectively; p = 0.74). On the contrary, stimulated PBMCs from chronically HIV-1-infected subjects underwent significantly higher levels of cell death compared with stimulated uninfected PBMCs (recovery ratio of 0.25 ± 0.10 and 0.41 ± 0.14, respectively; p = 0.0002). Thus, all data are expressed as ASC/106 viable PBMCs at the end of the culture period.
HIV envelope peptides
To detect epitope-specific ASCs in the ELISPOT assay we conjugated biotinylated peptides of the desired specificity to streptavidin to generate tetramerized peptides for increased sensitivity, to be used as capture reagents in plate-based assays. Diamond and colleagues previously reported that, in a murine model, monomeric peptides are not suitable in B cell ELISPOT assays to detect epitope-specific responses (18). We compared monomeric and tetramerized MN V3 gp120 Env (MN V3) peptide (TRPNYNKRKRIHIGPGRAFYTTKNIKGTIRQAH) and membrane-proximal external region (MPER) gp41 cluster II 2F5-epitope (SP62) peptide (QQEKNEQELLELDKWASLWN), as well as tetramerized peptides with the same amino acid content but with scrambled sequences (MN V3Scr peptide, AIKRHKRINITQRPKPGYRTHRNKGITGTANYF; SP62Scr peptide, NKEQDQAEESLQLWEKLNWL) for their ability to detect ASCs using mAb-producing cell lines. We confirmed that spots were detectable with tetramers but not with the respective monomers and that the spots obtained using tetramers were Ag-specific, as the scrambled versions of the tetramers failed to capture the secreted Abs and hence did not form spots. All the tetramerized peptides used in this study were routinely controlled for their ability to block binding of fluorochrome-conjugated homologous tetramerized peptide to Ab-coated beads by flow cytometry. The fluorochrome-conjugated tetramerized peptides had been previously tested for specific binding to both mAb-producing cell lines and Ab-coated beads. Additionally, we performed a checkerboard ELISA on cell lines producing mAbs of known specificity and confirmed that the tetramers only bound to the appropriate mAbs when used as capture reagents in plate-based assays.
Memory B cell ELISPOT assay
We performed a modified version of the memory B cell ELISPOT assay (17) in two ways. First, 96-well Immobilon P flat-bottom plates (Millipore), pretreated with 70% ethanol for 30 s and washed twice with PBS, were coated overnight at 4°C with 100 μl of goat polyvalent anti-human Ig (Invitrogen; catalog no. H17000), Imject mariculture keyhole limpet hemocyanin (Pierce Biotechnology; catalog no, PI77600), HIV-1 consensus B gp140CFI envelope, HIV-1 MN-V3 tetrameric peptides, and HIV-1 2F5-epitope tetrameric peptides at 10 μg/ml in PBS. The gp140 consensus B gp140CFI envelope protein was produced and purified as previously described (19). The plates were washed twice with PBS and blocked for 2 h at 37°C with PBS/1% BSA. Cells were added at 4 × 106 viable PBMCs/ml in a volume of 100 μl for Ag-specific tests and at 4 × 104 and 4 × 103 viable PBMCs/well for total IgG detection. Plates were loosely wrapped in aluminum foil (20) and incubated for 3 h at 37°C in a humidified atmosphere at 5% CO2. The plates were then washed four times with PBS/0.05% Tween 20 using the ELx405 automated plate washer (BioTek Instruments), and 100 μl of mouse biotin-conjugated anti-human IgG Ab (Hybridoma Reagent Laboratory; catalog no. HP6043B) diluted 1/4000 in PBS/1% BSA/0.05% Tween 20 was added for 1 h at room temperature. The detection Ab had been previously checked for isotype specificity and optimal concentration. Plates were washed four times with PBS/0.05% Tween 20, and 100 μl of HRP (Vector Laboratories; catalog no. A2004) diluted 1/1000 in PBS/1% BSA/0.05% Tween 20 was added to each well for 1 h at room temperature. Plates were washed twice with PBS/0.05% Tween 20 and twice with PBS. Spots were developed adding 100 μl/well of 3-amino-9-ethylcarbazole at 0.3 mg/ml diluted in 0.1 M sodium acetate (pH 5.0) and 0.03% hydrogen peroxide for 5 min in the dark. Plates were extensively washed with distilled water and let dry overnight. Spots were counted with the ImmunoSpot series 3B analyzer and ImmunoSpot 4.0 software (Cellulat Technology). Spot counts were then reported as ASCs ± SEM/106 viable PBMCs at the end of culture. In the present report, the term ASC refers to memory B cells detected with the ELISPOT assay. Results were background subtracted as follows: total IgG counts from un-stimulated cells were subtracted from total IgG counts of stimulated cells, and responses obtained against the KLH protein were subtracted from Ag-specific tests. IgG spots against KLH were rare events: among the 26 chronically HIV-1-infected subjects, 4 of them had 1 ASC/106 viable PBMCs, 3 had 2 ASCs/106 viable PBMCs, and 3 had 5, 6, and 11 ASCs/106 viable PBMCs, respectively.
To determine how our memory B cell assay performed compared with previous reports with regard to total and tetanus toxoid-specific memory IgG detection, we assayed total and tetanus toxoid-specific IgG ASC frequencies and found in uninfected and chronically HIV-1-infected subjects frequencies that were similar in magnitude to recent reports by others (21, 22). In particular, PBMCs from nine HIV-1-negative donors were tested for tetanus toxoid-specific memory B cells. All but two individuals had detectable tetanus toxoid-specific ASCs with a frequency over the total IgG ASCs ranging from 0.02% to 0.37% and an average of 0.11%. Tetanus toxoid-specific memory B cells have been previously shown to have a frequency of ~0.1% of total IgG memory B cells in adults vaccinated longer than 12 years (21). Also, this is in accordance with other reports focusing on circulating memory B cells specific for hepatitis B virus and smallpox (23, 24). Finally, we tested a further subset of PBMC samples from chronically HIV-1-infected subjects for reactivity against the bare tetramer (tetramerized biotin with streptavidin with no peptide) and no spots were formed (data not shown).
Second, we extended our observations on MPER-specific memory B cells to an additional 13 chronically HIV-1-infected subjects using a reversed format of ELISPOT assay with anti-IgG Ig as capture reagent (Hybridoma Reagent Laboratory; catalog no HP6046P) and the biotin-ylated SP62 gp41 MPER peptide (2.5 μM) as detector. Total IgG ASCs from stimulated and unstimulated cells and KLH-specific ASCs were enumerated and used as controls as described above. Data obtained were identical to the data obtained with the direct ELISPOT assay using peptide tetramers.
Direct binding ELISA
Ninety-six-well ELISA plates (Corning; catalog no. 3369) were coated with 0.2 μg/well Ag in 0.1 M sodium bicarbonate and blocked with assay diluent (PBS containing 4% (w/v) whey protein/15% normal goat serum/0.5% Tween 20/0.05% sodium azide). Sera were incubated for 1 h in 2-fold serial dilutions beginning at 1/400 followed by washing with PBS/0.1% Tween 20. One hundred microliters of alkaline phosphate-conjugated goat anti-human secondary Ab (Sigma-Aldrich; catalog no. A9544) was incubated for 1 h, washed, and detected with 100 μl substrate (CBC buffer plus 2 mM MgCl2 plus 1 mg/ml p-npp (4-nitrophenyl phosphate di(2-amino-2-ethyl-1,3-propanediol) salt)). Plates were developed for 45 min and read with a plate reader at 405 nm. Alternatively, binding to tetanus toxoid (EMD/Calbiochem; catalog no. 5882231) and 2007 Fluzone vaccine were tested in 384-well ELISA plates (Corning; catalog no. 3700) using 3-fold sera dilutions starting at 1/25 and developed with 30 μl/well of both secondary Ab and substrate. Results were reported as OD405 values at the same plasma dilution across patients. In every case the OD405 reading at the dilution used was within the linear range of the assay. The results shown did not change when other plasma dilutions within the linear range of the assay were used for analysis (data not shown).
Ab binding inhibition assay
Ninety-six-well ELISA plates were coated with 0.2 μg/well HIV-1 gp140 JRFL envelope in 0.1 M sodium bicarbonate and blocked with assay diluent (PBS containing 4% (w/v) whey protein/15% normal goat serum/0.5% Tween 20/0.05% sodium azide). All assay steps were conducted in assay diluent (except substrate step) and incubated for 1 h at room temperature (for 2F5) or at 37°C (for 13H11) followed by washing with PBS/0.1% Tween 20. Sera were diluted 1/50 and incubated in triplicate wells. Fifty microliters of biotinylated target mAb was added at the EC50 (determined by direct binding curve of biotinylated mAb to JRFL). The extent of biotin-mAb binding was detected with streptavidin-alkaline phosphatase at 1/1000 (Promega; catalog no. V5591) followed by substrate (CBC buffer plus 2 mM MgCl2 plus 1 mg/ml p-npp (4-nitrophenyl phosphate di(2-amino-2-ethyl-1,3-propanediol) salt)). Plates were developed for 45 min and read with a plate reader at 405 nm. Triplicate wells were background subtracted and averaged. Percentage inhibition was calculated as follows: 100 − (sera triplicate mean/no inhibition control mean)100.
Luminex assay
A sensitive Luminex assay was performed as previously described (13). Briefly, carboxylated fluorescent beads (Luminex) covalently coupled to p55 Gag, gp41 Env, or gp120 Env proteins were incubated with plasma serial dilutions ranging from 1/50 to 1/102,400. Beads were then washed and acquired on a Bio-Plex instrument (Bio-Rad). Purified IgG protein (Sigma-Aldrich) and a constant HIV-positive serum titration were utilized as positive controls in every assay. Background values (beads in the absence of detection Ab) and normal human plasma were utilized as the negative controls. Results were background subtracted and expressed as mean fluorescence intensity.
Statistical analysis
Statistical tests and the apparent half-life models were performed using SAS v9.1 (SAS Institute). Midpoint titers were determined with a 4-point fit using both SoftMax Pro 5 (Molecular Devices) and GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA). To address possible biases due to therapeutic failures affecting the HIV-1-specific Ab titers and the estimated Ab level half-lives, we monitored the viral load throughout the follow-up period for each patient and considered the removal of a specific time point and those from the next 3 years in case a 10-fold increment between two contiguous time points was observed. No significant changes in viral load were observed over time, and therefore all the data were included in the analysis. One subject (PID 835) received a tetanus toxoid boost early after initiation of ART. To ensure that the resulting spike in Ab titers for this patient did not affect the estimate of the overall duration of the Ab level half-life, we excluded from the analysis of the tetanus toxoid-specific Ab titer half-life all of the data points before week 105 from initiation of ART. Additionally, we repeated the analysis just excluding this subject and obtained comparable results (data not shown). The apparent Ab half-lives of gp120 and gp41 were estimated using a single exponential decay model (y = (β0 − β1)e−χtβ2 + β1, where β0 is the level at time 0, β1 is the distance to the lower asymptote, xt is time, β2 is the exponential rate of decay such that ln(2)/β2 is the computed half-life). This model was chosen based on its a priori compatibility to the data collected (i.e., true 0) as well as better fit than other two- and three-phase exponential decay models. Apparent half-lives for p55 Gag, anti-tetanus toxoid, and anti-influenza Abs were estimated using the mixed model method described by Amanna et al. (11).
Results
Detection of levels of memory B cells producing anti-whole Env and anti-gp120 V3 loop IgG Abs in vaccinated subjects and correlation with plasma Ab levels
We first evaluated the levels of HIV-1 Ag- and epitope-specific circulating memory B cells in a setting in which we could identify B cells producing Abs commonly made, such as anti-gp140 Env and anti-V3 loop, and where we could match the V3 sequences in our indicator peptides with an immunogen in a human vaccine trial. We therefore analyzed samples from the VaxGen VAX004 gp120 trial for detection of gp120 and V3 peptide-specific memory B cells using consensus B gp140 oligomer envelope and HIV MN V3 peptides, respectively. PBMC samples obtained 2 wk after the fourth immunization were stimulated in vitro for 6 days with pokeweed mitogen, CpG-oligodeoxynucleotides, and IL-15 to induce differentiation of memory B cells into ASCs (21, 25–27).
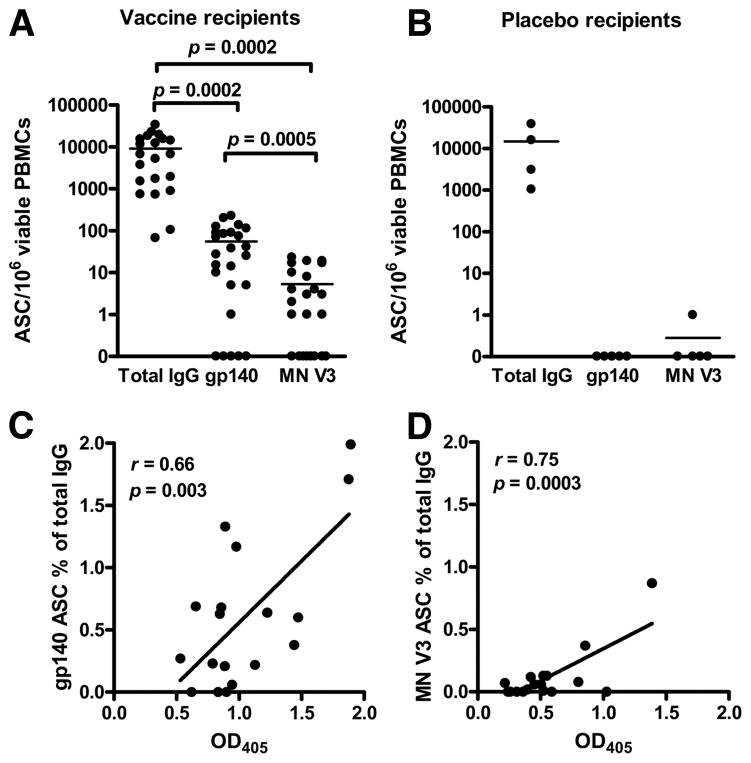
Total IgG-producing B cells, consensus B whole Env- and MN V3-specific IgG-secreting cells were measured in a B cell ELIS-POT assay. IgG ASCs were detected in all the samples (9140 ± 1997 ASCs/106 viable stimulated PBMCs) (Fig. 1A). Consensus B Env-specific IgG memory B cells were detected in 80% of the subjects. Among these positive subjects, the number of spots ranged from 1 to 228 ASCs/106 viable stimulated PBMCs, with a mean of 70 ± 15 ASCs/106 viable stimulated cells. Overall, consensus B Env-specific responses represented 1.79% of the total IgG ASCs (Fig. 1A).
FIGURE 1.
Frequency of total, gp140 envelope-specific, and MN V3 epitope-specific circulating IgG memory B cells in VaxGen VAX004 rgp120 vaccine and placebo recipients, and correlation between plasma Ab levels and epitope-specific IgG memory B cells. A, ELISPOT assay on stimulated PBMCs from 25 Vaxgen VAX004 rgp120 vaccine recipients. Circulating IgG memory B cells were detected in all tested samples. HIV-1 consensus B gp140 Env-specific and MN V3-specific ASCs were detected in 20 of 25 samples and 15 of 25 samples, respectively. Anti-gp140 Env-and anti MN V3-specific ASC frequencies were 2 and 3 orders of magnitude lower than total IgG ASCs, respectively (line at mean). The difference was statistically significant in-between all the groups (two-tailed paired Student’s t tests). B, The mean total IgG ASCs (line) in placebo recipients was comparable to that in vaccinees. No HIV-specific responses were detected (n = 5). One spot against MN V3 was detected in one placebo recipient and could be due to cross-reactivity. C, Statistical linear correlation (r = 0.66, p = 0.003) between the plasma Ab levels (1/40 plasma dilution is shown; x-axis) and the percentage of consensus B gp140-spe-cific ASCs over total IgG ASCs assay (y-axis). D, Seemingly, the correlation between the plasma Ab levels (OD405 at 1/12.5 plasma dilution is shown) and the percentage of MN V3-specific ASCs over total IgG ASCs was statistically significant (r = 0.75, p = 0.003). All plasmas were assayed at the same time and the same dilution was used for each OD reading across patients; in every case, the OD at the dilution used was within the linear range of the ELISA.
HIV MN V3-specific ASCs were detected in 60% of the gp120 vaccine recipients, with V3-specific ASC frequency an order of magnitude lower than whole Env ASC frequency (V3 = 0.13% of total IgG spots) and a mean of 9 ± 2 ASCs/106 viable stimulated PBMCs (Fig. 1A). No HIV-1 envelope-specific and HIV MN V3-specific ASCs were detected in placebo recipients (Fig. 1B). These data demonstrated that our assay was adequately sensitive and specific to detect HIV whole Env and epitope-specific ASCs in the context of vaccinated individuals.
Plasma Abs may be derived from short-lived plasma cells, either matured from the naive compartment or differentiated by resting memory B cells upon reencounter with Ag, or derived from long-lived plasma cells that reside in the bone marrow (5). When plasma Ab levels are predominantly maintained by long-lived plasma cells, then the plasma levels of Ab do not correlate with the levels of memory B cells (11). Thus, we asked if there was a correlation between the frequency of circulating HIV-specific gp140 or Env V3 memory B cells and levels of plasma anti-Env Abs of the same specificity in the VaxGen rgp120 recipients. Fig. 1C shows the analysis of ratios of anti-gp140 Env ASCs vs total IgG memory B cells and levels of anti-gp140 Env plasma Abs, performed as previously reported (11, 22, 27), in 18 samples for which paired observations were available. We found a significant correlation between gp140 Env ASC ratios and anti-gp140 Env plasma Ab levels (r = 0.66, p = 0.003). Similarly, a strong correlation between HIV MN V3-specific circulating memory B cell ratios of total ASCs and anti-HIV MN V3 Ab levels was also present (r = 0.75, p = 0.0003; Fig. 1D). The direct correlation between anti-gp140 and anti-Env V3 memory B cell ratios and plasma Ab levels implied that the contribution of long-lived plasma cells to plasma Ab levels was not predominant in this vaccination setting and that most of the detectable Ab response was maintained by shorter lived memory B cells. However, note that some patients had no detectable Ag-specific circulating memory B cells, yet they did have detectable plasma Ab titers. We cannot rule out the notion that in some patients the plasma Ab levels are indeed maintained by cells other than memory B cells, that is, bone marrow plasma cells.
Importantly, when analyses were performed using absolute numbers of Ag-specific ASCs and not the ratio of Ag-specific ASCs to total ASCs, no correlation with Ab levels was found (data not shown), most likely due to the wide variability (>2 logs) in frequencies of memory B cells among patients. Thus, as others have suggested (11, 22, 27), it appears that the biologically important comparison of ASCs is the ratio of Ag-specific to total ASCs vs plasma Ab levels. Ab level half-lives could not be calculated from VaxGen plasma because of the frequent dosing regimen in the trial (14).
Detection of IgG-producing total and Env-specific memory B cells in chronically HIV-1-infected subjects
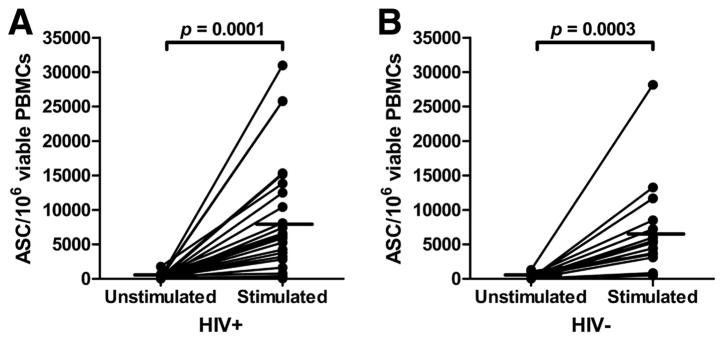
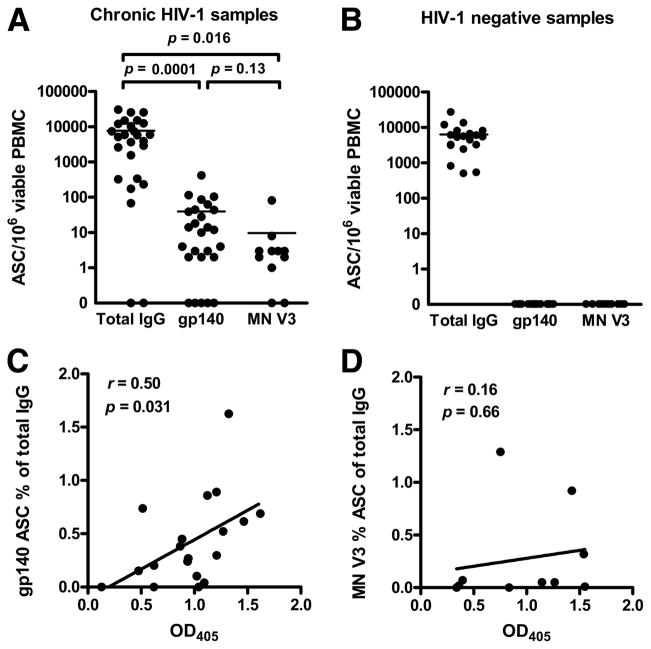
We next determined anti-gp140 Env and anti-HIV MN V3 IgG memory B cell levels in 26 chronically HIV-1-infected and 23 uninfected subjects from the United States. Fig. 2A shows that, upon stimulation of HIV-1-infected PBMCs, the total memory B cell level increased from a mean of 239 ± 70 ASCs/106 viable PBMCs with no stimulation to 7963 ± 1702 ASCs/106 viable PBMCs after 6 days in culture (p = 0.0001). Similarly, memory B cells from HIV-1 uninfected subjects increased from 256 ± 86 ASCs/106 viable PBMCs with no stimulation to 6457 ± 1426 ASCs/106 cultured PBMCs (p = 0.0003; Fig. 2B). We next assayed for anti-gp140 consensus B Env memory B cells and found detectable ASCs in 81% of HIV-1 chronically infected subjects. These samples averaged 49 ± 20 ASCs/106 viable stimulated PBMCs (range, 2– 417; Fig. 3A). Among chronically HIV-1-infected subjects with detectable responses, the ratio of anti-gp140 Env IgG ASCs vs total IgG-secreting cells was 0.64% (see supplemental Table S1).4 Anti-Env V3 IgG memory B cells were similarly detected in 82% of chronically HIV-1-infected patients using the HIV-1 MN V3 peptide. The Env V3-specific memory B cell response averaged 12 ± 9 ASCs/106 viable stimulated PBMCs (Fig. 3A). Overall, the levels of gp140 Env- and HIV MN V3 epitope-specific memory B cells in chronically infected subjects were comparable with observations in vaccinated individuals (Fig. 1A), being ~2 (p = 0.0001) and 3 (p = 0.016) orders of magnitude lower than total IgG, respectively (Fig. 3A). No HIV-1 envelope-specific or HIV MN V3-specific ASCs were detected in HIV-1-negative subjects (Fig. 3B).
FIGURE 2.
Production of IgG ASCs is induced by in vitro stimulation with pokeweed mitogen, CpG-oligodeoxynucleotides and IL-15 in both HIV-positive and HIV-negative samples. IgG ASCs were enumerated by ELISPOT assay after a 6-day culture with or without pokeweed mitogen, CpG-oligodeoxynucleotides and IL-15 to measure the effect of the stimulation in driving resting memory B cells into ASCs. The results are expressed as ASCs per million viable PBMCs at the end of culture. Both HIV-positive (n = 26; A) and HIV-negative (n = 19; B) samples showed a statistically significant (two-tailed paired Student’s t test) increased number of IgG-secreting cells upon stimulation (line at mean). ASCs from unstimulated cultured cells (spontaneously secreting cells) were subtracted as background from the total IgG memory B cell ASC counts in subsequent analyses.
FIGURE 3.
Frequency of total, gp140 Env-specific, and MN V3 epitope-specific circulating IgG memory B cells in chronically HIV-1-infected or HIV-1-negative subjects, and correlation between plasma Ab levels and epitope-specific circulating IgG memory B cells. A, Circulating IgG memory B cells were detected in 24 of 26 samples from HIV-1-infected subjects (line at mean). HIV-1 consensus B gp140 Env-specific responses were detected in 21 of 26 samples, and MN V3-specific memory responses were detected in 9 of 11 samples. Comparison among consensus B gp140-and MN V3-specific frequencies of IgG ASCs shows a pattern that is similar to vaccinated individuals. Statistical differences were calculated with paired Student’s t tests. B, The frequency of total IgG in 19 HIV-negative individuals was comparable to that in HIV-positive samples. No HIV-specific responses were detected. C, The plasma Ab levels detected by ELISA (OD405 at 1/800 dilution; x-axis) of 19 HIV-positive samples were correlated to the percentage of consensus B gp140-specific ASCs over total IgG ASCs detected by ELISPOT assay (y-axis) for which paired observations were available. A significant linear correlation was detected (r = 0.5, p = 0.031). All plasmas were assayed at the same time and the same dilution was used for each OD reading across patients; in every case, the OD at the dilution used was within the linear range of the ELISA. D, On the contrary, no correlation was found in the same subjects between MN V3-specific ASCs and plasma Ab levels.
The analysis of memory B cells and plasma Ab levels in HIV-1 chronically infected subjects also showed a correlation between gp140 memory B cell levels and plasma gp140 Env Ab levels (r = 0.50, p = 0.031; Fig. 3C). In contrast, no significant correlation was found between the memory Env V3-positive B cells and plasma Env V3 Ab levels (Fig. 3D), possibly relating to the heterogeneity of the Env V3 loop in chronically infected subjects and/or the inability of the V3 peptide to capture all Env V3-reactive memory B cells. The positive correlation of gp140 memory B cell ratio and plasma gp140 Ab, however, is similar to data obtained in gp120-immunized subjects, suggesting that the plasma Env Ab was maintained predominantly by the shorter lived memory B cell response to Env and not by a long-lived plasma cell pool (11).
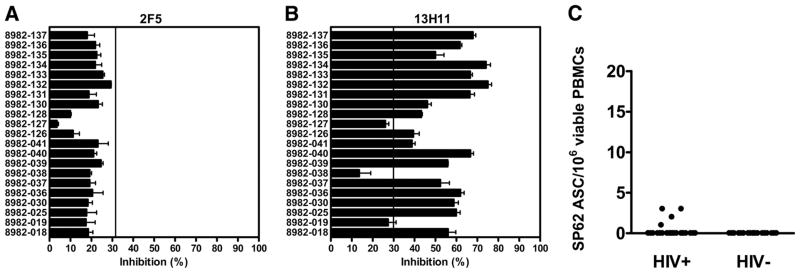
The ability of the MPER of gp41 to induce Abs is a key question for the development of an HIV-1 envelope-based vaccine (28), since a cluster of epitopes in the MPER is the target for broadly neutralizing Abs (28, 29). We could not assay for HIV-1 Env gp41 memory B cells in the gp120 Env vaccination trial subjects since they were immunized with only gp120. However, as another example of HIV-1 epitope-specific memory B cell response, it was of interest to determine whether we could identify any gp41 MPER memory B cells in chronically infected and uninfected subjects. We first assayed for gp41 MPER cluster II 2F5-related epitope Abs in two ways: direct binding ELISA to the nominal epitope, and competitive inhibition of patient plasma of biotinylated 2F5 mAb and the non-neutralizing MPER mAb 13H11 (30). We found MPER 2F5 region-related epitope-specific plasma Abs in 5 of 21 subjects (23.8%) in direct ELISA using the gp41 MPER cluster II peptide QQEKNEQELLELDKWASLWN (SP62) as Ag. Three subjects had titers of 100, 50, and 25, respectively, and two had lower titers of 6.25 (see supplemental Table S2). We and others have previously shown that Abs that competitively block non-neutralizing gp41 cluster II Abs are commonly present in the plasma of HIV-1-positive subjects as detected in competitive inhibition assays (31, 32). Thus, using competitive inhibition assays, we found that none of the subjects studied had Abs that could block the binding of biotinylated 2F5 mAb to the 2F5 nominal neutralizing epitope on a clade B oligomer (Fig. 4A) (30). In contrast, 87% of subjects had Abs to a closely related MPER non-neutralizing epitope that blocked the binding of the non-neutralizing mAb 13H11 (Fig. 4B) (30). Both 2F5 neutralizing and 13H11 non-neutralizing MPER mAbs bind to the SP62 peptide (30). However, while the 2F5 epitope represented in the SP62 peptide was highly conserved among clade B HIV-1 isolates (28) and 87% of the subjects studied had Abs that could competitively block the binding of mAb 13H11, only 16.7% of chronically HIV-1-infected subjects had circulating memory B spot-forming cells that recognized the SP62 peptide (Fig. 4C). The magnitude of the MPER 2F5 epitope-specific memory response was 1–3 ASCs/106 viable stimulated PBMCs (see Fig. 4C and supplemental Table S2). Interestingly, all four MPER ASC responders were on ART at the time of study, and only one subject, 8982-135, had both detectable MPER 2F5 epitope-specific memory B cells and plasma Abs (see supplemental Table S2). No ASCs specific for the 2F5 epitope were detected in HIV-1-negative subjects (Fig. 4C).
FIGURE 4.
IgG memory B cells and plasma Ab levels against the MPER gp41 cluster II 2F5 epitope. A and B, As a sensitive measurement of the plasma Abs binding to the MPER cluster II gp41 2F5 epitope, we tested the ability of plasma (at 1/50 dilution) to block the binding of either the broadly neutralizing 2F5 (A) or the non-neutralizing 13H11 (B) mAbs, both of which bind to the MPER gp41 cluster II 2F5 epitope, to the clade B JRFL gp140 oligomer by comparing the extent of biotin-mAb binding detected with or without preincubating the JRFL envelope with plasma. The data shown are expressed as follows: 100 − (sera triplicate mean/no inhibition control mean)100. Triplicate wells were background subtracted and averaged. The threshold (vertical line) was conservatively calculated as 3 times the mean readout of multiple HIV-negative samples + 3 SEM. C, Only 4 (16.7%) of the 24 HIV-positive samples tested had detectable IgG ASCs against the MPER cluster II gp41 2F5 epitope. The magnitude of the response was significantly lower than that against the MN V3 epitope (p < 0.0001, Mann-Whitney U test). No ASCs against the MPER cluster II gp41 2F5 epitope were detected in 18 HIV-negative samples.
To confirm these findings, we tested an additional 13 chronically HIV-1-infected subjects using the reverse ELISPOT assay format for the detection of epitope-specific ASCs (see Materials and Methods). In this cohort, the frequency and numbers of IgG ASCs were comparable to those of the 26 chronically HIV-1-infected subjects previously studied: 11 of 13 samples had circulating IgG memory B cells with an average of 6309 ± 2372 ASCs/106 viable PBMCs, but only 3 of 13 subjects (23%) had MPER cluster II gp41 epitope-specific memory responses with 10, 13, and 41 ASCs/106 viable PBMCs, respectively (data not shown).
Taken together, these results demonstrated that most chronically HIV-1-infected patients did not have detectable levels of circulating IgG memory B cells against the MPER cluster II gp41 peptide containing the 2F5-related neutralizing or the 13H11-related non-neutralizing B cell epitopes. Since a subset of anti-gp41 MPER cluster II-specific Abs bind to conformational epitopes (30), the magnitude of the response against the 2F5/13H11-related epitope defined by the SP62 peptide is likely to represent only a fraction of the overall responses. Nonetheless, memory B cells capable of making 13H11-related MPER non-neutralizing Ab (defined by Abs that block the binding of non-neutralizing mAb 13H11 that are commonly made in chronic HIV-1 infection) should have been more readily detected by the SP62 peptide ELISPOT assay, yet they were not. These data implied that MPER specificities of memory B cells were rare in blood.
Half-life of anti-gp120 and anti-gp41 Ab titers in chronically HIV-1-infected subjects with ART-suppressed viremia
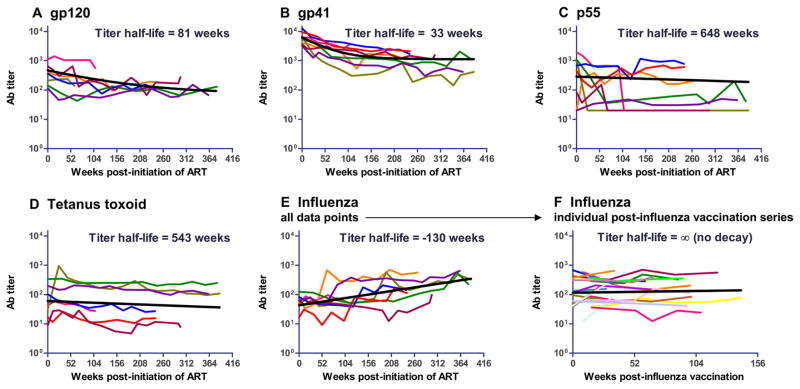
To further characterize the duration of anti-HIV-1-specific responses during chronic HIV-1 infection, we determined the time of decay of anti-gp120 and anti-gp41 Env IgG plasma Ab levels in eight chronically HIV-1-infected subjects followed for up to 387 wk (>7 years) after successful suppression of viremia upon initiation of ART. Both anti-gp120 (Fig. 5A) and anti-gp41 (Fig. 5B) Abs declined rapidly during the time of study on ART (4.5-fold and 5.5-fold decline in anti-gp120 and anti-gp41 titers, respectively) and then stabilized to a lower baseline level. We estimated the apparent half-life of Ab titers for each Ag and compared them to that for HIV-1 p55 Gag. Additionally, we also determined the plasma Ab titer for two non-HIV-1-related Ags: tetanus toxoid and 2007 influenza vaccine Fluzone. Exposure to tetanus toxoid occurred years before the period of observation, whereas repeated boost vaccinations were documented for influenza. As mentioned, a short estimated Ab level half-life implies a greater dependency of the Ab titer to active viral replication and persistent antigenic stimulation, and thus indicates a higher contribution of short-lived memory B cell responses to the Ag-specific Ab pool. Anti-gp120 Ab level half-life was ~81 wk (95% confidence interval, 30 –∞ wk; Fig. 5A) and anti-gp41 Env IgG Ab level half-life was similarly short at 33 wk (95% confidence interval, 23–58 wk; Fig. 5B). Similar findings for rapid decline of gp120 plasma Abs on ART have been reported previously (33). Conversely, overall anti-p55 Ab titers did not consistently decline over time (Fig. 5C); the variable patterns of the eight subjects precluded a precise definition of a half-life of p55 Ab levels, although an apparent titer half-life of 648 wk was estimated by a special linear mixed model (see Materials and Methods). This finding provides direct evidence that the rapid decay observed in the anti-gp120 and anti-gp41 Ab titers is not consistently observed, under the same conditions, for other non-HIV-1 envelope-related Ags, such as p55 Gag.
FIGURE 5.
Ab responses in chronically HIV-1-infected subjects upon initiation of anti-retroviral therapy. Eight HIV-positive subjects were followed-up for up to 378 wk upon initiation of ART and suppression of viremia. For each of them the (A) anti-gp120, (B) anti-gp41, (C) anti-p55, (D) anti-tetanus toxoid, and (E) anti-influenza plasma IgG Ab levels are shown over the entire 7-year period (colored lines), and (F) the plasma anti-influenza Ab half-lives of the individual intervals of time after each documented boost of influenza vaccine are shown. The calculated plasma Ab level half-lives have been modeled as described in Materials and Methods and are shown with the black line.
Our chronic HIV-1 subjects received annual or biennial influenza vaccinations, and Ab titers spiked in response to these immunizations (Fig. 5E). As a consequence, the overall Ab levels to the 2007 influenza vaccine Fluzone rose over time, as expected (6). To better characterize the decay of the anti-influenza Ab levels after vaccination, we estimated the anti-influenza titer half-life of each period in-between documented influenza vaccine boosts as independent longitudinal series. Again, as opposed to anti-envelope titers and similarly to p55 titers, anti-influenza titers did not fall after each vaccination and no significant decay was observed (Fig. 5F).
Finally, the Ab level half-life against tetanus toxoid, an Ag that has been shown to elicit predominantly long-lived plasma cell responses (11), was estimated at 543 wk (~11 years; Fig. 5D), consistent with prior observations of Amanna et al. (11) in HIV-negative individuals.
Taken together, these data provided evidence for the inability of HIV-1 Env molecule to induce long-lived duration of Ab in chronic HIV-1 infection at levels comparable to those induced by other HIV-1 molecules (i.e., p55 Gag) or vaccines (tetanus toxoid or influenza).
Discussion
HIV-1 Env is the target for all known neutralizing epitopes of HIV-1 (29, 34). A critical goal of HIV-1 vaccine development is to understand how to design and formulate HIV-1 Env to induce broadly reactive, protective Ab responses that are durable and of sufficient titer. In this study we show that in both Env gp120 vaccination and HIV-1 chronic infection, plasma levels of induced Abs correlated with the levels of blood memory B cells, directly implying that short-lived, Ag-driven B cells are primarily responsible for anti-Env plasma Ab levels. We were able to confirm this notion in ART-treated chronically HIV-1-infected subjects with near complete suppression of plasma viremia by showing that the half-life of anti-Env Abs was relatively short (33 wk for gp41 and 81 wk for gp120) compared with the half-life of anti-p55 Gag (648 wk) and postvaccination anti-influenza Ab levels. Most if not all successful anti-infectious agent vaccines have neutralizing Abs as a correlate of immunity and, in contrast to HIV-1 Env, the apparent half-lives of vaccine-induced Ab levels range from 10 to 19 years for protein-induced Abs (e.g., tetanus, diphtheria) to 30 or more years for live vector-induced Abs (e.g., polio, measles) (11). We showed that the tetanus toxoid-specific Ab titer half-life, which has been previously described to be predominantly sustained in HIV-1-negative subjects by long-lived plasma cells rather than memory B cells (11), was maintained at ~11 years in chronically HIV-1-infected subjects.
Our study is important in that it directly demonstrates that, in addition to the inability of HIV-1 Env to routinely induce broadly neutralizing protective Abs (29), HIV-1 Env is also limited in its ability to induce long-lived B cell responses by persistent plasma cells. Moreover, this is the first study to our knowledge that examines the frequency of epitope-specific memory B cell levels in HIV-1-infected or vaccinated subjects.
The generation of immunological memory is a hallmark of successful vaccination (5, 6). Such protection is generated and exerted by two independently regulated mechanisms that lead to the development of reservoirs of long-lived plasma cells and memory B cells (5, 8, 11, 12, 15, 35). Long-lived plasma cells are terminally differentiated, nondividing cells generated upon first encounter with an Ag or derived from previously generated memory B cells that reside in the bone marrow for many years and constantly secrete low levels of highly specific Abs without the need of further contact with the same Ag (5, 7, 9). Since Ag-specific Abs are already circulating by the time of reexposure, long-lived plasma cells represent the very first line of defense exerted by humoral memory (5).
As opposed to long-lived plasma cells, memory B cells do not prevent infection per se, but they proliferate and differentiate into ASCs upon Ag reexposure, resulting in a rise of high-affinity Ag-specific plasma Ab levels (7). Moreover, memory B cell levels in peripheral blood have been suggested to represent the overall pool of memory B cells since they constantly recirculate through the blood and secondary lymphoid organs, such as tonsils, spleen, peripheral lymph nodes, and Peyer’s patches (11, 15, 35). Therefore, the detection of circulating memory B cells with a sensitive ELIS-POT assay upon polyclonal in vitro stimulation allows the detection of epitope-specific memory B cells representative of the overall population of memory B cells produced upon vaccination or during chronic infection that are functionally able to differentiate into ASCs and are therefore capable of providing protection against reexposure to the cognate Ag.
The correlation between the ratio of HIV-1 Env-specific vs total memory B ASCs and plasma Ab levels describes the relative contribution of memory B cells to the maintenance of immunological memory after vaccination or during chronic infection (11, 22, 27). Whenever immunological memory is sustained by memory B cells, a correlation with plasma Ab levels is predicted to be maintained after reexposure to the same Ag (11, 21, 35).
In the present report, a correlation between ratios of circulating memory B cells and plasma Ab levels against gp140 as well as to the Env gp120 V3 region was detected in vaccinees and for gp140 Abs in chronically infected individuals. These findings imply that the contribution of long-lived plasma cells to the maintenance of immunological memory against the HIV-1 envelope after vaccination with rgp120 and during chronic infection may be limited, and that HIV-1 envelope-specific plasma Abs are more short- than long-lived in both settings. However, several subjects with no anti-gp140 or anti-V3 region memory B cells still had detectable Ab titers, implying that these specificities were maintained by cells other than memory B cells. Therefore, it is entirely likely that Env in some patients is able to induce a degree of long-lived plasma cells to Env. It will be of interest to determine any correlation of long-lived Ab responses with maintenance of T follicular helper T cells in peripheral generative B cell microenvironments such as Peyer’s patches in gut.
The direct analysis of anti-gp41 and gp120 Ab level half-lives in chronically HIV-1-infected subjects in which viremia was suppressed by ART confirmed the hypothesis that HIV-1 envelope-specific plasma Abs are more short- than long-lived. Additionally, the lack of a consistent rapid decay in HIV-1 p55 Ab titers after initiation of ART and influenza after booster vaccinations suggested that, in the setting of chronic HIV-1 infection, the regulation of anti-p55 Gag Abs may differ from that of anti-Env Abs. In particular, it is possible that ART might have different effects on envelope Ags, which are HIV-1 protease-processed Ags, and p55 Gag, which is a polypeptide precursor, that would lead to differential regulation of these B cell responses.
Our half-life estimate of anti-gp120 Ab level is longer than that previously reported by Morris and colleagues (33), most likely due to the prolonged length of our observations (up to 7 years vs 1 year), allowing the Ab titers to reach a plateau at a lower level in most cases, such that the relative contribution of sustained Ab responses was reflected in the estimated Ab half-life. Note that the estimate of the plasma Ab level half-lives reflects the balance between at least two distinct components: sustained production of Abs by long-lived plasma cells, with very slow decline over several years, and the faster Ab level decay shortly after antigenic exposure (11). Additionally, other elements can contribute to extending the predicted 3-wk IgG half-life (36) and prolong plasma Ab levels even in absence of long-lived plasma cells: (1) Ag-specific IgG production is not immediately stopped at initiation of therapy when the viral load is still high; (2) residual Ag produced during antiretroviral therapy could circulate and continue to stimulate Ab production; (3) Ags could be trapped on follicular dendritic cells and in other compartments that are not in equilibrium with peripheral blood; and (4) other infection-related abnormalities might alter the normal clearance of IgGs from plasma (33).
The drop of anti-gp120 and anti-gp41 IgG Ab titers was rapid and dramatic, implying direct dependence of plasma anti-gp120 and anti-gp41 Env IgG Ab levels on antigenic stimulation. In contrast, anti-p55 Gag Ab responses did not decay consistently over time nor did anti-influenza Ab titers after each documented influenza vaccine boost. Since the specific contribution of long-lived plasma cells to plasma Ab levels can only be directly studied by analyzing bone marrow samples, our data imply, but do not prove, that the pool of circulating anti-gp120 and anti-gp41 IgG Abs was predominantly supported by short-lived plasma cells.
Previous work has suggested a selectively short duration of various HIV-1 Env Abs after vaccination (14, 37–39). A number of studies have also suggested a decrease in Env Abs in subjects followed for short periods on ART (33, 40 – 43). Recently, evidence for short-lived envelope responses were seen in an HIV-1-positive individual with acute leukemia transplanted with a CCR5Δ32 bone marrow stem cell transplant (44). Both his leukemia and HIV-1 were controlled posttransplant, and with a fall in plasma HIV-1 viral load to undetectable, his anti-Env Ab level continually fell over the ensuing 1.5 years (44). However, follow-up of Ab levels over years is needed for accurate determinations of duration of plasma Abs (33).
The present study is novel in that the levels of HIV-1 Env-specific memory B cells vs plasma Abs were analyzed in both the settings of chronic infection and vaccination, and in that it demonstrates that the mechanism of short duration of plasma anti-Env Abs in chronic HIV-1 infection and upon vaccination is dependence on memory B cell Ab production.
Finally, it was surprising that gp41 cluster II MPER 2F5/13H11-related epitope-specific memory B cells were present in only a small fraction of chronically HIV-1-infected patients and did not correlate with plasma Ab levels. These observations warrant additional studies of the persistence of 13H11-related epitope Abs, as this non-neutralizing MPER gp41 epitope, against which Abs are commonly made in HIV-1-infected subjects (30), may induce long-lived plasma cells.
The different biologies of memory B cells and long-lived plasma cells should be taken in account in the design of an AIDS vaccine (4). A preventive HIV-1 vaccine needs to extinguish infection before viral latency is established in CD4+ T cells (4, 13, 45), and to do this, high levels of long-lived protective Ab needs to be present before HIV-1 infection. In fact, while viral latency is certainly established by the time of seroconversion (46), several studies suggest that the window of opportunity to prevent the establishment of a reservoir of latent viruses may be as short as few days after transmission (4, 13, 47). Therefore, memory B cells may not have the time to differentiate into ASCs within the window of opportunity, and neutralizing Abs may need to be available at the time of transmission (4), implying that the induction of long-lived plasma cells is required for an AIDS vaccine to be effective. Previous studies correlating Ag-specific memory B cell frequencies in peripheral blood with plasma Ab levels after booster vaccination suggest that circulating memory B cells peak at 1–2 wk postre-vaccination and drive the Ag-specific effector response for only 2– 4 wk after revaccination (8, 35, 48).
A number of recent observations provide strong indications regarding why HIV-1 Env does not routinely induce longer lived Ab responses and why memory B cell function is disrupted upon infection (6): we have recently demonstrated very early damage to B cell germinal centers in Peyer’s patches early on in acute HIV-1 infection (49); HIV patients contain a subset of “exhausted”-like FCRH4+ memory B cells enriched for HIV specificities (50); and, certainly, the early loss of robust CD4+ T cell help in early HIV-1 infection is an obvious mechanism for lack of induction of long-lived B cell responses (5, 6, 51), However, in the context of vaccination, in which the immune system of the recipients is not impaired by HIV infection, potential factors responsible for the lack of long-term responses should be focused on the characteristics of the immunogen: the biological activity of HIV-1 Env gp160 (52), T cell suppression by gp120 binding to CD4 (53), and the ability of the carbohydrates of gp120, which comprise ~50% of the Env mass, to bind to mannose receptors on both dendritic cells (54) and B cells (55), hence suppressing Ag-specific immune responses.
One mechanism for overcoming the short duration of induced HIV-1 envelope Abs is the formulation of HIV-1 Env in potent adjuvants. In this regard, Goepfert and colleagues have reported immunization of humans with the adjuvant AS02A (QS-21 and 3-deacylated monophosphoryl lipid A in an oil-in-water emulsion), which induced anti-gp120 Env Abs lasting up to 9 mo after final boost, although a half-life of Ab levels was not determined (56).
In conclusion, we have shown that anti-HIV-1 envelope plasma levels are correlated with envelope blood memory B cell levels both in the setting of vaccination and during chronic infection. With the rapid induction of immune suppression and CD4+ T cell loss as well as germinal center damage by transmitted/founder HIV-1 (47, 49, 57– 60), it will be critical to be able to induce long-lived plasma and mucosal Abs against protective epitopes of HIV-1 Env that are present before contact with HIV-1. Even if the problem of induction of rare specificities of broadly neutralizing Abs is solved, the problem of inducing Abs of high titers and sufficient duration will still need to be solved for a HIV-1 vaccine to be effective. Thus, understanding how to design HIV-1 Env immunogens that are capable of inducing not only memory B cells but also long-lived plasma cells will be critical for the development of a successful HIV-1 vaccine.
Supplementary Material
Acknowledgments
The authors thank Dr. Jon Warren (Vaccine Research Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services) for providing the Vaxgen VAX004 clinical trial samples, Judith T. Lucas and Vicki C. Ashley for expert technical assistance, and Judy L. Stein for coordinating sample collection and providing clinical data.
Footnotes
This study has been supported by the National Institute of Allergy and Infectious Diseases Grant U19 AI067854 (Center for HIV/AIDS Vaccine Immunology) and by a Collaboration for Vaccine Discovery Grant to B.F.H. from the Bill and Melinda Gates Foundation.
Abbreviations used in this paper: rgp120, recombinant gp120; ART, antiretroviral therapy; ASC, Ab-secreting cell; KLH, keyhole limpet hemocyanin; MPER, membrane-proximal external region.
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Johnston MI, Fauci AS. An HIV vaccine: challenges and prospects. N Engl J Med. 2008;359:888–890. doi: 10.1056/NEJMp0806162. [DOI] [PubMed] [Google Scholar]
- 2.Hall HI, Song R, Rhodes P, Prejean J, An Q, Lee LM, Karon J, Brookmeyer R, Kaplan EH, McKenna MT, Janssen RS for the HIV Incidence Surveillance Group. Estimation of HIV incidence in the United States. J Am Med Assoc. 2008;300:520–529. doi: 10.1001/jama.300.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fauci AS, Johnston MI, Dieffenbach CW, Burton DR, Hammer SM, Hoxie JA, Martin M, Overbaugh J, Watkins DI, Mahmoud A, Greene WC. HIV vaccine research: the way forward. Science. 2008;321:530–532. doi: 10.1126/science.1161000. [DOI] [PubMed] [Google Scholar]
- 4.Wong SBJ, Siliciano RF. Biology of early infection and impact on vaccine design. In: Wayne PK, Koff C, Gust ID, editors. AIDS VaccineDevelopment: Challenges and Opportunities. Caister Academic Press; Wymondham, UK: 2007. pp. 17–22. [Google Scholar]
- 5.Dörner T, Radbruch A. Antibodies and B cell memory in viral immunity. Immunity. 2007;27:384–392. doi: 10.1016/j.immuni.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Cagigi A, Nilsson A, De Milito A, Chiodi F. B cell immunopathology during HIV-1 infection: lessons to learn for HIV-1 vaccine design. Vaccine. 2008;26:3016–3025. doi: 10.1016/j.vaccine.2007.11.063. [DOI] [PubMed] [Google Scholar]
- 7.Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KG, Dorner T, Hiepe F. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat Rev Immunol. 2006;6:741–750. doi: 10.1038/nri1886. [DOI] [PubMed] [Google Scholar]
- 8.Shokrgozar MA, Sam MR, Amirkhani A, Shokri F. Frequency analysis of HBsAg-specific B lymphocytes in high-responder individuals to recombinant hepatitis B vaccine: comparison of LDA and ELISPOT assays. Scand J Immunol. 2006;64:536–543. doi: 10.1111/j.1365-3083.2006.01838.x. [DOI] [PubMed] [Google Scholar]
- 9.Manz RA, Lohning M, Cassese G, Thiel A, Radbruch A. Survival of long-lived plasma cells is independent of antigen. Int Immunol. 1998;10:1703–1711. doi: 10.1093/intimm/10.11.1703. [DOI] [PubMed] [Google Scholar]
- 10.Slifka MK, Ahmed R. Long-lived plasma cells: a mechanism for maintaining persistent antibody production. Curr Opin Immunol. 1998;10:252–258. doi: 10.1016/s0952-7915(98)80162-3. [DOI] [PubMed] [Google Scholar]
- 11.Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357:1903–1915. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- 12.Leyendeckers H, Odendahl M, Löhndorf A, Irsch J, Spangfort M, Miltenyi S, Hunzelmann N, Assenmacher M, Radbruch A, Schmitz J. Correlation analysis between frequencies of circulating antigen-specific IgG-bearing memory B cells and serum titers of antigen-specific IgG. Eur J Immunol. 1999;29:1406–1417. doi: 10.1002/(SICI)1521-4141(199904)29:04<1406::AID-IMMU1406>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 13.Tomaras GD, Yates NL, Liu P, Qin L, Fouda GG, Chavez LL, Decamp AC, Parks RJ, Ashley VC, Lucas JT, et al. Initial B cell responses to transmitted HIV-1: virion-binding IgM and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J Virol. 2008;82:12449–12463. doi: 10.1128/JVI.01708-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilbert PB, Peterson ML, Follmann D, Hudgens MG, Francis DP, Gurwith M, Heyward WL, Jobes DV, Popovic V, Self SG, et al. Correlation between immunologic responses to a recombinant glycoprotein 120 vaccine and incidence of HIV-1 infection in a phase 3 HIV-1 preventive vaccine trial. J Infect Dis. 2005;191:666–677. doi: 10.1086/428405. [DOI] [PubMed] [Google Scholar]
- 15.Blink EJ, Light A, Kallies A, Nutt SL, Hodgkin PD, Tarlinton DM. Early appearance of germinal center-derived memory B cells and plasma cells in blood after primary immunization. J Exp Med. 2005;201:545–554. doi: 10.1084/jem.20042060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, Zheng NY, Mays I, Garman L, Helms C, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453:667–671. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crotty S, Aubert RD, Glidewell J, Ahmed R. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J Immunol Methods. 2004;286:111–122. doi: 10.1016/j.jim.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 18.Newman J, Rice JS, Wang C, Harris SL, Diamond B. Identification of an antigen-specific B cell population. J Immunol Methods. 2003;272:177–187. doi: 10.1016/s0022-1759(02)00499-4. [DOI] [PubMed] [Google Scholar]
- 19.Liao HX, Sutherland LL, Xia SM, Brock ME, Scearce RM, Vanleeuwen S, Alam SM, McAdams M, Weaver EA, Camacho Z, et al. A group M consensus envelope glycoprotein induces antibodies that neutralize subsets of subtype B and C HIV-1 primary viruses. Virology. 2006;353:268–282. doi: 10.1016/j.virol.2006.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalyuzhny A, Stark S. A simple method to reduce the background and improve well-to-well reproducibility of staining in ELISPOT assays. J Immunol Methods. 2001;257:93–97. doi: 10.1016/s0022-1759(01)00451-3. [DOI] [PubMed] [Google Scholar]
- 21.Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298:2199–2202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 22.Titanji K, De Milito A, Cagigi A, Thorstensson R, Grutzmeier S, Atlas A, Hejdeman B, Kroon FP, Lopalco L, Nilsson A, Chiodi F. Loss of memory B cells impairs maintenance of long-term serologic memory during HIV-1 infection. Blood. 2006;108:1580–1587. doi: 10.1182/blood-2005-11-013383. [DOI] [PubMed] [Google Scholar]
- 23.Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171:4969–4973. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 24.Tuaillon E, Tabaa YA, Petitjean G, Huguet MF, Pajeaux G, Fondere JM, Ponseille B, Ducos J, Blanc P, Vendrell JP. Detection of memory B lymphocytes specific to hepatitis B virus (HBV) surface antigen (HBsAg) from HBsAg-vaccinated or HBV-immunized subjects by ELISPOT assay. J Immunol Methods. 2006;315:144–152. doi: 10.1016/j.jim.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 25.Crotty S, Aubert RD, Glidewell J, Ahmed R. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J Immunol Methods. 2004;286:111–122. doi: 10.1016/j.jim.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 26.Kacani L, Sprinzl GM, Erdei A, Dierich MP. Interleukin-15 enhances HIV-1-driven polyclonal B-cell response in vitro. Exp Clin Immunogenet. 1999;16:162–172. doi: 10.1159/000019108. [DOI] [PubMed] [Google Scholar]
- 27.Crompton PD, Mircetic M, Weiss G, Baughman A, Huang CY, Topham DJ, Treanor JJ, Sanz I, Lee FE, Durbin AP, et al. The TLR9 ligand CpG promotes the acquisition of Plasmodium falciparum-specific memory B cells in malaria-naive individuals. J Immunol. 2009;182:3318–3326. doi: 10.4049/jimmunol.0803596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montero M, van Houten NE, Wang X, Scott JK. The membrane-proximal external region of the human immunodeficiency virus type 1 envelope: dominant site of antibody neutralization and target for vaccine design. Microbiol Mol Biol Rev. 2008;72:54–84. doi: 10.1128/MMBR.00020-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haynes BF, Montefiori DC. Aiming to induce broadly reactive neutralizing antibody responses with HIV-1 vaccine candidates. Expert Rev Vaccines. 2006;5:579–595. doi: 10.1586/14760584.5.4.579. [DOI] [PubMed] [Google Scholar]
- 30.Alam SM, Scearce RM, Parks RJ, Plonk K, Plonk SG, Sutherland LL, Gorny MK, Zolla-Pazner S, Vanleeuwen S, Moody MA, et al. Human immunodeficiency virus type 1 gp41 antibodies that mask membrane proximal region epitopes: antibody binding kinetics, induction, and potential for regulation in acute infection. J Virol. 2008;82:115–125. doi: 10.1128/JVI.00927-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alam SM, Scearce RM, Parks RJ, Plonk K, Plonk SG, Sutherland LL, Gorny MK, Zolla-Pazner S, Vanleeuwen S, Moody MA, et al. Human immunodeficiency virus type 1 gp41 antibodies that mask membrane proximal region epitopes: antibody binding kinetics, induction, and potential for regulation in acute infection. J Virol. 2008;82:115–125. doi: 10.1128/JVI.00927-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu JY, Gorny MK, Palker T, Karwowska S, Zolla-Pazner S. Epitope mapping of two immunodominant domains of gp41, the transmembrane protein of human immunodeficiency virus type 1, using ten human monoclonal antibodies. J Virol. 1991;65:4832–4838. doi: 10.1128/jvi.65.9.4832-4838.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morris L, Binley JM, Clas BA, Bonhoeffer S, Astill TP, Kost R, Hurley A, Cao Y, Markowitz M, Ho DD, Moore JP. HIV-1 antigen-specific and -nonspecific B cell responses are sensitive to combination antiretroviral therapy. J Exp Med. 1998;188:233–245. doi: 10.1084/jem.188.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walker BD, Burton DR. Toward an AIDS vaccine. Science. 2008;320:760–764. doi: 10.1126/science.1152622. [DOI] [PubMed] [Google Scholar]
- 35.Nanan R, Heinrich D, Frosch M, Kreth HW. Acute and long-term effects of booster immunisation on frequencies of antigen-specific memory B-lymphocytes. Vaccine. 2001;20:498–504. doi: 10.1016/s0264-410x(01)00328-0. [DOI] [PubMed] [Google Scholar]
- 36.Zuckier LS, Rodriguez LD, Scharff MD. Immunologic and pharmacologic concepts of monoclonal antibodies. Semin Nucl Med. 1989;19:166–186. doi: 10.1016/s0001-2998(89)80012-1. [DOI] [PubMed] [Google Scholar]
- 37.Hart MK, Palker TJ, Matthews TJ, Langlois AJ, Lerche NW, Martin ME, Scearce RM, McDanal C, Bolognesi DP, Haynes BF. Synthetic peptides containing T and B cell epitopes from human immunodeficiency virus envelope gp120 induce anti-HIV proliferative responses and high titers of neutralizing antibodies in rhesus monkeys. J Immunol. 1990;145:2677–2685. [PubMed] [Google Scholar]
- 38.Haynes BF, Torres JV, Langlois AJ, Bolognesi DP, Gardner MB, Palker TJ, Scearce RM, Jones DM, Moody MA, McDanal C. Induction of HIVMN neutralizing antibodies in primates using a prime- boost regimen of hybrid synthetic gp120 envelope peptides. J Immunol. 1993;151:1646–1653. [PubMed] [Google Scholar]
- 39.Anderson KP, Lucas C, Hanson CV, Londe HF, Izu A, Gregory T, Ammann A, Berman PW, Eichberg JW. Effect of dose and immunization schedule on immune response of baboons to recombinant glycoprotein 120 of HIV-1. J Infect Dis. 1989;160:960–969. doi: 10.1093/infdis/160.6.960. [DOI] [PubMed] [Google Scholar]
- 40.Adalid-Peralta L, Grangeot-Keros L, Rudent A, Ngo-Giang-Huong N, Krzysiek R, Goujard C, Deveau C, Le Gall M, Meyer L, Emilie D, Rouzioux C. Impact of highly active antiretroviral therapy on the maturation of anti-HIV-1 antibodies during primary HIV-1 infection. HIV Med. 2006;7:514–519. doi: 10.1111/j.1468-1293.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- 41.Markowitz M, Vesanen M, Tenner-Racz K, Cao Y, Binley JM, Talal A, Hurley A, Ji X, Chaudhry MR, Yaman M, et al. The effect of commencing combination antiretroviral therapy soon after human immunodeficiency virus type 1 infection on viral replication and antiviral immune responses. J Infect Dis. 1999;179:527–537. doi: 10.1086/314628. [DOI] [PubMed] [Google Scholar]
- 42.Montefiori DC, Hill TS, Vo HTT, Walker BD, Rosenberg ES. Neutralizing antibodies associated with viremia control in a subset of individuals after treatment of acute human immunodeficiency virus type 1 infection. J Virol. 2001;75:10200–10207. doi: 10.1128/JVI.75.21.10200-10207.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Voltersvik P, Albrektsen G, Ulvestad E, Dyrhol-Riise AM, Sorensen B, Asjo B. Changes in immunoglobulin isotypes and immunoglobulin G (IgG) subclasses during highly active antiretroviral therapy: anti-p24 IgG1 closely parallels the biphasic decline in plasma viremia. J Acquired Immune Defic Syndr. 2003;34:358–367. doi: 10.1097/00126334-200312010-00002. [DOI] [PubMed] [Google Scholar]
- 44.Hutter G, Nowak D, Mossner M, Ganepola S, Mussig A, Allers K, Schneider T, Hofmann J, Kucherer C, Blau O, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360:692–698. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 45.Johnston MI, Fauci AS. An HIV vaccine-evolving concepts. N Engl J Med. 2007;356:2073–2081. doi: 10.1056/NEJMra066267. [DOI] [PubMed] [Google Scholar]
- 46.Chun TW, Engel D, Berrey MM, Shea T, Corey L, Fauci AS. Early establishment of a pool of latently infected, resting CD4+ T cells during primary HIV-1 infection. Proc Natl Acad Sci USA. 1998;95:8869–8873. doi: 10.1073/pnas.95.15.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gasper-Smith N, Crossman DM, Whitesides JF, Mensali N, Ottinger JS, Plonk SG, Moody MA, Ferrari G, Weinhold KJ, Miller SE, et al. Induction of plasma (TRAIL), TNFR-2, Fas ligand, and plasma microparticles after human immunodeficiency virus type 1 (HIV-1) transmission: implications for HIV-1 vaccine design. J Virol. 2008;82:7700–7710. doi: 10.1128/JVI.00605-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams JL, Christensen CJ, McMahon BJ, Bulkow LR, Cagle HH, Mayers JS, Zanis CL, Parkinson AJ, Margolis HS. Evaluation of the response to a booster dose of hepatitis B vaccine in previously immunized healthcare workers. Vaccine. 2001;19:4081–4085. doi: 10.1016/s0264-410x(01)00112-8. [DOI] [PubMed] [Google Scholar]
- 49.Levesque MC, Moody MA, Hwang K, Marshall DJ, Whitesides JF, Amos JD, Gurley TC, Allgood S, Haynes BB, Vandergrift NA, et al. Polyclonal B cell differentiation and loss of gastrointestinal tract germinal centers in the earliest stages of HIV-1 infection. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000107. Epub ahead of print July 7, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moir S, Ho J, Malaspina A, Wang W, DiPoto AC, O’Shea MA, Roby G, Kottilil S, Arthos J, Proschan MA, et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med. 2008;205:1797–1805. doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Malaspina A, Moir S, Orsega SM, Vasquez J, Miller NJ, Donoghue ET, Kottilil S, Gezmu M, Follmann D, Vodeiko GM, et al. Compromised B cell responses to influenza vaccination in HIV-infected individuals. J Infect Dis. 2005;191:1442–1450. doi: 10.1086/429298. [DOI] [PubMed] [Google Scholar]
- 52.Chirmule N, V, Kalyanaraman S, Saxinger C, Wong-Staal F, Ghrayeb J, Pahwa S. Localization of B-cell stimulatory activity of HIV-1 to the carboxyl terminus of gp41. AIDS Res Hum Retroviruses. 1990;6:299–305. doi: 10.1089/aid.1990.6.299. [DOI] [PubMed] [Google Scholar]
- 53.Weinhold KJ, Lyerly HK, Stanley SD, Austin AA, Matthews TJ, Bolognesi DP. HIV-1 GP120-mediated immune suppression and lymphocyte destruction in the absence of viral infection. J Immunol. 1989;142:3091–3097. [PubMed] [Google Scholar]
- 54.Shan M, Klasse PJ, Banerjee K, Dey AK, Iyer SP, Dionisio R, Charles D, Campbell-Gardener L, Olson WC, Sanders RW, Moore JP. HIV-1 gp120 mannoses induce immunosuppressive responses from dendritic cells. PLoS Pathog. 2007;3:e169. doi: 10.1371/journal.ppat.0030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He B, Qiao X, Klasse PJ, Chiu A, Chadburn A, Knowles DM, Moore JP, Cerutti A. HIV-1 envelope triggers polyclonal Ig class switch recombination through a CD40-independent mechanism involving BAFF and C-type lectin receptors. J Immunol. 2006;176:3931–3941. doi: 10.4049/jimmunol.176.7.3931. [DOI] [PubMed] [Google Scholar]
- 56.Goepfert PA, Tomaras GD, Horton H, Montefiori D, Ferrari G, Deers M, Voss G, Koutsoukos M, Pedneault L, Vandepapeliere P, et al. Durable HIV-1 antibody and T-cell responses elicited by an adjuvanted multi-protein recombinant vaccine in uninfected human volunteers. Vaccine. 2007;25:510–518. doi: 10.1016/j.vaccine.2006.07.050. [DOI] [PubMed] [Google Scholar]
- 57.Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, Nguyen PL, Khoruts A, Larson M, Haase AT, Douek DC. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guadalupe M, Reay E, Sankaran S, Prindiville T, Flamm J, McNeil A, Dandekar S. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol. 2003;77:11708–11717. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mehandru S, Poles MA, Tenner-Racz K, Horowitz A, Hurley A, Hogan C, Boden D, Racz P, Markowitz M. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004;200:761–770. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Veazey RS, Marx PA, Lackner AA. The mucosal immune system: primary target for HIV infection and AIDS. Trends Immunol. 2001;22:626–633. doi: 10.1016/s1471-4906(01)02039-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.