Abstract
Cancer chemotherapy is believed to be impeded by multidrug resistance (MDR). Pluronic (triblock copolymers of poly(ethylene oxide) (PEO) and poly(propylene oxide) (PPO), PEO-b-PPO-b-PEO) were previously shown to sensitize MDR tumors to antineoplastic agents. This study uses animal models of Lewis lung carcinoma (3LL-M27) and T-lymphocytic leukemia (P388/ADR and P388) derived solid tumors to delineate mechanisms of sensitization of MDR tumors by Pluronic P85 (P85) in vivo. First, non-invasive single photon emission computed tomography (SPECT) and tumor tissue radioactivity sampling demonstrate that intravenous co-administration of P85 with a Pgp-substrate, 99Tc-sestamibi, greatly increases the tumor uptake of this substrate in the MDR tumors. Second, 31P magnetic resonance spectroscopy (31P-MRS) in live animals and tumor tissue sampling for ATP suggest that P85 and doxorubicin (Dox) formulations induce pronounced ATP depletion in MDR tumors. Third, these formulations are shown to increase tumor apoptosis in vivo by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay and reverse transcription polymerase chain reaction (RT-PCR) for caspases 8 and 9. Altogether, formulation of Dox with P85 results in increased inhibition of the growth solid tumors in mice and represents novel and promising strategy for therapy of drug resistant cancers.
INTRODUCTION
Appearance of MDR is a serious problem [1–5] that at least in some cases is believed to impede outcomes of chemotherapeutic regimens in treatment of cancer [6]. New antineoplastic agents and combination chemotherapies have produced limited success for MDR tumors [3]. Dose-intensified and high-dose regimens are active in certain cases but such regimens are accompanied with increased treatment-related morbidity and mortality [3, 4]. Limited success was also achieved with chemosensitizing agents that inhibit the drug efflux transporter P-glycoprotein (Pgp/ABCB1) [7, 8]. Several generations of Pgp-inhibitors were developed and evaluated in clinical trials [8–10]. The early agents such as cyclosporine A and verapamil had relatively low affinity to Pgp and were toxic. The more potent and less toxic second generation agents (valspodar, biricodar and others) have shown success, but their use has been impeded by their effects on non-targeted proteins. Notably, they also inhibit cytochrome P450 resulting in increased blood drug levels [10]. Thus, new agents are currently in development that would improve drug pharmacokinetics [7, 8].
Apart from these approaches using low molecular mass compounds to modulate Pgp is the use of triblock copolymers of poly(ethylene oxide) (PEO) and poly(propylene oxide) (PPO), (PEO-b-PPO-b-PEO) also known as Pluronics or poloxamers. Pluronic block copolymers are listed in the U.S. and British Pharmacopoeia under the name “poloxamers” as excipients and are widely used in a variety of clinical applications [11]. One formulation containing doxorubicin (Dox) and a mixture of Pluronic L61 and F127, SP1049C, that is particularly relevant for the present study, has successfully completed Phase II human trials in advanced esophageal adenocarcinoma [12]. Contrary to most low molecular mass inhibitors of Pgp, that are tailored to interact specifically with the transport system protein, Pluronics have a broad spectrum of activities. First, they inhibit Pgp drug efflux pump [12], which involves interaction of Pluronic molecules with MDR cell membranes, decrease in membrane microviscosity and inhibition of Pgp ATPase activity [13, 14]. Second, they inhibit respiratory chain complexes in mitochondria of MDR cells and thus deplete ATP that deprives the MDR cells of the energy source [14]. Third, they promote generation of reactive oxygen species (ROS) and simultaneously inhibit the glutathione/glutathione S-transferase (GSH/GST) detoxification by decreasing GSH and inhibiting GST activity [13]. Fourth, they attenuate drug sequestration in acidic vesicles, which may increase drug bioavailability within the cancer cell [15]. Finally, they decrease membrane potential in mitochondria of MDR cells, promote release of cytochrome C and overall enhance pro-apoptotic signaling and mitigate anti-apoptotic cellular defense of MDR cells [16]. It is also remarkable that despite rather simple structure and lack of precise spatial arrangement of pharmacophoric groups, Pluronics appear to be selective with respect to the MDR cell phenotype [14]. This is most noticeably seen in ATP depletion by Pluronic, which correlates with the level of Pgp expression in the cancer cells [17].
The current study investigates the effects of Pluronic P85 (P85) in mouse models of MDR solid tumors. Formulation of Dox with P85 resulted in increased inhibition of Lewis lung carcinoma (3LL-M27) and T-lymphocytic leukemia (P388/ADR and P388) tumors in mice. Three major effects of Pluronic formulations observed in MDR tumors in vivo include 1) significant increase of tumor accumulation of a Pgp substrate, 99Tc-sestamibi (shown by non-invasive single photon emission computed tomography (SPECT) and tumor tissue radioactivity sampling), 2) ATP depletion (shown by 31P magnetic resonance spectroscopy (31P-MRS) in live animals and tumor tissue sampling for ATP) and 3) enhanced apoptosis (shown by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay and reverse transcription polymerase chain reaction (RT-PCR) for caspases 8 and 9). Thus present in vivo studies confirmed major pathways for P85 chemosensitization as they affect MDR cancer cells and support the notion that formulation of drugs with Pluronic represents a novel and promising strategy for drug resistant cancers. At the same time significant difference in dose-dependence of inhibition of tumor growth in immunocompetent and immunodefficient mice is noted, which for the first time suggests involvement of the immunological component(s) in the antitumor activities of drug and Pluronic formulations. Overall, such formulations display superior antitumor activity in MDR and non-MDR tumors, and their clinical application may be broader than it was initially suggested for Pgp overexpressing cancers.
MATERIALS AND METHODS
Drugs and Chemicals
The present study used P85 (lot # WPOP-587A) provided by BASF Corp. (Parispany, NJ). The molecular mass of the polypropylene-oxide (PO) segment in this copolymer sample was approximately 2,500 Daltons, and the content of the polyethylene-oxide (EO) chains was ~50 % (w/w). The physicochemical characteristics of Pluronic copolymers have been previously reported [18]. Dox was purchased from Sigma Chemical Co. (St. Louis, MO, USA). 99Tc-Sestamibi (Cardiolite) was received from Cardinal Health (Omaha, NE, USA). The tritium labeled copolymer (3H-P85) was obtained by exposure of P85 to tritium gas (NEN Life Science Products, Boston, MA).
Cell Culture
Lewis lung carcinoma 3LL-M27 cells were cultured in DMEM with 10 % FBS, 10 mM HEPES and 1 % penicillin/streptomycin. The murine leukemia P388 and P388/ADR cells were cultured in RPMI 1640 with 10 % FBS (fetal bovine serum), 10 mM HEPES and 1 % penicillin/streptomycin. All other tissue culture reagents were obtained from Gibco Life Technologies, Inc. (Grand Island, NY, USA). Cells were cultured at 37°C in a humidified atmosphere with 5 % CO2.
Animals
The experiments were performed with female C57BL/6 or BDF1 mice at 11–12 weeks of age (Taconic Laboratories, Germantown, NY). The animals were kept at 4–5 per cage with a filter cover under light (12 h light/dark cycle) and handled according to institutional guidelines. All manipulations with the animals were performed under a sterilized laminar hood. Food and water were given ad libitum. Homozygous B6.CB17-Prkdcscid/SZJ mice with the severe combined immune deficiency spontaneous mutation characterized by absence of functional T-cells and B- cells were employed to evaluate involvement of immune system. All procedures involving animals were carried out under a protocol approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Nebraska Medical Center (UNMC).
Preparation of Dox/Pluronic Formulations
P85 was dissolved at various concentrations (0.0002–1 % wt) in saline at 4°C and then sterilized by filtration through a 0.2 μm filter. Dox/P85 compositions were obtained by addition of a sterile isotonic solution of Doxorubicin HCl (2 mg/ml) to the copolymer solutions. These compositions were incubated at 37°C for at least one hour prior to their use in the experiments.
Evaluation of mdr1 levels by reverse transcription polymerase chain reaction (RT-PCR)
To assess the level of mdr1 gene in solid tumors, C57BL/6 mice or BDF1 mice were injected subcutaneously (s.c.) with 3LL-M27 (C57BL/6), P388/ADR (BDF1) or P388 (BDF1) cells (106 cells/mouse in 50 μl saline), and tumors were allowed to grow for 7–10 days. When solid tumors reached ca. 300 mm2, tumor tissues were dissected and RNA was extracted using Reagent® (Molecular Research Center, Inc. Cincinnati, OH) according to the manufacturer’s protocol. RNA quality was determined via ethidium bromide staining following agarose/formaldehyde (1.2 %) gel electrophoresis. Quantity of extracted RNA was determined by ratio of absorbance at 260 and 280 nm recorded in spectrophotometer. 100 ng of total RNA was reverse-transcribed with AccessQuick RT-PCR system (Promega, Madison, WI, USA). For PCR amplification, 100 ng of RNA was included in a total of 50 μl reaction mixture, containing 1× Access Quick Master Mix, sense and antisense primers, 1 μM each (the sequences are shown in Table 1) and 5 Units AMV reverse transcriptase. PCR amplification was performed at 48°C for 45 min (reverse transcription), 94°C for 2 min (initial denaturation) followed by 30 cycles at 94°C for 60 s (denaturation), 55°C for 45 s, (annealing) 70°C for 30 s (extension) and lastly 72°C for 5 min (final extension). PCR products were run along with a DNA ladder (Promega) on 2 % agarose gels and stained with ethidium bromide (0.5 μg/mL). Gadph and β-actin were used as housekeeping genes. The running gels were captured using the GEL DOC 2000 (Bio-Rad, Hercules, CA, USA) and images were analyzed using Image QuaNT version 5.1 Software (Molecular Dynamics, GE Healthcare, NJ, USA).
Table 1.
Summary of primer sequences for mdr1, gadph, caspase 8 and 9; and b-actin
| No | Gene | GenBank # | Primer for RT-PCR | Band size |
|---|---|---|---|---|
| 1 | mdr1 | NM-011075 | Sense ACTCGGGAGCAGAAGTTTGA Antisense GCACCAAAGACAACAGCAGA |
224 bp |
| 2 | gadph | NM-001001303 | Sense AAGTTGTCATGGATGACCTTGG Antisense AAGGTGAAGGTCGGAGTCAACG |
497 bp |
| 3 | b-actin | NM-007393 | Sense AGCCATGTACGTAGCCATCC Antisense CTCTCAGCTGTGGTGGTGAA |
228 bp |
| 4 | caspase 8 | NM-009812 | Sense GGCCTCCATCTATGACCTGA Antisense GCAGAAAGTCTGCCTCATCC |
212 bp |
| 5 | caspase 9 | NM-015733 | Sense GATGCTGTCCCCTATCAGGA Antisense GGGACTGCAGGTCTTCAGAG |
205 bp |
Tumor treatment
To evaluate antitumor effect of Dox/P85 compositions in vivo we used murine leukemia P388 (sensitive) or P388/ADR (resistant) cells in BDF1 mice, and Lewis lung carcinoma 3LL-M27 cells in C57/BL or B6.CB17-Prkdcscid/SZJ mice. The P388/ADR and 3LL-M27 cell lines produce aggressive solid tumors that overexpress Pgp in vivo. Mice were injected s.c. with 106 cells/mouse in 50 μl saline, and tumors were allowed to grow for 7–10 days. When solid tumors reached ca. 100 mm2, mice were randomly divided into groups and treated with a) saline; b) various concentrations P85 alone (0.0002–1 % wt); c) Dox (2.5 mg/kg) in saline; or d) Dox (2.5 mg/kg) with various concentrations P85 (same as in b); and e) Dox (2.5 mg/kg) with cyclosporine A (CSA) (15 mg/kg). The drug formulations were given intravenously (i.v.) in a volume of 10 mL/kg on days 1, 4, and 7 after random group assignment. Tumor length (L) and width (W) were measured and tumor weight (WR) was calculated twice a week as follows:
The data were expressed in relative weight (RW) calculated using the formula:
where Wo is the mean tumor weight at the beginning of treatment and Wi is the mean tumor weight at any subsequent time point. The rate of tumor inhibition was determined on day 22 after group assignment for 3LL-27M tumors and day 11 for P388/ADR tumors using the following formula:
where RWt and RWc are relative weights in the treated and control groups, respectively. Both the RW and TI indexes were considered not measurable if at least one animal in the treated group died by the day of measurement (on 22nd day for 3LL-27M bearing mice, and on 11th day for P388/ADR bearing mice).
Single photon emission computed tomography (SPECT)
To visualize the biodistribution of a radiolabeled Pgp substrate, 99Tc-sestamibi in solid tumors, C57BL/6 mice were injected with 3LL-M27 cells (106 cells/mouse in 50 μl phosphate buffer saline (PBS)/mouse) s.c. at the nuchal. When tumors reach approximately 300 mm2, mice were randomly divided into three groups and injected via the tail vein with 100 μl of 99Tc-sestamibi (100 μCi/mouse) in saline 99Tc-sestamibi in 0.02 % P85, or 99Tc-Sestamibi in 1% P85. At 1.75 hrs post injection, mice were anesthetized with 1–1.5 % isoflurane delivered in 66% NO/33 % O2. Anesthetized mice were positioned in the custom build holder with ear bars and bite bar, and taped into the bed. By two hrs post-injection, 99Tc-sestamibi was evaluated by SPECT analysis (Gamma Medica-Ideas, Northridge, CA, USA). For each animal, 64 × 60 second exposures were acquired to obtain a 360° rotational image of neck area. Individual exposures were reconstructed to yield a 3 dimensional tomogram. Areas of nuchal tumors were encompassed by electronic bitmaps into regions of interest (ROI) and each ROI was electronically sectioned into transverse slices. Total isotopic counts for 99Tc-sestamibi were calculated by the summation of counts for all bit-mapped transverse slices. Quantification was achieved by normalizing tissue counts to external standards of known concentration of 99Tc-sestamibi and radioactivity was adjusted for 99Tc decay.
99Tc-sestamibi biodistribution by γ-Scintillation spectrometry
Mice with 3LL-M27 tumors were injected with 10 μCi of 99Tc-sestamibi per mouse in the same three formulations. Every group consisted of five animals. Five hrs post-injection, tumors were harvested, solubilized and evaluated for 99Tc-sestamibi incorporation using γ-scintillation spectrometry (Wizard 1480 Automatic γ-counter, Perkin Elmer, Life Sciences, Shetton, CT, USA). The radioactivity amount was normalized for the tissue weight.
P85 biodistribution
A tracer dose of [3H]-P85 (5 μCi; 8.5 μg/kg) mixed with 0.02 %, 0.2 % or 1 % wt P85 solution (100 μL) was injected via the tail vein in C57BL/6 mice with 3TLL solid tumors. At each sampling time point (from 0.5 to 192 hrs post-injection) animals were sacrificed and the tumor tissue was dissected, rinsed in the ice-cold saline, blotted and weighed. Four mice were used for each time point. The samples were supplemented with 0.5 ml of a tissue solubilizer and then homogenized in a glass tissue TearorTM homogenizer (BioSpec Products, Inc., Bartlesville, OK). Each sample was mixed with 5 μl of 30 % hydrogen peroxide and incubated at 4° C overnight for decolorization. Then, 100 μL of the serum or 100 μL of the tissue homogenate were placed into 4 ml of a liquid scintillation cocktail, and the radioactivity levels were determined using a Tricarb 4000 (Packard, Meriden, CT, USA). The results obtained in these experiments represent average concentrations of P85 without discrimination of its interstitial or intracellular localization in the solid tumors. The area under the P85 concentration–time curve from time zero to time infinity (AUC) was calculated by the trapezoidal rule–extrapolation method.
ATP levels in MDR solid tumors in vivo
To determine whether P85 is causing metabolic changes (a loss of ATP, pH shifts or ionic gradient shifts) within an MDR tumor, BDF1 mice with P388/ADR resistant solid tumors were injected with Dox alone, Dox formulated with 0.2 % wt P85, or 0.2 % wt P85 alone as described above on days 1, 4, and 7 post group assignment. Before and during the third injection, animals were anesthetized as described above and placed into a 7 Tesla Bruker Biospec MRI/MRS system (Bruker, Karlsure, Germany) using a custom built holder with 1 cm diameter 31P transmit/receive surface coil built into the base of the bed and oriented orthogonal to the 1H volume coil. The 1H volume coil was used for image acquisition and localized shimming (PRESS) [19]. Image-selected in vivo spectroscopy (ISIS) [20] was used to obtain a spectrum from a region prescribed on a contiguous series of T1 weighted images (Figure 5G). The tumor was placed into the surface coil to maximize sensitivity and volume discrimination. The volume selected was approximately 7 mm X 10 mm X 10 mm, TR (repetition time) = 4s, spectral bandwidth = 8KHz, inversion pulse = 2 ms hyperbolic secant set −3000 Hz off resonance to minimize chemical shift effects, BW = 8800 Hz, excitation pulse = 60 μs block pulse, 4K data points, 256 averages, and acquisition time of 30 min. Spectroscopic analysis was performed by a time domain fit (AMARES in the jMRUI package) of each spectrum. Metabolite concentrations, pH, and metabolite ratios were determined for each animal from the 31P-spectra. Peak areas from 31P metabolite spectra were normalized to total phosphate within the spectrum.
Fig. 5.
Metabolic response to i.v. injection of 2.5 mg/kg Dox formulated with 0.2 % P85 in P388/ADR tumors. (A) Pre-injection spectrum and fit, (B) First spectrum after injection and fit, 2.5 hr time course of: (C) ATP and ADP levels, (D) pH, (E) inorganic phosphate (Pi), and (F) total PME. (G) Image guided selection of the tumor volume for ISIS spectroscopic acquisition.
For the luciferin/luciferase assay, C57BL/6 mice with 3LL-M27 solid tumors and BDF1 mice with P388/ADR and P388 solid tumors were treated with Dox formulated with various concentrations of P85 as described above. Two hrs after the last injection mice were sacrificed and tumors were isolated. Each tumor sample was supplemented with 3 mL/g tissue PBS and homogenized. ATP content was determined using a CellTiter-Glo® Luminescent Cell Viability Assay (Promega, Madison, WI, USA). For this purpose, 100 μL aliquots of homogenized cells were mixed with 100 μL of CellTiter-Glo® Reagent in 96-well plate. Light emission was measured with a Packard FusionTM luminometer (PerkinElmer, Waltham, MA, USA). Raw data were collected as relative light units integrated over 20 sec, and converted to ATP concentrations with the aid of a standard calibration curve obtained using ATP standard (# FL-AAS, Sigma). ATP levels were normalized for protein content, and each data point represented the mean ± SEM of a minimum of five replicates.
Apoptotic DNA degradation levels in solid tumors by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
C57BL/6 mice with 3LL-M27 solid tumors were treated with Dox formulated with various concentrations of P85 as described above. Twenty four hrs after the last injection, mice were sacrificed and solid tumors were isolated. Tumors were dissected, fixed in 10 % formalin overnight, dehydrated, and embedded in paraffin blocks. Tissue sections 6 μm thick were placed on silane-coated slides and processed according to the manufacturer’s protocol (ApopTag fluorescent in situ Apoptosis Detection Kit, Chemicon International, Inc.). Immunoreactivity was evaluated by fluorescent analysis. Apoptosis level was measured as a function of positive area using ImageJ software (National Institute of Health).
RT-PCR assays for pro-apoptotic genes levels
C57BL/6 mice with 3LL-M27 solid tumors were treated with Dox formulated with various concentrations of P85 as described above. Twenty four hrs after the last injection, mice were sacrificed and tumor tissues were isolated. RNA samples were prepared as described above and reverse-transcribed with AccessQuick RT-PCR system. The sequences of sense and antisense primers are presented in Table 1.
Total toxicity of P85 injections in mice
Healthy C57BL/6 mice were injected three times i.v. with various concentrations P85 solutions. Relative weight was recorded over 5 weeks.
Statistical analysis
For the all experiments, data are presented as the mean ± SEM. Tests for significant differences between the groups were done using one-way ANOVA with multiple comparisons (Fisher’s pair-wise comparisons) using GraphPad Prism 4.0 (GraphPad software, San Diego, CA, USA). A minimum p value of 0.05 was estimated as the significance level for all tests.
RESULTS
Expression of mdr1 gene in tumor tissues in vivo
The levels of mdr1 gene expression in solid tumors grown in mice were assayed by RT-PCR. The drug resistant tumors, 3LL-27M (lane 2), and P388/ADR (lane 3), showed high expression levels of mdr1 gene (Figure 1A), which corresponded to 1.01 and 1.84 relative units (normalized to the housekeeping gene, β-actin) (Figure 1B). In contrast, P388 cells (lane 4) displayed lower endogenous mdr1 expression (0.19 relative units).
Fig. 1.
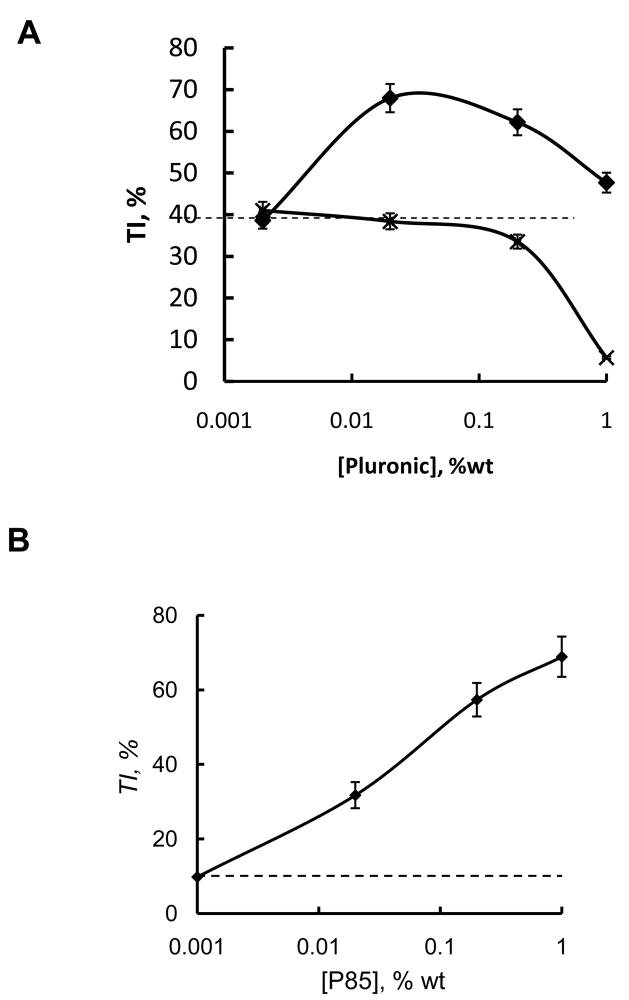
(A, B) The expression levels of mdr1 in solid tumors by RT-PCR: (1) ladder (2) Lewis lung carcinoma 3LL-M27 (grown in C57BL/6 mice), (3) Dox-resistant murine leukemia P388/ADR, and (4) Dox-sensitive murine leukemia P388 (both grown in BDF1 mice). (C–F) Antitumor effects of Dox, P85 and Dox/P85 formulations in mice with (C, D) 3LL-27M and (E, F) P388/ADR tumors. Panels (C, E) represent tumor relative weight (RW) vs. time. Mice with tumors implanted s.c. and grown for 7–10 days were treated with saline (empty diamonds), Dox in saline (empty squares), Dox in 0.002 % P85 (filled squares), Dox in 0.02 % P85 (filled circles), Dox in 0.2 % P85 (filled triangles), or Dox in 1 % P85 (filled diamonds). Concentration of Dox in all injections was 2.5 mg/kg and i.v. injections were performed on days 1, 4, and 7 after tumor sized assessment and random group assignment. Panels (D, F) present tumor inhibition (TI) vs. concentration of P85 for mice treated with Dox/P85 (filled diamonds) or P85 alone (crosses). Dashed lines in (D, F) represent TI for Dox alone.
Antitumor activity of Dox/P85 formulations in mouse models
The antitumor effects of Dox formulated with various concentrations of P85 (as well as Dox and P85 alone) were first evaluated in immunocompetent mouse models of 3LL-27M and P388/ADR tumors (Figure 1C–F). Dox alone administered in saline was shown to inhibit tumor growth compared to non-treated controls in both tumor models (Figure 1C,E). The TI values of the free drug were ca. 29 % and 35 % in 3LL-27M and P388/ADR tumors, respectively. However, co-administration of the same dose of Dox with P85 allowed further increase in the anti-tumor effects compared to the free drug. Interestingly, the maximal values of TI were achieved at intermediate concentrations of the copolymer (Figure 1D,F). Specifically, the TI values were ca. 79 % at 0.02 % P85 in 3LL-27M tumor and 61 % at 0.02 % P85 in P388/ADR tumor. In both tumors the TI values were considerably decreased when Dox was formulated with higher concentrations of P85 (1 % wt). Interestingly, P85 alone also displayed some antitumor effect and its dose dependence in both tumors was characterized by the bell-shaped curve (Figures 1D,F). In case of P388/ADR tumor the maximal TI with P85 was comparable to that of Dox/P85 formulation but it was observed at nearly 10 times higher dose of the copolymer (Figure 1F). In case of 3LL-27M tumor the maximal TI with P85 never reached the value observed with Dox/P85 treatment, although the optimal concentration of the copolymer alone was still about 10 times greater than that in the formulation. Furthermore, 1 % P85 even accelerated the P388/ADR tumor growth compared to non-treated controls (TI value negative). Finally, optimal Dox/P85 formulation demonstrated significantly higher TI (p<0.05) compared to the drug administered with the low molecular mass Pgp inhibitor, CSA (TI = 29 % for 3LL-27M solid tumors) (Figure 1S, Supplementary material).
We further examined drug sensitive P388 tumor in BDF1 mice (Figure 2A). Interestingly, in this case the antitumor effects of the block copolymer formulations were similar to those observed in MDR tumors. First, the block copolymer alone displayed high antitumor activity with TI reaching nearly the same levels as Dox alone (TI ca. 39 %). However, the TI decreased as the P85 concentration increased to 1 % wt. The combination therapy using Dox/P85 was the most efficient resulting in nearly 68 % TI values at the optimal P85 concentration of 0.02 % wt. However, there was also a bell-shaped copolymer dose dependence of the TI.
Fig. 2.
Antitumor effects of Dox/P85 in (A) P388 tumor in BDF1 mice and (B) 3LL-27M tumors in immunodeficient B6.CB17-Prkdcscid/SZJ mice. Data points represent TI values vs. P85 concentration in Dox/P85 formulation. Dashed lines represent TI for Dox alone. Mice were injected three times with Dox/P85 formulations as described in Figure 1.
Finally, we evaluated the antitumor activity of Dox/P85 in 3LL-27M tumors in B6.CB17-Prkdcscid/SZJ mice deficient in T and B cells (Figure 2B). In this case formulation of Dox with P85 also enhanced the antitumor effect compared to the free drug. However, the TI values of Dox/P85 at 0.02 % P85 were considerably less than those observed with 3LL-27M tumors in C57BL/6 mice at the same copolymer and Dox doses. Furthermore, contrary to the immunocompetent mice, no bell-shaped dose dependence was observed and the TI monotonously increased as the P85 concentration increased in iommunodeficient mice. The highest TI value of nearly 70 % was observed at 1 % wt P85.
Overall, Dox with the optimal doses of P85 produced superior antitumor activity compared the drug alone, the copolymer alone or the drug with the conventional Pgp inhibitor. Interestingly, Dox/P85 was more efficient than the drug alone in both resistant and non-resistant tumors. The antitumor effects of Dox/P85 in immunocompetent mice at the optimal doses of the copolymer were greater than those effects in the immunodefficient mice. However, in the immunodefficient mice the anti-tumor effect of the formulation increased as P85 concentration increased and ultimately reached values comparable to those observed in the immunocompetent mice at lower doses of P85.
Effect of P85 on 99Tc-sestamibi accumulation in MDR tumor in vivo
We used 99Tc-sestamibi as an in vivo Pgp probe to determine whether P85 inhibits Pgp in 3LL-M27 tumor [8, 21–26]. First, we examined the effect of P85 on tumor accumulation of 99Tc-sestamibi by SPECT (Figure 3A–C).
Fig. 3.
Effect of P85 on 99Tc-sestamibi accumulation in solid tumors in C57BL/6 mice with 3LL-M27 tumors. Mice were injected i.v. with (A) 99Tc-sestamibi (100 μCi/mouse) in saline; (B) 99Tc-sestamibi in 0.02 % P85, or (C) 99Tc-sestamibi in 1 % P85. SPECT scans were performed two hrs after the injection. (D) Total isotopic counts for 99Tc-sestamibi were calculated by the summation of counts for all bit-mapped transverse slices. (E) Tumor accumulation of 99Tc-sestamibi formulated with various conc. of P85 and injected i.v. in tumor-bearing mice 5 hrs post-injection by γ-scintillation spectrometry. Data presented are means ± SEM of five animals per time point, ** p < 0.005.
Total isotopic counts for Tc-sestamibi calculated by the summation of counts for all bitmapped transverse slices suggested that co-administration of low (0.02 % wt) and high (1 % wt) doses of P85 considerably increased accumulation of 99Tc-sestamibi in the tumor (Figure 3D). At 1 % P85, the amount of 99Tc-sestamibi detected in the tumor by SPECT was nearly 40 times greater than that in animals injected with 99Tc-sestamibi alone. Second, to validate the SPECT findings the amounts of 99Tc-sestamibi in isolated tumor tissues were quantified by γ–scintillation spectrometry (Figure 3E). The data demonstrate that formulation of 99Tc-sestamibi with 0.02 % and 1 % P85 increased 99Tc-sestamibi accumulation in the tumor by 5 and 20 fold, respectively. Therefore, both methods suggest that P85 considerably increases the accumulation of the Pgp substrate in MDR tumor in vivo.
Accumulation of P85 in MDR tumors in vivo
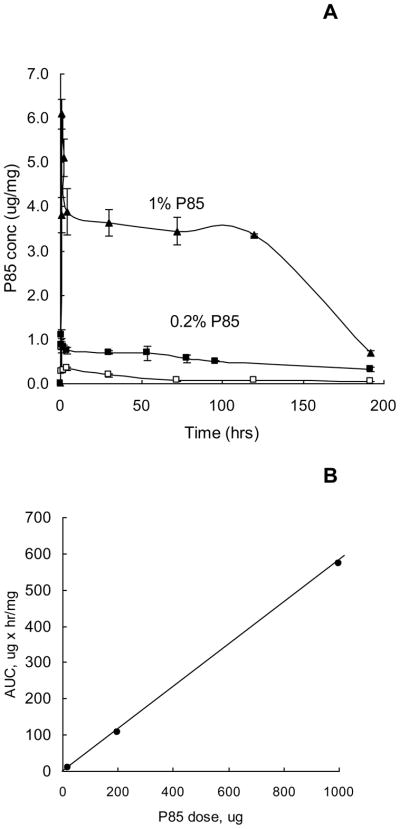
Figure 4A presents the time courses of P85 accumulation in the solid tumors at three different doses of the copolymer, 0.02 %, 0.2 % and 1 % wt. At each dose significant amounts of the block copolymer were detected in the tumor for at least 100 hrs post-injection. The AUC values for P85 in the solid tumors appeared to increase linearly as the block copolymer concentration increased (Figure 4B).
Fig. 4.
Accumulation of radiolabeled P85 in MDR solid tumors in vivo. (A) The time course of P85 concentrations in C57BL/6 mice with 3LL-27M solid tumors following i.v. administration of various doses of P85: 0.02 % (empty squares), 0.2 % (filled squares) and 1 % (filled triangles). Each point represents the mean ± SEM of four animals per time point. (B) The relationship between the P85 concentration and AUC in the tumor for data shown in panel A.
Inhibition of metabolism in MDR tumors by Dox/P85 formulations
In-vivo 31P-MRS of tumors was used for non-invasive detection of metabolic responses to therapy [27]. MRS provides the relative or absolute concentrations of ATP, phosphomonoesters (PME), inorganic phosphate (Pi) and pH. Metabolic responses observed within minutes to hours after injection of drugs usually correlate with tumor shrinkage and histological determination of necrosis after resection measured on the time scale of weeks to months [28–30]. To test the hypothesis that P85 can induce ATP depletion in MDR tumors in vivo, mice with P388/ADR tumors were treated with various Dox/P85 formulations and spectra from the implanted tumors were acquired by localized 31P-MRS. Figure 5A shows three well-defined peaks of ATP in nuclear magnetic resonance spectra in solid tumor prior the injection of Dox/P85 formulation. These peaks almost disappeared 2.5 hrs after the drug was administered (Figure 5B). Almost immediately after the injection of Dox/P85 the ATP levels (Figure 5C) and pH (Figure 5D) dropped dramatically and continued to decrease slowly over the 4 hr time course. Furthermore, increase in Pi was also detected (Figure 5E), while the level of PME was not altered (Figure 5F). Noteworthy, injections of Dox alone or P85 alone did not induce changes in ATP, pH, Pi or PME (Figure 2S, Supplementary Material).
To validate these findings, the effects of i.v. injections of various Dox/P85 formulations on ATP levels were quantified by luciferin/luciferase assay in cells isolated from tumor tissues
Data suggest that Dox formulated with high concentrations of P85 (0.02–1 % wt) caused significant decrease in ATP levels in P388/ADR (Figure 6A, black bars) and 3LL-27M (Figure 6C) tumors. A similar pattern, but at higher doses of the block copolymer (1 % wt) was found in case of injections of P85 alone (Figure 6B, D black bars). In contrast, sensitive tumors (P388) were not affected by injections of Dox/P85 (Figure 6A, white bars) or P85 alone (Figure 6B, white bars). This suggests that both Dox/P85 and P85 alone can cause selective ATP depletion in vivo in the MDR solid tumors but not in the non-MDR tumors. Co-administration Dox with P85 enhances the ATP depletion.
Fig. 6.
Effect of Dox/P85 formulations on ATP levels in BDF1 mice (A, B) bearing solid tumors: P388/ADR (black bars) and P388 (white bars); and C57BL/6 mice (C, D) bearing 3LL-27M solid tumors. Mice were injected three times with Dox alone or Dox/P85 formulations with various P85 concentrations (A, C) or P85 alone (B, D) on days 1, 4, and 7 after group assignment. One hr after the third injection, mice were sacrificed, solid tumors were isolated, and ATP levels were measured as described in Materials and Methods section. Each point represents the mean ± SEM of five animals per time point. Statistical comparisons are made between the treated groups and the control groups injected with the same volume of the saline: * p < 0.05, ** p < 0.005.
Effect of P85 on apoptosis in MDR tumors
To evaluate whether injections of Dox/P85 induced apoptosis in MDR 3LL-27M solid tumors, TUNEL staining of tumors tissues was performed (Figure 7A–C). Quantification of fluorescence levels is presented on Figure 7D. The data suggested that Dox formulated with P85 caused a considerable increase in the amount of DNA fragmentation resulting from apoptotic signaling cascades in tumor tissue, while effect of the same dose of Dox alone was insignificant.
Fig. 7.
Effect of Dox/P85 formulations on apoptosis in C57BL/6 mice with 3LL-27M solid tumors. Mice were treated with (A) saline, (B) Dox alone, (C) Dox/0.02 % P85. Twenty four hrs after the last injection, tumors were isolated, embedded in paraffin, and apoptotic DNA degradation levels were assessed by TUNEL method. (D) Apoptosis level was measured for samples in Panels A–C as the function of TUNEL positive area using ImageJ software. (E) RT-PCR data for expression of caspase 8 (white bars) and caspase 9 (black bars) in 3LL-27M solid tumors treated with Dox and Dox/P85 formulations at different P85 concentrations. Each point represents the mean ±SEM of four animals per time point. Statistical comparisons are made between the treated and the control groups injected with the same volume of the saline: * p< 0.05, ** p < 0.005.
To further validate the effect of Dox/P85 formulations for MDR tumor apoptosis, the expression levels of two pro-apoptotic genes, caspase 8 and caspase 9, were evaluated by RT-PCR. These data confirmed that Dox alone induced apoptosis in the tumors (Figure 7E). Co- administration of P85 with the drug significantly (about six times in case of caspase 8) increased pro-apoptotic signals. Noteworthy, maximal increases in pro-apoptotic signals after administration of Dox/P85 formulations were recorded at the intermediate P85 concentrations, which were the most efficient in tumor inhibition (at 0.002 % – 0.02 % P85).
Body weight of P85 injected mice
To assess whether injections of P85 can cause toxic effects in mice, relative body weight was recorded in control (with saline injections) and copolymer-treated animals for 5 weeks. No effect on total body weight was observed in any animal group indicating absence of toxicity of P85 in mice (data not shown). This is consistent with our previous report that P85 injections in mice do not cause histological changes in main organs (liver, kidney, and brain [31]).
DISCUSSION
Pluronic block copolymers are potent chemosensitizers of MDR tumors [12]. One formulation containing doxorubicin and mixture of Pluronics L61 and F127, SP1049C, has completed the Phase II human trials and demonstrated high level of activity in patients with advanced adenocarcinoma of the esophagus [32]. In addition, it was shown that Pluronic prevents development of MDR phenotype in breast cancer and leukemia cells in vitro [33, 34] and leukemic ascites in vivo [34]. The present study focused on the mechanism of Pluronic sensitization of MDR solid tumors in vivo.
Based on prior cell culture studies the mechanism of Pluronic sensitization effects in MDR cancer cells can be narrowed down to three major interrelated effects: (1) inhibition of Pgp drug efflux system, (2) depletion of ATP, and (3) enhancement of pro-apoptotic signaling [14–16]. The inhibition of Pgp enhances accumulation of anticancer agents in MDR cells, which results in the increased cytotoxicity and sensitization of these cells to the drug. We also reported that P85 inhibits Pgp in blood brain barrier (BBB) in vivo [35]. Thus, brain delivery of a Pgp substrate, digoxin, in the wild-type mice expressing functional Pgp, was greatly enhanced in the presence of P85 and reached the same levels as in mdr1-knockout mice.
To assess Pgp activity in MDR tumors in vivo, we used 99mTc-sestamibi, a lipophilic imaging probe for tumor detection in patients [36, 37] that is also a Pgp substrate [8, 21–26]. Two methods, a non-invasive SPECT [38] and conventional tumor tissue sampling with radioactive counts, were employed to assess effects of P85 on 99mTc-sestamibi accumulation in tumors. SPECT real time analysis indicated that co-administration of P85 drastically increased accumulation of 99Tc-sestamibi in the MDR tumors. This was corroborated by quantitative measurement of radioactive counts in homogenized tumor tissue. Interestingly, both methods suggested that 99mTc-sestamibi accumulation in the tumor increased as the copolymer dose increased. Furthermore, the amount of P85 accumulated in the tumor also increased nearly proportionally to the copolymer dose. Overall, the more P85 accumulates in the tumor the greater the accumulation of the Pgp substrate in the tumor is.
Essential role of Pluronic interaction with mitochondria in the sensitization of MDR tumors has been previously shown in cell culture models [14]. Within minutes after administration to cells Pluronic transports to the endoplasmic reticulum (ER) and then accumulates in mitochondria, which serves as its final destination [39]. In mitochondria of MDR cells Pluronic disrupts oxidative phosphorylation by inhibiting respiratory complexes I and IV, decreases mitochondria membrane potential, promotes release of cytochrome C and triggers accumulation of the ROS [40], The inhibition of cellular respiration leads to ATP depletion observed within 15 min after exposure of MDR cells to Pluronic [41]. This in turn contributes to the inhibition of the Pgp drug efflux function. The essential role of metabolic effects in sensitization of MDR cells with Pluronic was demonstrated earlier by restoration of the Pgp function in these cells by ATP supplementation [41].
Overall, malignant cells are believed to have unique metabolism, which leads to upregulation of phospholipid metabolic intermediates resulting in elevated PME peaks in the 31P-MRS spectrum [42]. Metabolic response to therapy depends on the tumor type, tumor grade, and therapeutic intervention, but in general, responses include reduced ATP, increased Pi, reduced PME, and/or reduced pH. Very similar effects were found in MDR tumor following i.v. injections of Dox/P85. The data obtained by 31P-MRS suggest that this formulation caused significant ATP depletion, accompanied by increased Pi, and decreased pH. The metabolic effects of Dox/P85 were further examined by measuring ATP in isolated tumor cells by luciferin/luciferase assay. We demonstrate that Dox/P85 causes significant reduction in ATP levels in P388/ADR tumors, although P388 tumors are not affected. Interestingly similar effects were observed with P85 alone although Dox/P85 is considerably more effective in ATP depletion. Overall our data suggest that both Dox/P85 and P85 “selectively” affect metabolism in the MDR tumors in vivo, which consistent with previous in vitro studies and is the first demonstration of such effect in animal models. Previously we reported that ATP depletion by P85 in vitro correlates with the expression of drug efflux transporters [14, 17]. Specifically, for all related pairs of MDR and non-MDR cells the overexpression of Pgp was accompanied by a significant increase in responsiveness of MDR cells to Pluronic. One of the reasons for the selectivity of Pluronics to MDR cell phenotype may be the differences in the mitochondria of sensitive and resistant tumors. Notably, P85 was shown to inhibit respiratory chain complexes I and IV in isolated mitochondria of MDR cells where its effect is more pronounced compared to mitochondria of non-MDR cells [40]. The MDR cells have lower mitochondria membrane potential, and more uncoupled respiration due to the mitochondria membrane leakage and higher level of uncoupling protein 2 (UCP2) [43]. As a result, mitochondria function in MDR cells is partially compromised in comparison to their sensitive counterparts and, therefore, may represent a viable target for chemosensitization.
The ability of Pluronic to induce ATP depletion in tumor cells is unique for chemosensitizers and may have further implications for the disease therapy [12]. In parallel studies, Krupka et al. investigated ATP depletion as a possible mechanism for potentiating intratumoral chemotherapy of colorectal adenocarcinoma using carboplatin with P85 or L61 [44]. Both copolymers were found to increase cytotoxic effects of intratumoral carboplatin in an experimental adenocarcinoma in rats. The ATP measurements in the carcinoma cells in vitro showed that the copolymers also reduced the levels of intracellular ATP. Furthermore, since many of the same pathways affected by Pluronic are connected to the cellular heat shock response these authors proposed that the copolymer would also be active in hyperthermia-induced cell injury. In support of this hypothesis both P85 and L61 were shown to improve the hyperthermic cancer treatment in vitro by potentiating heat-induced cytotoxicity [45]. Furthermore, P85 was shown to increase cytotoxic effects of carboplatin on nonresistant cancer in vivo when administered i.v. prior to radiofrequency ablation [46]. Finally, combined treatment with low frequency ultrasound and Pluronic was shown to enhance cytotoxic effects of Dox in tumors [47, 48]. The authors suggested that the acoustic treatment promotes disintegration of Pluronic micelles and enhances accumulation of both Pluronic and drug in tumor cells [49, 50]. However, one cannot exclude a synergy between the stress responses induced by heat or acoustic treatment and Pluronic affects on cell metabolism. Noteworthy, in addition to Pgp overexpressing tumors Pluronics were shown to induce ATP depletion in some other types of cancer cells, in particular those expressing the multidrug resistant proteins (MRPs) [13]
As already mentioned above, Pluronic interactions in mitochondria promote the release of cytochrome C and accumulation of ROS in MDR cells. This is a likely reason for enhanced apoptosis observed in MDR cells in response to the drug [16]. In the present study we demonstrate that P85 administered with Dox can significantly increase the apoptosis in MDR tumor in a mouse model. However, the dependence of the caspase 8 and 9 levels on the P85 concentration in the Dox/P85 formulations is not monotonous and appears to mirror the bell-shaped dose response of the TI discussed below. The highest levels of these caspases are observed at P85 of ca. 0.002 % to 0.02 % when the greatest anti-tumor effects of Dox/P85 are also observed.
In general, the antitumor activity studies presented in this paper reinforce previous findings that combinational treatment with Dox and Pluronic increases antitumor effects of the drug against both MDR and non-MDR tumors [44, 51, 52]. However, for first time we report here that the dependences of Dox/P85 antitumor effects on P85 dose in immunocompetent mouse exhibit bell-shaped patterns in all tumor models. The maximal efficiency is observed at relatively low concentration of the block copolymer of 0.02 % wt. This concentration is below the critical micelle concentration (CMC) of P85 (≈0.03 % wt), which suggests that antitumor effects of drug/copolymer formulations in vivo are promoted by P85 single chains (unimers). Noteworthy, the optimal dose of the copolymer in our study is ca. 2 mg/kg body weight, which approximately corresponds to the copolymers doses in SP1049C during treatment of patients. It is also interesting that some antitumor activity of P85 alone was found in all tumors. In this case the dose dependence of TI also revealed the bell-shaped pattern, but the maximal antitumor effect of P85 was observed at considerably higher doses of the copolymer than in the case of Dox/P85 formulation.
It is very interesting that the bell-shaped dose dependence was not observed in the immunodeficient mice, where anti-tumor activity of Dox/P85 almost linearly increased as the P85 dose increased. Therefore, we speculate that the bell-shaped dose dependence in immunocompetent mice may be related to affects of the excess dose of the block copolymer on the immune system. Immunomodulating effects of water-soluble synthetic polymers were previously reported including the activation or suppression of natural killer (NK) cells, lymphokine-activated killers (LAK) and macrophages [53]. Some Pluronics (such as L121) were reported to induce long-lasting antibody responses, activate complement, induce cell-mediated immunity, lymphokine production by CD4+ T cells, cytotoxic response by CD8+ specific cytotoxic T lymphocytes (CTLs), and expression of MHC class II glycoprotein in antigen-presenting cells [54–56]. These effects in principle may contribute to anti-tumor activities of the Pluronic-based formulations at the optimal doses of the copolymer. Conversely, high doses of P85 (dozens of mg/kg body weight) administered intramuscularly in mice were implicated in activation of NFkB signaling pathway [57]. The activation of this pathway may have adverse effect in therapy by stimulating tumor survival and growth [58]. Interestingly, this hypothesis is consistent with almost complete disappearance of caspases activation at the high doses of the copolymer as well as with some increase in ATP levels observed at 1 % wt P85, which may be due to the increase in the overall tumor mass. Altogether, ability of synthetic polymers to suppress or activate tumor growth through immune responses may depend on a number factors, including dose, route and timing of administration, as well as the site of their action [59]. In case of Pluronics it is also a function of the length of the copolymer hydrophobic PPO chain [60]. Thus, while we cannot exclude that immunomodulating activity of Pluronic may be partially responsible for the observed bell-shaped effects of Dox/P85 and P85 on tumor growth, these effects would require more detailed characterization in the future.
CONCLUSIONS
This study for the first time demonstrated that Pluronic can 1) increase tumor accumulation of the Pgp substrate; 2) induce ATP depletion and 3) promote apoptosis in animal models of MDR tumors. Furthermore, the copolymer can increase the anti-tumor effect of the drug both in MDR and non-MDR tumors. The effects of the copolymer on the tumor growth as well as activation of apoptosis and ATP depletion in immunocompetent mice reveal unusual bell-shaped dependencies with the optimal concentration of P85 being approximately 0.02 % wt. In contrast, in immunodefficient mice the anti-tumor effect monotonously increases as the copolymer dose increases. Clearly, achieving the optimal dose of the copolymer in Dox/P85 formulation is essential for chemotherapy of tumors using Pluronic-based drug formulations. Furthermore, the data of this publication as well as previous studies by our and other groups suggest that these formulations are superiorly active in both MDR and non-MDR tumors. This suggests that the potential therapeutic use of such formulations is broader than it was initially thought and in addition to Pgp expressing tumors may be beneficial for treatment of other cancers. This may be significant in consideration of the ongoing and planned clinical trials of SP1049.
Supplementary Material
Fig. 1S. Antitumor effects of Dox/CSA and Dox/P85 formulations in mice with 3LL-27M tumors. Mice with tumors implanted s.c. and grown for 7–10 days were treated with saline (empty diamonds), Dox with CSA (filled squares), and Dox in 0.02 % P85 (filled circles). Concentration of Dox in all injections was 2.5 mg/kg and i.v. injections were performed on days 1, 4, and 7 after tumor size assessment and random group assignment.
Fig. 2S. Metabolic response to i.v. injection of 2.5 mg/kg Dox alone (green line) or P85 alone (black line) in P388/ADR tumors. (A) Pre-injection spectrum and fit; (B) First spectrum after injection and fit, 2.5 hr time course of 0.2 % P85 alone; (C) ATP and ADP levels, (D) pH, (E) inorganic phosphate (Pi), and (F) total PME.
Acknowledgments
We appreciate the support by the National Institutes of Health grants CA89225 (to A.V.K.) and the Bioimaging Core of the Center of Biomedical Research Excellence (COBRE): Nebraska Center for Nanomedicine (RR021937). The authors (E.V.B., V.Y.A. and A.V.K.) are co-developers of SP1049C and have interest in Supratek Pharma Inc. (Canada), which undertakes commercial development of this new drug.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5(3):219–234. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 2.Fojo T, Bates S. Strategies for reversing drug resistance. Oncogene. 2003;22(47):7512–7523. doi: 10.1038/sj.onc.1206951. [DOI] [PubMed] [Google Scholar]
- 3.Naito S, Yokomizo A, Koga H. Mechanisms of drug resistance in chemotherapy for urogenital carcinoma. Int J Urol. 1999;6:427–439. doi: 10.1046/j.1442-2042.1999.00088.x. [DOI] [PubMed] [Google Scholar]
- 4.Kroger N, Achterrath W, Hegewisch-Becker S, Mross K, Zander AR. Current options in treatment of anthracycline-resistant breast cancer. Cancer Treat Rev. 1999;25(5):279–291. doi: 10.1053/ctrv.1999.0137. [DOI] [PubMed] [Google Scholar]
- 5.Baird RD, Kaye SB. Drug resistance reversal-are we getting closer? Eur J Cancer. 2003;39(17):2450–2461. doi: 10.1016/s0959-8049(03)00619-1. [DOI] [PubMed] [Google Scholar]
- 6.Abbott NJ, Khan EU, Rollinson CM, Reichel A, Janigro D, Dombrowski SM, Dobbie MS, Begley DJ. Drug resistance in epilepsy: the role of the blood-brain barrier. Novartis Found Symp. 2002;243:38–47. discussion 47–53, 180–185. [PubMed] [Google Scholar]
- 7.Liscovitch M, Lavie Y. Cancer multidrug resistance: a review of recent drug discovery research. IDrugs. 2002;5(4):349–355. [PubMed] [Google Scholar]
- 8.Thomas H, Coley HM. Overcoming multidrug resistance in cancer: an update on the clinical strategy of inhibiting p-glycoprotein. Cancer Control. 2003;10(2):159–165. doi: 10.1177/107327480301000207. [DOI] [PubMed] [Google Scholar]
- 9.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2(1):48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 10.Sikic BI, Fisher GA, Lum BL, Halsey J, Chen BOLG. Modulation and prevention of multidrug resistance by inhibitors of P-glycoprotein. Cancer Chemother Pharmacol. 1997;40(Suppl):S13–S19. doi: 10.1007/s002800051055. [DOI] [PubMed] [Google Scholar]
- 11.Schmolka IR. Poloxamers in the pharmaceutical industry. CRC Press; Boca Ratin, FL: 1991. [Google Scholar]
- 12.Batrakova EV, Kabanov AV. Pluronic block copolymers: evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J Control Release. 2008;130(2):98–106. doi: 10.1016/j.jconrel.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Batrakova EV, Li S, Alakhov VY, Elmquist WF, Miller DW, Kabanov AV. Sensitization of cells overexpressing multidrug-resistant proteins by pluronic P85. Pharm Res. 2003;20(10):1581–1590. doi: 10.1023/a:1026179132599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Batrakova EV, Li S, Elmquist WF, Miller DW, Alakhov VY, Kabanov AV. Mechanism of sensitization of MDR cancer cells by Pluronic block copolymers: Selective energy depletion. Br J Cancer. 2001;85(12):1987–1997. doi: 10.1054/bjoc.2001.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venne A, Li S, Mandeville R, Kabanov A, Alakhov V. Hypersensitizing effect of Pluronic L61 on cytotoxic activity, transport, and subcellular distribution of doxorubicin in multiple drug-resistant cells. Cancer Res. 1996;56:3626–3629. [PubMed] [Google Scholar]
- 16.Minko T, Batrakova EV, Li S, Li Y, Pakunlu RI, Alakhov VY, Kabanov AV. Pluronic block copolymers alter apoptotic signal transduction of doxorubicin in drug-resistant cancer cells. J Control Release. 2005;105(3):269–278. doi: 10.1016/j.jconrel.2005.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kabanov AV, Batrakova EV, Alakhov VY. An essential relationship between ATP depletion and chemosensitizing activity of Pluronic block copolymers. J Control Release. 2003;91(1–2):75–83. doi: 10.1016/s0168-3659(03)00211-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kozlov MY, Melik-Nubarov NS, Batrakova EV, Kabanov AV. Relationship between Pluronic block copolymer structure, critical micellization concentration and partitioning coefficients of low molecular mass solutes. Macromolecules. 2000;33:3305–3313. [Google Scholar]
- 19.Bottomley PA. Spatial localization in NMR spectroscopy in vivo. Ann N Y Acad Sci. 1987;508:333–348. doi: 10.1111/j.1749-6632.1987.tb32915.x. [DOI] [PubMed] [Google Scholar]
- 20.Ordidge RJ, Bowley RM, McHale G. A general approach to selection of multiple cubic volume elements using the ISIS technique. Magn Reson Med. 1988;8(3):323–331. doi: 10.1002/mrm.1910080309. [DOI] [PubMed] [Google Scholar]
- 21.Cordobes MD, Starzec A, Delmon-Moingeon L, Blanchot C, Kouyoumdjian JC, Prevost G, Caglar M, Moretti JL. Technetium-99m-sestamibi uptake by human benign and malignant breast tumor cells: correlation with mdr gene expression. J Nucl Med. 1996;37(2):286–289. [PubMed] [Google Scholar]
- 22.Peck RA, Hewett J, Harding MW, Wang YM, Chaturvedi PR, Bhatnagar A, Ziessman H, Atkins F, Hawkins MJ. Phase I and pharmacokinetic study of the novel MDR1 and MRP1 inhibitor biricodar administered alone and in combination with doxorubicin. J Clin Oncol. 2001;19(12):3130–3141. doi: 10.1200/JCO.2001.19.12.3130. [DOI] [PubMed] [Google Scholar]
- 23.Kao CH, Tsai SC, Liu TJ, Ho YJ, Wang JJ, Ho ST, ChangLai SP. P-Glycoprotein and multidrug resistance-related protein expressions in relation to technetium-99m methoxyisobutylisonitrile scintimammography findings. Cancer Res. 2001;61(4):1412–1414. [PubMed] [Google Scholar]
- 24.Cayre A, Cachin F, Maublant J, Mestas D, Feillel V, Ferriere JP, Kwiaktowski F, Chevillard S, Finat-Duclos F, Verrelle P, Penault-Llorca F. Single static view 99mTc-sestamibi scintimammography predicts response to neoadjuvant chemotherapy and is related to MDR expression. Int J Oncol. 2002;20(5):1049–1055. [PubMed] [Google Scholar]
- 25.Palmedo H. What can we expect from MDR breast cancer imaging with sestamibi? J Nucl Med. 2002;43(4):526–530. [PubMed] [Google Scholar]
- 26.Cayre A, Cachin F, Maublant J, Mestas D, Penault-Llorca F. Does 99mTc-sestamibi uptake discriminate breast tumors? Cancer Invest. 2004;22(4):498–504. doi: 10.1081/cnv-200026388. [DOI] [PubMed] [Google Scholar]
- 27.Arias-Mendoza F, Brown TR. In vivo measurement of phosphorous markers of disease. Dis Markers. 2003;19(2–3):49–68. doi: 10.1155/2004/419095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griffiths JR, McSheehy PM, Robinson SP, Troy H, Chung YL, Leek RD, Williams KJ, Stratford IJ, Harris AL, Stubbs M. Metabolic changes detected by in vivo magnetic resonance studies of HEPA-1 wild-type tumors and tumors deficient in hypoxia-inducible factor-1beta (HIF-1beta): evidence of an anabolic role for the HIF-1 pathway. Cancer Res. 2002;62(3):688–695. [PubMed] [Google Scholar]
- 29.Lemaire LP, McSheehy PM, Griffiths JR. Pre-treatment energy status of primary rat tumours as the best predictor of response to 5-fluorouracil chemotherapy: a magnetic resonance spectroscopy study in vivo. Cancer Chemother Pharmacol. 1998;4(23):201–209. doi: 10.1007/s002800050806. [DOI] [PubMed] [Google Scholar]
- 30.Sijens PE. Phosphorus MR spectroscopy in the treatment of human extremity sarcomas. NMR Biomed. 1998;11(7):341–353. doi: 10.1002/(sici)1099-1492(1998110)11:7<341::aid-nbm516>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 31.Batrakova EV, Zhang Y, Li Y, Li S, Vinogradov SV, Persidsky Y, Alakhov VY, Miller DW, Kabanov AV. Effects of pluronic P85 on GLUT1 and MCT1 transporters in the blood-brain barrier. Pharm Res. 2004;21(11):1993–2000. doi: 10.1023/b:pham.0000048189.79606.6e. [DOI] [PubMed] [Google Scholar]
- 32.Armstrong A, Brewer J, Newman C, Alakhov V, Pietrzynski G, Campbell S, Corrie P, Ranson MJW. SP1049C as first-line therapy in advanced (inoperable or metastatic) adenocarcinoma of the oesophagus: a phase II window study. J Clin Oncology; ASCO Annual Meeting Proceedings Part I; 2006. p. 4080. [Google Scholar]
- 33.Batrakova EV, Kelly DL, Li S, Li Y, Yang Z, Xiao L, Alakhova DY, Sherman S, Alakhov VY, Kabanov AV. Alteration of genomic responses to doxorubicin and prevention of MDR in breast cancer cells by a polymer excipient: pluronic P85. Mol Pharm. 2006;3(2):113–123. doi: 10.1021/mp050050g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma AK, Zhang L, Li S, Kelly DL, Alakhov VY, Batrakova EV, Kabanov AV. Prevention of MDR development in leukemia cells by micelle-forming polymeric surfactant. J Control Release. 2008;131(3):220–227. doi: 10.1016/j.jconrel.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Batrakova E, Miller D, Li S, Alakhov V, Kabanov A, Elmquist W. Pluronic P85 enhances the delivery of digoxin to the brain: in vitro and in vivo studies. J Pharmacol Exp Ther. 2001;296(2):551–557. [PubMed] [Google Scholar]
- 36.Fukumoto M. Single-photon agents for tumor imaging: 201Tl, 99mTc-MIBI, and 99mTc-tetrofosmin. Ann Nucl Med. 2004;18(2):79–95. doi: 10.1007/BF02985098. [DOI] [PubMed] [Google Scholar]
- 37.Mele A, Offidani M, Visani G, Marconi M, Cambioli F, Nonni M, Catarini M, Brianzoni E, Berbellini A, Ascoli G, Brunori M, Agostini V, Corvatta L, Isidori A, Spinelli A, Gradari M, Leoni P. Technetium-99m sestamibi scintigraphy is sensitive and specific for the staging and the follow-up of patients with multiple myeloma: a multicentre study on 397 scans. Br J Haematol. 2007;136(5):729–735. doi: 10.1111/j.1365-2141.2006.06489.x. [DOI] [PubMed] [Google Scholar]
- 38.Kostakoglu L, Elahi N, Uzal D, Khademi B, Atahan L, Bekdik CF. Double-phase Tc-99m-SestaMIBI SPECT imaging in a case of glomus jugulare tumor. Radiat Med. 1997;15(5):331–334. [PubMed] [Google Scholar]
- 39.Sahay G, Gautam V, Luxenhofer R, Kabanov AV. The utilization of pathogen-like cellular trafficking by single chain block copolymer. Biomaterials. 2009 doi: 10.1016/j.biomaterials.2009.11.020. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alakhova DY, Rapoport NY, Batrakova EV, Timoshin AA, Li S, Nicholls D, Alakhov VY, Kabanov AV. Differential metabolic responses to pluronic in MDR and non-MDR cells: A novel pathway for chemosensitization of drug resistant cancers. J Control Release. 2009 doi: 10.1016/j.jconrel.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Batrakova E, Li S, Alakhov V, Kabanov A. Selective energy depletion and sensitization of multiple drug resistant cancer cells by Pluronic block copolymers. Polym Prepr. 2000;41(2):1639–1640. [Google Scholar]
- 42.Redmond OM, Stack JP, O’Connor NG, Carney DN, Dervan PA, Hurson BJ, Ennis JT. 31P MRS as an early prognostic indicator of patient response to chemotherapy. Magn Reson Med. 1992;25(1):30–44. doi: 10.1002/mrm.1910250104. [DOI] [PubMed] [Google Scholar]
- 43.Harper ME, Antoniou A, Villalobos-Menuey E, Russo A, Trauger R, Vendemelio M, George A, Bartholomew R, Carlo D, Shaikh A, Kupperman J, Newell EW, Bespalov IA, Wallace SS, Liu Y, Rogers JR, Gibbs GL, Leahy JL, Camley RE, Melamede R, Newell MK. Characterization of a novel metabolic strategy used by drug-resistant tumor cells. Faseb J. 2002;16(12):1550–1557. doi: 10.1096/fj.02-0541com. [DOI] [PubMed] [Google Scholar]
- 44.Krupka TM, Weinberg BD, Wu H, Ziats NP, Exner AA. Effect of intratumoral injection of carboplatin combined with pluronic P85 or L61 on experimental colorectal carcinoma in rats. Exp Biol Med (Maywood) 2007;232(7):950–957. [PubMed] [Google Scholar]
- 45.Krupka TM, Dremann D, Exner AA. Time and dose dependence of pluronic bioactivity in hyperthermia-induced tumor cell death. Exp Biol Med (Maywood) 2009;234(1):95–104. doi: 10.3181/0807-RM-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weinberg BD, Krupka TM, Haaga JR, Exner AA. Combination of sensitizing pretreatment and radiofrequency tumor ablation: evaluation in rat model. Radiology. 2008;246(3):796–803. doi: 10.1148/radiol.2463070228. [DOI] [PubMed] [Google Scholar]
- 47.Nelson JL, Roeder BL, Carmen JC, Roloff F, Pitt WG. Ultrasonically activated chemotherapeutic drug delivery in a rat model. Cancer Res. 2002;62(24):7280–7283. [PubMed] [Google Scholar]
- 48.Gao ZG, Fain HD, Rapoport N. Controlled and targeted tumor chemotherapy by micellar-encapsulated drug and ultrasound. J Control Release. 2005;102(1):203–222. doi: 10.1016/j.jconrel.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 49.Gao Z, Fain HD, Rapoport N. Ultrasound-enhanced tumor targeting of polymeric micellar drug carriers. Mol Pharm. 2004;1(4):317–330. doi: 10.1021/mp049958h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Husseini GA, Pitt WG. The use of ultrasound and micelles in cancer treatment. J Nanosci Nanotechnol. 2008;8(5):2205–2215. doi: 10.1166/jnn.2008.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Batrakova EV, Dorodnych TY, Klinskii EY, Kliushnenkova EN, Shemchukova OB, Goncharova ON, Arjakov SA, Alakhov VY, Kabanov AV. Anthracycline antibiotics non-covalently incorporated into the block copolymer micelles: in vivo evaluation of anti-cancer activity. Br J Cancer. 1996;74(10):1545–1552. doi: 10.1038/bjc.1996.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alakhov V, Klinski E, Li S, Pietrzynski G, Venne A, Batrakova EV, Bronitch T, Kabanov A. Block copolymer-based formulation of Doxorubicin. From cell screen to clinical trials. Colloids Surf B: Biointerfaces. 1999;16:113–134. [Google Scholar]
- 53.Rihova B. Immunomodulating activities of soluble synthetic polymer-bound drugs. Adv Drug Deliv Rev. 2002;54(5):653–674. doi: 10.1016/s0169-409x(02)00043-1. [DOI] [PubMed] [Google Scholar]
- 54.Hunter RL, McNicholl J, Lal AA. Mechanisms of action of nonionic block copolymer adjuvants. AIDS Res Hum Retroviruses. 1994;10(Suppl 2):S95–98. [PubMed] [Google Scholar]
- 55.Brey RN. Development of vaccines based on formulations containing nonionic block copolymers. Pharm Biotechnol. 1995;6:297–311. doi: 10.1007/978-1-4615-1823-5_11. [DOI] [PubMed] [Google Scholar]
- 56.Ke Y, McGraw CL, Hunter RL, Kapp JA. Nonionic triblock copolymers facilitate delivery of exogenous proteins into the MHC class I and class II processing pathways. Cell Immunol. 1997;176(2):113–121. doi: 10.1006/cimm.1997.1084. [DOI] [PubMed] [Google Scholar]
- 57.Gaymalov ZZ, Yang Z, Pisarev VM, Alakhov VY, Kabanov AV. The effect of the nonionic block copolymer pluronic P85 on gene expression in mouse muscle and antigen-presenting cells. Biomaterials. 2009;30(6):1232–1245. doi: 10.1016/j.biomaterials.2008.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gurova KV, Hill JE, Guo C, Prokvolit A, Burdelya LG, Samoylova E, Khodyakova AV, Ganapathi R, Ganapathi M, Tararova ND, Bosykh D, Lvovskiy D, Webb TR, Stark GR, Gudkov AV. Small molecules that reactivate p53 in renal cell carcinoma reveal a NF-kappaB-dependent mechanism of p53 suppression in tumors. Proc Natl Acad Sci U S A. 2005;102(48):17448–17453. doi: 10.1073/pnas.0508888102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tzianabos AO. Polysaccharide immunomodulators as therapeutic agents:structural aspects and biologic function. Clin Microbiol Rev. 2000;13:523–533. doi: 10.1128/cmr.13.4.523-533.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hunter R, Olsen M, Buynitzky S. Adjuvant activity of non-ionic block copolymers. IV. Effect of molecular weight and formulation on titre and isotype of antibody. Vaccine. 1991;9(4):250–256. doi: 10.1016/0264-410x(91)90108-i. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. 1S. Antitumor effects of Dox/CSA and Dox/P85 formulations in mice with 3LL-27M tumors. Mice with tumors implanted s.c. and grown for 7–10 days were treated with saline (empty diamonds), Dox with CSA (filled squares), and Dox in 0.02 % P85 (filled circles). Concentration of Dox in all injections was 2.5 mg/kg and i.v. injections were performed on days 1, 4, and 7 after tumor size assessment and random group assignment.
Fig. 2S. Metabolic response to i.v. injection of 2.5 mg/kg Dox alone (green line) or P85 alone (black line) in P388/ADR tumors. (A) Pre-injection spectrum and fit; (B) First spectrum after injection and fit, 2.5 hr time course of 0.2 % P85 alone; (C) ATP and ADP levels, (D) pH, (E) inorganic phosphate (Pi), and (F) total PME.