Abstract
We used a nested PCR with Borrelia flagellin gene (flaB) primers and DNA sequencing to determine if Borrelia lonestari was present in Amblyomma americanum ticks removed from military personnel and sent to the Tick-Borne Disease Laboratory of the U.S. Army Center for Health Promotion and Preventive Medicine. In our preliminary investigation, we detected Borrelia sequences in 19 of 510 A. americanum adults and nymphs from Ft. A. P. Hill, Va. During the 2001 tick season, the flaB primers were used to test all A. americanum samples as they were received, and 29 of 2,358 A. americanum samples tested individually or in small pools were positive. PCRs with 2,146 A. americanum samples in 2002 yielded 26 more Borrelia-positive samples. The positive ticks in 2001 and 2002 were from Arkansas, Delaware, Kansas, Kentucky, Maryland, New Jersey, North Carolina, Tennessee, and Virginia. The last positive sample of the 2001 season was a pool of larvae. To further investigate larval infection, we collected and tested questing A. americanum larvae from Aberdeen Proving Ground, Md.; 4 of 33 pools (40 larvae per pool) were positive. Infection of unfed larvae provides evidence of the maintenance of B. lonestari by means of transovarial transmission. Sequence analysis revealed that the amplicons were identical to sequences of the B. lonestari flaB gene in GenBank. Despite the low prevalence of infection, the risk of B. lonestari transmission may be magnified because A. americanum is often abundant and aggressive, and many tick bite victims receive multiple bites.
Tick-transmitted infection is an occupational health threat to military personnel (10, 24, 31). Effective arthropod repellents are available through the military supply system and are actively promulgated (Armed Forces Pest Management Board, technical guide 36 [http://www.afpmb.org/coweb/guidance_targets/ppms/TG36/TG36.htm]); nevertheless, compliance is inadequate and many soldiers in the field experience tick bites (8). In response to the threat of tick-borne diseases, the Department of Defense operates the Human Tick Test Kit Program, administered by the Tick-Borne Disease Laboratory (TBDL) of the Entomological Sciences Program of the U.S. Army Center for Health Promotion and Preventive Medicine (USACHPPM) (37). Ticks removed from military personnel are mailed in the kits to the TBDL for identification and analysis by PCR. Results are reported back to the tick bite patient's health care provider, and cumulative results are used to assess the threat of tick-borne diseases at specific military installations. Amblyomma americanum, the lone star tick, is the species most frequently submitted, with many patients experiencing multiple concurrent tick bites. Recent evidence has linked an A. americanum-borne Borrelia species, provisionally named Borrelia lonestari, with a case of erythema migrans (9), and it may be responsible for cases of a Lyme disease-like illness associated with bites of A. americanum called Southern tick-associated rash illness (STARI), or Master's disease (3, 4). To determine if this organism was present in the A. americanum ticks removed from military personnel and sent to the TBDL, we began performing PCRs with A. americanum, using broadly reactive Borrelia flagellin gene (flaB) primer pairs FlaLL-FlaRL and FlaLS-FlaRS (3). Here we report the results of our ongoing investigation.
MATERIALS AND METHODS
Ticks.
We first screened, with Borrelia flaB primers, the DNAs of 510 individual A. americanum ticks (299 adults and 211 nymphs) removed in 2000 from humans at Ft. A. P. Hill, Va. All were negative in Borrelia burgdorferi-specific PCRs when they were previously tested in 2000. We pooled the DNAs of five individual ticks for the initial PCR screen. The individual DNAs in any positive pool were subsequently tested to identify the positive individual tick(s). In 2001, the Borrelia flaB primers were incorporated into the TBDL protocol and used to test all A. americanum ticks as they were received. A total of 2,358 A. americanum samples were tested individually or in small pools (ticks of the same species, removed from the same patient at the same time, were pooled). Interestingly, the last positive sample of the 2001 season was a pool of larval A. americanum. To further investigate larval infection, we collected 1,320 A. americanum larvae in the field in September 2001 at Aberdeen Proving Ground, Md., triturated them in pools of 40 for DNA isolation, and tested the pools with the flaB primers. In 2002, Borrelia flaB PCRs of all A. americanum ticks received by the TBDL continued, and 2,146 A. americanum samples were tested individually or in small pools.
DNA extraction.
All ticks except the field-collected larvae were individually bisected with sterile 18-gauge hypodermic needles, and total DNA was extracted by use of the IsoQuick nucleic acid extraction kit (ORCA Research, Bothell, Wash.) according to the manufacturer's instructions, with one modification: the amount of lysis solution was increased to 200 μl (37). The final pellet was resuspended in 25 μl of nuclease-free water. Each group of extractions included a blank extraction (no tick) which was tested as a contamination control for the extraction process.
The larvae were processed, with one well of a Coors ceramic well plate (Spot Plate; Adolphe Coors Co., Golden, Colo.) as a mortar and a 16- by 75-mm borosilicate culture tube as a pestle. Larvae that had been killed by freezing were counted under a dissecting microscope and 40 at a time were placed into the well. A mixture of 110 μl of IsoQuick reagent A and 220 μl of IsoQuick reagent 1 (10% extra for waste and evaporation) was prepared in a 1-ml microcentrifuge tube. A 20-μl aliquot of this mixture was then pipetted into the well containing the larvae, where they were quickly and easily crushed with the convex end of the culture tube. The rest of the mixture was used to rinse any remaining tick debris from the end of the culture tube into the well. The entire contents of the well were pipetted back into the microcentrifuge tube, where IsoQuick extraction was completed. The well plate and culture tube were flame sterilized between pools. We used a large pipette tip (1 ml) to mix and transfer the pool from well to tube.
PCR.
Nested PCR was performed in 25-μl reaction volumes prepared with Ready-To-Go PCR beads (Amersham Pharmacia Biotech, Piscataway, N.J.), which contain 10 mM Tris-HCl (pH 9.0), 1.5 mM MgCl2, a 200 μM concentration of each deoxynucleoside triphosphate, and 1.5 U of Taq DNA polymerase. The primary reaction contained 1 μl of tick DNA as the template and a 1.0 μM concentration (each) of primers FlaLL (5′-ACATATTCAGATGCAGACAGAGGT-3′) and FlaRL (5′-GCAATCATAGCCATTGCAGATTGT-3′). The nested reaction mixture contained 0.5 μl of the primary PCR product as the template, plus a 1.0 μM concentration (each) of primers FlaLS (5′-AACAGCTGAAGAGCTTGGAATG-3′) and FlaRS (5′-CTTTGATCACTTATCATTCTAATAGC-3′). Cycling conditions for both reactions involved an initial 3-min denaturation at 95°C and then 40 cycles, with each cycle consisting of a 1-min denaturation at 95°C, a 1-min annealing at 55°C, and a 1-min extension at 75°C (3). All ticks positive by this PCR were then tested with nested p66 gene primers (a-a′ and f-f′) specific for B. burgdorferi (29). The positive control for both assays was B. burgdorferi strain B31 (a gift of W. Wirtz, Centers for Disease Control and Prevention, Atlanta, Ga.). All PCRs were performed under strict conditions to minimize the risk of amplicon contamination. Extraction of tick DNA, reaction setup, and gel analysis of PCR products were performed in physically separate areas with dedicated pipettes and aerosol-resistant filter pipette tips. Each PCR set included at least one negative control, with water substituted for the DNA template. Reaction products were analyzed by agarose gel electrophoresis using 2% agarose gel cassettes (E-Gel; Invitrogen Corp., Carlsbad, Calif.) stained with ethidium bromide and visualized by UV transillumination.
Enzymatic removal of primers from PCR products.
Primers were removed from amplicons by enzymatic digestion using ExoSAP-IT (USB Corporation, Cleveland, Ohio). Enzymatic treatment was performed by adding 4 μl of ExoSAP-IT to 23 μl of PCR mixture containing the generated product. Samples were mixed gently and collected at the bottom of a thin-walled microcentrifuge tube before incubation for 15 min at 37°C. Inactivation of the enzyme was accomplished by heating the sample at 80°C for 15 min. Samples were held at 4°C until use.
Cycle sequencing of PCR products.
Purified amplicons were cycle sequenced as specified by the Applied Biosystems, Inc., protocol, using an ABI Prism dRhodamine terminator cycle sequencing ready reaction kit (Applied Biosystems, Inc., Foster City, Calif.). Unincorporated primers and dye terminators were removed by using Centri-Sep columns (Princeton Separations, Inc., Adelphia, N.J.) per the manufacturer's instructions. Purified cycle sequencing products were suspended in 25 μl of template suppression reagent (Applied Biosystems, Inc.), electrophoretically separated, and detected on an ABI Prism 310 genetic analyzer (Applied Biosystems, Inc.), and data were collected by ABI Prism sequencing analysis software, version 3.7.
Sequence analysis.
Sequence analysis was performed by using Sequencher, version 4.1.4 (Gene Codes Corporation, Ann Arbor, Mich.), and edited sequence was prepared for submission to GenBank by using Sequin, version 4.28 (National Center for Biotechnology Information).
Nucleotide sequence accession numbers.
The GenBank accession numbers for the B. lonestari flaB gene sequences identified from A. americanum reported here are AY237656, AY237657, AY237658, AY237659, AY237660, AY237661, AY237662, AY237663, AY237664, AY237665, AY237666, AY237667, AY237668, AY237669, AY237670, AY237671, AY237672, AY237673, AY237674, AY237675, AY237676, AY237677, AY237678, AY237679, AY237680, AY237681, AY237682, AY237683, AY237684, AY237685, AY237686, AY237687, AY237688, AY237689, AY237690, AY237691, AY237692, AY237693, AY237694, AY237695, AY237696, AY237697, AY237698, AY237699, AY237700, AY237701, AY237702, AY237703, AY237704, AY237705, AY237706, AY237707, AY237708, AY237709, AY237710, AY237711, AY237712, AY237713, AY237714, AY237715, AY237716, AY237717, AY237718, AY237719, AY237720, and AY237721.
RESULTS
Genus-specific PCR of Borrelia in tick samples.
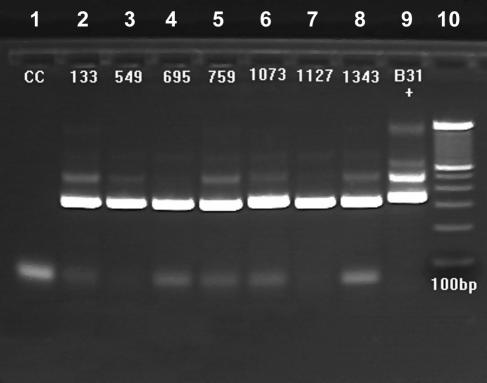
In the Borrelia flaB PCRs of pooled A. americanum ticks from Ft. A. P. Hill, Va., 17 pools were positive, and the five DNAs that comprised each positive pool were then tested individually. Fifteen pools yielded 1 positive tick each and two pools had 2 positives each, for a total of 19 (19 of 510; 3.7%) individual positives (Table 1). Six (of 211) of the positives were nymphs, and 13 (of 299) were adults. PCR in 2001 with 2,358 ticks individually or in pools produced 29 positives, for a minimum infection rate of 1.2%, assuming only 1 positive tick per pool. The Borrelia-positive ticks were from Kentucky, Maryland, New Jersey, Tennessee, and Virginia (Table 2). PCRs of 33 larval pools (1,320 ticks) from Aberdeen Proving Ground, Md., resulted in 4 positive pools, APGEA 21 (not sequenced), APGEA 23 (AY237719), APGEA 26 (AY237720), and APGEA 31 (AY237721). PCRs in 2002 of 2,146 ticks individually or in pools produced 26 positives (minimum infection rate, 1.2%), from Arkansas, Delaware, Kansas, Kentucky, Maryland, New Jersey, North Carolina, Tennessee, and Virginia (Table 3). All of these Borrelia flaB-positive ticks were subsequently negative in B. burgdorferi p66 gene PCRs. Figure 1 presents examples of agarose gel electrophoresis of positive A. americanum DNAs.
TABLE 1.
B. lonestari-infected A. americanum samples received from Ft. A. P. Hill, Va., by the Department of Defense Human Tick Test Kit Program, 2000
| Log no. | GenBank accession no. | Sex or stagea |
|---|---|---|
| 000133 | AY237656 | M |
| 000549 | AY237657 | F |
| 000695 | AY237658 | F |
| 000748 | NSb | F |
| 000759 | AY237659 | N |
| 001073 | AY237660 | F |
| 001127 | AY237661 | F |
| 001140 | AY237662 | N |
| 001343 | AY237663 | M |
| 001669 | AY237664 | M |
| 001680 | AY237665 | N |
| 001694 | AY237666 | F |
| 001707 | AY237667 | F |
| 001711 | AY237668 | N |
| 001738 | AY237669 | N |
| 001812 | AY237670 | F |
| 001832 | AY237671 | F |
| 002016 | AY237672 | F |
| 002017 | NS | N |
F, female; M, male; N, nymph.
NS, not sequenced.
TABLE 2.
B. lonestari-infected A. americanum samples received by the Department of Defense Human Tick Test Kit Program, 2001
| Log no. | GenBank accession no. | Site acquired | Sex and/or stage (no.)a |
|---|---|---|---|
| 010096 | AY237673 | Ft. Knox, Ky. | F |
| 010107 | AY237674 | Ft. A. P. Hill, Va. | F, N |
| 010199 | AY237675 | Ft. A. P. Hill, Va. | N |
| 010201 | AY237676 | Ft. A. P. Hill, Va. | M, N |
| 010269 | AY237677 | Ft. A. P. Hill, Va. | F, M (2), N |
| 010298 | AY237678 | Ft. A. P. Hill, Va. | F (2), M, N |
| 010335 | NSb | Montgomery Co., Tenn. | N (6) |
| 010500 | AY237679 | Ft. A. P. Hill, Va. | F, M |
| 010662 | AY237680 | Ft. Belvoir, Va. | N (2) |
| 010799 | AY237681 | Ft. Lee, Va. | M |
| 010937 | NS | Ft. A. P. Hill, Va. | M |
| 011149 | AY237682 | Ft. Knox, Ky. | F |
| 011164 | AY237683 | Ft. A. P. Hill, Va. | N |
| 011166 | AY237684 | Ft. A. P. Hill, Va. | N |
| 011235 | AY237685 | Ft. Dix, N.J. | F |
| 011292 | AY237686 | Unknown, Ky. | F |
| 011323 | AY237687 | Ft. A. P. Hill, Va. | N (3) |
| 011543 | AY237688 | Newport News, Va. | F |
| 011685 | AY237689 | Ft. Dix, N.J. | N |
| 011686 | AY237690 | Ft. Dix, N.J. | N |
| 011693 | NS | Ft. Dix, N.J. | F |
| 011768 | NS | Ft. Knox, Ky. | F, N |
| 011872 | AY237691 | Ft. A. P. Hill, Va. | F |
| 012186 | AY237692 | Ft. Pickett, Va. | N |
| 012453 | NS | Ft. Dix, N.J. | M |
| 012518 | AY237693 | Ft. Belvoir, Va. | F |
| 012522 | AY237694 | Aberdeen Proving Ground, Md. | N |
| 012659 | AY237695 | Greenville, Ky. | N (2) |
| 012762 | AY237696 | Ft. Monmouth, N.J. | L (14) |
F, female; M, male; N, nymph; L, larva.
NS, not sequenced.
TABLE 3.
B. lonestari-infected A. americanum samples received by the Department of Defense Human Tick Test Kit Program, 2002
| Log no. | GenBank accession no. | Site acquired | Sex and/or stage (no.)a |
|---|---|---|---|
| 020119 | AY237697 | Aberdeen Proving Ground, Md. | F |
| 020181 | NSb | Ft. Knox, Ky. | F |
| 020289 | NS | Ft. Knox, Ky. | N (3) |
| 020589 | AY237698 | Montgomery Co., Tenn. | M |
| 020675 | AY237699 | Ft. A. P. Hill, Va. | M |
| 020772 | AY237700 | Aberdeen Proving Ground, Md. | N (4) |
| 020890 | AY237701 | Ft. Pickett, Va. | N |
| 020981 | AY237702 | Ft. Campbell, Ky. | N (6) |
| 021090 | NS | Aberdeen Proving Ground, Md. | N |
| 021473 | AY237703 | Greenville, Ky. | N |
| 021477 | AY237704 | Ft. Dix, N.J. | F |
| 021506 | AY237705 | Cherry Point, N.C. | F, M, N (2) |
| 021745 | AY237706 | Ft. Riley, Kans. | F (4), N (4) |
| 021846 | AY237707 | Ft. Dix, N.J. | N |
| 021853 | AY237708 | Ft. Monmouth, N.J. | F, M (3), N (4) |
| 021888 | AY237709 | Ft. A. P. Hill, Va. | N (6) |
| 021935 | AY237710 | Ft. Monmouth, N.J. | M (5) |
| 021937 | AY237711 | Kent Co., Del. | M |
| 022070 | AY237712 | Fairfax Co., Va. | F |
| 022072 | AY237713 | Little Rock Air Force Base, Ark. | N |
| 022102 | AY237714 | Ft. A. P. Hill, Va. | N |
| 022128 | AY237715 | Ft. A. P. Hill, Va. | M (2), N (7), L (8) |
| 022180 | AY237716 | Aberdeen Proving Ground, Md. | N |
| 022376 | AY237717 | Camp Robinson, Ark. | N |
| 022433 | AY237718 | Ft. Eustis, Va. | N |
| 022553 | NS | Aberdeen Proving Ground, Md. | L (63) |
F, female; M, male; N, nymph; L, larva.
NS, not sequenced.
FIG. 1.
Examples of ∼350-bp Borrelia sp. flaB PCR products detected with primers FlaLL, FlaLS, FlaRS, and FlaRL in A. americanum ticks from Ft. A. P. Hill, Va. Lane 1, contamination control (water); lane 2, male 000133; lane 3, female 000549; lane 4, female 000695; lane 5, nymph 000759; lane 6, female 001073; lane 7, female 001127; lane 8, female 001343; lane 9, B. burgdorferi strain B31; lane 10, 100-bp ladder.
DNA sequence analysis.
Sequences from 66 amplicons were aligned with all known B. lonestari flaB gene sequences from GenBank. In comparison, our sequence data fall roughly into four sets (Table 4). These data vary from the reference full-length flaB gene sequence (AY166716) (2) by the absence or presence of a single nucleotide triplet immediately downstream of nucleotide position 851, but they are identical to B. lonestari sequences from a tick and a human patient (AF273670 and AF273671) (9) at those positions. However, the same amplicons differ with respect to these two sequences (AF273670 and AF273671) at three nucleotide positions near the terminal ends. The nucleotide substitutions cause a change in the predicted amino acid sequence of flaB at two residues. The primers FlaLL, FlaLS, FlaRS, and FlaRL, used to generate the amplicons, were derived from a Borrelia flagellin gene consensus sequence (3); therefore, a nucleotide sequencing reaction using the PCR product as the template will reflect the primer sequence and not the actual template.
TABLE 4.
Relevant nucleotide comparison of B. lonestari flagellin gene sequences amplified from A. americanum ticks with those listed in GenBank
| GenBank accession no. | Nucleotide at position:a
|
Reference | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 663 | 664 | 665 | 851.1 | 851.2 | 851.3 | 867 | 868 | 869 | 978 | 979 | 980 | 981 | 982 | 983 | ||
| AY166716 | A | C | T | — | — | — | G | A | A | G | T | A | A | C | T | 2 |
| U26705 | A | C | T | — | — | — | G | A | A | G | T | A | A | C | T | 3 |
| U26704 | A | C | T | — | — | — | G | A | G | G | T | A | A | C | T | 3 |
| AF273670 | A | C | A | G | A | A | G | A | A | A | T | A | A | G | T | 9 |
| AF273671 | A | C | A | G | A | A | G | A | A | A | T | A | A | G | T | 9 |
| AF408410 | * | * | * | G | A | A | G | A | A | * | * | * | * | * | * | 36 |
| AF298653 | * | * | * | — | — | — | G | A | A | * | * | * | * | * | * | 4 |
| AF298654 | * | * | * | — | — | — | G | A | A | * | * | * | * | * | * | 4 |
| AF538846 | * | * | * | — | — | — | G | A | A | * | * | * | * | * | * | 20 |
| AF538847 | * | * | * | G | A | A | G | A | A | * | * | * | * | * | * | 20 |
| AF538848 | * | * | * | G | A | A | G | A | A | * | * | * | * | * | * | 20 |
| AF538849 | * | * | * | — | — | — | G | A | A | * | * | * | * | * | * | 20 |
| AF538850 | * | * | * | G | A | A | G | A | A | * | * | * | * | * | * | 20 |
| AF538851 | * | * | * | — | — | — | G | A | A | * | * | * | * | * | * | 20 |
| AF538852 | * | * | * | G | A | A | G | A | A | * | * | * | * | * | * | 20 |
| Data set 1b | A | C | T | G | A | A | G | A | A | G | T | A | A | C | T | This study |
| Data set 2c | * | * | * | G | A | A | G | A | A | * | * | * | * | * | * | This study |
| Data set 3d | A | C | T | — | — | — | G | A | A | G | T | A | A | C | T | This study |
| Data set 4e | * | * | * | — | — | — | G | A | A | * | * | * | * | * | * | This study |
Nucleotide positions correspond to B. lonestari complete flaB gene sequence, GenBank accession number AY166716. —, no corresponding nucleotide base; *, no corresponding sequence data for comparison. Bold nucleotides indicate nucleotide differences with respect to the complete B. lonestari flaB gene sequence AY166716.
Corresponds to sequence data generated in this study, under GenBank accession numbers AY237656, AY237667, AY237671, AY237677, AY237678, AY237679, and AY237707.
Corresponds to sequence data generated in this study, under GenBank accession numbers AY237657, AY237663, AY237664, AY237665, AY237666, AY237668, AY237670, AY237672, AY237674, AY237681, AY237683, AY237688, AY237689, AY237690, AY237691, AY237692, AY237693, AY237695, AY237696, AY237699, AY237701, AY237702, AY237703, AY237705, AY237706, AY237712, AY237714, AY237715, AY237716, AY237717, AY237718, AY237719, AY237720, and AY237721.
DISCUSSION
B. lonestari appears to have widespread distribution and low infection prevalence in A. americanum in the U.S. We found low levels of the spirochete in A. americanum ticks from Arkansas, Delaware, Kansas, Kentucky, Maryland, New Jersey, North Carolina, Tennessee, and Virginia, and similar low rates have been detected by PCR with A. americanum ticks from Alabama (4), Maryland (28), Missouri (2), and Tennessee (36) and in deer blood from Arkansas, Georgia, North Carolina, and South Carolina (20). The molecular variations that we identified in the flaB sequence (Table 4) do not appear to indicate geographic differences for B. lonestari; e.g., both the sequence containing the triplet downstream of nucleotide 851 and the sequence without the triplet appeared in ticks from Aberdeen Proving Ground, Md., Ft. Dix, N.J., Ft. A. P. Hill, Va., and Ft. Knox, Ky. The characteristic low infection level contrasts with rates of B. burgdorferi infection in Ixodes scapularis, which are typically much higher, e.g., >50% for adults (14). To date, all of the published B. lonestari PCR studies have been done with B. burgdorferi-specific primers that were designed to also amplify other species of Borrelia. Further studies using primers based specifically on B. lonestari sequences might reveal a greater prevalence of infection in ticks and mammal hosts. Despite a low prevalence of infection, the risk of B. lonestari transmission by A. americanum is magnified because the tick is often abundant and aggressive and many tick bite victims receive multiple bites.
The discovery of B. lonestari sequences in A. americanum larvae was not unexpected. Borrelia spp. have been detected in unfed, questing A. americanum larvae from New Jersey (34) and in larvae removed from raccoons in Virginia (15). Furthermore, phylogenetic analysis has grouped flagellin gene sequences of A. americanum-borne Borrelia spp. with those of a veterinary pathogen, Borrelia theileri, which is transovarially transmitted by Boophilus and Rhipicephalus ticks that are classified with Amblyomma in subfamily Metastriata (3, 28). The presence of B. lonestari in larvae has human health consequences because larvae typically attach to hosts in large clusters of potentially infected cohorts and thus the risk of pathogen transmission may be magnified.
The natural history of B. lonestari in A. americanum is unknown; however, infection of unfed larvae may provide evidence of the maintenance of B. lonestari by means of transovarial (vertical) transmission. Additional evidence may be found by comparing nymph and adult infection rates. We did not detect an increase in the prevalence of B. lonestari infection in adult A. americanum ticks in our analysis of 299 adults and 211 nymphs from Ft. A. P. Hill, Va.; there was no significant difference in infection rates between nymphs (6 of 211; 2.8%) and adults (13 of 299; 4.3%) (χ2 = 0.78; P = 0.3770; 1 degree of freedom). This contrasts with the pattern of horizontal amplification of B. burgdorferi in I. scapularis nymphs and adults, by which adult infection rates are typically twice those for nymphs (14). The lack of an increase of B. lonestari infection prevalence in adult A. americanum ticks might indicate maintenance by vertical transmission; however, it may be the result of immune modulation of infectivity by ticks or hosts or the absence of immunosuppressive properties in A. americanum saliva (13). The role of vertebrate hosts in maintaining B. lonestari is also unknown, but the discovery of flaB sequences in the blood of an important host of A. americanum, the white-tailed deer (Odocoilus virginianus), indicates that this species might be a reservoir host for the spirochete (20). It is likely that B. lonestari is maintained in A. americanum ticks both transovarially and transtadially; most vector-borne diseases cannot be maintained by transovarial transmission alone (7).
Borrelia infection has been detected in numerous studies of populations of A. americanum over the last 20 years (Table 5). At first, analysis targeted B. burgdorferi, because A. americanum was suspected as a vector of Lyme disease. However, vector competency studies indicated that B. burgdorferi is rarely transmitted by A. americanum (19, 21, 22, 25, 26, 30, 32), and in 1996, phylogenetic analysis of Borrelia DNA sequences amplified from A. americanum identified a species distinct from B. burgdorferi, B. lonestari (3). Subsequently, this new species became the focus of research. It is possible that spirochetes identified in studies using polyclonal antibodies were actually B. lonestari; nevertheless, detection of B. burgdorferi with assays using monoclonal antibodies, species-specific PCR, and culturing with Barbour-Stoenner-Kelley medium indicates that A. americanum can be infected with both Borrelia species. Despite numerous attempts, A. americanum-borne Borrelia spp. have been largely refractory to the culture medium that supports B. burgdorferi. B. lonestari has never been cultured (3, 9), and few instances of culture of B. burgdorferi from A. americanum have been reported in the literature (5, 23, 29, 34, 38). The relationship of B. lonestari to its tick and vertebrate hosts, to other Borrelia spp., and to human disease awaits explanation.
TABLE 5.
Detection of Borrelia spp. in A. americanum ticks
| Yr(s) of collection | Location(s) | Target organism | Prevalence of infection (% [no. detected/total]) | Analysis method(s)a | Reference(s) |
|---|---|---|---|---|---|
| 1983 | N.J. | B. burgdorferi | 9.1 (4/44) | Darkfield, DFA (MAb) | 33 |
| 1983-1984 | N.C. | B. burgdorferi | 2.0 (7/343) | DFA (PAb) | 18 |
| 1984 | N.J. | B. burgdorferi | 4.6 (35/756), 5/32 larval pools | Darkfield, DFA (PAb), culture | 34 |
| 1987 | N.C., Va. | B. burgdorferi | 1.8 (4/218) | Darkfield, IFA (MAb) | 15 |
| 1988-1989 | Ala. | B. burgdorferi | 4.1 (6/144) | DFA (PAb) | 16 |
| 1988-1989 | Tex. | B. burgdorferi | 3/354 pools | Darkfield, culture, IFA (MAb), SDS-PAGE, PCR, sequencing | 29, 38 |
| 1988-1990 | Ala. | Borrelia spp. | 1.7 (8/482) | DFA, IFA (PAb) | 17 |
| 1989 | Mo. | B. burgdorferi | 1.9 (33/1,752) | IFA (MAb), PCR, sequencing | 6 |
| 1989-1992 | Mo., N.J., N.Y., N.C. | B. lonestari | 2.3 (20/875) | DFA (PAb), PCR, sequencing | 3 |
| 1990-1992 | Tex. | Borrelia spp. | 1.0 (53/5,141) | DFA (PAb), culture | 27 |
| 1991-1994 | Va. | B. burgdorferi | 0.2 (1/546) | Darkfield, IFA (MAb) | 35 |
| 1993-1995 | Mo. | B. burgdorferi | 1 larvab | Culture, IFA (MAb), SDS-PAGE, PCR, HMA | 11, 12, 23 |
| 1994-1995 | Md. | Borrelia spp. | 1.0 (7/685) | Darkfield, IFA (PAb), culture, PCR | 1, 29 |
| 1997 | Continental U.S. | B. burgdorferi | 11.7 MIRc (26/222) | PCR | 37 |
| 1997-1999 | Ga. | B. burgdorferi | 3.3 (1/30) | Culture | 5 |
| 1999 | Ala. | B. lonestari | 10.5 (2/19) | PCR, sequencing | 4 |
| 1999 | Md. or N.C. | B. lonestari | 1 female only tested | PCR, culture, darkfield, sequencing | 9 |
| 1999-2000 | Tenn. | B. lonestari | 0.4 MIR (2/550) | PCR, dot blotting, sequencing | 36 |
| 2000 | Va. | B. lonestari | 3.7 (19/510) | PCR, sequencing | This study |
| 2001 | Mo. | B. lonestari | 5.6 MIR (12/214) | PCR, sequencing | 2 |
| 2001 | Continental U.S. | B. lonestari | 1.2 MIR (29/2,358) | PCR, sequencing | This study |
| 2001 | Md. | B. lonestari | 4/33 larval pools | PCR, sequencing | This study |
| 2002 | Continental U.S. | B. lonestari | 1.2 MIR (26/2,146) | PCR, sequencing | This study |
Darkfield, darkfield microscopy; DFA, direct fluorescent antibody test; MAb, monoclonal antibody; PAb, polyclonal antibody; IFA, indirect immunofluorescence assay; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis; HMA, heteroduplex mobility assay.
Prevalence not reported.
MIR, minimum infection rate.
Acknowledgments
We thank Barbara Johnson (Centers for Disease Control and Prevention, Ft. Collins, Colo.) for providing primers and Sara Garrett and Heather Werneke (USACHPPM) for technical assistance.
This project was supported by USACHPPM grant no. F187GJ-01 to the Uniformed Services University of the Health Sciences, administered by the Henry M. Jackson Foundation for the Advancement of Military Medicine, and by an appointment to the Internship/Research Participation Program for the USACHPPM administered by the Oak Ridge Institute for Science and Education through an agreement between the U.S. Department of Energy and the USACHPPM.
REFERENCES
- 1.Armstrong, P. M., L. R. Brunet, A. Spielman, and S. R. Telford III. 2001. Risk of Lyme disease: perceptions of residents of a Lone Star tick-infested community. Bull. W. H. O. 79:916-925. [PMC free article] [PubMed] [Google Scholar]
- 2.Bacon, R. M., R. D. Gilmore, Jr., M. Quintana, J. Piesman, and B. J. B. Johnson. DNA evidence of Borrelia lonestari in Amblyomma americanum (Acari: Ixodidae) in Southeast Missouri. J. Med. Entomol., in press. [DOI] [PubMed]
- 3.Barbour, A. G., G. O. Maupin, G. J. Teltow, C. J. Carter, and J. Piesman. 1996. Identification of an uncultivable Borrelia species in the hard tick Amblyomma americanum: possible agent of a Lyme disease-like illness. J. Infect. Dis. 173:403-409. [DOI] [PubMed] [Google Scholar]
- 4.Burkot, T. R., G. R. Mullen, R. Anderson, B. S. Schneider, C. M. Happ, and N. S. Zeidner. 2001. Borrelia lonestari DNA in adult Amblyomma americanum ticks, Alabama. Emerg. Infect. Dis. 7:471-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durden, L. A., J. H. Oliver, Jr., and A. A. Kinsey. 2001. Ticks (Acari: Ixodidae) and spirochetes (Spirochaetaceae: Spirochaetales) recovered from birds on a Georgia barrier island. J. Med. Entomol. 38:231-236. [DOI] [PubMed] [Google Scholar]
- 6.Feir, D., C. R. Santanello, B. Li, C. Xie, E. Masters, R. Marconi, and G. Weil. 1994. Evidence supporting the presence of Borrelia burgdorferi in Missouri. Am. J. Trop. Med. Hyg. 51:475-482. [PubMed] [Google Scholar]
- 7.Fine, P. E. M. 1981. Epidemiological principles of vector-mediated transmission, p. 77-91. In J. J. McKelvey, Jr., B. F. Eldredge, and K. Maramorosch (ed.), Vectors of disease agents: interactions with plants, animals, and man. Praeger Publishers, New York, N.Y.
- 8.Gamble, J. M., J. F. Brundage, R. A. Kuschner, and P. W. Kelley. 1998. Deployed U.S. Army soldiers' knowledge and use of personal protective measures to prevent arthropod-related casualties. J. Travel Med. 5:217-220. [DOI] [PubMed] [Google Scholar]
- 9.James, A. M., D. Liveris, G. P. Wormser, I. Schwartz, M. A. Montecalvo, and B. J. B. Johnson. 2001. Borrelia lonestari infection after a bite by an Amblyomma americanum tick. J. Infect. Dis. 183:1810-1814. [DOI] [PubMed] [Google Scholar]
- 10.Kardatzke, J. T., K. Neidhardt, D. P. Dzuban, J. L. Sanchez, and A. F. Azad. 1992. Cluster of tick-borne infections at Fort Chaffee, Arkansas: Rickettsiae and Borrelia burgdorferi in ixodid ticks. J. Med. Entomol. 29:669-672. [DOI] [PubMed] [Google Scholar]
- 11.Kollars, T. M., Jr., J. H. Oliver, Jr., and E. J. Masters. 2000. Phenotypic variation in Borrelia burgdorferi sensu lato in ticks (Acari: Ixodidae) and isolates from Missouri. Int. J. Acarol. 26:167-172. [Google Scholar]
- 12.Kollars, T. M., Jr., J. H. Oliver, Jr., and K. Wongkalasin. 2003. Variation in the fla gene detected by heteroduplex mobility assay of five Borrelia burgdorferi sensu lato isolated from ticks (Acari: Ixodidae) collected in Missouri. Int. J. Acarol. 29:1-3. [Google Scholar]
- 13.Lane, R. S. 1994. Competence of ticks as vectors of microbial agents with an emphasis on Borrelia burgdorferi, p. 45-61. In D. E. Sonenshine and T. N. Mather (ed.), Ecological dynamics of tick-borne zoonoses. Oxford University Press, New York, N.Y.
- 14.Lane, R. S., J. Piesman, and W. Burgdorfer. 1991. Lyme borreliosis: relation of its causative agent to its vectors and hosts in North America and Europe. Annu. Rev. Entomol. 36:587-609. [DOI] [PubMed] [Google Scholar]
- 15.Levine, J. F., D. F. Sonenshine, W. L. Nicholson, and R. T. Turner. 1991. Borrelia burgdorferi in ticks (Acari: Ixodidae) from coastal Virginia. J. Med. Entomol. 28:668-674. [DOI] [PubMed] [Google Scholar]
- 16.Luckhart, S., G. R. Mullen, and J. C. Wright. 1991. Etiologic agent of Lyme disease, Borrelia burgdorferi, detected in ticks (Acari: Ixodidae) collected at a focus in Alabama. J. Med. Entomol. 28:652-657. [DOI] [PubMed] [Google Scholar]
- 17.Luckhart, S., G. R. Mullen, L. A. Durden, and J. C. Wright. 1992. Borrelia sp. in ticks recovered from white-tailed deer in Alabama. J. Wildl. Dis. 28:449-452. [DOI] [PubMed] [Google Scholar]
- 18.Magnarelli, L. A., J. F. Anderson, C. S. Apperson, D. Fish, R. C. Johnson, and W. A. Chappell. 1986. Spirochetes in ticks and antibodies to Borrelia burgdorferi in white-tailed deer from Connecticut, New York state, and North Carolina. J. Wildl. Dis. 22:178-188. [DOI] [PubMed] [Google Scholar]
- 19.Mather, T. N., and M. E. Mather. 1990. Intrinsic competence of three ixodid ticks (Acari) as vectors of the Lyme disease spirochete. J. Med. Entomol. 27:646-650. [DOI] [PubMed] [Google Scholar]
- 20.Moore, V. A., A. S. Varela, M. J. Yabsley, W. R. Davidson, and S. E. Little. 2002. Detection of Borrelia lonestari, putative agent of Southern tick-associated rash illness, in white-tailed deer (Odocoilus virginianus) from the southeastern United States. J. Clin. Microbiol. 41:424-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mukolwe, S. W., A. A. Kocan, R. W. Barker, K. M. Kocan, and G. L. Murphy. 1992. Attempted transmission of Borrelia burgdorferi (Spirochaetales: Spirochaetacae) (JDI strain) by Ixodes scapularis (Acari: Ixodidae), Dermacentor variabilis, and Amblyomma americanum. J. Med. Entomol. 29:673-677. [DOI] [PubMed] [Google Scholar]
- 22.Oliver, J. H., F. W. Chandler, Jr., M. P. Luttrell, A. M. James, D. E. Stallknecht, B. S. McGuire, H. J. Hutcheson, G. A. Cummins, and R. S. Lane. 1993. Isolation and transmission of the Lyme disease spirochete from the Southeastern United States. Proc. Natl. Acad. Sci. USA 90:7371-7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oliver, J. H., T. M. Kollars, Jr., F. W. Chandler, Jr., A. M. James, E. J. Masters, R. S. Lane, and L. O. Huey. 1998. First isolation and cultivation of Borrelia burgdorferi sensu lato from Missouri. J. Clin. Microbiol. 36:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peterson, L. R., L. A. Sawyer, D. B. Fishbein, P. W. Kelley, R. J. Thomas, L. A. Magnarelli, M. Redus, and J. E. Dawson. 1989. An outbreak of ehrlichiosis in members of an army reserve unit exposed to ticks. J. Infect. Dis. 159:562-568. [DOI] [PubMed] [Google Scholar]
- 25.Piesman, J., and C. M. Happ. 1997. Ability of the Lyme disease spirochete Borrelia burgdorferi to infect rodents and three species of human-biting ticks (blacklegged tick, American dog tick, lone star tick) (Acari: Ixodidae). J. Med. Entomol. 34:451-456. [DOI] [PubMed] [Google Scholar]
- 26.Piesman, J., and R. J. Sinsky. 1988. Ability of Ixodes scapularis, Dermacentor variabilis, and Amblyomma americanum (Acari: Ixodidae) to acquire, maintain and transmit Lyme disease spirochetes (Borrelia burgdorferi). J. Med. Entomol. 25:336-339. [DOI] [PubMed] [Google Scholar]
- 27.Rawlings, J. A., and G. J. Teltow. 1994. Prevalence of Borrelia (Spirochaetaceae) in Texas ticks. J. Med. Entomol. 31:297-301. [DOI] [PubMed] [Google Scholar]
- 28.Rich, S. M., P. M. Armstrong, R. D. Smith, and S. R. Telford III. 2001. Lone Star tick-infecting borreliae are most closely related to the agent of bovine borreliosis. J. Clin. Microbiol. 39:494-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosa, P. A., D. Hogan, and T. G. Schwan. 1991. Polymerase chain reaction analyses identify two distinct classes of Borrelia burgdorferi. J. Clin. Microbiol. 29:524-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ryder, J. W., R. R. Pinger, and T. Glancy. 1992. Inability of Ixodes cookei and Amblyomma americanum nymphs (Acari: Ixodidae) to transmit Borrelia burgdorferi. J. Med. Entomol. 29:525-530. [DOI] [PubMed] [Google Scholar]
- 31.Sanchez, J. L., W. H. Candler, D. B. Fishbein, C. R. Greene, T. R. Cote, D. J. Kelly, D. P. Driggers, and B. J. Johnson. 1992. A cluster of tick-borne infections: association with military training and asymptomatic infections due to Rickettsia rickettsii. Trans. R. Soc. Trop. Med. Hyg. 86:321-325. [DOI] [PubMed] [Google Scholar]
- 32.Sanders, F. H., and J. H. Oliver, Jr. 1995. Evaluation of Ixodes scapularis, Amblyomma americanum and Dermacentor variabilis (Acari: Ixodidae) from Georgia as vectors of a Florida strain of the Lyme disease spirochete, Borrelia burgdorferi. J. Med. Entomol. 32:402-406. [DOI] [PubMed] [Google Scholar]
- 33.Schulze, T. L., G. S. Bowen, E. M. Bosler, M. F. Lakat, W. E. Parkin, R. Altman, B. G. Ormiston, and J. K. Shisler. 1984. Amblyomma americanum: a potential vector of Lyme disease in New Jersey. Science 224:601-603. [DOI] [PubMed] [Google Scholar]
- 34.Schulze, T. L., M. F. Lakat, W. E. Parkin, J. K. Shisler, D. J. Charette, and E. M. Bosler. 1986. Comparison of rates of infection by the Lyme disease spirochete in selected populations of Ixodes dammini and Amblyomma americanum (Acari: Ixodidae). Zentbl. Bakteriol. Mikrobiol. Hyg. A 263:72-78. [DOI] [PubMed] [Google Scholar]
- 35.Sonenshine, D. E., R. E. Ratzlaff, J. Troyer, S. Demmerle, E. R. Demmerle, W. E. Austin, S. Tan, B. A. Annis, and S. Jenkins. 1995. Borrelia burgdorferi in Eastern Virginia: comparison between a coastal and inland locality. Am. J. Trop. Med. Hyg. 53:123-133. [DOI] [PubMed] [Google Scholar]
- 36.Stegall-Faulk, T., D. C. Clark, and S. M. Wright. 2003. Detection of Borrelia lonestari in Amblyomma americanum (Acari: Ixodidae) from Tennessee. J. Med. Entomol. 40:100-102. [DOI] [PubMed] [Google Scholar]
- 37.Stromdahl, E. Y., S. R. Evans, J. J. O'Brien, and A. G. Gutierrez. 2001. Prevalence of infection in ticks submitted to the Human Tick Test Kit Program of the U.S. Army Center for Health Promotion and Preventive Medicine. J. Med. Entomol. 38:67-71. [DOI] [PubMed] [Google Scholar]
- 38.Teltow, G. J., P. V. Fournier, and J. A. Rawlings. 1991. Isolation of Borrelia burgdorferi from arthropods collected in Texas. Am. J. Trop. Med. Hyg. 44:469-474. [DOI] [PubMed] [Google Scholar]