Abstract
We have identified based on gene cluster analysis that the genes between Rv3799–Rv3807 in M. tuberculosis have orthologs in Corynebacteria, Mycobacteria and Nocardia (CMN) genomes. Therefore, this gene cluster possibly corresponds to the ‘Ancient Conserved Region’ of CMN mycolyltransferases. The evolutionary trace analysis suggests that twelve amino acid residues; Leu39, Trp51, Pro71, Trp82, Trp97, Phe100, Gly124, Ser126, Asp192, Glu230, Gly260 and Trp264 are ‘absolutely conserved’. These amino acid residues constitute the active site and conserved hydrophobic tunnel in CMN mycolyltransferases.
Keywords: Corynebacteria, Mycobacteria, Nocardia, Mycolyltransferases, Gene cluster, Evolutionary trace analysis
Introduction
The organisms belonging to the Corynebacterium, Mycobacterium and Nocardia (or ‘CMN’) genera have been grouped together based on factors that distinguish them individually. These include; complex cell wall components, presence and type of mycolic acids, adjuvant activity, presence of cord factor, sulfolipids, iron-chelating compounds, polyphosphate, and serological cross-reactivity. The cell wall of the CMN group organisms consist interconnected peptidoglycan and polysaccharide-mycolate complex and are characterized by the presence of mycolic acid on their surface (Cocito and Delville 1985). Mycolic acids are long chain fatty acids that form a part of the unique cell envelope responsible for the pathogenesis and the survival of the organism inside the host. These mycolic acids are identified by different names depending upon the genus and comprise different carbon chain lengths; corynomycolic acids from the genus Corynebacterium has 22–36 long carbon chain, mycolic acids from the genus Mycobacterium has nearly 60–90 long carbon chain and nocardomycolic acids from the genus Nocardia has 40–60 long carbon chain (Collins et al 1982; Minnikin 1982; Daffé M and Draper 1992).
In M. tuberculosis, the antigen 85 complex enzymes constitute three secreted proteins (Wiker and Harboe 1992); Ag85A (gene identifier: Rv3804), Ag85B (Rv1886) and Ag85C (Rv0129) that comprise a signal peptide at the N-terminus followed by a carboxylesterase domain. These are known to catalyse the transfer of mycolic acids to the α,α’- trehalosemonomycolate (TMM) and arabionogalactan. It has been demonstrated that Ag85 complex enzymes catalyse the transfer of mycolyl residue from one molecule of TMM to another TMM leading to the formation of α,α’- trehalosedimycolate (TDM) and hence these enzymes are termed mycolyltransferases (Belisle et al 1997). Also, in Corynebacterium and Nocardia, orthologous proteins synthesize trehalosedicorynomycolate (TDCM) and trehalosedino-cardiomycolate (TDNM), respectively. Mycolyltransferases are also termed fibronectin binding proteins, since they are involved in binding fibronectin that aids entry of the organism into host cells (Abou-Zeid et al 1988; Ratliff et al 1988). The compound TDM commonly known as “cord factor” extracted from M. tuberculosis has been shown to be toxic in mouse model (Kato 1968). Hence a study related to the analysis of the structure, function and evolution of proteins responsible for the synthesis of TDM in these species is important.
The three dimensional structures of Ag85A (PDB code:1SFR; Ronning et al 2004), Ag85B (1F0P; Anderson et al 2001) and Ag85C (1DQZ, 1VA5; Ronning et al 2000) are known for both native and substrate bound forms and comprises a α/β hydrolase fold. The catalytic triad constituting S126, G230 and H262 (numbering is according to PDB code: 1F0P) is responsible for the mycolyltransferase activity. The structural comparison of these mycolyltransferases revealed that the active sites are virtually identical indicating that they share the same substrate. However, in contrast to the high level conservation within the substrate-binding and active site, it was observed that surface residues disparate from the active site are quite variable indicating that all three Ag85 enzymes are needed to evade the host immune system (Ronning et al 2004). The multiple sequence alignment (see Appendix-A in supplementary data) suggests that the three sequences corresponding to Ag85A, Ag85B and Ag85C share more than 69% sequence identity. In our previous work (Adindla et al 2004a), we identified mycolyltransferases in the C. glutamicum and C. efficiens genome and analyzed the three-dimensional computer models that were constructed based on comparative modeling methods. The mycolyltransferases are restricted to the CMN genera and the complete genome sequences of M. tuberculosis (Cole et al 1998), C. glutamicum (Kalinowski et al 2003), C. efficiens (Kawarabayasi et al 2002), C. diphtheria (Cerdeno-Tarraga et al 2003) and Nocardia farcinica (Ishikawa et al 2004) are now available. Therefore, we intended to identify and analyse all the mycolyltransferases from various species in order to get an insight into their substrate specificity. Since there are several isoforms in each genome, we intended to understand the origin of their evolution and therefore carried out the evolutionary trace analysis. Further, synteny or colinearity of gene order is observed when a group of genes are present in the same order in two or more genomes as a cluster. Two species that have recently diverged from a common ancestor might be expected to share a similar set of genes present in the same order. During evolution the sequence of each pair of genes is accompanied by changes, such as, gene duplication and gene loss. Genetic analyses reveal that genes with related function are frequently clustered at one chromosomal location in evolution. Despite M. tuberculosis being an ancient species compared to Corynebacterium and Nocardia, evolution has maintained the conservation of proteins involved in the synthesis of the cell envelope. Also, it is interesting to note that there are varying numbers of mycolyltransferases in these different species. We therefore intended to identify the evolutionary origin of CMN mycolyltransferases based on the computational analysis of ‘gene neighborhood’ and to identify the role of conserved amino acid residues using the evolutionary trace analysis.
Materials and methods
The amino acid sequences corresponding to the mycolyltransferases; Ag85A, Ag85B and Ag85C were obtained from the website at www.srs.ebi.ac.uk/. The homologous proteins from C. glutamicum, C. efficiens, C. diptheria and N. farcinica completed genome database were identified using the BLASTP and PSI-BLAST (Altschul et al 1990; 1997) programs available at the website www.ncbi.nlm.nih.gov/BLAST/ and by using the sequence corresponding to Ag85B as query. The blosum62 matrices were used and the results were sorted based on p-value. The analysis of gene clusters was carried out by performing BLAST searches using mycolyltransferases and their neighbouring proteins as query on all the finished and unfinished genomes. The evolutionary trace (ET) analysis was carried out using TraceSuite II server (Innis et al., 2000) available at the website http://www.cryst.bioc.cam.ac.uk/~jiye/evoltrace/evoltrace.html by submitting the sequences corresponding to the carboxylesterase domain of CMN mycolyltransferases and the crystal structure of Ag85B (PDB code: 1F0P). A trace is generated by comparing the consensus sequences for groups of proteins that originate from a common node in a phylogenetic tree and is characterized by a common evolutionary time cut-off (ETC).
Results and discussion
Identification of CMN mycolyltransferases
We identified 32 mycolyltransferases in the genera of CMN group; 4 proteins in M. tuberculosis, 4 in C. diphtheria, 6 in C. glutamicum, 5 in C. efficiens, and 13 in N. farcinica. The four mycolyltransferases corresponding to each of the mycobacterial species; M. tuberculosis, M. leprae and M. bovis are highly similar, therefore only the mycolyltransferases from M. tuberculosis is referred to in the discussion. There are between 350–480 amino acid residues in these proteins. However, proteins corresponding to gene identifiers; Ncgl2777, Ce2709 and Dip2193 (in Corynebacterium) and Nfa1840 (in N. farcinica) are associated with an additional ∼300 amino acid residue domain towards the C-terminus that is not a part of the carboxylesterase domain. The carboxylesterase domain that is responsible for the mycolyltransferase activity, corresponds to approximately 280 amino acid residues. The multiple sequence alignment is attached as supplementary data along with this manuscript in Appendix-A. The N. farcinica proteins; Nfa1820 and Nfa1810, comprise a ‘long insertion’ sequence of 22 and 27 amino acid residues that is rich in glycine and serine respectively relative to the other CMN proteins. This region is located between the ‘absolutely conserved’ W82 and W97 residues. The glycine/serine rich sequences are often known to be associated with cell-surface proteins. Another insertion region in some corynomycolyltransferases and nocardiomycolyl transferases relative to mycolyltransferases is located between the ‘absolutely conserved’ D192 and E230 amino acid residues. We predict this loop to be close to the substrate binding site based on a comparison with the crystal structure of Ag85B.
Gene cluster analysis
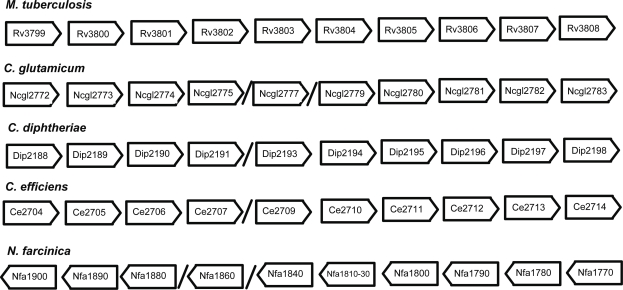
The analysis of all mycolyltransferases and their neighbouring proteins revealed that genes between Rv3799 – Rv3807 in the M. tuberculosis genome has corresponding orthologs in Corynebacterium and Nocardia genera as shown in Figure 1. The ten protein orthologs shown in Figure 1 share high sequence similarity in the five different species analyzed. In addition to mycolyltransferase (Rv3804) and its precursor protein (Rv3803) this cluster also comprises propionyl CoA carboxylase (Rv3799), polyketide synthase (Rv3800), acyl CoA synthase (Rv3801), membrane proteins (Rv3806, Rv3807), and hypothetical proteins (Rv3802, Rv3805). We observed that the Nocardia proteins are arranged in the reverse order relative to the other species. We report that this set of genes represents the only mycolyltransferase comprising gene cluster during divergence of a common ancestoral organism into individual genera, such as, Corynebacterium, Mycobacterium and Nocardia (CMN group). Therefore, we propose that this gene cluster corresponds to the “Ancient Conserved Regions - ACR’s” among the mycolyltransferases across the CMN genera. It was reported that Rv3800 (pks13) is involved in the final condensation step in mycolic acid synthesis (Damien et al 2004). It was also reported that the genes; Rv3799, Rv3800 and Rv3801 (accD4-pks13-fadD32) play an essential role in the biosynthesis of mycolic acids (Gande et al 2004). This indicates that the proteins in this cluster are important for the mycolic acid synthesis and its transfer to trehalose. Since, functionally related genes are often clustered, we suggest that the other “uncharacterized” proteins belonging to the ACR gene cluster may also have a role in associated functions. Further, we observed that the mycolyltransferase gene neighbours; Rv0129 and Rv1886 are conserved among M. tuberculosis and M. bovis suggesting that gene duplication events have occurred before speciation.
Figure 1.
Schematic representation of genes corresponding to the ‘Ancient Conserved Regions’ in five completed genomes based on gene neighbourhood analysis. ‘/’ indicates insertion of gene. Nfa1810-30 indicates three genes Nfa1810, Nfa1820 and Nfa1830.
Evolutionary Trace Analysis
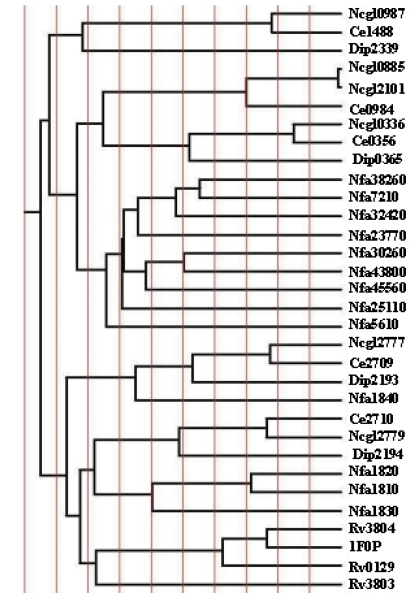
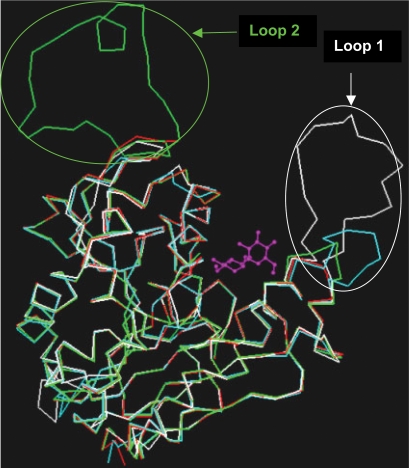
The TraceSuite II server generates a phylogenetic tree split into 10 evenly distributed partitions (P01–P10) in the order of increasing evolutionary time cut-off (ETC) as shown in Figure 2a. The conserved amino acid residues associated with each partition is shown in Figure 2b. Analysis of amino acid residues corresponding to P01 partition (see Figure 2b) revealed that 12 amino acid residues are “absolutely conserved”. By examining the equivalent residues in the crystal structure of the protein (PDB code: 1F0P), we infer that the residues; L39, P71, W82, W97 and F100 constitute the ‘hydrophobic tunnel’ as shown in Figure 3a. This figure also indicates the amino acid residues involved in the catalytic triad. The residues in the ‘hydrophobic tunnel’ are needed in order to accommodate the alkyl chain of mycolic acid indicating a functional conservation in these proteins. The invariant S126 and G260 are close to the catalytic active site comprising the amino acid residue E230. The indole side chains of the W51 and W264 are perpendicular to each other and are in proximity to G124 associated with the β 5 strand. The amino acid residue D192 is away from the active site indicating that the conservation extends beyond the catalytic site in CMN mycolyltransferases. According to Figure 2a, the 14 proteins indicated in the lower half, from Corynebacterium, Mycobacterium, and Nocardia represent the ‘Ancient Conserved Region’. The 18 proteins in the upper half, comprise only the Nocardia and Corynebacterium. From the multiple sequence alignment, we observed that the proteins in the upper half of Figure 2a are associated with an insertion loop of variable length between 4 to 20 amino acid residues and this loop is close to the active site. The positions of these insertion loops are shown in Figure 3b. Further, the amino acid residues comprising the specificity pockets defined by interactions with trehalose substrate in the protein with PDB code: 1F0P are mutated in these proteins. Primarily, the mutations associated with the substrate binding sites in some Corynebacterium (Adindla et al., 2004a) and Nocardia proteins accompanied by the presence of ‘insertion loops’ close to the active site suggest that these may interfere with trehalose binding. These Corynebacterium and Nocardia proteins are possibly a result of divergent evolution accompanied by gene duplication and mutation events in order to accommodate different substrates in the binding site. This suggests that the ancient proteins form a distinct cluster and are different from proteins that evolved later.
Figure 2(a).
TraceSuite II analysis representing partition based on evolutionary time cut off. (b) ‘absolutely conserved’ residues corresponding to P01 partition.
Figure 2(b).
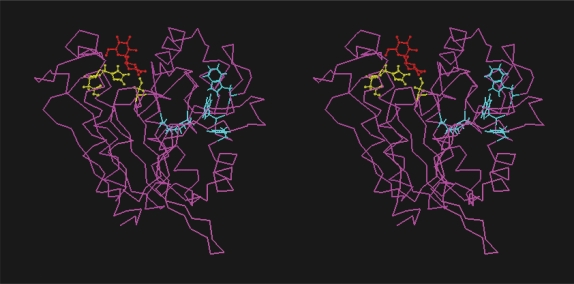
Figure 3(a).
Stereo-view showing three-dimensional model corresponding to the protein with gene identity Nfa1840. The amino acid residues comprising the catalytic triad (pink), hydrophobic tunnel (blue) and trehalose (red) are also indicated.
Figure 3(b).
Structural overlay corresponding to the proteins with PDB code: 1F0P and gene identities: Nfa1810, Ncgl0336 and Ncgl0987 indicating the position of the two loops; loop 1 (D192 – E230 loop) and loop 2 (W82-W97 loop).
We previously reported that the corynemy colyltransferase Ncgl2777 gene in C. glutamicum (protein with ∼300 amino acid residue C-terminal extension) is associated with a 55 amino acid residue ‘LGFP’ tandem repeat that is likely to be associated with maintaining cell-wall integrity (Adindla et al 2004b). Our hypothesis was based on the work of Brand et al., 2003 who have demonstrated that the deletion of Ncgl2777 gene in C. glutamicum resulted in a 10-fold increase in cell volume of the organism thereby suggesting its involvement in cell shape formation. In this work, we observed that the ‘LGFP’ tandem repeats are also present in the C-terminal region of Nocardia (NfaA1840) and C. diphtheria (Dip2193) proteins and accordingly may be involved in maintaining cell wall integrity.
Conclusions
The comparative analysis of mycolyltransferase proteins from different genomes suggested that the gene cluster corresponding to the ten gene families located between Rv3799 and Rv3807 in Mycobacterium tuberculosis genome represents the ‘Ancient Conserved Region’ in CMN genera. According to the evolutionary trace analysis twelve amino acid residues are ‘absolutely conserved’ in all CMN proteins analyzed. These CMN proteins fall into two distinct clusters in the phylogeny that correlates with the presence or absence of insertion loop close to the active site. Some Corynebacterium and Nocardia proteins with extra C-terminal 300 amino acid residues are associated with the LGFP tandem repeats.
Acknowledgments
HGR thanks UGC, New Delhi for a JRF fellowship. SA thanks CSIR New Delhi for a SRF fellowship. LGP thanks DBT, New Delhi for research funding.
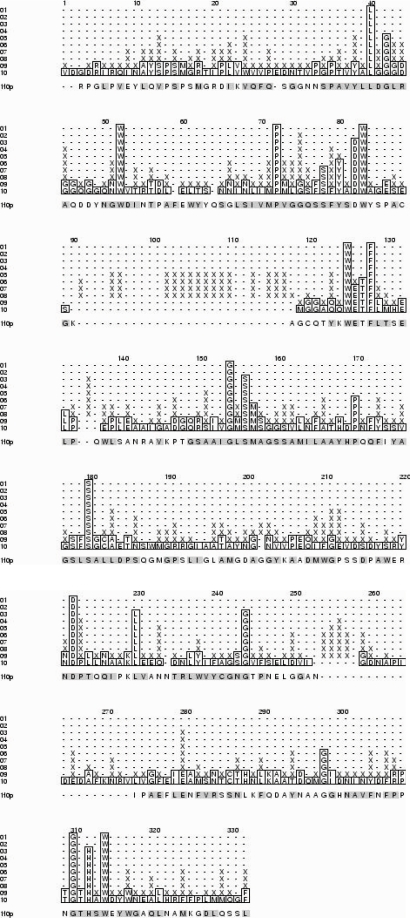
Appendix-A
Multiple sequence alignment corresponding to carboxylesterase domain in CMN mycolyltransferases
Note: The codes are according to the gene identity for individual genomes. The conserved residues are indicated by ‘*’ and the amino acid residue numbering is according to the PDB code:1F0P.
References
- Abou-Zeid C, Ratliff TL, Wiker HG, et al. Characterisation of fibronectin-binding antigens released by Mycobacterium tuberculosis and Mycobacterium bovis BCG. Infect Immun. 1988;56:3046–3051. doi: 10.1128/iai.56.12.3046-3051.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adindla S, Guruprasad K, Guruprasad L. Three-dimensional models and structure analysis of corynemycolyltransferases in Corynebacterium glutamicum and Corynebacterium efficiens. Int J Biol Macromol. 2004a;34:181–189. doi: 10.1016/j.ijbiomac.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Adindla S, Inampudi KK, Guruprasad K, et al. Identification and analysis of novel repeats in the cell surface proteins of archaeal and bacterial genomes using computational tools. Comp Funct Genom. 2004b;5:2–16. doi: 10.1002/cfg.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, et al. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schäffer AA, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DH, Harth G, Horwtiz MA, et al. An interfacial mechanism and a class of inhibitors inferred from two crystal structures of the Mycobacterium tuberculosis 30 kDa Major secretory protein (antigen 85B), a mycolyl transferase. J Mol Biol. 2001;307:671–681. doi: 10.1006/jmbi.2001.4461. [DOI] [PubMed] [Google Scholar]
- Belisle JT, Vissa VD, Sievert T, et al. Role of the major antigen of Mycobacterium tuberculosis in the cell wall biogenesis. Science. 1997;276:1420–1422. doi: 10.1126/science.276.5317.1420. [DOI] [PubMed] [Google Scholar]
- Brand S, Niehaus K, Puhler A, et al. Identification and functional analysis of six mycolyltransferase genes of Corynebacterium glutamicum ATCC 13032: the genes cop1, cmt1, and cmt2 can replace each other in the synthesis of trehalose dicorynomycolate, a component of the mycolic acid layer of the cell envelope. Arch Microbiol. 2003;180:33–44. doi: 10.1007/s00203-003-0556-1. [DOI] [PubMed] [Google Scholar]
- Cocito A, Delville J. Biological, chemical, immunological and staining properties of bacteria isolated from tissues of leprosy patients. Eur J. Epidemiol. 1985;1:202–231. doi: 10.1007/BF00234095. [DOI] [PubMed] [Google Scholar]
- Cole ST, Brosch R, Parkhill J, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393(6685):537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- Cerdeno-Tarraga AM, Efstratiou A, Dover LG, et al. The complete genome sequence and analysis of Corynebacterium diphtheriae NCTC13129. Nucleic Acids Res. 2003;31(22):6516–6523. doi: 10.1093/nar/gkg874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins MD, Goodfellow M, Minnikin DE. A survey of the structures of mycolic acids in Corynebacterium and related taxa. J Gen. Microbiol. 1982;128:129–149. doi: 10.1099/00221287-128-1-129. [DOI] [PubMed] [Google Scholar]
- Daffé M, Draper P. The envelope layers of mycobacteria with reference to their pathogenicity. Adv Microb Phys. 1998;39:131–203. doi: 10.1016/s0065-2911(08)60016-8. [DOI] [PubMed] [Google Scholar]
- Damien P, Célia de Sousa-D'Auria, Christine H, et al. A polyketide synthase catalyzes the last condensation step of mycolic acid biosynthesis in mycobacteria and related organisms. Proc Natl Acad Sci USA. 2004;101:314–319. doi: 10.1073/pnas.0305439101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gande R, Gibson KJC, Brown AK, et al. Acyl-CoA Carboxylases (accD2 and accD3), Together with a Unique Polyketide Synthase (Cg-pks), Are Key to Mycolic Acid Biosynthesis in Corynebacterianeae Such as Corynebacterium glutamicum and Mycobacterium tuberculosis. J. Biol. Chem. 2004;279:44847–44857. doi: 10.1074/jbc.M408648200. [DOI] [PubMed] [Google Scholar]
- Innis CA, Shi J, Blundell TL. Evolutionary trace analysis of TGF-β and related growth factors: implications for site-directed mutagenesis. Prot Engin. 2000;13:839–847. doi: 10.1093/protein/13.12.839. [DOI] [PubMed] [Google Scholar]
- Ishikawa J, Yamashita A, Mikami Y, et al. The complete genomic sequence of Nocardia farcinica IFM 10152. Proc Natl Acad Sci USA. 2004;101:14925–14930. doi: 10.1073/pnas.0406410101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinowski J, Bathe B, Bartels D, et al. The complete Corynebacterium glutamicum ATCC 13032 genome sequence and its impact on the production of L-aspartate-derived amino acids and vitamins. J Biotechnol. 2003;104(1–3):5–25. doi: 10.1016/s0168-1656(03)00154-8. [DOI] [PubMed] [Google Scholar]
- Kawarabayasi Y, Yamazaki J, Hino Y, et al. The entire genomic sequence of Corynebacterium efficiens YS-314. 2002 Unpublished. [Google Scholar]
- Minnikin DE. The Biology of the Mycobacteria. In: Ratledge C, Stanford JL, editors. London: Academic Press; 1982. pp. 95–184. [Google Scholar]
- Ratliff TL, McGarr JA, Abou-Zeid C, et al. Attachment of mycobacteria to fibronectin-coated surfaces. J Gen Microbiol. 1988;134:1307–1313. doi: 10.1099/00221287-134-5-1307. [DOI] [PubMed] [Google Scholar]
- Ronning DR, Klabunde T, Besra GS, et al. Crystal structure of the secreted form of antigen 85C reveals potential targets mycobacterial drugs and vaccines. Nat. Struc. Biol. 2000;7(2):141–146. doi: 10.1038/72413. [DOI] [PubMed] [Google Scholar]
- Ronning DR, Vissa V, Gurdyal B, et al. Mycobacterium tuberculosis antigen 85A and 85C structures confirm binding orientation and conserved substrate specificity. J Biol Chem. 2004;279:36771–36777. doi: 10.1074/jbc.M400811200. [DOI] [PubMed] [Google Scholar]
- Wiker HG, Harboe M. The antigen 85 complex: a major secretion product of Mycobacterium tuberculosis. Microbiol. Rev. 1992;56:648–661. doi: 10.1128/mr.56.4.648-661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]