Abstract
The current study evaluated a new series of N,N′-alkane-diyl-bis-3-picolinium (bAPi) analogs with C6–C12 methylene linkers as nicotinic receptor (nAChR) antagonists, for nicotine-evoked [3H]dopamine (DA) overflow, for blood-brain barrier choline transporter affinity and for attenuation of discriminative stimulus and locomotor stimulant effects of nicotine. bAPi analogs exhibited little affinity forα4β2* andα7* high affinity ligand binding sites, nor for nAChRs modulating DA transporter function. With the exception of C6, all analogs inhibited nicotine-evoked [3H]DA overflow (IC50=2 nM–6μM; Imax=54–64%), with N,N′-dodecane-1,12-diyl-bis-3-picolinium dibromide (C12, bPiDDB) being most potent. bPiDDB did not inhibit electrically-evoked [3H]DA overflow, suggesting specific nAChR inhibitory effects and a lack of toxicity to DA neurons. Schild analysis suggested that bPiDDB interacts in an orthosteric manner at nAChRs mediating nicotine-evoked [3H]DA overflow. To determine if bPiDDB interacts with α-conotoxin MII-sensitive α6β2-containing nAChRs, slices were exposed concomitantly to maximally-effective concentrations of bPiDDB (10 nM) and α-conotoxin MII (1 nM). Inhibition of nicotine-evoked [3H]DA overflow was not different with the combination compared with either antagonist alone, suggesting that bPiDDB interacts with α6β2-containing nAChRs. C7, C8, C10 and C12 analogs exhibited high affinity for the blood-brain barrier choline transporter in vivo, suggesting brain bioavailability. Although, none of the analogs altered the discriminative stimulus effect of nicotine, C8, C9, C10 and C12 analogs decreased nicotine-induced hyperactivity in nicotine-sensitized rats, without reducing spontaneous activity. Further development of nAChR antagonists that inhibit nicotine-evoked DA release and penetrate brain to antagonize DA-mediated locomotor stimulant effects of nicotine as novel treatments for nicotine addiction is warranted.
INTRODUCTION
Nicotine, the principal tobacco alkaloid, is an agonist at neuronal nicotinic receptor (nAChR) subtypes modulating dopamine (DA) release. Habitual tobacco smoking is maintained via rapid nicotine delivery to brain (Le Foll and Goldberg, 2005) and results from nicotine’s intrinsic rewarding properties, believed due to increased DA release. Classical nAChR antagonists, mecamylamine and dihydro-β-erythroidine (DHβE), inhibit nicotine-evoked DA release and decrease the locomotor stimulant and reinforcing effects of nicotine in rats (Corrigall et al., 1994; Watkins et al., 1999; Rahman et al., 2004), suggesting a role for nAChR-mediated DA release in these abuse-related behavioral effects of nicotine. However, evidence supporting a primary role for DA in nicotine discrimination is lacking (Corrigall and Coen, 1994).
Substantia nigra DA neurons express mRNA for α3, α4, α5, α6, α7, β2, β3 and β4 subunits (Klink et al., 2001;Azam et al., 2002). While β2-containing nAChRs play a critical role in mediating nicotine-evoked striatal DA release (Picciotto et al., 1998; Salminen et al., 2004; Scholze et al., 2007), a role for α6- and β3-containing subtypes also has been implicated (Cui et al., 2003). A comprehensive molecular genetics study, in which an individual gene (α4, α5, α7,β2, β3 orβ4) was deleted, suggested that six different subtypes mediate nicotine-evoked DA release from mouse striatum, including α-conotoxin MII (α-CtxMII)-sensitive (α6β2β3*,α4α6β2β3*,α6β2* and α4α6β2*) and α-CtxMII-resistant (α4β2* and α4α5β2*) nAChRs, whereas deletion of β4 and α7 had no effect (Salminen et al., 2004; Gotti et al., 2005). More recently, striatal synaptosomes from α4 and α4/β3 knockout mice were used to isolate α6-containing nAChRs to determine their involvement in nicotine-evoked DA release (Salminen et al., 2007). These results showed an increased EC50 value for nicotine to evoke DA release with α4 deletion, and combined deletion of α4 and β3 subunits resulted in a further 4–7-fold increase in EC50 value. Results suggest that the α4α6β2β3* subtype constitutes ~50% of α6-containing nAChRs on striatal DA terminals of wild-type mice, and that this subtype is most sensitive to nicotine activation and is a major subtype mediating nicotine-evoked DA release and behavior.
One approach to developing effective tobacco cessation pharmacotherapies is to discover molecules that selectively inhibit nicotine-evoked DA release. Although mecamylamine may have clinical efficacy as a cessation agent, adverse side effects due to inhibition of peripheral nAChRs limit its use (Rose et al., 1994). Development of antagonists selective for DA release-regulating nAChRs should retain therapeutic efficacy without producing peripheral side effects; unfortunately, no clinically-available compounds have this profile. In this regard, N-n-alkylnicotinium analogs with methylene linkers of C7-C12 potently inhibit nicotine-evoked [3H]DA overflow from rat striatal slices in an orthosteric manner and inhibit high affinity [3H]nicotine binding to rat brain membranes (Wilkins et al., 2002, 2003). Structurally-related N-n-pyridinium analogs with C10-C20 were also potent inhibitors of nicotine-evoked [3H]DA overflow, and longer chain analogs (C15 and C20) showed incomplete inhibition (Imax=50%), supporting the involvement of more than one nAChR subtype (Grinevich et al., 2003). These pyridinium analogs had little affinity for the [3H]nicotine binding site (Grinevich et al., 2003), indicating enhanced selectivity for nAChRs mediating nicotine-evoked DA release.
The bis-quaternary ammonium compounds, hexamethonium and decamethonium, which are considered to be simplified analogs of d-tubocurarine, have been used to differentiate muscle and ganglionic nAChRs (Koelle, 1975). The current study exploited the bis-quaternary ammonium structural framework to enhance nAChR subtype selectivity and afford a new class of N,N′-alkane-diyl-bis-3-picolinium (bAPi) analogs (Fig. 1; Dwoskin et al., 2004). In microdialysis studies using rats, the lead analog, N,N′-dodecane-1,12-diyl-bis-3-picolinium dibromide (C12, bPiDDB), decreased extracellular DA levels in nucleus accumbens following systemic nicotine (Rahman et al., 2007) and decreased intravenous nicotine self-administration (Neugebauer et al., 2006). Herein, we evaluated the bAPi series for their in vitro effects on nicotine-evoked DA release at striatal nAChRs and for their ability to inhibit the discriminative stimulus and/or locomotor stimulant centrally-mediated effects of nicotine. These polar and charged analogs are predicted to have poor brain bioavailability following systemic administration. However, previous work with structurally-related polar mono-nicotinium analogs revealed good affinity for the blood-brain barrier (BBB) choline transporter and active transport into brain (Allen et al., 2003). Therefore, an additional goal was to characterize these analogs for their affinity for the BBB choline transporter.
Fig. 1.
Chemical structures, full chemical names and abbreviations of N,N′-alkane-diyl-bis-3-picolinium (bAPi) salts.
METHODS
Chemicals
[3H]Dopamine ([3H]DA; 3,4-ethyl-2[N-3H] dihydroxyphenylethylamine; specific activity 28.0 Ci/mmol) and S-(−)-[3H]nicotine (S-(−)-[N-methyl-3H]; specific activity 81.0 Ci/mmol) were purchased from PerkinElmer Life Sciences Inc. (Boston, MA). L-Ascorbic acid, 1-[2-(bis[4-fluorophenyl]methoxy)ethyl]-4-[3-phenylpropyl]piperazine dihydrochloride (GBR 12909), bovine serum albumin (BSA), catechol, cytisine, decamethonium bromide (DEC), α-D-glucose, N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid) (HEPES), hexamethonium chloride (HEX), methyllycaconitine citrate (MLA), S(−)-nicotine ditartrate, nomifensine maleate, nudicauline, pargyline hydrochloride and sucrose were obtained from Sigma Chemical Co. (St. Louis, MO). [3H] Methyllycaconitine ([3H]MLA) ([1α,4(S),6β,14α,16β]-20-ethyl-1,6,14,16-tetramethoxy-4-([{2-([3-3H]-methyl-2,5-dioxo-1-pyrrolidinyl)benzoyl}oxy]methyl)aconitane-7,8-diol; specific activity 25.8 Ci/mmol) was purchased from Tocris Cookson Ltd. (Bristol, UK). TS-2 Tissue solublizer and scintillation cocktail were purchased from Research Products International Corp. (Mount Prospect, IL). d-Tubocurarine (TBC) was purchased from Aldrich Chemical Co. (Milwaukee, WI). All other chemicals used in the in vitro assay buffers were purchased from Fisher Scientific (Pittsburgh, PA).α-CtxMII was synthesized as described previously (Cartier et al., 1996). bAPi salts (Fig. 1, structures 1–7) were synthesized by reacting 3-picoline with a variety of diiodo or dibromoalkanes (Dwoskin et al., 2004). All compounds were characterized by 1H- and 13C-NMR spectroscopy, mass spectroscopy and elemental analysis. Chemical purity of all products was > 98%. bAPi analogs, S(−)-nicotine ditartrate, mecamylamine HCl and DHβE hydrobromide (Sigma, St. Louis, MO) were prepared in 0.9% NaCl (saline) for the behavioral experiments. Dilute NaOH was added to the nicotine solution to obtain a pH of ~7.4. All injections were administered sc in a volume of 1 ml/kg body weight. Doses of nicotine and the bAPi analogs are expressed as free base weights, and doses of mecamylamine and DHβE are expressed as salt weights.
Neurochemical Experiments
Subjects
Male Sprague-Dawley rats (200–225g) were obtained from Harlan (Indianapolis, IN) and were housed two per cage with free access to food and water in the Division of Laboratory Animal Resources, University of Kentucky. Experimental protocols involving the rats were in accordance with the NIH 1996 Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of Kentucky.
[3H]Nicotine Binding Assay
Striata from two rats were dissected, pooled and homogenized in 10 vol of ice-cold modified Krebs-HEPES buffer (in mM: 20 HEPES, 118 NaCl, 4.8 KCl, 2.5 CaCl2, 1.2 MgSO4, pH 7.5). Homogenates were incubated at 37°C for 5 min, and centrifuged (27,000g for 20 min at 4°C). Pellets were resuspended in 10 vol of ice-cold MilliQ water and incubated for 5 min at 37°C, followed by centrifugation (27,000g for 20 min at 4°C). Second and third pellets were resuspended in 10 vol of fresh, ice-cold 10% Krebs-HEPES buffer, incubated for 5 min at 37°C, and centrifuged. Final pellets were stored in 10% Krebs-HEPES buffer at −70 °C until assay. Upon assay, final pellets were resuspended in 20 vol of ice-cold MilliQ water, which afforded ~200 μg membrane protein/100 μl, using BSA as the protein standard. Binding assays were performed in duplicate in a final vol of 200 μl Krebs-HEPES buffer containing 200 mM Tris (pH 7.5 at 4°C). Assays were initiated by addition of 100μl membrane suspension to tubes containing final concentrations of 3 nM [3H]nicotine (50 μl) and 1 of 9 analog concentrations (50 μl; 10 nM–1 mM). Cytisine was used as a positive control for these experiments. Nonspecific binding was defined using 10 μM nicotine or cytisine (50 μM). After a 90 min incubation at 4 °C, reactions were terminated by dilution with 3 ml of ice-cold Krebs-HEPES buffer, followed by filtration through Schleicher and Schuell #32 glass fiber filters (presoaked in 0.5% PEI) using a BRANDEL cell harvester (Biomedical Research and Development Laboratories, Inc., Gaithersburg, MD). Filters were rinsed 3 times with 3 ml of ice-cold Krebs-HEPES buffer and transferred to scintillation vials. Scintillation cocktail (3 ml) was added and radioactivity was determined by liquid β-scintillation spectrometry (Packard model B1600 TR Scintillation Counter, Packard Instrument Company, Inc., Downer’s Grove, IL).
[3H]MLA Binding Assay
Rat brain (minus cortex and cerebellum) was homogenized in 20 vol of ice-cold hypotonic buffer (in mM: 2 HEPES, 14.4 NaCl, 0.15 KCl, 0.2 CaCl2 and 0.1 MgSO4, pH 7.5). Homogenates were incubated at 37 °C for 10 min and centrifuged (29,000g for 17 min at 4°C). Pellets were washed 3 times by resuspension in 20 vol of the same buffer, followed by centrifugation. Final pellets were resuspended in the incubation buffer to yield ~150 μg membrane protein/100 μl. Assays were performed in duplicate, in a final vol of 250 μl of incubation buffer (in mM: 20 HEPES, 144 NaCl, 1.5 KCl, 2 CaCl2, 1 MgSO4 and 0.05% BSA, pH 7.5) initiated by addition of 100 μl membrane suspension to 150 μl of sample containing 2.5 nM [3H]MLA and one of nine analog concentrations (10 nM – 1000μM), and incubated for 2 h at room temperature. Nudicauline was used as a positive control for these experiments. Nonspecific binding was defined in the presence of 1 mM nicotine. Assays were terminated by dilution of samples with 3 ml of ice-cold incubation buffer followed by filtration through Schleicher and Schuell #32 glass fiber filters (presoaked in 0.5% PEI) using the cell harvester. Filters were rinsed and processed as previously described.
[3H]DA Overflow Assay
Coronal slices of rat striata (500 μm, 6–8 mg) were incubated for 30 min at 34 °C in Krebs’ buffer (in mM: 118 NaCl, 4.7 KCl, 1.2 MgCl2, 1.0 NaH2PO4, 1.3 CaCl2, 11.1 α-D-glucose, 25 NaHCO3, 0.11 L-ascorbic acid and 0.004 EDTA, pH 7.4, saturated with 95% O2/5% CO2). Slices were incubated for 30 min in buffer containing 0.1 μM [3H]DA and then transferred to glass chambers and superfused (1 ml/min) with buffer containing nomifensine (10 μM), a DA uptake blocker, and pargyline (10 μM), a monoamine oxidase inhibitor, to ensure that [3H]overflow primarily represented [3H]DA, rather than [3H]metabolites. Following superfusion for 60 min, three samples were collected to determine basal [3H]outflow. Slices were then superfused for 60 min in the absence (0 nM; control) or presence of analog (1 nM–10 μM) to determine the ability of the analog to evoke [3H]overflow using a within-subject design. Each slice was exposed to only one analog concentration, which remained in the buffer until the end of the experiment; all concentrations (including the 0 concentration) were exposed to striatal slices from each rat (i.e., within-subject design). Following 60 min of superfusion, nicotine (10 μM) was added to the buffer and samples collected for 60 min to determine the ability of the analog to inhibit nicotine-evoked [3H]DA overflow. At least one striatal slice in each experiment was superfused for 60 min in the absence of analog, followed by superfusion with 10 μM nicotine to determine nicotine-evoked [3H]DA overflow in the absence of analog (nicotine control).
To determine if the analog inhibitory effects were specific for nicotine, the ability of bPiOI and bPiDDB (1 nM–10 μM) to inhibit electrically-evoked [3H]DA overflow was determined. Each slice was exposed to only one analog concentration and samples collected for 60 min. Electrical stimulation (1 Hz, 2 ms duration for 2 min; total 120 unipolar, rectangular pulses; SD9 stimulator; Grass Instruments, Quincy, MA) was applied and samples collected for 30 min. Stimulation parameters were chosen from a previous parametric analysis to provide [3H]DA overflow similar to that evoked by 10 μM nicotine, and resulting [3H]DA overflow was shown to be calcium-dependent in previous studies (Dwoskin and Zahniser, 1986).
A Schild analysis was performed to determine if the interaction of the representative analog bPiDDB with nAChRs mediating nicotine-evoked DA release was orthosteric or allosteric. Using a repeated measures design, concentration-response curves for nicotine were determined in the absence and presence of one of three bPiDDB concentrations (1, 3 or 10 nM), based on IC50 value for bPiDDB-induced inhibition of nicotine-evoked [3H]DA overflow. Slices from a single rat were superfused for 60 min in both the absence and presence of one of the bPiDDB concentrations, followed by a 60-min period of superfusion in which one of six nicotine concentrations (0.1–100 μM) was added to the buffer.
Experiments were conducted to determine if bPiDDB interacts with α-CtxMII-sensitive nAChRs. The concentration response for α-CtxMII was determined. Slices were superfused for 60 min followed by collection of two 4-min samples (2.4 ml/sample) to determine basal [3H]outflow. Then, slices were superfused for 36 min in the absence (0 nM; control) or presence of α-CtxMII (0.1 nM – 30 nM). Following superfusion in the absence or presence of α-CtxMII, nicotine (10 μM) was added to the buffer and samples collected for 36 min to determine the ability of the α-CtxMII to inhibit nicotine-evoked [3H]DA overflow. In subsequent experiments, striatal slices were superfused for 36 min either with buffer only, a maximal concentration of bPiDDB (10 nM), a maximal concentration of α-CtxMII (1 nM), or to concomitant bPiDDB and α-CtxMII at their maximal inhibitory concentrations. Nicotine (10 μM) was added to the buffer in the absence and presence of bPiDDB and/or α-CtxMII and superfusion continued for 36 min.
[3H]DA Uptake Assay
Since nAChRs modulate DA transporter (DAT) function (Zhu et al., 2007), the ability of the analogs to interact with these receptors to alter DAT function was assessed. Also, since nomifensine was included in the superfusion buffer in [3H]DA overflow assays, potential interactions with DAT would not be detected in the DA release assays. Striatum from an individual rat was homogenized in 20 ml of ice-cold 0.32 M sucrose containing 5 mM NaHCO3 (pH 7.4) with 16 up and down strokes of a Teflon pestle homogenizer (clearance ~0.003 inches). Homogenates were centrifuged at 2000g for 10 min at 4°C and resulting supernatants centrifuged at 20,000g for 17 min at 4°C. Resulting pellets were resuspended in 2.4 ml of ice-cold assay buffer (in mM: 125 NaCl, 5 KCl, 1.5 MgSO4, 1.25 CaCl2, 1.5 KH2PO4, 10 α-D-glucose, 25 HEPES, 0.1 EDTA, 0.1 pargyline and 0.1 ascorbic acid, saturated with 95% O2/5% CO2, pH 7.4). Final protein concentration was ~400 μg/ml, using BSA as a standard. Assays were performed in duplicate in a total volume of 500 μl. Synaptosomes (50 μl containing 20 μg of protein) were added to tubes containing 350 μl of buffer and 50 μl of one of nine concentrations of analog (final concentration, 0.001 nM–100 μM). GBR 12909 was used as a positive control for these experiments. Samples were incubated at 34°C for 5 min and 50μl of 0.1 μM [3H]DA was added. Incubations were continued at 34°C for 10 min. Reactions were terminated by addition of 3 ml of ice-cold assay buffer. Samples were filtered through Schleicher and Schuell #32 glass fiber filters (presoaked with 1 mM catechol buffer). Filters were washed 3 times with ice-cold buffer containing 1 mM catechol, transferred to vials, 10 ml of cocktail added, and radioactivity determined by liquid s-scintillation spectrometry.
Choline Transporter Experiments
Male Fischer 344 rats (250–300 g; Charles River Laboratories, Kingston, NY) were used to allow comparison with previous results obtained with these methods (Lockman et al., 2004). Unpublished results from our laboratory confirmed that results obtained using these assays do not differ significantly between Fischer-344 and Sprague Dawley rats. The in situ rat brain perfusion technique used to evaluate the effect of each bAPi analog on BBB transporter uptake of [3H]choline has been described previously (Lockman et al., 2004). Briefly, a PE-60 catheter filled with heparinized saline (100 units/ml) was placed into the left common carotid artery after ligation of the left external carotid, occipital, and common carotid arteries (common carotid artery ligation was accomplished caudally to the catheter implantation site). The pterygopalatine artery was left open. Rat body temperature was monitored and maintained at 37 °C by a heating pad and feedback device (YSI Indicating Controller, Yellow Springs, Ohio). Buffered physiologic perfusion fluid was titrated to pH 7.4 (~290 mOsm) containing (in mM): 128 NaCl, 2.4 NaPO3, 29.0 NaHCO3, 4.2 KCl, 1.5 CaCl, 0.9 MgCl and 9.0 d-glucose. [3H-Methyl]-choline chloride (80 Ci/mmol; final concentration, 14 nM) and [14C]-sucrose (4.75 mCi/mmol; final concentration, 650 nM) were obtained from Dupont-New England Nuclear (Boston, MA) and added to the perfusion buffer.
Immediately prior to perfusion, the perfusion fluid containing drug was filtered, warmed to 37 °C and gassed with 95% air and 5% CO2. Perfusion fluid was infused into the left carotid artery via infusion pump for 60 sec at 10 ml/min (Harvard Apparatus, South Natick, MA). This level of flow maintained carotid artery pressure at ~120 mm Hg. At time T, rats were decapitated, brain rapidly removed from the skull and perfused hemisphere dissected on ice after removal of the arachnoid membrane and meningeal vessels. Brain regions and perfusion fluid samples were digested overnight at 50 °C in 1 ml of 1.0 M piperidine. Scintillation fluid (10 ml, Scintisafe, Fisher Scientific, Pittsburgh, PA) was added to each vial and dual labeled scintillation counting of brain and perfusate samples accomplished with correction for quench, background and efficiency (LS 6500; Beckman Coulter, Fullerton, CA). Experiments were approved by the Texas Tech Animal Care and Use Committee and were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals (1996).
Behavioral Experiments
Behavioral Apparatus
Nicotine discrimination experiments were conducted using six standard operant conditioning chambers (ENV-008, MED Associates, St. Albans, VT). Each chamber was housed in a sound-attenuating enclosure (ENV-018M, Med Associates) and operated by a personal computer interface (SG-502, MED Associates). A 5 × 4.2 cm opening that allowed access to a recessed food tray was located on the front panel of each operant conditioning chamber. Two metal response levers were located on either side of the food tray 7.3 cm above a metal-grid floor. A 28 V, 3-cm diameter, white cue light was centered 6 cm above each response lever.
Locomotor activity experiments were conducted using an automated Digiscan animal activity monitoring system (AccuScan Instruments, Columbus, OH). The system consisted of 12 clear chambers (42 × 42 × 30 cm) made opaque by attaching sheets of white plastic to their outer surface. Each chamber incorporated a horizontal 16 × 16 grid of photo beam sensors interfaced to a personal computer operating Digipro System software (v. 1.40, AccuScan Instruments); each beam was spaced 2.5 cm apart and 7.0 cm above the chamber floor. Horizontal activity was measured as photo beam interruptions and expressed as total distance traveled (cm).
Drug Discrimination
Adult male Sprague-Dawley rats (250–275 g at the beginning of the experiment; Harlan Industries), food-restricted to 85–90% of normal weight, were trained initially to respond for sucrose pellets (45 mg Noyes Precision Pellets, Research Diets, Inc., New Brunswick, NJ) under a fixed-ratio (FR) 1 schedule of reinforcement. Sprague-Dawley rats were used for these behavioral experiments to permit comparison to our previous work (Neugebauer et al., 2006). Only one lever was presented each daily session, and the lever presented (i.e., left or right) alternated each day. Over subsequent sessions, the FR value required for reinforcement was incremented to a terminal FR 25. Following 2 sessions of responding under the FR 25 schedule, nicotine discrimination training began during daily, 15-min sessions, occurring 6 days per week (Mon–Sat). From this point on, both levers were present in the chamber each session. Rats were trained to discriminate between injections of nicotine (0.2 mg/kg, sc) and saline by reinforcing responding on one lever (i.e., the nicotine-appropriate lever) following nicotine injections, and by reinforcing responding on the other lever (i.e., the saline-appropriate lever) following saline injections. Responding on the incorrect lever was recorded, but had no programmed consequence. Nicotine or saline was administered 5 min prior to each experimental session. After the rats were placed in the chamber, the cue lights were illuminated to signal the onset of the session. For half of the rats, the left lever was designated as the nicotine-appropriate lever and the right lever was designated the saline-appropriate lever; the reverse was true for the other half of the rats. Daily injections of nicotine and saline were administered according to a double-alternation sequence (i.e., NNSSNN or SSNNSS) that was counterbalanced across rats. Training sessions continued until rats met the following acquisition criteria on 8 of 10 consecutive sessions: (1) the first ratio of the session was completed on the injection-appropriate lever with ≤ 3 responses emitted on the incorrect lever; and (2) ≥85% of the total responses emitted on the injection-appropriate lever.
Once acquisition criteria were met, test sessions were conducted twice per week (Tuesday and Friday). Baseline training sessions with the nicotine training dose or saline (random order) were conducted on intervening days. Stimulus conditions during test sessions were identical to training sessions, except that each test session was only 2 min in duration and no reinforcement was available. Prior to testing any novel pretreatments, rats were required to correctly complete 4 test sessions, 2 with nicotine and 2 with saline (counterbalanced order across rats). For all rats (n=6), a dose-response curve for nicotine (0.025, 0.05, 0.1, 0.2 or 0.4 mg/kg, sc) was first determined, followed by antagonism tests that determined the dose-response curves for the noncompetitive nAChR antagonist mecamylamine (0.1, 0.3, 0.56, 1.0 or 3.0 mg/kg, sc), the competitive nAChR antagonist DHβE (0.1, 0.3, 0.56, 1.0 or 3.0 mg/kg, sc) and each of the bAPi analogs (0.19–5.8 μmoles/kg, sc). Each antagonist was administered as a pretreatment 15 min prior to the 0.2 mg/kg training dose of nicotine using a Latin Square design to control for potential dose order effects. The 15-min pretreatment interval and dose range for the bAPi pretreatments was based on prior work from our laboratory (Neugebauer et al., 2006). Finally, substitution tests were conducted in which the 5.8 μmoles/kg dose of each N,N′-alkane-diyl-bis-3-picolinium analog was administered prior to saline to assess whether any of these analogs produced nicotine-like discriminative stimulus effects. Two dependent measures were collected during each test session: (1) percentage of total responses occurring on the nicotine-appropriate lever (calculated as the number of responses on the nicotine-appropriate lever divided by the total number of responses on both levers × 100); and (2) rate of responding in sec (calculated as the total number of responses on both levers divided by 120 sec). Lever selection data were excluded for rats that failed to complete one full ratio (FR 25) during a test session; however, all data were included for analysis of response rates.
Locomotor Activity
Adult male Sprague-Dawley rats (225–250 g at the beginning of the experiment; Harlan Industries) were sensitized initially to the locomotor stimulant effect of nicotine by administering nicotine (0.4 mg/kg, sc) on 21 consecutive days; locomotor activity was monitored for 60 min immediately following each nicotine injection. After completion of this repeated nicotine treatment phase, a test phase commenced on the following day (Day 22). For this phase of the experiment, rats (n=6 per group) were assigned randomly to receive pretreatment with mecamylamine (0.3–3 mg/kg, sc), DHβE (0.3–3 mg/kg, sc) or one of the bAPi analogs (0.58–5.8 μmoles/kg, sc). All doses were administered according to a within-subject Latin Square design to control for potential dose order effects. On the first day of the test phase (Day 22), the first pretreatment dose for a given rat was administered 15 min prior to nicotine (0.4 mg/kg), and locomotor activity was assessed for 60 min after nicotine administration. Over the next two days, rats were injected with nicotine (0.4 mg/kg) only; locomotor activity was assessed only during the second of these two nicotine maintenance days. On the following day (Day 25), the next pretreatment dose was administered; this 3-day cycle was repeated until testing of each analog dose was completed. Each rat was tested with only mecamylamine, DHβE or one of the bAPi analogs.
In a separate group of rats, the effect of each bAPi analog (n=6 per group) given alone following nicotine sensitization (21 days) was also assessed. The procedure was identical to that described above, with the exception that the bAPi analogs were administered prior to saline (no nicotine) on the test days. The dependent measure for locomotor activity experiments was the total horizontal distance traveled (cm). Protocols for all behavioral experiments were in accordance with the NIH Guide for the Care and Use of Laboratory Animals (1996) and were approved by the Institutional Animal Care and Use Committee at the University of Kentucky.
Data Analysis
For analog-induced inhibition of [3H]nicotine and [3H]MLA binding, data are expressed as fmol/mg protein and shown as a percent of control, i.e., specific [3H]nicotine or [3H]MLA binding in the absence of analog. Data were fit by a one-site, variable slope model, using nonlinear least-squares regression, such that Y = Bt + ((Tp − Bt)/(1 + 10) (log IC50 − X) · n), where Y = specific [3H]nicotine or [3H]MLA binding, X = logarithm of analog concentration, Bt and Tp = minimum and maximum specific [3H]nicotine or [3H]MLA binding respectively, log IC50 = log[analog] which decreased [3H]nicotine or [3H]MLA binding by 50%, and n = the pseudo-Hill coefficient. Analog affinity constants (Ki values) were calculated from IC50 values using the Cheng-Prusoff equation.
In [3H]DA overflow assays, fractional release in each sample was expressed as a percentage of total tissue tritium. Total [3H]overflow was calculated by summing the increases of [3H] above the basal [3H]outflow resulting from exposure to the analog, α-CtxMII, nicotine, or the combination, and subtracting the basal [3H]outflow for an equivalent period of analog exposure. Data were expressed as percent of nicotine control and were fitted by nonlinear least-squares regression using a variable slope, sigmoidal function. IC50 values for analog- or α-CtxMII-induced inhibition of nicotine-evoked [3H]DA overflow were determined using PRISM version 4.0 (GraphPad Software, Inc., San Diego, CA). The mechanism by which bPiDDB inhibited nicotine-evoked [3H]DA overflow was determined using Schild analysis. Nicotine concentration response curves in the absence and presence of bPiDDB were generated by nonlinear fit of the data to a sigmoidal dose-response equation (variable slope): response = Bt + (Tp − Bt)/[1 + 10(log EC50 − X) · n], where X is the logarithm of the nicotine concentration and n is the Hill slope. Linear regression was used to generate the slopes and x-intercepts of the lines, which were compared statistically for parallelism. For each experiment, the dose ratio (dr) for each concentration of bPiDDB was calculated as that producing an equivalent response in the absence and presence of bPiDDB. The log of dr −1 was plotted as a function of log of bPiDDB concentration to provide the Schild regression. Data were fit by linear regression, the slope determined, and linearity assessed. The effect of analog on [3H]DA overflow and its ability to inhibit nicotine-evoked [3H]DA overflow were analyzed by one-way repeated-measures analysis of variance (ANOVA), where analog or α-CtxMII concentration was a within-subjects factor (SPSS version 12.0, Chicago, IL). A two-way repeated measures ANOVA was performed to analyze the time course of analog or α-CtxMII inhibition of nicotine-evoked increase in the fractional release. If the two-way ANOVA revealed a significant concentration × time interaction, then one-way repeated measures ANOVAs were performed to determine the specific time points at which a concentration-dependent effect occurred. The ability of analogs to inhibit electrical field stimulation-evoked [3H]DA overflow was analyzed via one-way repeated-measures ANOVA. The inhibitory effect of the combination of bPiDDB and α-CtxMII were compared to the inhibition produced by analog or α-CtxMII alone using a one-way ANOVA. For all analyses, differences were considered significant when p < 0.05
For [3H]DA uptake assays, data are expressed as pmol/min/mg protein as a percent of control ([3H]DA uptake in the absence of analog) and were fit using a one-site, variable slope model, and a non-linear, non-weighted least-squares regression. The log IC50= log[analog] required to inhibit [3H]DA uptake into striatal synaptosomes by 50%. Analog affinity constants (Ki values) were calculated from IC50 values using the Cheng-Prusoff equation.
In the choline transporter experiments, concentrations of [3H]choline in brain and perfusion fluid were expressed as dpm/g brain or dpm/ml perfusion fluid, respectively. [3H]Choline uptake into brain was unidirectional for at least 60 sec; Kin was quantified in single time-point experiments using the equation: Kin = [Q*−VoC*]/C*T], where Q* is the quantity of [3H]tracer in brain (dpm/g) at the end of perfusion, C* is the perfusion fluid concentration of [3H]choline (dpm/ml), T is the perfusion time (sec) and Vo is the extrapolated intercept (T = 0 sec; “vascular volume” in ml/g). In addition, [14C]-sucrose was incorporated into the perfusion fluid as a small molecular weight impermeant vascular marker to verify integrity of the BBB in each experiment. Vascular bound [3H]choline was removed by a brief intravascular wash (15 sec) with tracer-free perfusion fluid. Kin values were converted to an apparent cerebrovascular permeability-surface area product (PS) using the Crone-Renkin equation: PS = −F ln (1−Kin/F). where F is the cerebral perfusion fluid flow. In all instances, PA differed by < 2% from Kin, because F exceeded Kin by > 40-fold. An apparent inhibition constant (Ki) for the analogs was determined from the equation: [(PSo−KD)/(PSi−KD)]=1+Ci/Ki assuming competitive kinetics where PSo is the [3H]choline PS in the absence of competitor, PSi is the [3H]choline PS in the presence of inhibitor, and Ci is the perfusate concentration of inhibitor (250 μM). Apparent Ki was defined as the analog concentration that reduced saturable brain [3H]choline influx by 50% at tracer choline concentration (Cpf ≪ Km).
For behavioral experiments, analyses of variance (ANOVA) for repeated measures, with dose as the within-subjects factor, were conducted on raw data. When the dose factor attained significance, a Dunnett’s post-hoc test was used to compare the effect of each drug dose to the saline control. For the drug discrimination experiment, ED50 and AD50 values (± 95% confidence interval, CI) were determined, where appropriate, using nonlinear regression and were expressed as the dose estimated to produce 50% nicotine-appropriate responding (ED50) or the antagonist dose estimated to reduce levels of nicotine-appropriate responding by the training dose to 50% (AD50). Data were analyzed with SPSS v.12 (SPSS Inc., Chicago, IL, USA) and Prism v.4 (GraphPad Software, Inc., San Diego, CA, USA). Statistical significance was declared at p < 0.05.
RESULTS
Neurochemical Experiments
Inhibition of [3H]Nicotine and [3H]MLA Binding
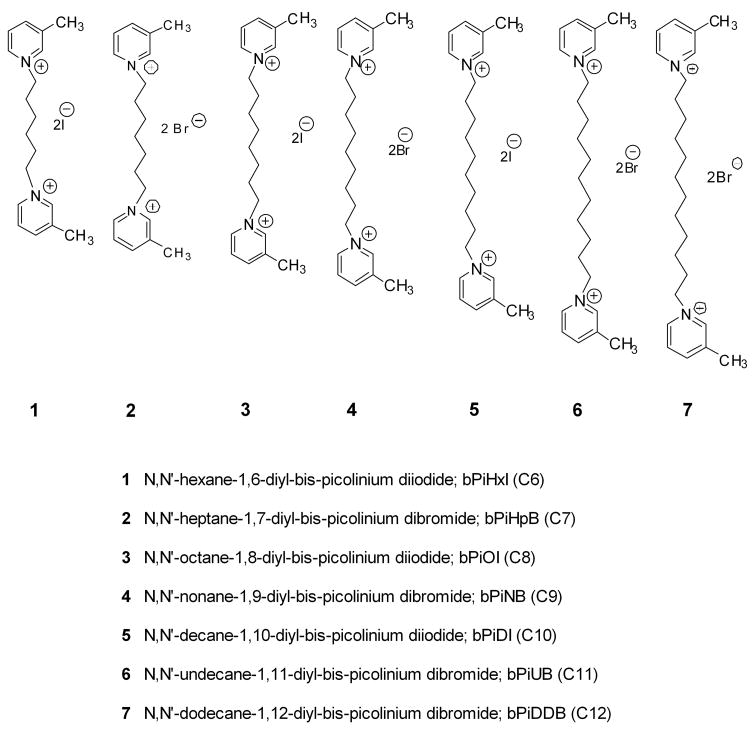
Analogs were evaluated for their ability to inhibit [3H]nicotine and [3H]MLA binding to rat striatal membranes to determine affinity for the ligand binding site onα4β2* and α7* nAChRs, respectively (Fig. 2). The bAPi analogs demonstrated low affinity for both α4β2* and α7* nAChRs (Ki = 49 μM to greater than 100 μM). Cytisine and nudicauline were used as positive controls for the [3H]nicotine and [3H]MLA binding assays, respectively, and were found to have a Ki values of 0.43 ± 0.06 nM and 1.58 ± 0.30 nM, respectively. The classical quaternary ammonium compounds, DEC, TBC and HEX also showed low affinity at the [3H]nicotine binding site (Ki = 5.7, 9.7 and 22 μM, respectively) and at the [3H]MLA binding site (Ki of >100, >100 and 4.8 μM, respectively); results not shown.
Fig. 2. At high concentrations, N,N′-alkane-diyl-bis-3-picolinium (bAPi) analogs inhibit [3H]nicotine binding (top panel) and [3H]MLA binding to rat brain membranes (bottom panel).
Analog abbreviations are provided in Fig. 1. Cytisine and nudicauline were used as a positive control for [3H]nicotine and [3H]MLA binding assays, respectively. Inhibition of the binding of 3 nM [3H]nicotine ([3H]NIC, top panel) or 2.5 nM [3H]MLA (bottom panel) was determined in separate series of experiments. Nonspecific binding was determined in the presence of 10 μM nicotine or cytisine for [3H]nicotine binding assays and 1 mM nicotine for [3H]MLA binding assays. Data are fmol/mg protein expressed as % of control. Control represents [3H]nicotine binding (85.2 ± 6.93 fmol/mg protein; mean ± S.E.M) and [3H]MLA binding (69.6 ± 9.72 fmol/mg protein; mean ± S.E.M), respectively, in the absence of analog; n= 3–5 rats/analog/binding assay. Curves were generated by nonlinear regression.
Inhibition of Nicotine-Evoked [3H]DA Overflow
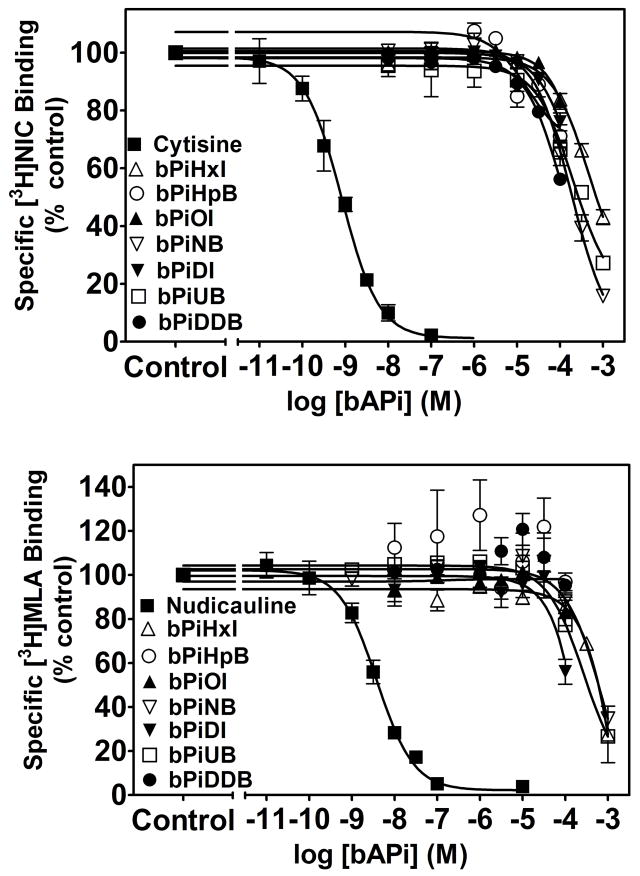
The ability of the analogs to evoke [3H]DA overflow from superfused rat striatal slices preloaded with [3H]DA was determined across a range of analog concentrations (1 nM–10 μM). None of the analogs evoked [3H]DA overflow across this concentration range (Table 1). Concentration response curves for the bAPi analogs (C6–12) to inhibit nicotine-evoked [3H]DA overflow from rat striatal slices are illustrated in Fig. 3 (top panel). With the exception of bPiHxI (C6), which showed no inhibition, one-way ANOVAs revealed significant concentration-dependent inhibition for each analog [bPiHpB (C7), F 7,33 = 15.534, P < 0.001; bPiOI (C8), F5,19 = 4.441, P = 0.007; bPiNB (C9), F5,19 = 4.053, P = 0.011; bPiDI (C10), F5,23 = 7.346, P < 0.001; bPiUB (C11), F5,28 = 11.750, P < 0.001; bPiDDB (C12), F5,23 = 13.282, P < 0.001]. Dunnett’s t-test revealed that bPiHpB (0.001 – 10 μM), bPiOI (1 and 10 μM), bPiNB (10 μM), bPiDI (1 and 10 μM), bPiUB (1 and 10 μM) and bPiDDB (0.001–10 μM) significantly inhibited nicotine-evoked [3H]DA overflow when compared to the within-subject control (i.e., nicotine-evoked [3H]DA overflow in the absence of analog). A wide range of IC50 values (2 nM to greater than 100 μM) was observed among the bAPi analogs (Table 2). The most potent analog, bPiDDB (C12), inhibited nicotine-evoked [3H]DA overflow with an IC50 of 2 nM. bPiHpB (C7) potently inhibited nicotine-evoked DA release with an IC50 value of 60 nM. bPiDI (C10) and bPiOI (C8) exhibited intermediate IC50 values of 180 and 300 nM, respectively; whereas bPiUB (C11) and bPiNB (C9) displayed lower potencies (IC50=1.12 and 5.81 μM, respectively). Thus, the overall rank order of potency for the seven bAPi analogs to inhibit nicotine-evoked [3H]DA overflow was bPiDDB>bPiHpB≫bPiDI=bPiOI≫bPiUB>bPiNB ⋙bPiHxI, revealing a lack of correlation with the length of the methylene groups in the n-alkane linker (r2 = 0.3782, p = 0.1416). Of note, these analogs did not inhibit nicotine-evoked [3H]DA overflow completely, and demonstrated maximal inhibition (Imax) in the range 54–64%.
TABLE 1. N,N′-Alkane-diyl-bis-3-picolinium (bAPi) analogs do not evoke [3H]DA overflow from superfused rat striatal slices.
Rat striatal slices were preloaded with [3H]DA. Superfusion buffer contained pargyline (10 μM) and nomifensine (10 μM). Slices were superfused with various concentrations (1 nM –10 μM) of bAPi analog. Each slice was exposed to only one concentration of analog. Data represent [3H]DA overflow during the 60-min superfusion period in the absence (Control) and presence of analog.
| bAPi Analog | Control | 0.001 μM | 0.01 μM | 0.1 μM | 1 μM | 10 μM |
|---|---|---|---|---|---|---|
| bPiHxI | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0a |
| bPiHpB | 0.09 ± 0.08 | 0.19 ± 0.13 | 0.13 ± 0.06 | 0.14 ± 0.05 | 0.23 ± 0.14 | 0.07 ± 0.04 |
| bPiOI | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0.08 ± 0.06 |
| bPiNB | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| bPiDI | 0 ± 0 | 0 ± 0 | 0.08 ± 0.05 | 0 ± 0 | 0 ± 0 | 0.29 ± 0.12 |
| bPiUB | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0.07 ± 0.06 |
| bPiDDB | 0 ± 0 | 0.14 ± 0.13 | 0.09 ± 0.07 | 0.05 ± 0.02 | 0.13 ± 0.07 | 0.14 ± 0.06 |
Data are mean ± S.E.M. total [3H]DA overflow expressed as a percentage of tissue tritium; n = 5–10 rats/analog.
Fig. 3. N,N′-Alkane-diyl-bis-3-picolinium (bAPi) analogs inhibit nicotine-evoked [3H]DA overflow from rat striatal slices (Top panel).
Analog abbreviations are provided in Fig. 1. Assay buffer contained nomifensine (10 μM) and pargyline (10 μM) throughout superfusion. Slices were superfused in the absence (control) or presence of analog for 60 min prior to nicotine addition to the buffer; superfusion continued for 60 min with nicotine added to the buffer. Control represents [3H]DA overflow in response to 10 μM nicotine (3.12 ± 0.16 total [3H]DA overflow as a percent of tissue [3H]content, mean ± S.E.M.). Data are expressed as % of nicotine control; n = 5–10 rats/analog. Curves for concentration response were generated by nonlinear regression. Time course of bPiDDB-induced inhibition of nicotine-evoked [3H]DA overflow is shown in the bottom panel. Time course data were used to generate [3H]DA overflow data for bPiDDB, shown in the top panel. Slices were superfused with a range of concentrations (1 nM–10 μM) of bPiDDB for 60 min. After collection of the third sample, nicotine (10 μM) was added to the superfusion buffer in the absence (control) and presence of bPiDDB and superfusion continued for 60 min. In each experiment, a control slice was superfused in the absence of bPiDDB and presence of nicotine to determine nicotine-evoked total [3H]DA overflow. The arrow indicates the time point at which nicotine was added to the superfusion buffer. Data are expressed as fractional release as a percent of basal (mean ± S.E.M.), n = 6 rats. Basal was 0.94 ± 0.04 fractional release as % tissue [3H]content.
TABLE 2.
Summary of inhibitory activities of N,N′-alkane-diyl-bis-3-picolinium analogs (bAPi) in [3H]nicotine and [3H]MLA binding assays, [3H]DA overflow assay and [3H]DA uptake assay.
| Compound | [3H]Nicotine Binding Assay Ki (μM) | [3H]MLA Binding Assay Ki (μM) | [3H]DA Overflow Assay |
[3H]DA Uptake Assay Ki (μM) | |
|---|---|---|---|---|---|
| IC50 (μM) | Imax (%) | ||||
| bPiHxI (C6) | >100a | >100 | >100 | - | >100 |
| bPiHpB (C7) | >100 | >100 | 0.06 ± 0.03 | 54 ± 5 | |
| bPiOI (C8) | >100 | >100 | 0.30 ± 0.18 | 57 ± 9 | >100 |
| bPiNB (C9) | 79.9 ± 16.6 | >100 | 5.81 ± 5.07 | 61 ± 11 | >100 |
| bPiDI (C10) | >100 | >100 | 0.18 ± 0.11 | 60 ± 9 | >100 |
| bPiUB (C11) | 69.2 ± 28.9 | >100 | 1.12 ± 0.65 | 62 ± 6 | >100 |
| bPiDDB (C12) | 48.6 ± 17.2 | >100 | 0.002 ± 0.001 | 64 ± 4 | >100 |
Data are mean ± S.E.M.; n = 5–10 rats/analog for [3H]DA overflow assay; n = 3–5 rats/analog for binding and uptake assays.
The time course of the bPiDDB-induced inhibition of nicotine-evoked [3H]DA overflow is illustrated in Fig. 3 (bottom panel). Fractional release evoked by 10 μM nicotine reached a maximum 10 min after the addition of nicotine to the buffer and then gradually decreased, despite the presence of nicotine in the buffer throughout the remainder of the experiment. Repeated measures two-way ANOVA revealed significant main effects of bPiDDB concentration (F5, 25 = 17.605, p < 0.001) and time (F11, 53 = 67.742, p < 0.001), and the concentration × time interaction was also significant (F55, 249 = 1.868, p = 0.001). Post hoc analysis revealed that 0.1, 1 and 10μM bPiDDB significantly inhibited nicotine-evoked fractional DA release across the time period from 15–60 min. Lower bPiDDB concentrations resulted in significant inhibition during a portion of the time course (at 25 min and 30 min with 1 nM; at 15–35 min and 50–55 min with 10 nM).
Field Stimulation-Evoked [3H]DA Overflow
The ability of two representative N,N′-alkane-diyl-bis-3-picolinium analogs, bPiOI (C8) and bPiDDB (C12), to inhibit electrical field stimulation-evoked [3H]DA overflow was determined to assess specificity of the inhibition of nicotine to evoke DA release. Consistent with the previous data, neither bPiOI or bPiDDB evoked [3H]DA overflow prior to electrical stimulation (results not shown). At a single concentration (0.1 μM), bPiOI inhibited electrically-evoked [3H]DA overflow; however, inhibition was not concentration-dependent (Table 3). Importantly, bPiDDB did not inhibit field stimulation-evoked [3H]DA overflow at any concentration examined.
TABLE 3. Effect of C8 and C12 N,N′-alkane-diyl-bis-3-picolinium analogs (bAPi) on field stimulation-evoked [3H]DA overflow from rat striatal slices.
Slices were superfused with a range of concentrations (0.001–10 μM) of analog for 60 min followed by field stimulation (2 min, 1 Hz, 120 pulses) and superfusion continued for 30 min. Superfusion buffer contained nomifensine (10μM) and pargyline (10 μM). Each slice was exposed to only one concentration of analog. Control represents the response to field stimulation in the absence of analog.
| bAPi Analog | Control | 0.001 μM | 0.01 μM | 0.1 μM | 1 μM | 10 μM |
|---|---|---|---|---|---|---|
| bPiOI (C8) | 2.48 ± 0.26 a | 1.87 ± 0.34 | 2.36 ± 0.34 | 1.23 ± 0.18* | 2.45 ± 0.31 | 2.45 ± 0.48 |
| bPiDDB(C12) | 2.75 ± 0.24 | 2.02 ± 0.24 | 2.27 ± 0.36 | 1.72 ± 0.37 | 1.91 ± 0.37 | 2.12 ± 0.42 |
Data are mean ± S.E.M. total [3H]DA overflow expressed as a percentage of tissue tritium. n = 11–12 rats/analog;
p < 0.05 different from control, Dunnett’s post hoc t-tests.
Mechanism of bPiDDB Inhibition of Nicotine-evoked [3H]DA Overflow
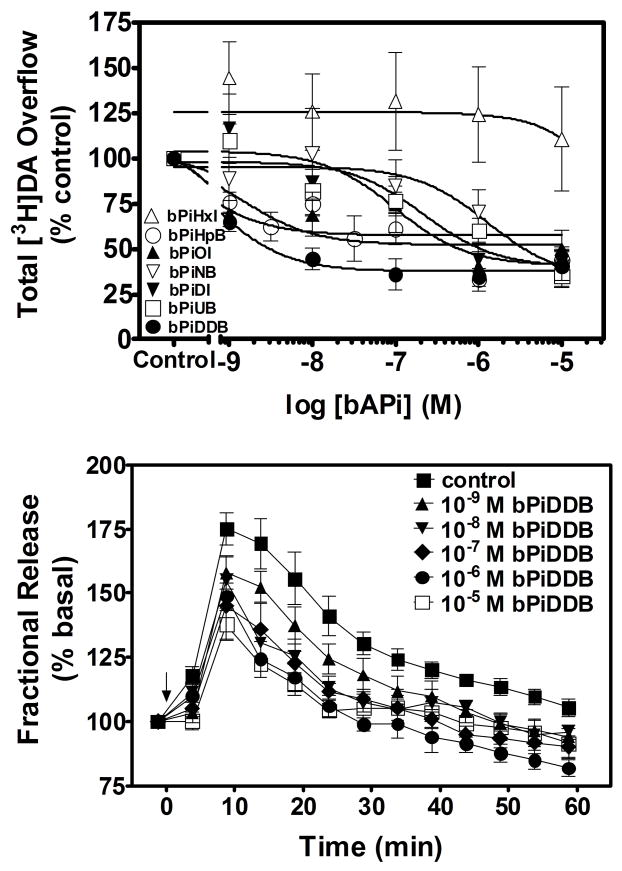
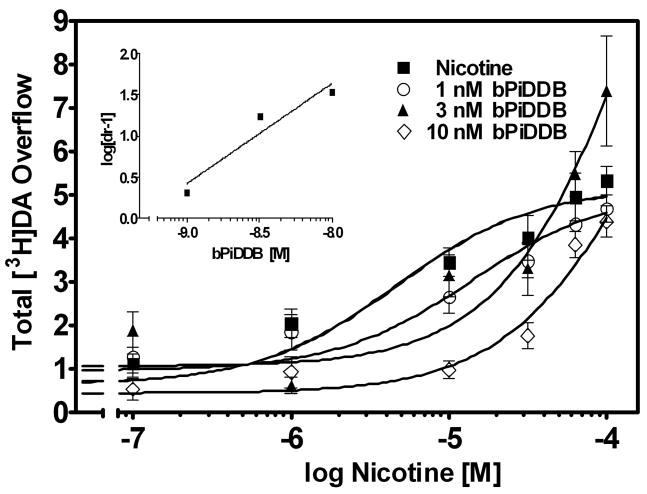
The mechanism of bPiDDB inhibition was assessed, as this analog was the most potent in the bAPi series for inhibition of nicotine-evoked [3H]DA overflow. A Schild analysis was performed, such that for each experiment, striatal slices from a single rat were superfused with each of six concentrations of nicotine (0.1–100 μM) in the absence and presence of one of three concentrations of bPiDDB (1 nM, 3 nM or 10 nM). Rightward shifts in the nicotine concentration-response curve were evident with increasing concentrations of bPiDDB, and inhibition was surmounted by increasing concentrations of nicotine (Fig. 4). The slopes of the linear portions of the curves were not different (F3,196 = 2.150, p = 0.0953), whereas the x-intercepts were different (F3,199 = 11.119, p < 0.0001), indicating that the curves are parallel. A linear fit (r2 = 0.92) to the Schild-transformed data revealed a slope (1.2 ± 0.36) not different from unity (Fig. 4, inset). Further analysis revealed that the dose ratios were independent of response (t 42 = 18.8, p < 0.0001). These results are consistent with bPiDDB acting as an orthosteric inhibitor of nAChRs mediating nicotine-evoked DA release.
Fig. 4. Schild analysis for bPiDDB inhibition of nicotine-evoked [3H]DA overflow from superfused rat striatal slices.
Assay buffer contained nomifensine (10 μM) and pargyline (10 μM) throughout superfusion. After collection of the third sample, slices were superfused with buffer in the absence and presence of bPiDDB (1, 3 or 10 nM) for 60 min prior to the addition of nicotine (0.1 nM–100 μM) to the buffer, and superfusion continued for an additional 60 min. For each nicotine concentration, the control response is that for nicotine in the absence bPiDDB. Data are presented as mean ± S.E.M. of total [3H]overflow during the 60 min exposure to nicotine in the absence and presence of bPiDDB; n = 5 rats. Concentration response curves were generated by nonlinear regression. Fig. 4 inset shows the Schild regression in which the log of dr −1 was plotted as a function of log of bPiDDB concentration. Data were fit by linear regression.
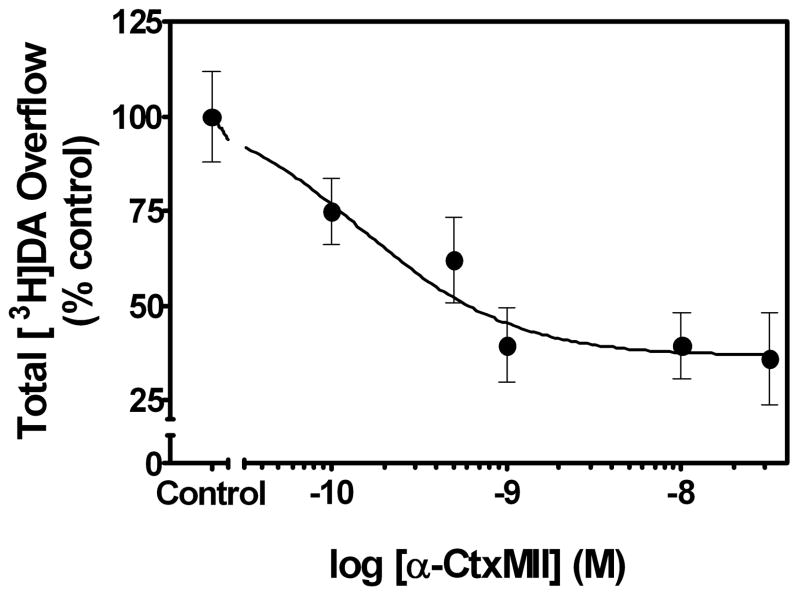
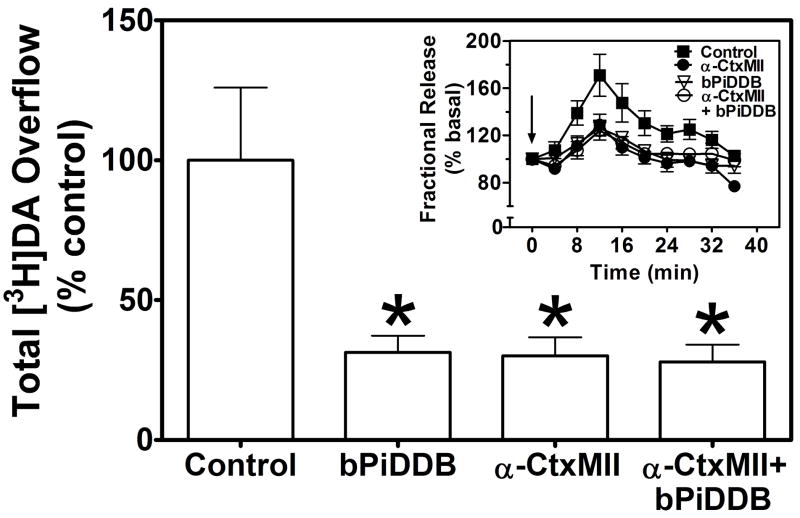
The bAPi analogs produced incomplete inhibition of nicotine-evoked [3H]DA overflow (Imax = 54–64%; Table 2). In order to test the hypothesis that the representative analog, bPiDDB, interacts with nAChR subtypes sensitive to α-CtxMII, experiments were conducted to compare responses to concentrations of bPiDDB and α-CtxMII, which maximally inhibited nicotine-evoked [3H]DA overflow, when each antagonist was presented alone and in combination. Initially, the concentration at which α-CtxMII produced maximal inhibition of nicotine-evoked [3H]DA overflow was determined. Under the current experimental conditions, α-CtxMII inhibited nicotine-evoked [3H]DA overflow in a concentration-dependent manner (F5,15 = 5.65, p < 0.005; IC50= 0.20 ± 0.12 nM, Imax = 62 ± 7.1%; Fig. 5). Post hoc tests revealed that 0.1 – 30 nM α-CtxMII significantly inhibited nicotine-evoked [3H]DA overflow compared to the within-subject control (i.e., nicotine-evoked [3H]DA overflow in the absence of the toxin). Concomitant exposure of rat striatal slices to maximally effective concentrations of bPiDDB (10 nM) and α-CtxMII (1 nM) resulted in significant inhibition of nicotine-evoked [3H]DA overflow compared to the within-subject control (F3,30= 5.27, p < 0.01; Fig. 6). Importantly, inhibition of nicotine-evoked [3H]DA overflow resulting from concomitant exposure was not different (p > 0.05) from that produced by either antagonist alone. The time course of the response to nicotine shows clearly that the inhibition produced by the antagonists presented concomitantly was not different from that following either bPiDDB or α-CtxMII alone (Fig. 6 inset).
Fig. 5. α-Conotoxin MII inhibition of nicotine (10 μM)-evoked [3H]DA overflow from superfused rat striatal slices is concentration-dependent, but incomplete.
The concentration was determined at which α-conotoxin (α-CtxMII) produced maximal inhibition of nicotine-evoked [3H]DA release from rat striatal slices using the current experimental conditions. Assay buffer contained nomifensine (10 μM) and pargyline (10 μM) throughout superfusion. Slices were superfused in the absence (control) or presence of α-CtxMII (0.1 – 30 nM) for 36 min, and superfusion continued for 36 min following addition of nicotine to the buffer. Control represents [3H]DA overflow in response to 10 μM nicotine (1.54 ± 0.18 total [3H]DA overflow as a percent of tissue [3H]content, mean ± S.E.M.). Data are expressed as mean ± S.E.M. of % of nicotine control for n=6 rats and the concentration-response curve was generated using nonlinear regression.
Fig. 6. Concomitant exposure to concentrations of bPiDDB and α-CtxMII produces inhibition of nicotine-evoked [3H]DA overflow not different from that following exposure to either antagonist alone.
Superfusion buffer contained nomifensine (10 μM) and pargyline (10 μM). Maximal inhibitory concentrations of α-CtxMII (1 nM) and bPiDDB (10 nM) were selected from previous concentration response analysis. After collection of the second sample, slices were superfused in the absence or presence of α-CtxMII, bPiDDB or α-CtxMII + bPiDDB for 36 min. Superfusion continued for 36 minutes following addition of nicotine (10 μM) to the buffer. Control represents nicotine-evoked [3H]DA overflow in the absence of antagonist (1.99 ± 0.52 total [3H]DA overflow as a percent of tissue [3H]content, mean ± S.E.M.). Data are expressed as mean ± S.E.M. of % control. * indicates different from control (p < 0.01). n = 8 rats. Inset: Time course of nicotine-evoked [3H]DA overflow in the absence and presence of α-CtxMII, bPiDDB or α-CtxMII + bPiDDB. Data are expressed as fractional release as a percent of basal outflow. Arrow indicates the time point at which nicotine was added to the superfusion buffer.
[3H]DA Uptake into Rat Striatal Synaptosomes
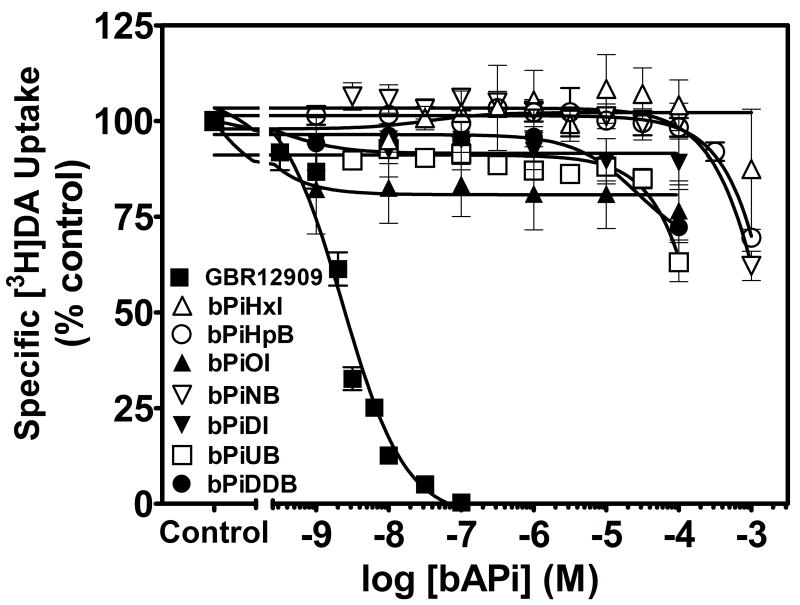
The ability of the bAPi analogs to inhibit [3H]DA uptake via DAT was determined using striatal synaptosomes (Fig. 7). None of bAPi analogs examined inhibited [3H]DA uptake across the concentration range 0.001μM–100 μM, although inhibition of [3H]DA uptake was observed with the positive control, GBR 12909 (Ki = 0.98 ± 0.10 nM). These results indicate that the ability of bAPi analogs to inhibit nicotine-evoked [3H]DA overflow did not result from inhibition of DAT function.
Fig. 7. N,N′-Alkane-diyl-bis-3-picolinium (bAPi) analogs do not inhibit [3H]DA uptake into rat striatal synaptosomes.
Analog abbreviations are provided in Fig. 1. GBR 12909 was used as a positive control for these experiments. Data are pmol/min/mg of specific [3H]DA uptake expressed as % of control; n= 3–4 rats. Control represents [3H]DA uptake in the absence of analog (33.6 ± 1.75 pmol/min/mg protein; mean ± S.E.M.). Nonspecific [3H]DA uptake was determined in the presence of 10 μM nomifensine. Curves were generated using nonlinear regression; analog abbreviations are provided in Fig. 1.
BBB Choline Transporter Experiments
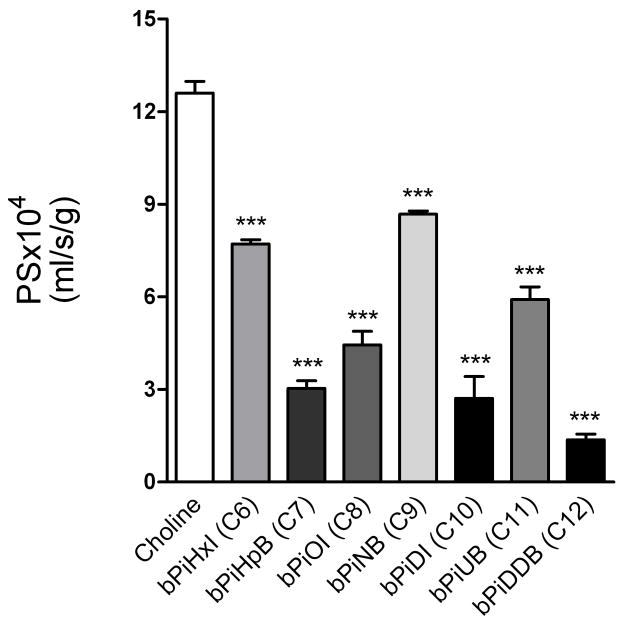
Fig. 8 shows that the addition of 250 μM of each of the bAPi analogs in separate experiments significantly reduced the permeability of [3H]choline (12.6 ± 0.38 × 10−4 mL/s/g). While all of the analogs produced greater inhibition than the natural substrate choline, there was no direct correlation between carbon chain linker length and ability to inhibit [3H]choline permeability. The [3H]choline PS reduction was lowest for bPiHxI (C6;7.7 ± 0.14 × 10−4 mL/s/g), bPiNB (C9;8.7 ± 0.1 × 10−4 mL/s/g), and bPiUB (C11;7.9 ± 0.4 × 10−4 mL/s/g), whereas it was greatest for the C12 chain linker bPiDDB (1.37 ± 0.19 × 10−4 mL/s/g). Intermediate inhibition of [3H]choline permeability was obtained with bPiHpB (C7), bPiOI (C8) and bPiDI (C10). Apparent inhibition constants presented in Table 4 were calculated from the reduction of [3H]choline BBB PS shown in Fig. 8, according to Equation 3, estimating the relative affinity of each analog for the choline transporter. The vascular volume of [14C]-sucrose with a 60-sec perfusion was 0.01 ml/g or less in each experiment.
Fig. 8. Reduction of cortical BBB [3H]choline permeability (PS) in the presence of N,N′-alkane-diyl-bis-3-picolinium (bAPi) analogs (250 μM) using the in-situ rat brain perfusion technique, suggesting interaction with the BBB choline transporter.
Data represent the mean (± SEM) for 3–6 independent observations (***p < 0.001).
TABLE 4.
BBB choline transporter inhibition constants for N,N′-alkane-diyl-bis-3-picolinium (bAPi) analogs.
| Substrate | CHT-Ki (μM) |
|---|---|
| Choline (Km) | 40 ± 3 |
| bPiHxI (C6) | 324 ± 15 |
| bPiHpB (C7) | 60 ± 7 |
| bPiOI (C8) | 123 ± 17 |
| bPiNB (C9) | 430 ± 13 |
| bPiDI (C10) | 79 ± 13 |
| bPiUB (C11) | 164 ± 21 |
| bPiDDB (C12) | 5 ± 3 |
The apparent inhibition constants (Ki) for choline and the bAPi analogs at the BBB choline transporter (CHT). Data represent the mean (± SEM) for 3–6 independent observations.
Behavioral Experiments
Drug Discrimination
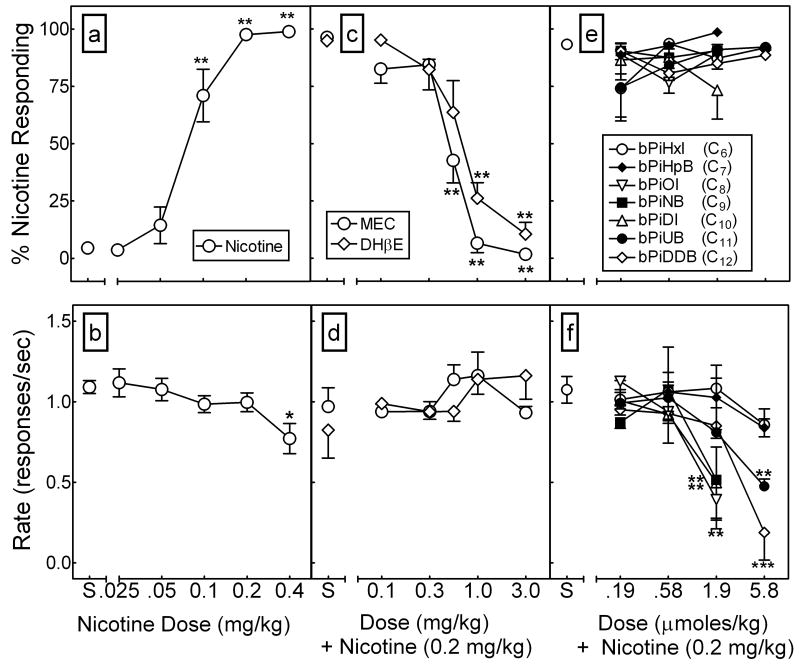
Figs. 9a and 9b illustrate the dose effect of nicotine for percent nicotine-appropriate responding and rate of responding. As expected, nicotine elicited dose-dependent substitution for the nicotine training dose (F5,35 = 61.79, p < 0.001), with an ED50 = 0.08 (0.06–0.09) mg/kg. Nicotine at doses of 0.1, 0.2 and 0.4 mg/kg produced significantly greater levels of nicotine-appropriate responding relative to saline (p < 0.01). Only the highest nicotine dose (0.4 mg/kg) significantly reduced the rate of responding (p < 0.05).
Fig. 9. In contrast to mecamylamine and DHβE, the bAPi analogs do not inhibit the discriminative stimulus effect of nicotine.
Discriminative stimulus and response rate effects of nicotine in rats (n=6) trained to discriminate nicotine (0.2 mg/kg, sc) from saline (panels a and b), and in nicotine-trained rats pretreated with either mecamylamine, DHβE (panels c and d), or bAPi analog (panels e and f). Analog abbreviations are provided in Fig. 1. Data points represent the mean (± SEM) percentage of total responses occurring on the nicotine-appropriate lever (upper panels) and rate of responding (lower panels) as a function of nicotine (panels a and b) or pretreatment (panels c–f) dose. Asterisks indicate a significant difference relative to saline (S) control (*p < 0.05, **p < 0.01).
Figs. 9c and 9d illustrate the dose effect of mecamylamine and DHβE administered 15 min prior to nicotine (0.2 mg/kg) for percent nicotine-appropriate responding and rate of responding. Mecamylamine dose-dependently and completely inhibited nicotine discrimination (F5,35 = 30.23, p < 0.001), with an AD50 = 0.57 (95% CI, 0.49–0.64) mg/kg. Similarly, DHβE dose-dependently and completely inhibited nicotine discrimination (F5,35 = 29.17, p < 0.001), with an AD50 = 0.66 (0.49–0.89) mg/kg. Complete inhibition of the nicotine cue was obtained with both mecamylamine and DHβE at doses that had no significant effect on the rate of responding.
In contrast to mecamylamine and DHβE, Figs. 9e and 9f illustrate that none of the bAPi analogs significantly altered nicotine discrimination, even when doses were administered that disrupted responding. Further, in substitution tests, none of the bAPi analogs at the 5.8 μmoles/kg dose elicited > 8% nicotine-appropriate responding (results not shown), indicating that these analogs lack nicotine-like discriminative stimulus effects.
Locomotor Activity
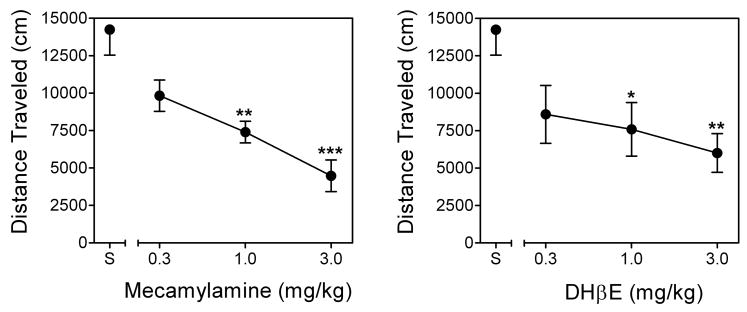
Across the initial 21-day repeated administration period, nicotine-induced hyperactivity increased progressively before reaching asymptotic levels on Day 16 (results not shown). Fig. 10 shows the dose effect of pretreatment with mecamylamine or DHβE on locomotor activity following nicotine administration in rats previously sensitized to the locomotor stimulant effect of nicotine. Nicotine-induced hyperactivity was evident by elevated activity levels in nicotine-treated rats given nicotine alone on the test day (i.e., no mecamylamine or DHβE pretreatment), compared to nicotine-treated rats given saline alone on the test day (results not shown). For mecamylamine, ANOVA revealed a significant main effect of dose (F3,15=11.50, p < 0.01), with 1 and 3 mg/kg of mecamylamine significantly reducing activity relative to saline pretreatment. For DHβE, ANOVA also revealed a significant main effect of dose (F3,15=5.20, p < 0.05), with 1 and 3 mg/kg of DHβE significantly reducing activity relative to saline pretreatment. Thus, acute administration of both of these classical nAChR antagonists inhibited the locomotor stimulant effect of nicotine following repeated nicotine treatment.
Fig. 10. Mecamylamine and DHβE inhibit the locomotor stimulant effect of nicotine.
Mecamylamine (MEC; 0.1–3.0 mg/kg, sc; left panel) or DHβE (0.1–3.0 mg/kg, sc; right panel) pretreatment was given prior to nicotine (0.4 mg/kg) administration in nicotine-sensitized rats. Points represent the mean (± SEM) total distance traveled (cm) as a function of pretreatment dose. Asterisks indicate a significant difference from the corresponding saline (S) control (*p < 0.05, **p < 0.01, ***p < 0.001).
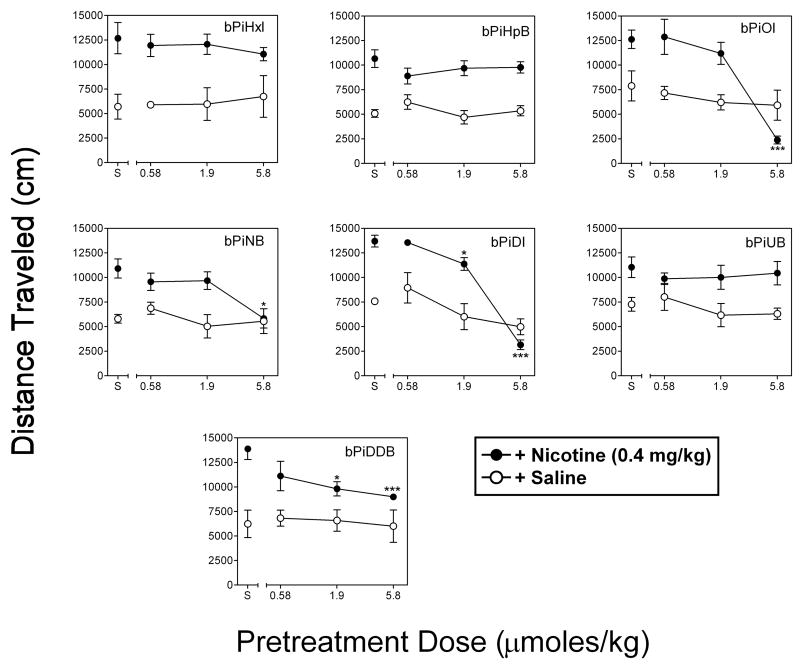
Fig. 11 shows the dose effect of pretreatment with each of the bAPi analogs on activity following nicotine or saline in rats sensitized previously to the locomotor stimulant effect of nicotine. Nicotine-induced hyperactivity was evident as a significant increase in activity in rats given nicotine alone (no analog pretreatment) compared to rats given saline on the test day. Separate ANOVAs of the data from each analog in the presence of nicotine revealed significant main effects of dose for bPiOI pretreatment (F3,15=53.79, p < 0.001), bPiNB pretreatment (F3,15=14.93, p < 0.001), bPiDI pretreatment (F3,15=119.14, p < 0.001), and bPiDDB pretreatment (F3,15=6.86, p < 0.01); there was no significant effect of dose for bPiHxI, bPiHpB or bPiUB. Dunnett’s tests revealed further that pretreatment with 5.8 μmoles/kg of bPiOI, 5.8 μmoles/kg of bPiNB, 1.9 and 5.8 μmoles/kg of bPiDI, and 1.9 and 5.8 μmoles/kg of bPiDDB significantly reduced activity relative to saline (p < 0.05). Within the dose range tested, inhibition of nicotine-induced hyperactivity was greatest with bPiOI and bPiDI. For both of these analogs, 5.8 μmoles/kg significantly decreased activity to a level below that observed in nicotine-sensitized rats given saline on the test day. In contrast to the effect of the analogs tested in the presence of nicotine, separate ANOVAs indicated no significant effects of any of the analogs tested following saline (Fig. 11, open circles).
Fig. 11. bAPi analogs differentially inhibit the locomotor stimulant effects of nicotine.
bAPi analogs (0.58–5.8 μmoles/kg, sc) were given prior to nicotine (0.4 mg/kg) or saline administration in nicotine-sensitized rats. Analog abbreviations are provided in Fig. 1. Points represent the mean (± SEM) total distance traveled (cm) as a function of pretreatment dose. Asterisks indicate a significant difference from corresponding saline (S) control (*p < 0.05, **p < 0.01, ***p < 0.001).
DISCUSSION
The current study describes the development of bAPi analogs with interconnecting alkane C6-C12 linkers as antagonists at nAChRs mediating nicotine-evoked DA release and their effects on nicotine-stimulated behaviors in rats. In contrast to the structurally-related C10 and C12 mono-quaternary nicotinium analogs, which exhibited high affinity for α4β2* nAChRs (Wilkins et al., 2003), the bAPi analogs displayed a lack of interaction at high affinity agonist binding sites on α4β2* orα7* nAChRs. Thus, incorporation of a second cationic head group into the mono-quaternary structure eliminates interaction with high affinity agonist binding sites at α4β2* and α7* nAChRs.
The bAPi analogs potently inhibited nicotine-evoked [3H]DA overflow, displaying a wide range of IC50 values and incomplete maximal inhibition. Rank order of inhibitory potency was bPiDDB>bPiHpB≫bPiDI=bPiOI≫bPiUB>bPiNB⋙bPiHxI. bPiDDB (C12) displayed the highest potency and incomplete maximal inhibition and was regarded as a lead compound for mechanistic evaluation. With the exception of bPiHxI, bAPi analogs were more potent than bis-quaternary ammonium antagonists, HEX and DEC, and the related compound, TBC (Grady et al., 1992). Schild analysis of bPiDDB inhibition revealed rightward shifts in the nicotine concentration-response curves and that increasing nicotine concentrations surmounted the inhibitory effect of bPiDDB. Slopes for the linear portions of the curves did not differ; however, x-intercepts were significantly different, indicating that parallelism was obtained. A linear fit to the Schild-transformed data revealed a slope not different from unity. At high nicotine concentrations (5 mM), mecamylamine inhibits DA release by ~50% (Grady et al., 1992), indicating that part of this effect is mediated by non-nAChR mechanisms; whereas nicotine concentrations of 500 μM were completely inhibited by mecamylamine. Thus, some caution must be exercised with respect to the interpretation of the current results across high nicotine concentrations. Despite this potential caveat, the results are consistent with the hypothesis that bPiDDB inhibits nAChRs mediating nicotine-evoked [3H]DA overflow in an orthosteric manner.
bAPi analogs exhibited incomplete maximal inhibition of nicotine-evoked DA release from rat striatal slices, similar to results reported with α-CtxMII using rat striatal synaptosomes (Kulak et al., 1997), suggesting that at least two subtypes of nAChRs mediate nicotine-evoked DA release. Previous studies using knockout mice have also shown the critical involvement of β2-containing subunits in subtypes mediating nicotine-evoked DA release (Picciotto et al., 1998). Six different β2-containing nAChR subtypes have been suggested to mediate nicotine-evoked DA release in mouse striatum: α4β2* andα4α5β2* (not α-CtxMII-sensitive), and α6β2*,α6β2β3*, α4α6β2* and α4α6β2β3* (α-CtxMII-sensitive) (Salminen et al., 2004). The predominant subtype constituting ~50% of α6-containing nAChRs mediating nicotine-evoked DA release in mouse striatum was α4α6β2β3* (Salminen et al., 2007). Some caution is needed in extrapolating these results from mice to rats, because the impact of nAChRs on catecholamine release in the terminal fields differs between these species (Scholze et al., 2007).
The current results support the interpretation that bPiDDB interacts with α-CtxMII-sensitive, α6β2-containing nAChR subtypes. In the current study, α-CtxMII inhibited (IC50=0.20 nM; Imax=62%) nicotine-evoked DA release from rat striatal slices and was ~5-fold more potent and produced greater inhibition than previously reported (Imax=34–49%, Kulak et al., 1997). Discrepancies between studies may be due to different striatal preparations (slice vs synaptosomes), duration of α-CtxMII exposure, and/or concentration and duration of nicotine exposure. Nevertheless, concomitant exposure of rat striatal slices to maximally effective bPiDDB and α-CtxMII concentrations resulted in inhibition of nicotine-evoked DA release no greater than that produced by either antagonist alone, suggesting that bPiDDB and α-CtxMII inhibit the same nAChR subtypes. The nonselective antagonist, mecamylamine was shown to nearly completely inhibit (Imax=91%) nicotine-evoked DA release (Teng et al., 1997). Thus, these results support the interpretation that similar to α-CtxMII, bPiDDB is a selective high potency antagonist at a subset of nAChR subtypes containingα6 and β2, and likely inhibits α6β2*, α6β2β3*, α4α6β2* and/or α4α6β2β3*.
Although α4- and β2-containing nAChRs have been implicated in nicotine-evoked striatal DA release (Picciotto et al., 1998; Marubio et al., 2003), bAPi analogs showed little affinity for high affinity nicotine binding sites on α4β2* nAChRs. Based on the Schild analysis, bAPi analogs may interact in an orthosteric manner at an alternative, lower affinity nicotine binding sites, perhaps in the channel of α4β2* nAChRs, which would not be detected by [3H]nicotine binding assays. However, results from the interaction study with α-CtxMII suggest that the subtypes mediating nicotine-evoked DA release are α-CtxMII-sensitive, which does not include α4β2* nAChRs.
bAPi analogs also do not appear to interact with α7* nAChRs, as these nAChRs likely are not involved in nicotine-evoked striatal DA release, although an indirect mechanism has been described (Kaiser and Wonnacott, 2000). α7* nAChRs, detected using [125I]α-bungarotoxin binding and immunoprecipitation, comprise only a small percentage of nAChRs in rat striatum (Zoli et al., 2002). Moreover, α7* gene deletion has no effect on nicotine-evoked [3H]DA release from striatal synaptosomes (Salminen et al., 2004). Moreover,α7* antagonists do not inhibit nicotine-evoked [3H]DA release from rat striatal slices (Cao et al., 2005), indicating that α7* nAChRs do not play a major role in mediating this response.
The specificity of bAPi analogs to inhibit nicotine-evoked DA release was assessed by determining their ability to inhibit field-stimulation evoked DA release. Field-stimulation parameters were chosen to provide calcium-dependent DA release in an amount similar to that evoked by nicotine (Dwoskin and Zahniser, 1986). bPiDDB did not inhibit field-stimulation evoked DA release, indicating that inhibition of nicotine-evoked DA release is likely specific for nAChRs. These results indicate that bPiDDB exposure did not result in toxicity at DA neurons, since subsequent field-stimulation evoked DA release was not different from control.
Nicotine has been shown to enhance DAT function (Zhu et al., 2007), and nAChR antagonists may alter DAT function. However, results from synaptosomal [3H]DA uptake assays revealed that bAPi analogs do not inhibit [3H]DA uptake, indicating that bAPi analogs do not alter DAT function.
The current results also show that bAPi analogs have affinity for the BBB choline transporter. Vascular volume following exposure to each analog was shown to be <0.01 ml/g, indicating no BBB disruption (Lockman et al., 2004). bPiDDB exhibited the highest affinity (Ki=5 μM) for the BBB choline transporter. bPiHpB, bPiOI and bPiDI were also potent (Ki<125 μM) and were within the range suggestive of transport into brain via the BBB choline transporter.
Although nonselective nicotinic antagonists have been shown to block nicotine discrimination (Zakharova et al., 2005), bAPi analogs had no effect. Nonselective antagonists block the full complement of β2-containing nAChRs mediating nicotine-evoked DA release, whereas bAPi analogs inhibit only a subset of β2-containing nAChRs, thus partially inhibiting nicotine-evoked DA release. Importantly, however, the discriminative stimulus effect of nicotine does not appear to depend on DA-mediated mechanisms. Pretreatment with DA receptor antagonists does not block the nicotine cue (Corrigall and Coen, 1994; Le Foll et al., 2005). Thus, the difference between nonselective nAChR antagonists and the bAPi analogs in altering the discriminative stimulus effects of nicotine is not likely explained by their differential ability to inhibit nicotine-evoked DA release.
In contrast to the discriminative stimulus effect of nicotine, previous work points to a critical role of DA systems in mediating the locomotor stimulant effect of nicotine. Nicotine-induced hyperactivity is inhibited by local infusion of the DA D2 antagonist eticlopride into nucleus accumbens (Boye et al., 2001) and 6-hydroxydopamine into nucleus accumbens or ventral tegmental area (Louis and Clarke, 1998). Locomotor sensitization produced by repeated nicotine administration is also associated with enhanced nicotine-evoked DA release in NAcc (Benwell and Balfour, 1992). In the current report, both mecamylamine and DHβE reversed nicotine-induced hyperactivity in nicotine-sensitized rats. Similarly, within the dose range tested, bPiOI, bPiNB, bPiDI and bPiDDB decreased nicotine-induced hyperactivity. Since none of the analogs reduced locomotor activity when co-administered with saline in nicotine-sensitized rats, the decrease in nicotine-induced hyperactivity was not likely due to nonspecific motor impairment. Instead, the attenuation in nicotine-induced hyperactivity produced by the bAPi analogs likely reflects a specific blockade of nicotine-evoked DA release. However, this conclusion must be tempered because a drug naive (no nicotine exposure) control group was not included in the experimental design to rule out completely nonspecific motor impairment.
Among the bAPi analogs evaluated, bPiOI and bPiDI administered with nicotine decreased nicotine-induced hyperactivity in sensitized rats to a level below the saline control value. In contrast, bPiOI and bPiDI did not alter activity when administered with saline in sensitized rats. Since both nicotine and nicotine-associated environmental cues may serve as conditioned stimuli (Murray and Bevins, 2007), the novel analogs may have had a greater effect in the presence of nicotine than in the presence of saline because they blocked the compound cue elicited by nicotine and the nicotine-associated locomotor context. This is interesting since both nicotine and nicotine-associated cues elicit nicotine-seeking behavior in rats (Liu et al., 2007). Future experiments should determine whether these novel bAPi analogs would be useful for preventing cue-dependent relapse to tobacco smoking in animal models and clinical populations.
Acknowledgments
The authors thank Agripina G. Deaciuc, Joshua Cutshall, David Eaves, Laura Fenton, Rajendar K. Mattapalli, Lisa Price and Carmen Enid Ruiz for their technical assistance. We also acknowledge the expert consultation of Terry P. Kenakin, Ph.D., William A. Corrigall, Ph.D and David D. Allen, Ph.D.
This research was supported by NIH grants K02 DA00399, T32 DA007304 and U19 DA017548.
Nonstandard Abbreviations
- ANOVA
analysis of variance
- bAPi
N,N′-alkane-diyl-bis-3-picolinium
- BBB
blood brain barrier
- bPiDDB
N,N′-dodecane-1,12-diyl-bis-3-picolinium dibromide
- bPiDI
N,N′-decane-1,10-diyl-bis-3-picolinium diiodide
- bPiHpB
N,N′-heptane-1,7-diyl-bis-3-picolinium dibromide
- bPiHxI
N,N′-hexane-1,6-diyl-bis-3-picolinium diiodide
- bPiNB
N,N′-nonane-1,9-diyl-bis-3-picolinium dibromide
- bPiOI
N,N′-octane-1,8-diyl-bis-3-picolinium diiodide
- bPiUB
N,N′-undecane-1,11-diyl-bis-3-picolinium dibromide
- BSA
bovine serum albumin
- CI
95% confidence intervals
- α-CtxMII
α-Conotoxin MII
- DA
dopamine
- DAT
dopamine transporter
- DEC
decamethonium bromide
- DHβE
dihydro-β-erythroidine
- HEPES
N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid)
- HEX
hexamethonium chloride
- MLA
methyllycaconitine
- nAChR
neuronal nicotinic acetylcholine receptor
- NDDNI
N-n-dodecylnicotinium iodide
- NDNI
N-n-decylnicotinium iodide
- NEcPB
N-n-eicosylpyridinium bromide
- NONI
N-n-octylnicotinium iodide
- NPDPB
N-n-pentadecylpyridinium bromide
- PEI
polyethylenimine
- S.E.M
standard error of the mean
- TBC
d-tubocurarine
- *
indicates putative nAChR subtype assignment
Footnotes
The University of Kentucky holds patents on the N,N′-alkane-diyl-bis-3-picolinium analogs described herein. A potential royalty stream to L.P.D., P.A.C. and J.T.A. may occur consistent with the University of Kentucky policy.
References
- Allen DD, Lockman PR, Roder KE, Dwoskin LP, Crooks PA. Active transport of high-affinity choline and nicotine analogs into the central nervous system by the blood-brain barrier choline transporter. J Pharmacol Exp Ther. 2003;304:1268–74. doi: 10.1124/jpet.102.045856. [DOI] [PubMed] [Google Scholar]
- Azam L, Winzer-Serhan UH, Chen YL, Leslie FM. Expression of neuronal nicotinic acetylcholine receptor subunit mRNAs within midbrain dopamine neurons. J Comp Neurol. 2002;444:260–274. doi: 10.1002/cne.10138. [DOI] [PubMed] [Google Scholar]
- Benwell ME, Balfour DJ. The effects of acute and repeated nicotine treatment on nucleus accumbens dopamine and locomotor activity. Br J Pharmacol. 1992;105:849–856. doi: 10.1111/j.1476-5381.1992.tb09067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boye SM, Grant RJ, Clarke PB. Disruption of dopaminergic neurotransmission in nucleus accumbens core inhibits the locomotor stimulant effects of nicotine and D-amphetamine in rats. Neuropharmacology. 2001;40:792–805. doi: 10.1016/s0028-3908(01)00003-x. [DOI] [PubMed] [Google Scholar]
- Cao Y, Surowy CS, Puttfarcken PS. Different nicotinic acetylcholine receptor subtypes mediating striatal and prefrontal cortical [3H]dopamine release. Neuropharm. 2005;48:72–79. doi: 10.1016/j.neuropharm.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Cartier GE, Yoshikami D, Gray WR, Luo S, Olivera BM, McIntosh JM. A new α-conotoxin which targets α3β2 nicotinic acetylcholine receptors. J Biol Chem. 1996;271:7522–7528. doi: 10.1074/jbc.271.13.7522. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 1994;653:278–284. doi: 10.1016/0006-8993(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Dopamine mechanisms play at best a small role in the nicotine discriminative stimulus. Pharmacol Biochem Behav. 1994;48:817–820. doi: 10.1016/0091-3057(94)90353-0. [DOI] [PubMed] [Google Scholar]
- Cui C, Booker TK, Allen RS, Grady SR, Whiteaker P, Marks MJ, Salminen O, Tritto T, Butt CM, Allen WR, Stitzel JA, McIntosh JM, Boulter J, Collins AC, Heinmann SF. The beta3 nicotinic receptor subunit: a component of alpha-conotoxin MII-binding nicotinic acetylcholine receptors that modulate dopamine release and related behaviors. J Neurosci. 2003;23:11045–11053. doi: 10.1523/JNEUROSCI.23-35-11045.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwoskin LP, Zahniser NR. Robust modulation of [3H]dopamine release from rat striatal slices by D-2 dopamine receptors. J Pharmacol Exp Ther. 1986;239:442–453. [PubMed] [Google Scholar]
- Dwoskin LP, Sumithran SP, Zhu J, Deaciuc AG, Ayers JT, Crooks PA. Subtype-selective nicotinic receptor antagonists: Potential as tobacco use cessation agents. Bioorg Med Chem Lett. 2004;14:1863–1867. doi: 10.1016/j.bmcl.2003.10.073. [DOI] [PubMed] [Google Scholar]
- Gotti C, Moretti M, Clementi F, Riganti L, McIntosh JM, Collins AC, Marks MJ, Whiteaker P. Expression of nigrostriatal alpha6-containing nicotinic acetylcholine receptors is selectively reduced, but not eliminated, by beta 3 subunit gene deletion. Mol Pharmacol. 2005;67:2007–2015. doi: 10.1124/mol.105.011940. [DOI] [PubMed] [Google Scholar]
- Grady SR, Marks MJ, Wonnacott S, Collins AC. Characterization of nicotinic receptor-mediated [3H]dopamine release from synaptosomes prepared from mouse striatum. J Neurochem. 1992;59:848–856. doi: 10.1111/j.1471-4159.1992.tb08322.x. [DOI] [PubMed] [Google Scholar]
- Grinevich VP, Crooks PA, Sumithran SP, Haubner AJ, Ayers JT, Dwoskin LP. N-n-Alkylpyridinium analogs, a novel class of nicotinic receptor antagonists: Selective inhibition of nicotine-evoked [3H]dopamine overflow from superfused rat striatal slices. J Pharmacol Exp Ther. 2003;306:1011–1020. doi: 10.1124/jpet.103.051789. [DOI] [PubMed] [Google Scholar]
- Kaiser S, Wonnacott S. alpha-Bungarotoxin-sensitive nicotinic receptors indirectly modulate [(3)]dopamine release in rat striatal slices via glutamate release. Mol Pharmacol. 2000;58:312–318. doi: 10.1124/mol.58.2.312. [DOI] [PubMed] [Google Scholar]
- Klink R, d’Exaerde AA, Zoli M, Changeux JP. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci. 2001;21:1452–1463. doi: 10.1523/JNEUROSCI.21-05-01452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelle GB. Neuromuscular blocking agents. In: Goodman LS, Gilman A, editors. The Pharmacological Basis of Therapeutic. MacMillan Pub Co; New York: 1975. pp. 575–588. [Google Scholar]
- Kulak JM, Nguyen TA, Olivera BM, McIntosh JM. α-Conotoxin MII blocks nicotine-stimulated dopamine release in rat striatal synaptosomes and slices. J Neurosci. 1997;17:5263–5270. doi: 10.1523/JNEUROSCI.17-14-05263.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Nicotine as a typical drug of abuse in experimental animals and humans. Psychopharmacology. 2005;184:367–381. doi: 10.1007/s00213-005-0155-8. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Sokoloff P, Stark H, Goldberg SR. Dopamine D3 receptor ligands block nicotine-induced conditioned place preferences through a mechanism that does not involve discriminative stimulus or antidepressant effects. Neuropsychopharmacol. 2005;30:720–730. doi: 10.1038/sj.npp.1300622. [DOI] [PubMed] [Google Scholar]
- Liu X, Caggiula AR, Yee SK, Nobuta H, Sved AF, Pechnick RN, Poland RE. Mecamylamine attenuates cue-induced reinstatement of nicotine-seeking behavior in rats. Neuropsychopharmacology. 2007;32:710–718. doi: 10.1038/sj.npp.1301129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockman PR, McAfee JH, Geldenhuys WJ, Allen DD. Cation transport specificity at the blood-brain barrier. Neurochem Res. 2004;29:2245–2250. doi: 10.1007/s11064-004-7032-4. [DOI] [PubMed] [Google Scholar]
- Louis M, Clarke PBS. Effect of ventral tegmental 6-hydroxydopamine lesions on the locomotor stimulant action of nicotine in rats. Neuropharmacology. 1998;37:1503–1513. doi: 10.1016/s0028-3908(98)00151-8. [DOI] [PubMed] [Google Scholar]
- Marubio LM, Gardier AM, Durier S, David D, Klink R, Arroyo-Jimenez MM, McIntosh JM, Rossi F, Champtiaux N, Zoli M, Changeux JP. Effects of nicotine in the dopaminergic system of mice lacking the alpha4 subunit of neuronal nicotinic acetylcholine receptors. Eur J Neurosci. 2003;17:1329–1337. doi: 10.1046/j.1460-9568.2003.02564.x. [DOI] [PubMed] [Google Scholar]
- Murray JE, Bevins RA. Behavioral and neuropharmacological characterization of nicotine as a conditional stimulus. Eur J Pharmacol. 2007;561:91–104. doi: 10.1016/j.ejphar.2007.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer NM, Zhang Z, Crooks PA, Dwoskin LP, Bardo MT. Effect of a novel nicotinic receptor antagonist, N,N′-dodecane-1,12-diyl-bis-3-picolinium dibromide, on nicotine self-administration and hyperactivity in rats. Psychopharmacology. 2006;184:426–434. doi: 10.1007/s00213-005-0163-8. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, Fuxe K, Changeux JP. Acetylcholine receptors containing the β2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Rahman S, Neugebauer NM, Zhang Z, Crooks PA, Dwoskin LP, Bardo MT. The effects of a novel nicotinic receptor antagonist N,N-dodecane1,12-diyl-bis-3-picolinium dibromide (bPiDDB) on acute and repeated nicotine-induced increases in extracellular dopamine in rat nucleus accumbens. Neuropharmacology. 2007;52:755–763. doi: 10.1016/j.neuropharm.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Rahman S, Zhang J, Corrigall WA. Local perfusion of nicotine differentially modulates somatodendritic dopamine release in the rat ventral tegmental area after nicotine preexposure. Neurochem Res. 2004;29:1687–1693. doi: 10.1023/b:nere.0000035803.64724.17. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Levin ED, Stein RM, Ripka GV. Mecamylamine combined with nicotine skin patch facilitates smoking cssation beyond nicotine patch treatment alone. Clin Pharmacol Ther. 1994;56:86–99. doi: 10.1038/clpt.1994.105. [DOI] [PubMed] [Google Scholar]
- Salminen O, Murphy KL, McIntosh JM, Drago J, Marks MJ, Collins AC, Grady SR. Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Mol Pharmacol. 2004;65:1526–1535. doi: 10.1124/mol.65.6.1526. [DOI] [PubMed] [Google Scholar]
- Salminen O, Drapeau JA, McIntosh JM, Collins AC, Marks MJ, Grady SR. Pharmacology of alpha-conotoxin MII-sensitive subtypes of nicotinic acetylcholine receptors isolated by breeding of null mutant mice. Mol Pharmacol. 2007;71:1563–1571. doi: 10.1124/mol.106.031492. [DOI] [PubMed] [Google Scholar]
- Scholze P, Orr-Urteger A, Changeux JP, McIntosh JM, Huck S. Catecholamine outflow from mouse and rat brain slice preparations evoked by nicotinic acetylcholine receptor activation and electrical field stimulation. Br J Pharmacol. 2007;151:414–422. doi: 10.1038/sj.bjp.0707236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng L, Crooks PA, Buxton ST, Dwoskin LP. Nicotinic-receptor mediation of S(−)-nornicotine-evoked [3H]-overflow from rat striatal slices preloaded with [3H]-dopamine. J Pharmacol Exp Ther. 1997;283:778–787. [PubMed] [Google Scholar]
- Watkins SS, Epping-Jordan MP, Koob GF, Markou A. Blockade of nicotine self-administration with nicotinic antagonists in rats. Pharmacol Biochem Behav. 1999;62:743–751. doi: 10.1016/s0091-3057(98)00226-3. [DOI] [PubMed] [Google Scholar]
- Wilkins LH, Haubner A, Ayers JT, Crooks PA, Dwoskin LP. N-n-Alkylnicotinium analogs, a novel class of nicotinic receptor antagonist: Inhibition of S(−)-nicotine-evoked [3H]dopamine overflow from superfused rat striatal slices. J Pharmacol Exp Ther. 2002;301:1088–1096. doi: 10.1124/jpet.301.3.1088. [DOI] [PubMed] [Google Scholar]
- Wilkins LH, Grinevich VP, Ayers JT, Crooks PA, Dwoskin LP. N-n-Alkylnicotinium analogs, a novel class of nicotinic receptor antagonists: Interaction with α4β2* and α7* neuronal nicotinic receptors. J Pharmacol Exp Ther. 2003;304:400–410. doi: 10.1124/jpet.102.043349. [DOI] [PubMed] [Google Scholar]
- Zakharova ES, Danysz W, Bespalov AY. Drug discrimination analysis of NMDA receptor channel blockers as nicotinic receptor antagonists in rats. Psychopharmacology. 2005;179:128–135. doi: 10.1007/s00213-004-2067-4. [DOI] [PubMed] [Google Scholar]
- Zhu J, Bardo MT, Green TA, Wedlund PJ, Dwoskin LP. Nicotine increases dopamine clearance in medial prefrontal cortex in rats raised in an enriched environment. J Neurochem. 2007 Oct 22; doi: 10.1111/j.1471-4159.2007.04951.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Zoli M, Moretti M, Zanardi A, McIntosh JM, Clementi F, Gotti C. Identification of the nicotinic receptor subtypes expressed on dopaminergic terminals in the rat striatum. J Neurosci. 2002;22:8785–8789. doi: 10.1523/JNEUROSCI.22-20-08785.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]