INTRODUCTION
Tobacco Alkaloids and Tobacco Use
Tobacco dependence is a significant health concern and the most preventable cause of death in the United States. Tobacco (Nicotiana tabacum) contains numerous pharmacologically active alkaloids of which nicotine is considered to be the primary alkaloid responsible for tobacco dependence (Balfour, 2002; Pomerleau & Pomerleau, 1992). Studies investigating the effects of minor tobacco alkaloids have demonstrated their pharmacological activity and interaction with nicotinic acetylcholine receptors (nAChRs; Dwoskin, Teng, Buxton, Ravard, Deo, & Crooks, 1995; Papke, Dwoskin & Crooks, 2007; Wei, Sumithran, Deaciuc, Burton, Bush, Dwoskin, & Crooks, 2005). In particular, the minor tobacco alkaloid and nicotine metabolite, nornicotine, likely contributes to tobacco dependence (Crooks & Dwoskin, 1997; Ghosheh, Dwoskin, Li, & Crooks, 1999; Ghosheh, Dwoskin, Miller, & Crooks, 2001) and warrants further investigation.
Nicotinic Receptor-Mediated Dopamine Release in Nicotine Reward
Nicotine acts as an agonist at all known nAChR subtypes. Primarily, nAChRs are located presynaptically, modulating neurotransmitter release (Wonnacott, 1997). Activation of nAChRs on dopamine (DA) terminals in the nucleus accumbens, medial prefrontal cortex, and striatum evokes the release of DA, which mediates the reward produced by nicotine leading to tobacco dependence (Bonci, Bernardi, Grillner, & Mercuri, 2003; Clarke & Pert, 1985; Corrigall, Franklin, Coen, & Clarke, 1992; Dani & De Biasi, 2001; Di Chiara, 2000; Koob, 1992; McGehee & Role, 1995; Pidoplichko, DeBiasi, Williams, & Dani, 1997; Pidoplichko, Noguchi, Areola, Liang, Peterson, Zhang, & Dani, 2004; Pontieri, Tanda, Orzi, & DiChiara, 1996; Spanagel & Weiss, 1999; Stolerman, & Jarvis, 1995; Teng, Crooks, Buxton, & Dwoskin, 1997; Wise, 2000; Wonnacott, 1997; Zhou, Liang, & Dani, 2001; also see Chapter 2, this Volume).
The mesolimbic DA system projects from the ventral tegmental area (VTA) to innervate medium spiny GABAergic neurons in the ventral striatum (Balfour, 2004; Bardo, 1998; Dani, 2003; Dani & Bertrand, 2007; Wonnacott, Sidhpura, & Balfour, 2005). The nucleus accumbens shell is responsible for the gating of primary appetitive stimuli associated with unconditioned drug reward, including nicotine (Bardo, 1998; Di Chiara, Bassareo, Fenu, De Luca, Spina, Cadoni, Acquas, Carboni, Valentini, & Lecca, 2004; Dani & De Biasi, 2001; Koob, 1999; Wise & Bozarth, 1987). The prefrontal cortex mediates secondary conditioned stimuli, i.e., cues paired with the drug of abuse that produces reward expectancy (Berridge & Robinson, 1998; Brody, Mandelkern, Lee, Smith, Sadeghi, Saxena, Jarvik, & London, 2004; Di Chiara et al., 2004; Rose & Behm, 2004; Shima & Tanji, 1998). Integration of this information from the prefrontal cortex occurs in part in the striatum and results in the initiation and execution of movement in reward expectancy and detection of reward (Martin-Soelch, Leenders, Chevalley, Missimer, Kunig, Magyar, Mino, & Schultz, 2001).
Sensorimotor and visual cues (e.g., a lit cigarette) act as secondary conditioned stimuli, which leads to an increase in nicotine self-administration in the dependent individual (Niaura, Shadel, Abrams, Monti, Rohsenow & Sirota, 1998; Perkins, Epstein, Grobe, & Fonte, 1994). Comparable findings have been shown in animal models. For example, rats show increased response rates for nicotine self-administration when nicotine is presented in combination with a secondary visual cue, suggesting that nicotine enhances the sensitivity of the neural system to the reward associated with the secondary stimulus (Caggiula, Donny, White, Chaudhri, Booth, Gharib, Hoffman, Perkins, & Sved, 2002; Palmatier, Evans-Martin, Hoffman, Caggiula, Chaudhri, Donny, Liu, Booth, Gharib, Craven, & Sved, 2006; for a detailed discussion of this research see Chapter 6, this Volume). Presentation of the cue may in itself produce some relatively low level of reward which is enhanced with noncontingent exposure to nicotine (Donny, Chaudhri, Caggiula, Evans-Martin, Booth, Gharib, Clements, & Sved, 2003). The primary reinforcing effects of nicotine depend on nAChR activity and an expectation of a reinforcing effect, while the reinforcing-enhancement effects of nicotine depend only on the acute action of nicotine at nAChRs (Palmatier, Liu, Caggiula, Donny, & Sved, 2007).
Nicotine interacts with the neural circuitry for mediating the experience of natural rewards, leading to habit formation, compulsive use, and ultimately abuse. Research from animal models employing intravenous nicotine self-administration demonstrates that nicotine lowers the threshold for intracranial self-stimulation, thereby increasing reward sensitivity in the neural circuitry responsible for integrating information on natural, biologically-relevant rewards (Kenny & Markou, 2006; Wise, 1996). Neurochemical correlates of the nicotine enhancement of the reinforcing efficacy of both primary and secondary conditioned reward have also been described using fast-scan cyclic voltammetry in rat striatal slices (Rice & Cragg, 2004). Results from these studies show that nicotine enhances DA release during phasic burst firing, but not during tonic neural activity. These findings suggest that nicotine desensitizes nAChRs to suppress DA release during non-reward low firing frequencies and selectively enhances reward-relevant DA release at higher burst-like, tonic firing frequencies. Thus, nicotine is thought to enhance the signal-to-noise when DA neuronal activity switches from tonic to phasic firing, possibly in response to primary rewarding stimuli as well as to conditioned stimuli associated with reward.
In in vivo microdialysis studies, nicotine has been shown to increase extracellular DA in the nucleus accumbens, and this nicotine-induced increase in DA release has been associated with reward (Benwell & Balfour, 1992; Di Chiara et al., 2004; Imperato, Mulas, & Di Chiara, 1986; Rahman, Zhang, & Corrigall, 2004). Additionally, 6-hydroxydopamine lesioning of mesolimbic DA neurons, as well as administration of DA receptor antagonists reduce nicotine self-administration, providing support for a primary role for DA in reward produced by nicotine (Corrigall, 1999; Corrigall & Coen, 1989; Corrigall et al., 1992; Di Chiara, 2000; Singer, Wallace, & Hall, 1982). The nicotine-induced increase in extracellular DA in nucleus accumbens has been shown to be inhibited by systemic or local VTA application of either noncompetitive or competitive nAChR antagonists, e.g., mecamylamine, and dihydro-β-erythroidine (DHβE), respectively, demonstrating the involvement of nAChRs in the VTA in mediating the effect of nicotine to increase DA release in nucleus accumbens (Benwell, Balfour, & Birrell, 1995; Brazell, Mitchell, Joseph, & Gray, 1990; Corrigall, Coen, & Adamson, 1994; Fu, Matta, Gao, & Sharp, 2000; Imperato et al., 1986; Nisell, Nomikos, & Svensson, 1994). Together, these results support a role for nAChR-mediated DA release in the rewarding effects of nicotine.
Nicotinic Receptor Subtypes
Activation of nAChRs modulates presynaptic neurotransmitter release by promoting calcium influx through nAChRs directly, or through subsequent indirect activation of voltage-sensitive calcium channels (Kulak, McIntosh, Yoshikami, & Olivera, 2001; McGehee & Role, 1995; Soliakov & Wonnacott, 1996; Wonnacott, 1997). The existence of 12 genes encoding α2-α10 and β2-β4 subunits leads to enormous complexities in receptor composition, as well as the potential for functional diversity and pharmacological response as a result of the numerous varieties of subunit compositions of nAChRs. nAChRs form pentameric protein structures with a stoichiometry of 2α and 3β (Anand, Conroy, Schoepfer, Whiting & Lindstrom, 1991; Cooper, Couturier & Ballivet, 1991), and these heteromeric nAChRs are the most commonly expressed in the central nervous system (CNS). Of these, α4β2* alone or perhaps in combination with other types of subunits are predominant in the CNS. Note, the asterisk following the subunit designation on nAChRs indicates that the precise composition of subunits is not known for these native receptors.
Immunoprecipitation studies reveal the overlapping CNS distribution of the various types of subunit mRNAs (Deneris, Boulter, Swanson, Patrick, & Heinemann, 1989; Wada, McKinnon, Heinemann, Patrick, & Swanson, 1990; Wada, Wada, Boulter, Deneris, Heinemann, Patrick, & Swanson, 1989). Individual neurons have been identified that express multiple nAChR subtypes and combinations of more than two different subunits can form functional nAChRs (Conroy, Vernallis, & Berg, 1992; Forsayeth & Kobrin, 1997; Poth, Nutter, Cuevas, Parker, Adams, & Luetje, 1997). Studies employing recombinant receptors have shown that when the ratio of subunit pairs is varied, different classes of nAChR subtypes can be formed and their function (e.g., sensitivity to receptor activation) depends on subunit ratio (Lopez-Hernandez, Sanchez-Padilla, Ortiz-Acevedo, Lizardi-Ortiz, Salas-Vincenty, Rojas, & Lasalde-Dominicci, 2004; Zwart & Vijverberg, 1998). Exposure to nicotine can also influence nAChR subtype stoichiometry, function, and maturity in recombinant receptor systems (Corringer, Sallette, & Changeux, 2006; Lopez-Hernandez et al., 2004; Nelson, Kuryatov, Choi, Zhou, & Lindstrom, 2003). In addition, individual nigral DA neurons can be categorized based upon the specific subtype compositions expressed (Azam, Winzer-Serhan, Chen, & Leslie, 2002). Thus, the presence of specific subunit mRNAs, the ratio of the expressed subunits and subtypes in individual DA neurons, and the pharmacological history of the organism are all important to neuronal function and may play an important role in the response to nicotine.
nAChR modulation of neurotransmitter release has been reviewed recently (Dani & Bertrand, 2007; Gotti, Zoli, & Clementi, 2006; also see Chapter 2, this Volume ). In the striatum and nucleus accumbens, heteromeric nAChRs containing the β2 subunit are predominant, and are expressed with either the α4 or α6 subunit (Jones, Bolam & Wonnacott, 2001; Wonnacott, Kaiser, Mogg, Soliakov, & Jones, 2000; Zoli, Moretti, Zanardi, McIntosh, Clementi, & Gotti, 2002). Homomeric pentamers comprised of α7* nAChRs are the second most abundant nAChRs in the brain (Anand et al., 1991; Flores, Rogers, Pabreza, Wolfe, & Kellar, 1992; Wada et al., 1989). Compared to heteromeric nAChRs, homomeric α7* subtypes are not as sensitive to nicotine. α7* nAChRs are located on glutamatergic presynaptic terminals in the VTA and substantia nigra, and as such may play a role in mediating nicotine-evoked DA release and reward (Mansvelder & McGehee, 2000; Wooltorton, Pidoplichko, Broide & Dani, 2003). Moreover, studies using β2 knockout mice implicate β2-containing nAChRs in nicotine-evoked DA release (Grady, Meinerz, Cao, Reynolds, Picciotto, Changeux, McIntosh, Marks, & Collins, 2001; Grady, Murphy, Cao, Marks, McIntosh & Collins, 2002; Picciotto, Zoli, Rimondini, Lena, Marubio, Pich, Fuxe & Changeux, 1998; Scholze, Orr-Urtreger, Changeux, McIntosh, & Huck, 2007; Whiteaker, Marks, Grady, Lu, Picciotto, Changeux & Collins, 2000; Zhou et al., 2001).
nAChR subtypes including α4β2*, α6β2*, and α4α6β2* also have been suggested to mediate the dopaminergic response to nicotine (Champtiaux, Gotti, Cordero-Erausquin, David, Przybylski, Lena, Clementi, Moretti, Rossi, Le Novere, McIntosh, Gardier, & Changeux, 2003). A comprehensive molecular genetics study in which an individual subunit gene (i.e., α4, α5, α7, β2, β3, and β4) was deleted suggested that at least six different nAChR subtypes mediate nicotine-evoked DA release from mouse striatum, including α-conotoxin-MII (α-CtxMII)-sensitive nAChRs (i.e., α6β2β3*, α4α6β2β3* and possibly a small amount of α6β2* or α4α6β2* subtypes) and α-CtxMII-resistant nAChRs (i.e., α4β2* and α4α5β2* subtypes), whereas deletion of β4 and α7 subunits had no effect (Gotti, Moretti, Clementi, Riganti, McIntosh, Collins, Marks, & Whiteaker, 2005; Salminen, Murphy, McIntosh, Drago, Marks, Collins, & Grady, 2004). nAChRs containing α6 and β3 subunits have also been implicated in nicotine-evoked DA release (Cui, Booker, Allen, Grady, Whiteaker, Marks, Salminen, Tritto, Butt, Allen, Stitzel, McIntosh, Boulter, Collins, & Heinemann, 2003; Kuryatov, Olale, Cooper, Choi, & Lindstrom, 2000; Le Novere, Zoli, & Changeux, 1996). Importantly, substantia nigra and VTA neurons express high levels of both α6 and β3 mRNA (Charpantier, Barneoud, Moser, Besnard, & Sgard, 1998; Cui et al., 2003; Deneris et al., 1989; Goldner, Dineley, & Patrick, 1997), consistent with the involvement of subtypes containing these subunits in mediating nicotine-evoked DA release. In a recent study, striatal synaptosomes from α4 and α4/β3 knockout mice were used to isolate nAChRs containing the α6 subunit and determine their involvement in the effects of nicotine on DA release (Salminen, Drapeau, McIntosh, Collins, Marks, & Grady, 2007). Results showed an increased EC50 value (i.e., the concentration required to produce 50% of the maximal agonist effect) for nicotine to evoke DA release with the deletion of the α4 subunit. Furthermore, results from the combined deletion of α4 and β3 subunits showed a 4-7 fold increase in EC50 value compared to deletion of only the α4 subunit. Taken together with previous reports in the literature, these results support the contention that the α4α6β2β3* nAChR subtype constitutes about 50% of α6-containing nAChRs on DA terminals of wild type mice and is the most sensitive to activation by nicotine, which strongly implicates the α4α6β2β3* subtype in nicotine-evoked DA release and nicotine reward.
nAChR Antagonists
Ligands that specifically inhibit nAChR subtypes have been investigated for their ability to inhibit nicotine-evoked [3H]DA release from synaptosomes and brain slices. DHβE is an antagonist at β2-containing nAChR subtypes, whereas methyllycaconitine (MLA) and α-bungarotoxin are relatively selective antagonists for α7* nAChRs (Alkondon, Pereira , Wonnacott, & Albuquerque, 1992; Castro & Albuquerque 1995; Gray, Rajan, Radcliffe, Yakehiro, & Dani, 1996). DHβE has been shown to decrease nicotine self-administration in rats, providing support for the involvement of β2-containing nAChRs in nicotine reward (Grottick, Trube, Corrigall, Huwyler, Malherbe, Wyler, & Higgins, 2000). Peptide neurotoxins isolated from the venom of cone snails have been shown to be selective ligands for a variety of nAChR subtypes (McIntosh, Olivera, & Cruz, 1999; McIntosh, Santos, & Olivera, 1999; Nicke, Wonnacott, & Lewis, 2004). For example, α-conotoxin MII (α-CtxMII) binds α6-containing nAChRs with high affinity and binds α3-containing nAChRs with lower affinity. It is important to note that α6-containing nAChRs comprise 25-30% of the presynaptic nAChRS in rodents, and as much as 70% in non-human primates (Kaiser, Soliakov, Harvey, Luetje, & Wonnacott, 1998; Kulak, Nguyen, Olivera, & McIntosh, 1997; McCallum, Parameswaran, Bordia, McIntosh, Grady, & Quik, 2005; Salminen et al., 2004;). α-CtxMII was found to partially inhibit nicotine-evoked DA release from striatal synaptosomes, supporting the involvement of at least two different subtypes of nAChRs, at least one of which containing α6 and/or α3 subunits (Grady, Grun, Marks, & Collins, 1997; Kulak et al.,1997).
Approved Smoking Cessation Pharmacotherapies
Currently, three smoking cessation pharmacotherapies have been approved by the FDA that validate the development of new therapeutic agents that target nAChRs. These are varenicline, marketed as Chantix®; bupropion, marketed as Zyban® and co-indicated as a treatment for depression in addition to smoking cessation; and finally nicotine replacement therapies (NRT), making use of oral and transdermal delivery. Nicotine replacement therapy provides nicotine to smokers attempting to quit, with the aim of precluding the need for tobacco consumption. Varenicline, has been shown to be a partial agonist at α4β2* nAChRs and a full agonist at α7* nAChRs (Coe, Brooks, Vetelino, Wirtz, Arnold, Huang, Sands, Davis, Lebel, Fox, Shrikhande, Heym, Schaeffer, Rollema, Lu, Mansbach, Chambers, Rovetti, Schulz, Tingley, & O’Neill, 2005; Mihalak, Carroll, & Luetje, 2006). In contrast, bupropion inhibits multiple nAChR subtypes, and also inhibits neurotransmitter transporters resulting in accumulation of extracellular DA in the nucleus accumbens among other effects (Nomikos, Damsma, Wenkstern, & Fibiger, 1989; Nomikos, Damsma, Wenkstern, & Fibiger, 1992; Rauhut, Neugebauer, Dwoskin, & Bardo, 2003; Slemmer, Martin, & Damaj, 2000; Miller, Sumithran, & Dwoskin, 2002; Vann, Rosecrans, James, Philibin, & Robinson, 2006).
Mecamylamine, a noncompetitive antagonist at nAChRs that lacks subtype selectivity, has been shown to reverse the positive and negative subjective effects resulting from nicotine use in smokers (Lundahl, Henningfield, & Lukas, 2000). The use of mecamylamine in combination with nicotine replacement therapies afforded extended smoking cessation outcomes in comparison with a nicotine patch alone (Rose, Behm, Westman, Levin, Stein, & Ripka, 1994). However, a major drawback of this therapy is the non-selective nature of mecamylamine, which leads to unwanted peripherally mediated side-effects such as constipation and dry-mouth that contribute to non-compliance and relapse (Rose et al., 1994; Rose, Westman, Behm, Johnson, & Goldberg, 1999). The above smoking cessation strategies have been shown to be limited in efficacy as indicated by high relapse rates. Thus, there remains a need for new pharmacotherapies that target specific nAChR subtypes that minimize side-effects and relapse (George & O’Malley, 2004; Hurt, Krook, Croghan, Loprinzi, Sloan, Novotny, Kardinal, Knost, Tirona, Addo, Morton, Michalak, Schaefer, Porter, & Stella, 2003; Irvin, Hendricks, & Brandon, 2003).
Novel nAChR Antagonists
Studies from our laboratory have focused on the hypothesis that selective antagonists targeting nAChRs that mediate nicotine-evoked DA release will be clinically effective smoking cessation agents, circumventing unwanted side-effects. Because nicotine interacts with all nAChR subtypes, our discovery of subtype-selective nAChR antagonists was initiated using nicotine as the structural scaffold. Simple addition of an N-n-alkyl group converts nicotine from an agonist to an antagonist, and surprisingly, subtype selectivity began to emerge based on the number of methylene groups in the n-alkyl chain (Ayers, Clauset, Schmitt, Dwoskin, & Crooks, 2005; Crooks, Ayers, Xu, Sumithran, Grinevich, Wilkins, Deaciuc, Allen & Dwoskin, 2004; Dwoskin, Sumithran, Zhu, Deaciuc, Ayers, & Crooks, 2004; Grinevich, Crooks, Sumithran, Haubner, Ayers, & Dwoskin, 2003; Sumithran, Crooks, Xu, Zhu, Deaciuc, Wilkins, & Dwoskin, 2005; Wilkins, Haubner, Ayers, Crooks, & Dwoskin, 2002; Zheng, Bayram, Sumithran, Ayers, Zhan, Schmitt, Dwoskin & Crooks, 2006). In the latter studies we also investigated structural modifications of the cationic nicotinium head group. The analog with the longest carbon chain, N-n-docecyl-nicotinium iodide (NDDNI, C12), was the most potent (IC50 = 9 nM) inhibitor of nicotine-evoked [3H]DA overflow, compared with that of DHβE (IC50 =1.6 μM). The IC50 indicates the analog concentration which decreased nicotine-evoked [3H]DA overflow by 50% of the maximal effect. The results revealed a significant correlation between N-n-alkyl chain length and nicotinium analog-induced inhibition of nicotine-evoked DA release (Wilkins et al., 2002). Unfortunately, with chain lengths of C9-C12, a loss of selectivity was observed, since these compounds also exhibited significant affinity (Ki = 0.23 – 2.1 μM) for α4β2* (Wilkins, Grinevich, Ayers, Crooks, & Dwoskin, 2003). Interestingly, further increases in affinity for the α4β2* nAChR subtype were observed with N-n-alkyl chain lengths of C13-C20 (unpublished data); however, none of the analogs had high affinity for the α7* subtype (Dwoskin et al., 2004; Crooks et al., 2004; Sumithran et al., 2005; Wilkins et al., 2003; Wilkins, Miller, Ayers, Crooks, & Dwoskin, 2006 and unpublished data). Importantly, these N-n-alkylnicotinium analogs exhibited high affinity for the blood-brain barrier choline transporter (Allen, Lockman, Roder, Dwoskin, & Crooks, 2003; Crooks et al., 2004). Subsequent studies with tritiated N-n-octylnicotinium iodide (NONI) showed that this mono-quaternary ammonium compound was actively transported into the CNS via the blood-brain barrier choline transporter. These studies indicate that the above mono-quaternary ammonium compounds penetrate the CNS and are considered brain bio-available molecules.
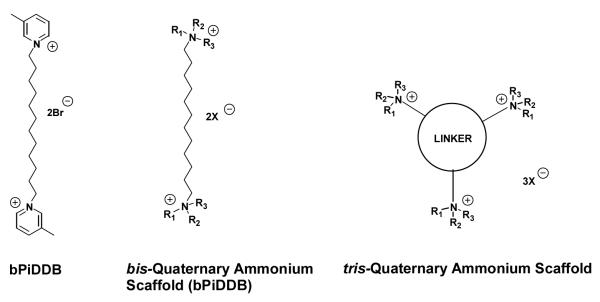
Taking into account the classical discovery that the bis-tri-alkylammonium channel blockers, hexamethonium and decamethonium, exhibited subtype selectivity between ganglionic and muscle type nAChRs, respectively, we adopted a similar approach and generated a sub-library of compounds centered around a bis-nicotinium analog structure and incorporating a variety of head groups and diverse linker units which varied in length, unsaturation, and polarity (Ayers, Dwoskin, Deaciuc, Grinevich, Zhu, & Crooks, 2002; Ayers et al., 2005; Crooks et al., 2004; Dwoskin et al., 2004; Rahman, Neugebauer, Zhang, Crooks, Dwoskin, & Bardo, 2007; Zheng et al., 2006; current Chapter). This approach afforded a new lead compound, N,N’-dodecyl-1,12-diyl-bis-3-picolinium dibromide (bPiDDB), which potently inhibited nicotine-evoked DA release both in vitro and in vivo, and moreover, decreased nicotine self-administration in rats (Ayers et al., 2002; Ayers et al., 2005; Crooks, Ayers, Haubner, Grinevich, Sumithran, Deaciuc, & Dwoskin, 2002; Crooks et al., 2004; Dwoskin et al., 2004; Rahman et al., 2007). The latter studies also showed that bPiDDB had no affinity for either the α4β2* or α7* binding sites in rat brain membranes. Utilizing [14C]-bPiDDB, we have also shown that this compound is brain bio-available and similar to the mono-quaternary ammonium compounds, utilizes the blood-brain barrier choline transporter for active transport into the CNS with similar affinity as the natural substrate choline (Zhang, Lockman, Geldenhuys, Allen, Dwoskin, & Crooks, 2005; Crooks et al., 2004.)
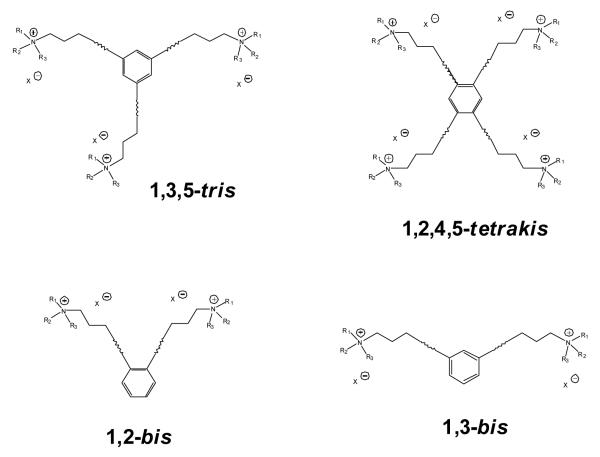
In the evolution of subsequent molecules within this series, we constructed a scaffold incorporating a central phenyl ring from which was appended three identical quaternary ammonium head groups in a 1, 3, 5-orientation, i.e., tris-analogs; these head groups were tethered to the phenyl ring via linker units to afford N-N’ inter-atomic distances approximating that in bPiDDB molecule (Pivavarchyk, Zhang, Crooks & Dwoskin, 2007; Stokes, Dwoskin, Crooks, Jacobs, McIntosh, & Papke, 2007; Zhang, Pivavarchyk, Deaciuc, Dwoskin & Crooks, 2007; current Chapter). The linker units were either saturated or contained unsaturation. Further structural elaboration led to the synthesis of the tetrakis compounds, in which four quaternary ammonium head groups were similarly arranged around a central phenyl ring in a 1, 2, 4, 5-orientation, incorporating saturated or unsaturated linkers. Each of the above sub-libraries, i.e., mono-, bis-, tris- and tetrakis-quaternary ammonium analogs, have provided structure activity (SAR) information of use in developing the antagonist pharmacophore for nAChRs that mediate nicotine-evoked DA release.
The current chapter illustrates the progressive structural approach and gradual improvement in the hit-rate associated with these novel nAChR antagonists. Using this approach, we have determined the ability of these analogs to alter DA release, and moreover, to inhibit nicotine-evoked DA release from superfused rat striatal slices. Additionally, herein, we report results demonstrating the ability of bPiDDB to decrease nicotine-induced reinstatement of nicotine self-administration in order to assess the effectiveness of this lead compound to inhibit nicotine-seeking behavior in rats.
METHODS
Animals
For DA release and behavioral assays, male Sprague-Dawley rats (200 to 225 g) from Harlan Industries (Indianapolis, IN) were used. For blood-brain barrier choline transporter assays, Fischer 344 rats (220-250 g) from Charles River Laboratories (Kingston, N.Y., U.S.A.) were used. Rats had unlimited access to food and water in the home cage, except as noted. Rats were maintained on a 14:10 h light/dark cycle in which the lights came on at 0600 h and went off at 2000 h. All experiments were conducted during the light phase of the cycle. In the behavioral studies, rats were acclimated to the animal colony for at least 5 days and were handled briefly on 3-5 consecutive days prior to the start of the experiment. The Institutional Animal Care and Use Committee of the University of Kentucky approved the conduct of the experiments described herein. The experiments conformed to the guidelines established by the NIH Guide for the Care and Use of Laboratory Animals (1996 Edition).
Synthesis of Analogs
The general strategy for the synthesis of the compounds described involved initial Sonagashira coupling of various halogenated benzenes with 4-pentyn-1-ol. Thus, 1,2-dibromobenzene, 1,3-dibromobenzene or 1,3,5-tribromobenzene was coupled with 4-pentyn-1-ol in the presence of bis-(triphenylphosphine)palladium dichloride and cuprous iodide in triethylamine to afford 1,2-benzene-bis-1-pentyn-5-ol, 1,3-benzene-bis-1-pentyn-5-ol or 1,3,5-benzene-tris-1-pentyn-5-ol, respectively. Due to the sluggish reactivity of 1,2,4,5-tetrabromobenzene under Sonagashira coupling conditions, the alternative synthon, 1,2,4,5-tetraiodobenzene, was utilized to afford the desired 1,2,4,5-benzene-tetrakis-1-pentyn-5-ol. The pentyn-5-ol side chains of these compounds were then either directly transformed into the corresponding pentynyl bromide derivative or catalytically reduced to the corresponding pentan-1-ol side chains, followed by bromination to afford the corresponding pentanyl bromide derivative. Bromination was achieved in high yield utilizing a mild bromination procedure employing triphenylphosphine and carbon tetrabromide. Thus, 4 precursors with pentynyl bromide side chains and 4 precursors with pentanyl bromide side chains were prepared accordingly. Each of these bromide precursors was reacted with a series of azaheterocycles to yield three sub-libraries of bis-, tris-, or tetrakis-quaternary ammonium analogs.
[3H]DA Release Assay
Nicotine-evoked overflow of [3H]DA (28 Ci/mmol, Perkin Elmer, Boston, MA) from striatal slices preloaded with [3H]DA was determined using a previously published method with minor modifications (Grinevich et al., 2003; Sumithran et al., 2005; Wilkins et al., 2002). Briefly, striatal slices were prepared using a McIlwain tissue chopper (Mickle Laboratory Engineering Co Ltd, Surrey, England). Slices were incubated at 34°C in Krebs’ buffer containing 118 mM NaCl, 4.7 mM KCl, 1.2 mM MgCl2, 1.0 mM NaH2PO4, 1.3 mM CaCl2, 11.1 mM α-D-glucose, 25 mM NaHCO3, 0.11 mM L-ascorbic acid, and 0.004 mM ethylenediaminetetraacetic acid (EDTA), pH 7.4, saturated with 95%O2/5%CO2 in a metabolic shaker for 30 min. Slices were transferred to fresh buffer, 0.1 μM [3H]DA added and incubation continued for 30 min. Subsequently, slices were rinsed with Krebs’ buffer and transferred to superfusion chambers maintained at 34°C (Brandel suprafusion system 2500, Gaithersburg, MD) and were superfused (flow rate = 0.6 ml/min) for 60 min with oxygenated Krebs’ buffer containing both nomifensine (10 μM), a DA uptake inhibitor, and pargyline (10 μM), a monoamine oxidase inhibitor. Subsequently, two samples (2.4 ml/sample, sample collection at 4-min intervals) were collected for determination of basal [3H]outflow. Slices were superfused for 36 min in the absence (0 nM; control) or presence of analog (1 nM -10 μM) to determine the effect of the compound alone. Nicotine (10 μM) was added to the buffer; superfusion continued and samples were collected for 36 min to determine the ability of analog to inhibit nicotine-evoked [3H]DA overflow. At the end of the experiment, slices were removed from the chambers and were solubilized with 1 ml of TS-2 tissue solubilizer (Research Products International Corp, Prospect, IL). Scintillation cocktail (4 mL) was added to superfusate and solubilized tissue samples. Radioactivity was determined by liquid scintillation spectrometry using a 1600 TR Tri Carb Liquid Scintillation Analyzer (Packard, Downer’s Grove, IL). Fractional release was determined by dividing the [3H] in each superfusate sample by the tissue-[3H] at the time of collection and expressed as percent of total tissue tritium. Basal [3H]outflow was defined as the mean of fractional release in the two basal samples collected prior to the introduction of analog in the superfusion buffer. Total [3H]overflow was the sum of fractional release above basal following addition of nicotine to the buffer. A one-way ANOVA with Dunnett’s post-hoc analysis was used to determine if analog inhibited nicotine-evoked total [3H]DA overflow.
Nicotine-Induced Reinstatement of Nicotine-Self-Administration
Experiments were conducted in operant conditioning chambers (ENV-001; Med Associates, St Albans, VT, USA), housed in sound-attenuated outer chambers using a Med Associates Interface model SG-503 with MED-IV software. The end walls of the operant conditioning chamber were aluminum, front and back walls were made of clear Plexiglas, and the floor consisted of 18 stainless steel rods (4.8 mm in diameter and placed 1.6 cm apart). Located in the bottom center of one of the end walls was an opening (5 × 4.2 cm) for a recessed food tray, into which a food hopper could dispense sucrose pellets individually. Located on either side of the food tray was a response lever. A 28-V white cue light was located 6 cm above each response lever. An infusion pump (Med-Associates, St. Albans, VT) delivered drug reinforcement via a silastic tube attached to a swivel mounted on the outside of the back wall.
For nicotine self-administration, rats were initially given brief lever-press training for food presentations (45 mg Precision Pellets, Bio-Serv, Frenchtown, NJ). Rats were food deprived to 85% of their ad libitum weights by restricting their intake of rat chow to 8-10 g per day for 5 days. Rats were then briefly trained to press a lever in a two-lever operant chamber using a fixed ratio 1 (FR 1) schedule, which was incrementally increased to a FR 5 across 7 sessions, during 15 min sessions. Rats were given 20 g/day of food following each lever-press training session.
After training for food reinforcement, rats were allowed ad libitum access to food in the home cage for 7 days and were then implanted with an indwelling jugular catheter. Rats were anesthetized by injections of ketamine (80 mg/kg, i.p.) and diazepam (5 mg/kg, i.p.) and a silastic catheter was inserted into the jugular vein. The free end of the catheter exited through the skin and was secured to an acrylic head mount attached to the skull. An infusion pump was attached to the head mount via silastic tubing that was protected by a metal spring leash during the self-administration sessions. The nicotine self-administration procedure was similar to that described previously (Corrigall & Coen, 1989). Following recovery from catheter surgery (7 days), rats were reintroduced to operant conditioning chambers for 60-min daily sessions; food restriction was maintained for the duration of the experiment (17-20 g/day, given in the home cage after the session). Responses made on one lever (active) were recorded and were followed by an infusion of nicotine (0.03 mg/kg/infusion, 100 μl delivered over 5.9 sec), whereas responses made on the other lever (inactive) were recorded, but had no scheduled consequence. The unit dose of nicotine (0.03 mg/kg/infusion) was chosen based on previously published work (Corrigall & Coen, 1989). Nicotine was administered i.v. and dose is expressed as the free base weight. This dose produces optimal responding on a FR schedule with limited access. Completion of the FR requirement resulted in simultaneous activation of the infusion pump and cue lights, which signaled a 20-sec time-out period during which responding on either lever had no consequence. The FR 1 schedule was gradually increased across sessions to a terminal FR 5 schedule. Rats were trained on the FR 5 schedule until stable responding was achieved, defined by the following criteria: (1) minimum of 10 infusions per session; (2) less than 20% variability in active lever responding for 3 consecutive sessions; and (3) minimum of 2:1 (active:inactive) response ratio.
After responding for nicotine stabilized, rats underwent at least 10 extinction sessions during which all cues remained the same and saline was substituted for nicotine. Animals were then assessed for reinstatement following systemic administration of nicotine. Mecamylamine or bPiDDB was administered 5 min prior to nicotine or saline. Animals were placed in the operant chamber 15 min after the nicotine or saline injection in all studies. All reinstatement dose regimens were counterbalanced using a Latin square design. Two separate groups of rats were assessed for nicotine-induced reinstatement (0.2 mg/kg), the effects of mecamylamine on nicotine (0.2 mg/kg)-induced reinstatement, and the effects of bPiDDB pretreatment on nicotine (0.2 mg/kg)-induced reinstatement. Data are expressed as the number of lever presses. Separate two-way ANOVAs were used to assess the effect of mecamylamine and bPiDDB on operant responding. For each ANOVA, dose of antagonist was a within-subject factor. Statistical significance was declared at p<0.05.
RESULTS AND DISCUSSION
[3H]DA Release Assay
Our research efforts have focused on identifying analogs which inhibit nicotine-evoked [3H]DA overflow from superfused rat striatal slices. Of the 283 novel analogs synthesized thus far, 205 analogs have been assayed using a probe concentration of 100 nM to assess inhibition of nicotine-evoked [3H]DA release. We defined a “hit” as an analog that inhibited nicotine-evoked [3H]DA release ≥ 40% at a concentration of 100 nM. Of the 205 analogs tested, 67 inhibited nicotine-evoked [3H]DA release ≥ 40%. Thus, 32% of the analogs tested were considered to be “hits”. An additional 36 analogs (17% of the total number of analogs assessed) inhibited nicotine-evoked [3H]DA release between 30-40%, and were considered to be lower priority “hits”.
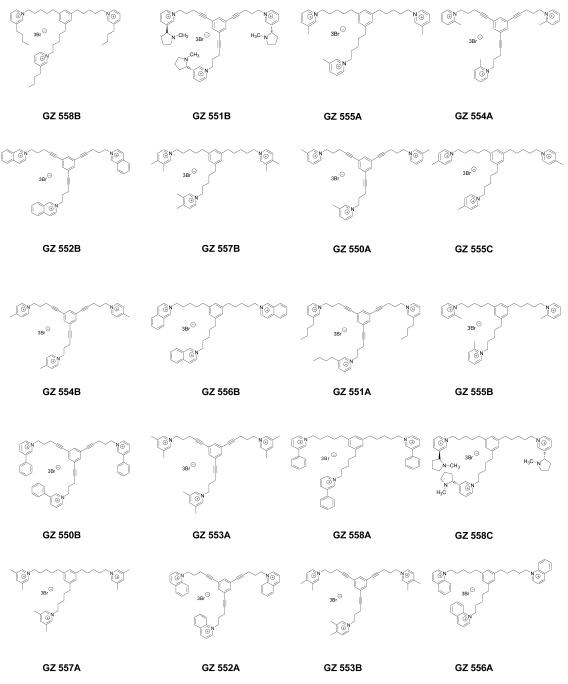
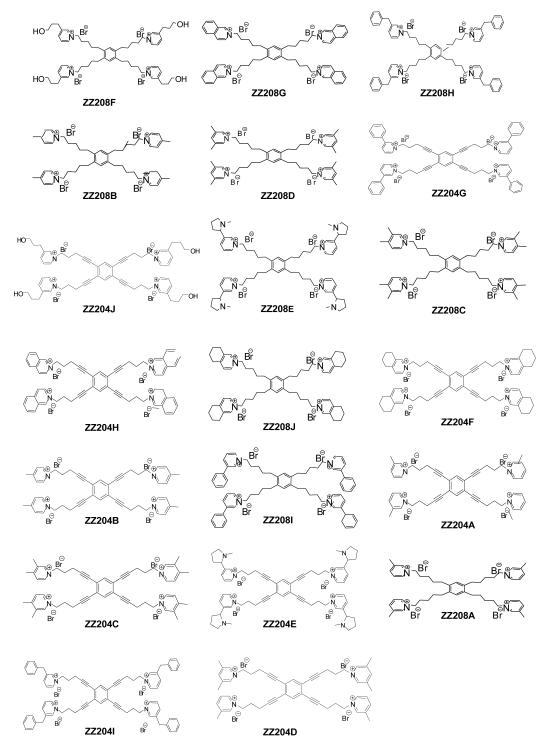
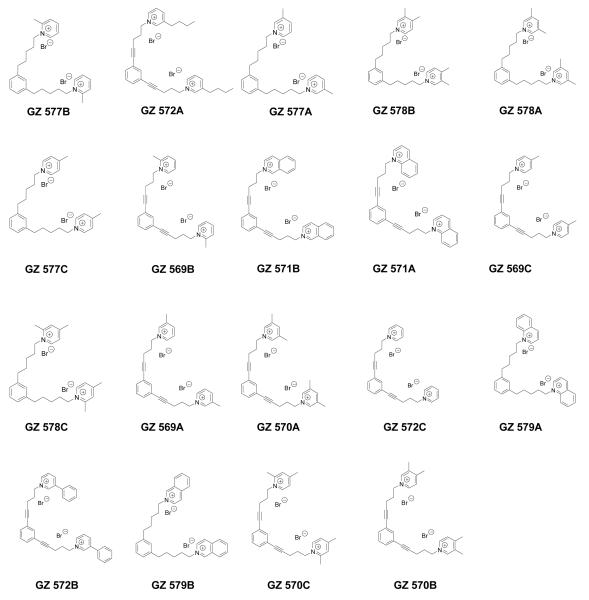
A subset of the above analogs constitutes a new structural motif, in which an additional quaternary ammonium head group is incorporated into the parent bis-quaternary ammonium structure, e.g., bPiDDB, to afford a tris-quaternary ammonium structure (Figure 4.1). These tris-molecules were constructed using a scaffold incorporating a central phenyl ring from which was appended three identical quaternary ammonium head groups in a 1, 3, 5-orientation, and tethered to the phenyl ring via linker units to afford N-N’ inter-atomic distances approximating that in bPiDDB (Figure 4.2; Stokes et al., 2007; Pivavarchyk et al., 2007; Zhang et al., 2007). Also, the linker units were either saturated or unsaturated; the unsaturated linkers incorporating a triple bond conjugated to the central phenyl ring. The rationale was based on the premise that increasing the number of quaternary ammonium head groups from 2 to 3 would result in an increase in the number of ionic interactions with putative negatively charged binding sites on nAChRs mediating nicotine-evoked DA release. GZ 551B and GZ 558C contain nicotinium head groups, whereas other analogs contain the 3-picolinium (GZ 555A and GZ 550A) moiety present in the lead candidate compound, bPiDDB, as well as related isomeric picolinium (GZ 554A, GZ 555B, GZ 554B, GZ 555C), and lutidinium (GZ553A, GZ 553B, GZ 557A, GZ 557B) moieties. More bulky quaternary ammonium head groups also were introduced, such as 4-butyl pyridinium (GZ 558B and GZ 551A), isoquinolinium (GZ 552B and GZ 556B), quinolinium (GZ 552A and GZ 556A), and phenylpyridinium groupings (GZ 550B and GZ 558A). The availability of both the saturated and unsaturated linker units in these tris-quaternary ammonium compounds provides information on the importance of conformational flexibility and the extent of the area of coplanarity in the center of the molecule with respect to the ability of these compounds to interact with critical nAChR binding sites to inhibit nicotine-evoked DA release.
Figure 4.1.
Structure of bPiDDB and the structural scaffolds of bis- and tris-quaternary ammonium analogs.
Figure 4.2.
Structures of tris-quaternary ammonium analogs evaluated in the nicotine-evoked dopamine release assay.
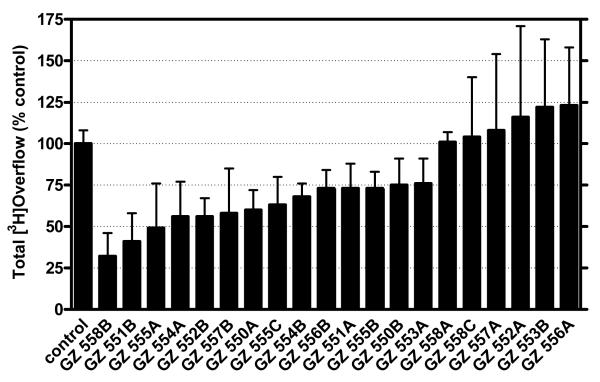
Figure 4.3 shows the tris-analog-induced inhibition of [3H]DA release evoked by 10 μM nicotine. Each of the 20 tris-analogs evaluated was assessed at 100 nM. tris-Analogs afforded decreases in nicotine-evoked [3H]DA release in the range of 24-70% of control (control represents the effect of nicotine in the absence of analog); however, 6 of these analogs, including two quinolinium analogs (GZ 552A and GZ 556A), a 3-phenylpyridinium analog (GZ 558A), a nicotinium analog (GZ 558C), and two isomeric lutidinium analogs (GZ 557A and GZ 553B), did not inhibit nicotine-evoked [3H]DA release. Nine analogs were identified as hits (≥30% inhibition). One commonality in the chemical structure of several of the hits (i.e., GZ 550A, GZ 554A, GZ 554B, GZ 555A, and GZ 555C) is the presence of a 2-, 3- or 4-picolinium head group, with a saturated or unsaturated linker. In terms of SAR, the position of the methyl group in these picolinium analogs did not appear to be critical. However, the 2-picolinium derivative with a saturated linker produced 25% inhibition and was not considered a hit. Generally, inclusion of a second methyl group into the pyridinium ring to afford a lutidinium analog appears to significantly reduce activity. The other four hits had more diversity in head group structure, in that they had either a nicotinium (GZ 551B), a 3,4-lutidinium (GZ 557B), an isoquinolinium (GZ 552B) or a 3-n-butylpyridinium head group (GZ 558B). Of note, the tris-analogs containing quinolinium head groups (GZ 552A and GZ 556A), incorporating either unsaturated or saturated linker units, respectively, produced no inhibition of nicotine-evoked [3H]DA release. Generally, the tris-picolinium analogs were pharmacologically active and the structural relationship to bPiDDB is apparent.
Figure 4.3.
The inhibitory activity of tris-quaternary ammonium analogs in the nicotine (10 μM)-evoked dopamine release assay. Compounds were evaluated at a probe concentration of 100 nM in triplicate, and results are expressed as percentage inhibition compared to control (absence of drug).
The most potent analog, GZ 558B, which contained a tris-3-n-butylpyridinium head group, exhibited 70% inhibition of nicotine-evoked DA release and incorporated a saturated linker connecting the head groups to the 1, 3, and 5 positions of the central phenyl ring. However, if this linker was changed to an unsaturated triple bond containing linker (i.e., GZ 551A), which increases the area of planarity of the central core moiety of the molecule, then inhibition was reduced to 25%. This indicates the importance of conformational flexibility in the linker unit. Surprisingly, the opposite was observed with the unsaturated isoquinolinium analog (GZ 552B), which exhibited about 48% inhibition of nicotine-evoked DA release and contained the more rigid triple bond linker units. When the triple bonds were reduced to give the saturated isoquinolinium analog (GZ 556B), inhibition was reduced to 26%. Similarly, the more rigid nicotinium analog (GZ 551B) was one of the most potent compounds in the series producing 60% inhibition; however, when the triple bonds were reduced to afford the more flexible nicotinium analog (GZ 558C), all inhibitory activity was lost, indicating the important role of linker geometry in combination with the nature of the head group in these molecules. In summary, tris-analog with 3-alkylpyridinium head groups containing saturated linkers exhibit robust inhibitory activity, whereas tris-analogs with quinolinium and nicotinium head groups containing saturated linkers exhibit poor inhibitory activity.
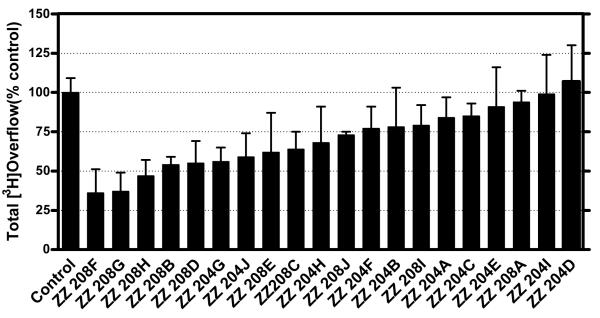
To further investigate the effect of increasing the number of quaternary ammonium head groups in the molecule, a series of novel tetrakis quaternary ammonium analogs was synthesized incorporating quaternary ammonium head groups at the 1, 2, 4, and 5 positions around the central phenyl ring (Figure 4.4). The tetrakis-analogs were pursued in order to increase the number of interactions with anionic binding sites on nAChRs mediating nicotine-evoked DA release. The same approach that was used in the tris-analog series was followed, and new quaternary ammonium head groups were incorporated into the molecules (Figure 4.5). These tetrakis-analogs were evaluated at 100 nM for inhibition of nicotine-evoked DA release. The effect of increasing the head groups from 3 to 4 resulted in a slightly greater number of hits, i.e., a 50% hit rate, compared to the tris series (Figure 4.6). Three of the most potent inhibitors contained fully saturated linker units and incorporated similar head groups to the active tris compounds, i.e., isoquinolinium (ZZ 208G), 4-picolinium (ZZ 208B) and 3,5-lutidinium (ZZ 208D). Additional saturated analogs with new head groups included a 1-([3-hydroxy]propyl)pyridinium analog (ZZ 208F), and a 3-benzylpyridinium analog (ZZ 208H). Other active saturated analogs contained nicotinium (ZZ 208E), and 3,4-lutidinium (ZZ 208C) head groups. Several unsaturated analogs were also active, including the 1-([3-hydroxy]propyl)pyridinium analog (ZZ 204J), the 3-phenylpyridinium analog (ZZ 204G), and the isoquinolinium analog (ZZ 204H). All of the above active saturated linker analogs lost their inhibitory activity on incorporation of a triple bond into the linker units. ZZ 204A, the tetrakis analog of GZ 550A (the most potent tris analog), exhibited only 16% inhibition at 100 nM. However, it must be emphasized that the tetrakis series do not contain within their structure the 1, 3, 5 phenyl substitution pattern of the tris scaffold (Figure 4.4). Thus, the spatial arrangement of the linkers around the central phenyl ring may also be important in determining activity. Nevertheless, it is clear that in the tetrakis series, the more potent compounds have more flexible linkers. Considering the higher number of ”hits” in this series, it will be important to perform full concentration response analyses to fully elucidate the SAR in this promising sub-library of compounds.
Figure 4.4.
Comparative structural scaffolds of tris-, tetrakis-, 1,2-bis-, and 1,3-bis-quaternary ammonium analogs.
Figure 4.5.
Structures of tetrakis-quaternary ammonium analogs evaluated in the nicotine-evoked dopamine release assay.
Figure 4.6.
The inhibitory activity of tetrakis-quaternary ammonium analogs in the nicotine (10 μM)-evoked dopamine release assay. Compounds were evaluated at a probe concentration of 100 nM in triplicate, and results are expressed as percentage inhibition compared to control (absence of drug).
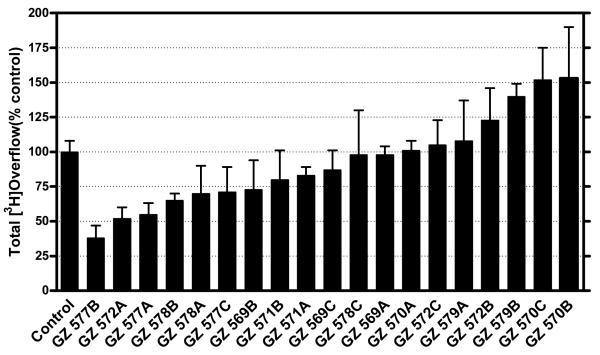
We have also evaluated 1,3-bis-analogs that are fragments of both the tris and tetrakis analogs to determine if the number of head groups and/or the spatial location of the head groups around the phenyl ring are important structural factors (Figure 4.4). In the 1,3-bis series (Figure 4.7), only 4 compounds exhibited inhibition greater than 30% at 100 nM (Figure 4.8). This demonstrates a significant reduction in the number of active compounds (lower “hit” rate) when the third quaternary ammonium head group was removed from the 5-position on the phenyl ring in the tris-series, or when the third and fourth quaternary ammonium head groups were removed from the 2,4-position on the phenyl ring in the tetrakis series (Figure 4.4). Again, this structural change dramatically decreases the hit rate compared to that observed in the tetrakis series. In the 1,3-bis series, three of the most active analogs had a picolinium or lutidinium head group and saturated linker units (i.e., GZ 577A, GZ 577B, GZ578B, Figures 4.7 and 4.8). Of note, GZ 577A is a close structure analog of the lead bis-compound, bPiDDB.
Figure 4.7.
Structures of the 1,3-bis-quaternary ammonium analogs [bis-1,3-(n-pentyl-4-ammonium) benzene and bis-1,3-(n-pent-1-ynyl-4-ammonium) benzene analogs] evaluated in the nicotine-evoked dopamine release assay.
Figure 4.8.
The inhibitory activity of the 1,3-bis-quaternary ammonium analogs [bis-1,3-(n-pentyl-4-ammonium) benzene and bis-1,3-(n-pent-1-ynyl-4-ammonium) benzene analogs] in the nicotine (10 μM)- evoked dopamine release assay. Compounds were evaluated at a probe concentration of 100 nM in triplicate, and results are expressed as percentage inhibition compared to control (absence of drug).
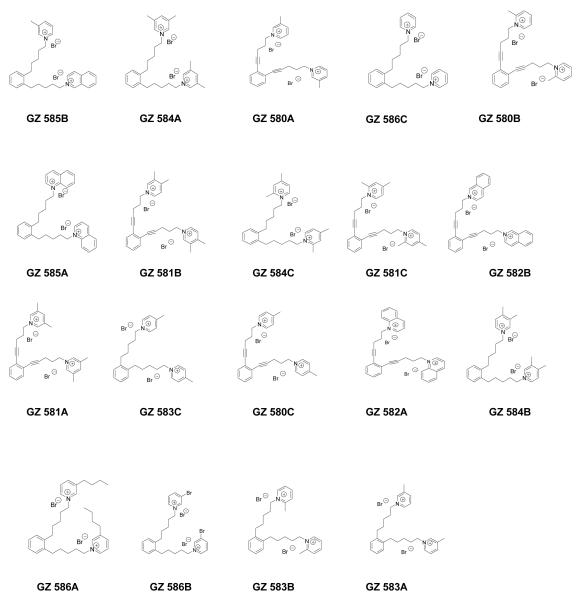
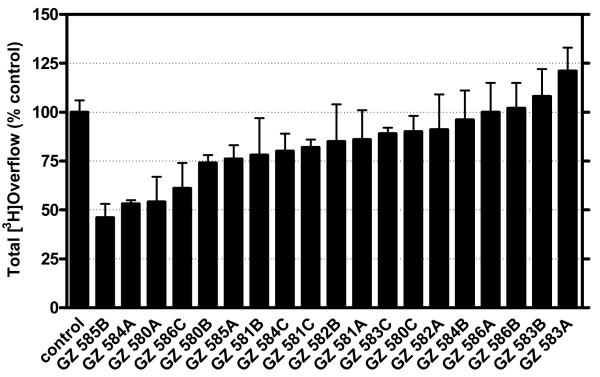
bis-Fragments of the tetrakis analogs containing head groups at the 1 and 2 positions around the central phenyl ring were also evaluated (Figure 4.4). Generally, the same approach was used in the 1,2- bis analogs series as in the 1,3-bis analog series, and new quaternary ammonium head groups were also employed (Figure 4.9). Similarly, the hit rate was also significantly reduced in this series compared to the tetrakis series (Figure 4.10). In the 1,2-bis series of compounds, the most active analogs were those containing isoquinolinium (GZ 585B), 3,5-lutidinium (GZ 584A), 3-picolinium (GZ 508A) and pyridinium (GZ 586C) head groups. Of note, the two most active compounds, GZ 585B and GZ 584A, contained head groups attached to saturated linkers, where the third most active compound, GZ 580A, contained a 3-picolinum head group and an unsaturated linker.
Figure 4.9.
Structures of the 1,2-bis-quaternary ammonium analogs [bis-1,2-(n-pentyl-4-ammonium) benzene and bis-1,2-(n-pent-1-ynyl-4-ammonium) benzene analogs] evaluated in the nicotine-evoked dopamine release assay.
Figure 4.10.
The inhibitory activity of the 1,2-bis-quaternary ammonium analogs [bis-1,2-(n-pentyl-4-ammonium) benzene and bis-1,2-(n-pent-1-ynyl-4-ammonium) benzene analogs] in the nicotine (10 μM)-evoked dopamine release assay. Compounds were evaluated at a probe concentration of 100 nM in triplicate, and results are expressed as percentage inhibition compared to control (absence of drug).
Thus, through an iterative process of structural modification and the use of a rapid through-put assay, we have identified a sub-set of quaternary ammonium molecules which affords a substantially improved hit rate for inhibition of nicotine-evoked DA release. The evolution of this discovery work has clearly indicated that as one progresses from the simple bis-quaternary ammonium compounds, such as bPiDDB, to the more complex tris- and tetrakis-sub-libraries of analogs, one obtains a more diverse selection of lead candidate molecules. A limitation of these SAR investigations is that thus far, only a single concentration of each candidate compound has been investigated, and a clear picture of the structure-activity relationships will only be evident once the full concentration response and IC50 values are generated. Nevertheless, the neurochemical data generated thus far suggests that both the number of quaternary ammonium head groups in the molecule and the spatial location of these head groups are critical, when there is a central planar phenyl core in the molecule. For example, with respect to the importance of spatial location, in the 1,3-bis series, the 2- and 3-picolinium analogs with saturated linkers were highly potent inhibitors of nicotine-evoked DA release (Figure 4.7), whereas the corresponding analogs in the 1,2-bis series were inactive (Figure 4.9). Obtaining IC50 values within these series of compounds, and further elucidating the mechanism of inhibition, will determine the importance of number and type of quaternary ammonium head groups, conformational requirements for the linker units, and the spatial arrangement of the quaternary ammonium moieties around the central phenyl core. These data can be utilized to optimize potency and subtype selectivity of the interaction of these novel nAChR antagonists with nAChRs mediating nicotine-evoked DA release.
Nicotine-Induced Reinstatement of Nicotine-Self-Administration
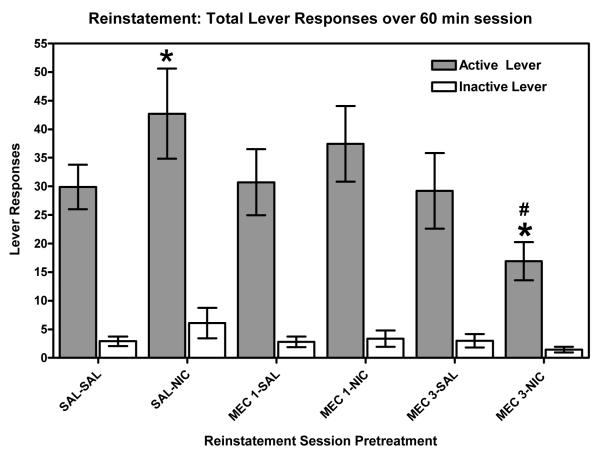
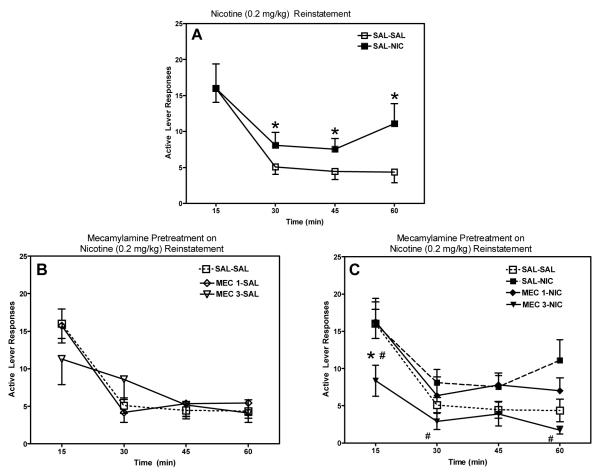
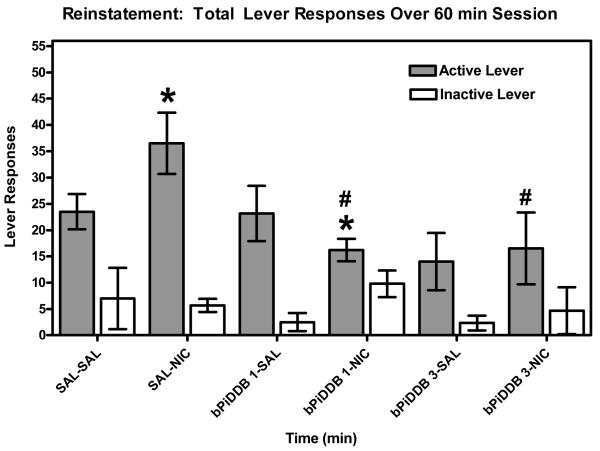
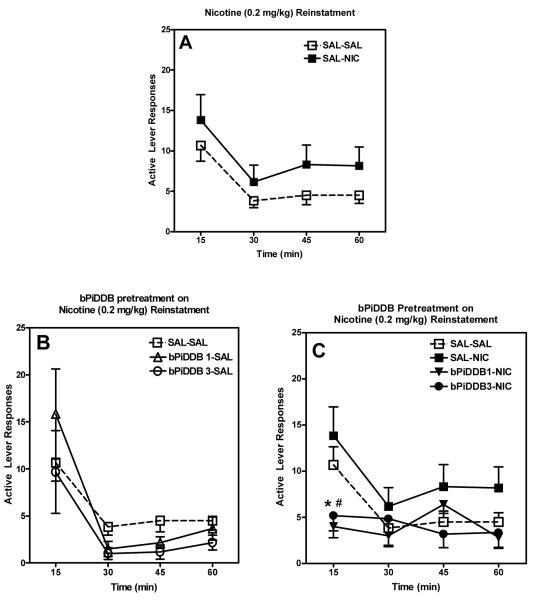
The current study establishes a rodent model of reinstatement in order to assess the potential of novel nicotinic receptor antagonists to attenuate relapse. Two groups of rats were used to assess the ability of a well characterized, classical nAChR antagonist (mecamylamine) and our lead compound (bPiDDB) to attenuate nicotine-induced reinstatement of extinguished nicotine self-administration (i.e., nicotine-seeking behavior). Mecamylamine (3 mg/kg) pretreatment significantly attenuated nicotine (0.2 mg/kg)-seeking behavior, while having no effect when administered alone (Figure 4.11). A time-course analysis of nicotine-induced reinstatement revealed that the reinstatement was more pronounced during the latter portion of the session, and that this effect was attenuated by mecamylamine (Figure 4.12). Nicotine (0.2 mg/kg) administration significantly increased responding at 30, 45, and 60 min time points compared to responding observed following administration of saline. Mecamylamine (3 mg/kg) pretreatment reduced responding at 15 min compared to saline-saline and saline-nicotine (0.2 mg/kg) groups, as well as significantly attenuating the nicotine-induced increase in responding at 30 and 60 min. Similar to the results observed following mecamylamine administration, pretreatment with bPiDDB significantly attenuated nicotine (0.2 mg/kg)-induced drug seeking across the 60-min session at both doses tested (1 and 3 mg/kg; Figure 4.13). A time-course analysis also revealed that bPiDDB (1 and 3 mg/kg) pretreatment significantly decreased the number of active lever responses during the first 15 min of the reinstatement session compared to saline-saline and saline-nicotine (0.2 mg/kg) groups (Figure 4.14). Interestingly, these doses of bPiDDB have been shown previously to decrease nicotine self-administration acutely (Neugebauer, Zhang, Crooks, Dwoskin, & Bardo, 2006). In addition, these results showing an attenuation of nicotine-induced reinstatement of nicotine seeking by both mecamylamine and bPiDDB extend previous work showing that mecamylamine attenuates cue-induced reinstatement (Liu, Caggiula, Yee, Nobuta, Sved, Pechnick, & Poland, 2007). Taken together, the effectiveness of bPiDDB in decreasing both nicotine self-administration (Neugebauer et al., 2006) and nicotine-stimulated reinstatement (current study) suggest that nicotinic receptor antagonists may be a useful tobacco dependence pharmacotherapy to reduce relapse.
Figure 4.11.
Mean (±SEM) number of responses on the active and inactive levers for each of six treatment conditions. SAL=saline; NIC=nicotine, 0.2 mg/kg; MEC 1= mecamylamine, 1 mg/kg; and MEC 3= mecamylamine, 3 mg/kg. * indicates significant difference from SAL-SAL active lever (p<0.05). # indicates significant difference from SAL-NIC active lever (p<0.05).
Figure 4.12.
Mean (±SEM) number of responses on the active lever for each treatment condition across 15-min time intervals within the session. Panel A: effect of nicotine (0.2 mg/kg) alone; Panel B: effect of mecamylamine (1 or 3 mg/kg) alone; and Panel C: effect of nicotine (0.2 mg/kg) in the presence and absence of mecamylamine (1 or 3 mg/kg). SAL=saline; NIC=nicotine, 0.2 mg/kg; MEC 1= mecamylamine, 1 mg/kg; and MEC 3= mecamylamine, 3 mg/kg. *indicates significant difference from SAL-SAL active lever (p<0.05). # indicates significant difference from SAL-NIC active lever (p<0.05).
Figure 4.13.
Mean (±SEM) number of responses on the active and inactive levers for each of six treatment conditions. SAL=saline; NIC=nicotine, 0.2 mg/kg; bPiDDB 1 indicates 1 mg/kg; and bPiDDB 3 indicates 3 mg/kg. *indicates significant difference from SAL-SAL active lever (p<0.05). # indicates significant difference from SAL-NIC active lever (p<0.05).
Figure 4.14.
Mean (±SEM) number of responses on the active lever for each treatment condition across 15-min time intervals within the session. Panel A: effect of nicotine (0.2 mg/kg) alone; Panel B: effect of bPiDDB (1 or 3 mg/kg) alone; and Panel C: effect of nicotine (0.2 mg/kg) in the presence and absence of bPiDDB (1 or 3 mg/kg). SAL=saline; NIC=nicotine, 0.2 mg/kg; bPiDDB 1 indicates 1 mg/kg; and bPiDDB 3 indicates 3 mg/kg.*indicates significant difference from SAL-SAL active lever (p<0.05). # indicates significant difference from SAL-NIC active lever (p<0.05).
The current approach in identifying nAChR antagonists that may have benefit in treating tobacco dependence employed the nicotine-evoked striatal DA release assay to assess analogs for their ability to inhibit the effect of nicotine. However, a relatively large literature minimizes the role of the presynaptic nAChRs located in the dopaminergic terminal regions as mediating nicotine-evoked DA release, and rather implicates nAChRs in the dopaminergic cell body regions as being critically involved. Our own findings, in part, support this contention. Similar to mecamylamine, bPiDDB (1 or 3 mg/kg) produced a dose-dependent blockade of the nicotine-induced increase in extracellular nucleus accumbens DA (Rahman et al., 2007). The anatomical localization of the critical bPiDDB-sensitive nAChRs involved in regulating nicotine-evoked DA release in nucleus accumbens was assessed using reverse dialysis (Rahman, Zhang, Crooks, Dwoskin & Bardo, 2007). The increase in extracellular DA in nucleus accumbens following systemic nicotine (0.4 mg/kg, sc) was blocked completely by bPiDDB (1 μM) infused directly into the VTA, but not by bPiDDB infused into the nucleus accumbens, suggesting that in vivo the nAChRs critical for mediating nicotine-stimulated DA release are located in the VTA.
Nicotine activates and rapidly desensitizes nAChRs on DA neurons in the VTA, and these nAChRs predominantly are high affinity α4β2* nAChRs (Mansvelder & McGehee, 2002; Picciotto et al., 1998; Pidoplichko et al., 1997; Tapper, McKinney, Nashmi, Schwarz, Deshpande, Labarca, Whiteaker, Marks, Collins, & Lester, 2004). Also, VTA DA neurons are activated indirectly through nicotine stimulation of α7* nAChRs located on glutamatergic presynaptic terminals leading to glutamate release and subsequent excitation of VTA DA neurons (Dani & Harris, 2005; Mansvelder & McGehee, 2000; Wooltorton et al., 2003). These α7* nAChRs have a lower affinity for nicotine and are not desensitized at nicotine concentrations experienced by tobacco smokers, which leads to long-term potentiation and prolonged excitation of the VTA DA neurons (Dani & Bertrand, 2007). Electrophysiological results demonstrate that nicotine enhances excitation of the DA neurons by interacting with α7* nAChRs on excitatory glutamatergic neurons that are not readily desensitized; nicotine also activates, but more importantly, desensitizes α4β2* nAChRs located on GABAergic terminals, which impinge on the DA neurons, leading to disinhibition of the DA neurons (Bonci et al., 2003; Dani, Ji, & Zhou, 2001; Klink, de Kerchove d’Exaerde, Zoli, & Changeux, 2001; McGehee, Heath, Gelber, Devay, & Role, 1995; Mansvelder, Keath, & McGehee, 2002; Mansvelder & McGehee, 2000; Mansvelder & McGehee, 2002).
While some data support the importance of α7* nAChRs on glutamatergic presynaptic terminals in mediating nicotine effects on DA release, other studies suggest that the role of α7* nAChRs is of lesser importance. Specifically, administration of α7* nAChR agonists (AR-R 17779 and DMAC) to non-tolerant and nicotine-sensitized rats failed to increase locomotion, in contrast with the hyperactivity observed following nicotine or an α4β2*-selective agonist (SIB 1765F). These results suggest that α7* nAChRs do not play a role in nicotine-induced hyperlocomotion (Grottick et al., 2000). Furthermore, the latter study showed that MLA did not decrease nicotine self-administration, in contrast to the effects of DHβE, which decreased nicotine self-administration, again suggesting that α7* nAChRs do not play a role in nicotine reward (also see Markou & Paterson, 2001). Another study reported that β2 subunit deletion or DHβE administration to wild type mice blocked nicotine conditioned place preference (CPP), whereas α7 subunit deletion or MLA administration to wild type mice did not alter nicotine CPP (Walters, Brown, Changeux, Martin, & Damaj, 2006). In a very recent microdialysis study, DHβE was shown to decrease nicotine-induced DA release when coadministered locally in the nucleus accumbens in freely-moving rats, implicating a role for presynaptic nAChRs in the DA terminal region (Quarta, Ciruela, Patkar, Borycz, Solinas, Lluis, Franco, Wise, Goldberg, Hope, Woods, & Ferre, 2007). In the latter study, the DA receptor agonist, quinpirole, blocked the nicotine-induced increase in DA release in accumbens, and interestingly, D2 receptors were shown to co-immunoprecipitate with β2 nAChR subunits from rat striatum, suggesting the close association of the D2 receptor with β2-containing nAChRs in striatum. Although the majority of studies implicates nAChRs in the dopaminergic cell body regions as regulating nicotine-evoked DA release in the terminal regions, the latter in vivo study provides support for nAChRs in the terminal regions as playing a role in nicotine-stimulated DA release, and further, supports the use of nAChRs in DA terminal regions as valid targets in the discovery of therapeutic candidates for the treatment of tobacco dependence. In summary, the effectiveness of the nAChR antagonist bPiDDB to decrease both nicotine self-administration (Neugebauer et al., 2006) and nicotine-stimulated reinstatement (current study) in rats provides in vivo preclinical evidence that bPiDDB and the subsequent generations of structurally related analogs such as those described in this Chapter will provide novel, clinically effective treatments for nicotine dependence.
ACKNOWLEDGMENTS
This work was supported by a National Cooperative Drug Discovery Group research grant NIH U19 DA017548.
REFERENCES
- Alkondon M, Pereira EF, Wonnacott S, Albuquerque EX. Blockade of nicotinic currents in hippocampal neurons defines methyllycaconitine as a potent and specific receptor antagonist. Molecular Pharmacology. 1992;41(4):802–808. [PubMed] [Google Scholar]
- Allen DD, Lockman PR, Roder KE, Dwoskin LP, Crooks PA. Active transport of high affinity choline and nicotine analogs into the central nervous system by the blood brain barrier choline transporter. Journal of Pharmacology and Experimental Therapeutics. 2003;304:1268–1274. doi: 10.1124/jpet.102.045856. [DOI] [PubMed] [Google Scholar]
- Anand R, Conroy WG, Schoepfer R, Whiting P, Lindstrom J. Neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes have a pentameric quaternary structure. Journal of Biological Chemistry. 1991;266(17):11192–11198. [PubMed] [Google Scholar]
- Ayers JT, Clauset A, Schmitt JD, Dwoskin LP, Crooks PA. Molecular modeling of mono- and bis-quaternary ammonium salts as ligands at the alpha4beta2 nicotinic acetylcholine receptor subtype using nonlinear techniques. The AAPS Journal. 2005;7(3):E678–E685. doi: 10.1208/aapsj070368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayers JT, Dwoskin LP, Deaciuc AG, Grinevich VP, Zhu J, Crooks PA. Bis-Azaaromatic quaternary ammonium analogues: ligands for alpha4beta2* and alpha 7* subtypes of neuronal nicotinic receptors. Bioorganic & Medicinal Chemistry Letters. 2002;12(21):2067–3071. doi: 10.1016/s0960-894x(02)00687-x. [DOI] [PubMed] [Google Scholar]
- Azam L, Winzer-Serhan UH, Chen Y, Leslie FM. Expression of neuronal nicotinic acetylcholine receptor subunit mRNAs within midbrain dopamine neurons. Journal of Comparative Neurology. 2002;444(3):260–274. doi: 10.1002/cne.10138. [DOI] [PubMed] [Google Scholar]
- Balfour DJ. The neurobiology of tobacco dependence: a commentary. Respiration. 2002;69:7–11. doi: 10.1159/000049362. [DOI] [PubMed] [Google Scholar]
- Balfour DJ. The neurobiology of tobacco dependence: a preclinical perspective on the role of the dopamine projections to the nucleus accumbens. Nicotine & Tobacco Research. 2004;6(6):899–912. doi: 10.1080/14622200412331324965. [DOI] [PubMed] [Google Scholar]
- Bardo MT. Neuropharmacological mechanisms of drug reward: beyond dopamine in the nucleus accumbens. Critical Reviews in Neurobiology. 1998;12(1-2):37–67. doi: 10.1615/critrevneurobiol.v12.i1-2.30. [DOI] [PubMed] [Google Scholar]
- Benwell ME, Balfour DJ. The effects of acute and repeated nicotine treatment on nucleus accumbens dopamine and locomotor activity. British Journal of Pharmacology. 1992;105(4):849–456. doi: 10.1111/j.1476-5381.1992.tb09067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benwell ME, Balfour DJ, Birrell CE. Desensitization of the nicotine-induced mesolimbic dopamine responses during constant infusion with nicotine. British Journal of Pharmacology. 1995;114(2):454–460. doi: 10.1111/j.1476-5381.1995.tb13248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Research Brain Research Reviews. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Bonci A, Bernardi G, Grillner P, Mercuri NB. The dopamine-containing neuron: maestro or simple musician in the orchestra of addiction? Trends in Pharmacological Sciences. 2003;24(4):172–177. doi: 10.1016/S0165-6147(03)00068-3. [DOI] [PubMed] [Google Scholar]
- Brazell MP, Mitchell SN, Joseph MH, Gray JA. Acute administration of nicotine increases the in vivo extracellular levels of dopamine, 3,4-dihydroxyphenylacetic acid and ascorbic acid preferentially in the nucleus accumbens of the rat: comparison with caudate-putamen. Neuropharmacology. 1990;29(12):1177–1185. doi: 10.1016/0028-3908(90)90042-p. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Lee G, Smith E, Sadeghi M, Saxena S, Jarvik ME, London ED. Attenuation of cue-induced cigarette craving and anterior cingulate cortex activation in bupropion-treated smokers: a preliminary study. Psychiatry Research. 2004;130(3):269–281. doi: 10.1016/j.pscychresns.2003.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Environmental stimuli promote the acquisition of nicotine self-administration in rats. Psychopharmacology. 2002;163:230–237. doi: 10.1007/s00213-002-1156-5. [DOI] [PubMed] [Google Scholar]
- Castro NG, Albuquerque EX. alpha-Bungarotoxin-sensitive hippocampal nicotinic receptor channel has a high calcium permeability. Biophysical Journal. 1995;68(2):516–524. doi: 10.1016/S0006-3495(95)80213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przybylski C, Lena C, Clementi F, Moretti M, Rossi FM, Le Novere N, McIntosh JM, Gardier AM, Changeux JP. Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. The Journal of Neuroscience. 2003;23(21):7820–7829. doi: 10.1523/JNEUROSCI.23-21-07820.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpantier E, Barneoud P, Moser P, Besnard F, Sgard F. Nicotinic acetylcholine subunit mRNA expression in dopaminergic neurons of the rat substantia nigra and ventral tegmental area. Neuroreport. 1998;9(13):3097–3101. doi: 10.1097/00001756-199809140-00033. [DOI] [PubMed] [Google Scholar]
- Clarke PB, Pert A. Autoradiographic evidence for nicotine receptors on nigrostriatal and mesolimbic dopaminergic neurons. Brain Research. 1985;348(2):355–358. doi: 10.1016/0006-8993(85)90456-1. [DOI] [PubMed] [Google Scholar]
- Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, Shrikhande A, Heym JH, Schaeffer E, Rollema H, Lu Y, Mansbach RS, Chambers LK, Rovetti CC, Schulz DW, Tingley FD, 3rd, O’Neill BT. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. Journal of Medicinal Chemistry. 2005;48(10):3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- Conroy WG, Vernallis AB, Berg DK. The alpha 5 gene product assembles with multiple acetylcholine receptor subunits to form distinctive receptor subtypes in brain. Neuron. 1992;9(4):670–691. doi: 10.1016/0896-6273(92)90031-8. [DOI] [PubMed] [Google Scholar]
- Cooper E, Couturier S, Ballivet M. Pentameric structure and subunit stoichiometry of a neuronal acetylcholine receptor. Nature. 1991;350(6315):235–238. doi: 10.1038/350235a0. [DOI] [PubMed] [Google Scholar]
- Corrigall WA. Nicotine self-administration in animals as a dependence model. Nicotine & Tobacco Research. 1999;1(1):11–20. doi: 10.1080/14622299050011121. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology (Berl) 1989;99(4):473–478. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Research. 1994;653(1-2):278–284. doi: 10.1016/0006-8993(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Franklin KB, Coen KM, Clarke PB. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology (Berl) 1992;107(2-3):285–289. doi: 10.1007/BF02245149. [DOI] [PubMed] [Google Scholar]
- Corringer PJ, Sallette J, Changeux JP. Nicotine enhances intracellular nicotinic receptor maturation: a novel mechanism of neural plasticity? Journal of Physiology, Paris. 2006;99(2-3):162–171. doi: 10.1016/j.jphysparis.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Crooks PA, Dwoskin LP. Contribution of CNS nicotine metabolites to the neuropharmacological effects of nicotine and tobacco smoking. Biochemical Pharmacology. 1997;54:743–753. doi: 10.1016/s0006-2952(97)00117-2. [DOI] [PubMed] [Google Scholar]
- Crooks PA, Ayers JT, Haubner AJ, Grinevich VP, Sumithran SP, Deaciuc AG, Dwoskin LP. The bis-picolinium salt, bPiDDB, is a potent and selective antagonist at nicotinic acetylcholine receptors mediating nicotine-evoked dopamine release in rat striatum. Drug and Alcohol Dependence. 2002;64:38. [Google Scholar]
- Crooks PA, Ayers JT, Xu R, Sumithran SP, Grinevich VP, Wilkins LH, Deaciuc AG, Allen DD, Dwoskin LP. Development of subtype-selective ligands as antagonists at nicotinic receptors mediating nicotine-evoked dopamine release. Bioorganic & Medicinal Chemistry Letters. 2004;14(8):1869–1874. doi: 10.1016/j.bmcl.2003.10.074. [DOI] [PubMed] [Google Scholar]
- Cui C, Booker TK, Allen RS, Grady SR, Whiteaker P, Marks MJ, Salminen O, Tritto T, Butt CM, Allen WR, Stitzel JA, McIntosh JM, Boulter J, Collins AC, Heinemann SF. The β3 nicotinic receptor subunit: A component of α-conotoxin MII-binding nicotinic acetylcholine receptors that modulate dopamine release and related behaviors. Journal of Neuroscience. 2003;23:11045–11053. doi: 10.1523/JNEUROSCI.23-35-11045.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JA. Roles of dopamine signaling in nicotine addiction. Molecular Psychiatry. 2003;8(3):255–256. doi: 10.1038/sj.mp.4001284. [DOI] [PubMed] [Google Scholar]
- Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annual Review of Pharmacology and Toxicology. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- Dani JA, De Biasi M. Cellular mechanisms of nicotine addiction. Pharmacology, Biochemistry and Behavior. 2001;70(4):439–446. doi: 10.1016/s0091-3057(01)00652-9. [DOI] [PubMed] [Google Scholar]
- Dani JA, Harris RA. Nicotine addiction and comorbidity with alcohol abuse and mental illness. Nature Neuroscience. 2005;8(11):1465–1470. doi: 10.1038/nn1580. [DOI] [PubMed] [Google Scholar]
- Dani JA, Ji D, Zhou FM. Synaptic plasticity and nicotine addiction. Neuron. 2001;31(3):349–352. doi: 10.1016/s0896-6273(01)00379-8. [DOI] [PubMed] [Google Scholar]
- Deneris ES, Boulter J, Swanson LW, Patrick J, Heinemann S. Beta 3: a new member of nicotinic acetylcholine receptor gene family is expressed in the brain. Journal of Biological Chemistry. 1989;264(11):6268–6272. [PubMed] [Google Scholar]
- Di Chiara G. Role of dopamine in the behavioural actions of nicotine related to addiction. European Journal of Pharmacology. 2000;393:295–314. doi: 10.1016/s0014-2999(00)00122-9. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, Acquas E, Carboni E, Valentini V, Lecca D. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47:227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Sumithran SP, Zhu J, Deaciuc AG, Ayers JT, Crooks PA. Subtype-selective nicotinic receptor antagonists: potential as tobacco use cessation agents. Bioorganic & Medicinal Chemistry Letters. 2004;14:1863–1867. doi: 10.1016/j.bmcl.2003.10.073. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Teng LH, Buxton ST, Ravard A, Deo N, Crooks PA. Minor alkaloids of tobacco release [3H]dopamine release from superfused rat striatal slices. European.Journal of.Pharmacology. 1995;276:195–199. doi: 10.1016/0014-2999(95)00077-x. [DOI] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Clements LA, Sved AF. Operant responding for a visual reinforcer in rats in enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology (Berl) 2003;169(1):68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Flores CM, Rogers SW, Pabreza LA, Wolfe BB, Kellar KJ. A subtype of nicotinic cholinergic receptor in rat brain is composed of alpha 4 and beta 2 subunits and is up-regulated by chronic nicotine treatment. Molecular Pharmacology. 1992;41(1):31–37. [PubMed] [Google Scholar]
- Forsayeth JR, Kobrin E. Formation of oligomers containing the beta 3 and beta 4 subunits of the rat nicotinic receptor. Journal of Neuroscience. 1997;17(5):1531–1538. doi: 10.1523/JNEUROSCI.17-05-01531.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Matta SG, Gao W, Sharp BM. Local alpha-bungarotoxin-sensitive nicotinic receptors in the nucleus accumbens modulate nicotine-stimulated dopamine secretion in vivo. Neuroscience. 2000;101(2):369–375. doi: 10.1016/s0306-4522(00)00371-7. [DOI] [PubMed] [Google Scholar]
- Ghosheh O, Dwoskin LP, Li WK, Crooks PA. Residence time and half-lives of nicotine metabolites in rat brain after acute peripheral administration of [2’-14C]nicotine. Drug Metabolism and Disposition. 1999;27:1448–1455. [PubMed] [Google Scholar]
- Ghosheh A, Dwoskin P, Miller K, Crooks A. Accumulation of nicotine and its metabolites in rat brain after intermittent or continuous peripheral administration of [2’-14C]-nicotine. Drug Metabolism and Disposition. 2001;29:645–651. [PubMed] [Google Scholar]
- George TP, O’Malley SS. Current pharmacological treatments for nicotine dependence. Trends in Pharmacological Sciences. 2004;25(1):42–48. doi: 10.1016/j.tips.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Goldner FM, Dineley KT, Patrick JW. Immunohistochemical localization of the nicotinic acetylcholine receptor subunit alpha 6 to dopaminergic neurons in the substantia nigra and ventral tegmental area. Neuroreport. 1997;8(12):2739–2742. doi: 10.1097/00001756-199708180-00019. [DOI] [PubMed] [Google Scholar]
- Gotti C, Moretti M, Clementi F, Riganti F, McIntosh JM, Collins AC, Marks MJ, Whiteaker P. Expression of nigrostriatal α6-containing nicotinic acetylcholine receptors is selectively reduced, but not eliminated, by β3 subunit gene deletion. Molecular Pharmacology. 2005;67:2007–2015. doi: 10.1124/mol.105.011940. [DOI] [PubMed] [Google Scholar]
- Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends in Pharmacological Sciences. 2006;27(9):482–491. doi: 10.1016/j.tips.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Grady SR, Grun EU, Marks MJ, Collins AC. Pharmacological comparisons of transient and persistent [3H]dopamine release from mouse striatal synaptosomes and response to chronic L-nicotine treatment. The Journal of Pharmacology and Experimental Therapeutics. 1997;282:32–43. [PubMed] [Google Scholar]
- Grady SR, Meinerz NM, Cao J, Reynolds AM, Picciotto MR, Changeux J-P, McIntosh JM, Marks MJ, Collins AC. Nicotinic agonists stimulate acetylcholine release from mouse interpeduncular nucleus: a function mediated by a different nAChR than dopamine release from striatum. Journal of Neurochemistry. 2001;76:258–226. doi: 10.1046/j.1471-4159.2001.00019.x. [DOI] [PubMed] [Google Scholar]
- Grady SR, Murphy KL, Cao J, Marks MJ, McIntosh JM, Collins AC. Characterization of nicotinic agonist-induced [(3)H]dopamine release from synaptosomes prepared from four mouse brain regions. Journal of Pharmacology and Experimental Therapeutics. 2002;301(2):651–660. doi: 10.1124/jpet.301.2.651. [DOI] [PubMed] [Google Scholar]
- Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature. 1996;383(6602):713–716. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- Grinevich VP, Crooks PA, Sumithran SP, Haubner AJ, Ayers JT, Dwoskin LP. N-n-Alkylpyridinium analogs, a novel class of nicotinic receptor antagonists: selective inhibition of nicotine-evoked [3H]dopamine overflow from superfused rat striatal slices. The Journal of Pharmacology and Experimental Therapeutics. 2003;306(3):1011–1020. doi: 10.1124/jpet.103.051789. [DOI] [PubMed] [Google Scholar]
- Grottick AJ, Trube G, Corrigall WA, Huwyler J, Malherbe P, Wyler R, Higgins GA. Evidence that nicotinic alpha(7) receptors are not involved in the hyperlocomotor and rewarding effects of nicotine. The Journal of Pharmacology and Experimental Therapeutics. 2000;294(3):1112–1119. [PubMed] [Google Scholar]
- Hurt RD, Krook JE, Croghan IT, Loprinzi CL, Sloan JA, Novotny PJ, Kardinal CG, Knost JA, Tirona MT, Addo F, Morton RF, Michalak JC, Schaefer PL, Porter PA, Stella PJ. Nicotine patch therapy based on smoking rat followed by bupropion for prevention of relapse to smoking. Journal of Clinical Oncology. 2003;21(5):914–920. doi: 10.1200/JCO.2003.08.160. [DOI] [PubMed] [Google Scholar]
- Imperato A, Mulas A, Dichiara G. Nicotine preferentially stimulates dopamine release in the limbic system of freely moving rats. European Journal of Pharmacology. 1986;132(2-3):337–338. doi: 10.1016/0014-2999(86)90629-1. [DOI] [PubMed] [Google Scholar]
- Irvin JE, Hendricks PS, Brandon TH. The increasing recalcitrance of smokers in clinical trials II: Pharmacotherapy trials. Nicotine & Tobacco Research. 2003;5(1):27–35. doi: 10.1080/1462220031000070534. [DOI] [PubMed] [Google Scholar]
- Jones IW, Bolam JP, Wonnacott S. Presynaptic localization of the nicotinic acetylcholine receptor beta 2 subunit immunoreactivity in rat nigrostriatal dopaminergic neurons. The Journal of Comparative Neurology. 2001;439(2):235–247. doi: 10.1002/cne.1345. [DOI] [PubMed] [Google Scholar]
- Kaiser SA, Soliakov L, Harvey SC, Luetje CW, Wonnacott S. Differential inhibition by α-conotoxin-MII of the nicotinic stimulation of [3H]dopamine release from rat striatal synaptosomes and slices. Journal of Neurochemistry. 1998;70:1069–1076. doi: 10.1046/j.1471-4159.1998.70031069.x. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity. Neuropsychopharmacology. 2006;31:1203–1211. doi: 10.1038/sj.npp.1300905. [DOI] [PubMed] [Google Scholar]
- Klink R, de Kerchove d’Exaerde A, Zoli M, Changeux JP. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. Journal of Neuroscience. 2001;21(5):1452–1463. doi: 10.1523/JNEUROSCI.21-05-01452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Neural mechanisms of drug reinforcement. Annals of New York Academy of Sciences. 1992;654:171–191. doi: 10.1111/j.1749-6632.1992.tb25966.x. [DOI] [PubMed] [Google Scholar]
- Koob GF. The role of the striatopallidal and extended amygdala systems in drug addiction. Annals of New York Academy of Sciences. 1999;877:445–46. doi: 10.1111/j.1749-6632.1999.tb09282.x. [DOI] [PubMed] [Google Scholar]
- Kulak JM, McIntosh JM, Yoshikami D, Olivera BM. Nicotine-evoked transmitter release from synaptosomes: functional association of specific presynaptic acetylcholine receptors and voltage-gated calcium channels. Journal of Neurochemistry. 2001;77:1581–1589. doi: 10.1046/j.1471-4159.2001.00357.x. [DOI] [PubMed] [Google Scholar]
- Kulak JM, Nguyen TA, Olivera BM, McIntosh JM. Alpha-conotoxin MII blocks nicotine-stimulated dopamine release in rat striatal synaptosomes. Journal of Neuroscience. 1997;17(14):5263–5270. doi: 10.1523/JNEUROSCI.17-14-05263.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuryatov A, Olale F, Cooper J, Choi C, Lindstrom J. Human alpha6 AChR subtypes: subunit composition, assembly and pharmacological responses. Neuropharmacology. 2000;38(13):2570–2590. doi: 10.1016/s0028-3908(00)00144-1. [DOI] [PubMed] [Google Scholar]
- Le Novere N, Zoli M, Changeux JP. Neuronal nicotinic receptor alpha 6 subunit mRNA is selectively concentrated in catecholaminergic nuclei of the rat brain. European Journal of Neuroscience. 1996;8(11):2428–2439. doi: 10.1111/j.1460-9568.1996.tb01206.x. [DOI] [PubMed] [Google Scholar]
- Liu X, Caggiula AR, Yee SK, Nobuta H, Sved AF, Pechnick RN, Poland RE. Mecamylamine attenuates cue-induced reinstatement of nicotine-seeking behavior in rats. Neuropsychopharmacology. 2007;32(3):710–718. doi: 10.1038/sj.npp.1301129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Hernandez GY, Sanchez-Padilla J, Ortiz-Acevedo A, Lizardi-Ortiz J, Salas-Vincenty J, Rojas LV, Lasalde-Dominicci JA. Nicotine-induced up-regulation and desensitization of alpha4beta2 neuronal nicotinic receptors depend on subunit ratio. Journal of Biological Chemistry. 2004;279(36):38007–38015. doi: 10.1074/jbc.M403537200. [DOI] [PubMed] [Google Scholar]
- Lundahl LH, Henningfield JE, Lukas SE. Mecamylamine blockade of both positive and negative effects of IV nicotine in human volunteers. Pharmacology, Biochemistry, and Behavior. 2000;66(3):637–643. doi: 10.1016/s0091-3057(00)00252-5. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33(6):905–919. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron. 2000;27(2):349–357. doi: 10.1016/s0896-6273(00)00042-8. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS. Cellular and synaptic mechanisms of nicotine addiction. Journal of Neurobiology. 2002;53(4):606–617. doi: 10.1002/neu.10148. [DOI] [PubMed] [Google Scholar]
- Markou A, Paterson NE. The nicotinic antagonist methyllycaconitine has differential effects on nicotine self-administration and nicotine withdrawal in the rat. Nicotine and Tobacco Research. 2001;3(4):361–373. doi: 10.1080/14622200110073380. [DOI] [PubMed] [Google Scholar]
- Martin-Soelch C, Leenders KL, Chevalley AF, Missimer J, Kunig G, Magyar S, Mino A, Schultz W. Reward mechanisms in the brain and their role in dependence: evidence from neurophysiological and neuroimaging studies. Brain Research Review. 2001;26(2-3):139–149. doi: 10.1016/s0165-0173(01)00089-3. [DOI] [PubMed] [Google Scholar]
- McCallum SE, Parameswaran N, Bordia T, McIntosh JM, Grady SR, Quik M. Decrease in alpha3*/alpha6* nicotinic receptors but not nicotine-evoked dopamine release in monkey brain after nigrostriatal damage. Molecular Pharmacology. 2005;68(3):737–746. doi: 10.1124/mol.105.012773. [DOI] [PubMed] [Google Scholar]
- McGehee DS, Heath MJ, Gelber S, Devay P, Role LW. Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science. 1995;269(5231):1681–1682. doi: 10.1126/science.7569895. [DOI] [PubMed] [Google Scholar]
- McGehee DS, Role LW. Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annual Review of Physiology. 1995;57:521–546. doi: 10.1146/annurev.ph.57.030195.002513. [DOI] [PubMed] [Google Scholar]
- McIntosh JM, Olivera BM, Cruz LJ. Conus peptides as probes for ion channels. Methods in Enzymology. 1999;294:605–624. doi: 10.1016/s0076-6879(99)94034-x. [DOI] [PubMed] [Google Scholar]
- McIntosh JM, Santos AD, Olivera BM. Conus peptides targeted to specific nicotinic acetylcholine receptor subtypes. Annual Review of Biochemistry. 1999;68:59–88. doi: 10.1146/annurev.biochem.68.1.59. [DOI] [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Molecular Pharmacology. 2006;70(3):801–805. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- Miller DK, Sumithran SP, Dwoskin LP. Bupropion inhibits nicotine-evoked [3H]overflow from rat striatal slices preloaded with [3H]dopamine and from rat hippocampal slices preloaded with [3H]norepinephrine. The Journal of Pharmacology and Experimental Therapeutics. 2002;203(3):1113–1122. doi: 10.1124/jpet.102.033852. [DOI] [PubMed] [Google Scholar]
- Nelson ME, Kuryatov A, Choi CH, Zhou Y, Lindstrom J. Alternate stoichiometries of alpha4beta2 nicotinic acetylcholine receptors. Molecular Pharmacology. 2003;63(2):332–341. doi: 10.1124/mol.63.2.332. [DOI] [PubMed] [Google Scholar]
- Neugebauer NM, Zhang Z, Crooks PA, Dwoskin LP, Bardo MT. Effect of a novel nicotinic receptor antagonist, N,N’dodecane-1,12-diyl-bis-3-picolinium dibromide, on nicotine self-administration and hyperactivity in rats. Psychopharmacology (Berl) 2006;184(3-4):426–434. doi: 10.1007/s00213-005-0163-8. [DOI] [PubMed] [Google Scholar]
- Niaura R, Shadel WG, Abrams DB, Monti PM, Rohsenow DJ, Sirota A. Individual differences in cue reactivity among smokers trying to quit: effects of gender and cue type. Addictive Behaviors. 1998;23(2):209–224. doi: 10.1016/s0306-4603(97)00043-9. [DOI] [PubMed] [Google Scholar]
- Nicke A, Wonnacott S, Lewis RJ. Alpha-conotoxins as tools for the elucidation of structure and function of neuronal nicotinic acetylcholine receptor subtypes. European Journal of Biochemistry. 2004;271(12):2305–2319. doi: 10.1111/j.1432-1033.2004.04145.x. [DOI] [PubMed] [Google Scholar]
- Nisell M, Nomikos GG, Svensson TH. Systemic nicotine-induced dopamine release in the rat nucleus accumbens is regulated by nicotinic receptors in the ventral tegmental area. Synapse. 1994;16(1):36–44. doi: 10.1002/syn.890160105. [DOI] [PubMed] [Google Scholar]
- Nomikos GG, Damsma G, Wenkstern D, Fibiger HC. Acute effects of bupropion on extracellular dopamine concentrations in rat striatum and nucleus accumbens studied by in vivo microdialysis. Neuropsychopharmacology. 1989;2(4):273–279. doi: 10.1016/0893-133x(89)90031-6. [DOI] [PubMed] [Google Scholar]
- Nomikos GG, Damsma G, Wenkstern D, Fibiger HC. Effects of chronic bupropion on interstitial concentrations of dopamine in rat nucleus accumbens and striatum. Neuropsychopharmacology. 1992;7(1):7–14. [PubMed] [Google Scholar]
- Palmatier MI, Evans-Martin FF, Hoffman A, Caggiula AR, Chaudhri N, Donny EC, Liu X, Booth S, Gharib M, Craven L, Sved AF. Dissociating the primary reinforcing and reinforcement-enhancing effects of nicotine using a rat self-administration paradigm with concurrently available drug and environmental reinforcers. Psychopharmacology. 2006;184:391–400. doi: 10.1007/s00213-005-0183-4. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Liu X, Caggiula AR, Donny EC, Sved AF. The role of nicotinic acetylcholine receptors in the primary reinforcing and reinforcement-enhancing effects of nicotine. Neuropsychopharmacology. 2007;32:1098–1108. doi: 10.1038/sj.npp.1301228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Dwoskin LP, Crooks PA. The pharmacological fingerprint of nAChRs subtypes utilizing nicotine and nornicotine: relevance to nicotine dependence and drug discovery. Journal of Neurochemistry. 2007;101(1):160–167. doi: 10.1111/j.1471-4159.2006.04355.x. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Epstein LH, Grobe J, Fonte C. Tobacco abstinence, smoking cues, and the reinforcing value of smoking. Pharmacology, Biochemistry, and Behavior. 1994;47(1):107–112. doi: 10.1016/0091-3057(94)90118-x. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, Fuxe K, Changeux JP. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391(6663):173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Pidoplichko VI, DeBiasi M, Williams JT, Dani JA. Nicotine activates and desensitizes midbrain dopamine neurons. Nature. 1997;390(6658):401–404. doi: 10.1038/37120. [DOI] [PubMed] [Google Scholar]
- Pidoplichko VI, Noguchi J, Areola OO, Liang Y, Peterson J, Zhang T, Dani JA. Nicotinic cholinergic synaptic mechanisms in the ventral tegmental area contribute to nicotine addiction. Learning and Memory. 2004;11(1):60–69. doi: 10.1101/lm.70004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivavarchyk M, Zhang Z, Crooks PA, Dwoskin LP. Tetrakis quaternary ammonium salts: novel nicotinic receptor antagonists which inhibit nicotine-evoked [3H]dopamine overflow from superfused rat striatal slices. Society for Neuroscience. 2007 Abstract. [Google Scholar]
- Pomerleau CS, Pomerleau OF. Euphoriant effects of nicotine in smokers. Pychopharmacology (Berl) 1992;108:460–465. doi: 10.1007/BF02247422. [DOI] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Orzi F, Di Chiara G. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature. 1996;382(6588):255–257. doi: 10.1038/382255a0. [DOI] [PubMed] [Google Scholar]
- Poth K, Nutter TJ, Cuevas J, Parker MJ, Adams DJ, Luetje CW. Heterogeneity of nicotinic receptor class and subunit mRNA expression among individual parasympathetic neurons from rat intracardiac ganglia. Journal of Neuroscience. 1997;17(2):586–596. doi: 10.1523/JNEUROSCI.17-02-00586.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarta D, Ciruela F, Patkar K, Borycz J, Solinas M, Lluis C, Frankco R, Wise RA, Goldberg SR, Hope BT, Woods AS, Ferre S. Heteromeric nicotinic acetylcholine-dopamine autoreceptor complexes modulate striatal dopamine release. Neuropsychopharmacology. 2007;32(1):35–42. doi: 10.1038/sj.npp.1301103. [DOI] [PubMed] [Google Scholar]
- Rahman S, Neugebauer NM, Zhang Z, Crooks PA, Dwoskin LP, Bardo MT. The effects of a novel nicotinic receptor antagonist N,N’-dodecane-1,12-diyl-bis-3-picolinium dibromide (bPiDDB) on acute repeated nicotine-induced increases in extracellular dopamine in rat nucleus accumbens. Neuropharmacology. 2007;52(3):755–763. doi: 10.1016/j.neuropharm.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Rahman S, Zhang J, Corrigall WA. Effects of nicotine preexposure on sulpiride-induced dopamine release in the nucleus accumbens. European Journal of Pharmacology. 2004;494(1):31–34. doi: 10.1016/j.ejphar.2004.04.044. [DOI] [PubMed] [Google Scholar]
- Rahman S, Zhang Z, Crooks PA, Dwoskin LP, Bardo MT. Local perfusion of the novel nicotinic receptor antagonist N,N’dodecane-1,12-diyl-bis-3-picolinium dibromide (bPiDDB) into the ventral tegmental area (VTA) or the nucleus accumbens (N.Acc), differentially regulates nicotine-induced increases in extracellular dopamine in the nucleus accumbens. American Association of Pharmaceutical Scientists Journal. 2007 in press. [Google Scholar]
- Rauhut AS, Neugebauer N, Dwoskin LP, Bardo MT. Effect of bupropion on nicotine self-administration in rats. Psychopharmacology (Berl) 2003;169:1–9. doi: 10.1007/s00213-003-1450-x. [DOI] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nature Neuroscience. 2004;7(6):583–584. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM. Extinguishing the rewarding value of smoke cues: pharmacological and behavioral treatments. Nicotine & Tobacco Research. 2004;6(3):523–532. doi: 10.1080/14622200410001696501. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Levin ED, Stein RM, Ripka GV. Mecamylamine combined with nicotine skin patch facilitates smoking cessation beyond nicotine patch treatment alone. Clinical Pharmacology and Therapeutics. 1994;56(1):86–99. doi: 10.1038/clpt.1994.105. [DOI] [PubMed] [Google Scholar]
- Rose JE, Westman EC, Behm FM, Johnson MP, Goldberg JS. Blockade of smoking satisfaction using the peripheral nicotinic antagonist trimethaphan. Pharmacology, Biochemistry, and Behavior. 1999;62(1):165–172. doi: 10.1016/s0091-3057(98)00153-1. [DOI] [PubMed] [Google Scholar]
- Salminen O, Drapeau JA, McIntosh JM, Collins AC, Marks MJ, Grady SR. Pharmacology of α-conotoxin MII-sensitive subtypes of nicotinic acetylcholine receptors isolated by breeding of null mutant mice. Molecular Pharmacology. 2007 doi: 10.1124/mol.106.031492. [Epub] [DOI] [PubMed] [Google Scholar]
- Salminen O, Murphy KL, McIntosh JM, Drago J, Marks MJ, Collins AC, Grady SR. Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Molecular Pharmacology. 2004;65:1526–1535. doi: 10.1124/mol.65.6.1526. [DOI] [PubMed] [Google Scholar]
- Scholze P, Orr-Urtreger A, Changeux JP, McIntosh JM, Huck S. Catecholamine outflow from mouse and rat brain slice preparations evoked by nicotinic acetylcholine receptor activation and electrical field stimulation. British Journal of Pharmacology. 2007;151(3):414–422. doi: 10.1038/sj.bjp.0707236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima K, Tanji J. Role for cingulated motor area cells in voluntary movement selection based on reward. Science. 1998;282:1335–1338. doi: 10.1126/science.282.5392.1335. [DOI] [PubMed] [Google Scholar]
- Singer G, Wallace M, Hall R. Effects of dopaminergic nucleus accumbens lesions on the acquisition of schedule induced self injection of nicotine in the rat. Pharmacology, Biochemistry, and Behavior. 1982;17(3):579–581. doi: 10.1016/0091-3057(82)90321-5. [DOI] [PubMed] [Google Scholar]
- Slemmer JE, Martin BR, Damaj MI. Bupropion is a nicotinic antagonist. The Journal of Pharmacology and Experimental Therapeutics. 2000;295(1):321–327. [PubMed] [Google Scholar]
- Soliakov L, Wonnacott S. Voltage-sensitive Ca2+ channels involved in nicotinic receptor-mediated [3H]dopamine release from rat striatal synaptosomes. Journal of Neurochemistry. 1996;67:163–170. doi: 10.1046/j.1471-4159.1996.67010163.x. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Weiss F. The dopamine hypothesis of reward: past and current status. Trends in Neuroscience. 1999;22(11):521–527. doi: 10.1016/s0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]
- Stokes C, Dwoskin LP, Crooks PA, Jacobs LB, McIntosh JM, Papke RL. The identification of selective competitive and noncompetitive nAChR antagonists using alpha6 nicotinic acetylcholine receptor chimeras. Society for Neuroscience. 2007 Abstract. [Google Scholar]
- Stolerman IP, Jarvis MJ. The scientific case that nicotine is addictive. Psychopharmacology. 1995;117(1):2–10. doi: 10.1007/BF02245088. [DOI] [PubMed] [Google Scholar]
- Sumithran SP, Crooks PA, Xu R, Zhu J, Deaciuc AG, Wilkins LH, Dwoskin LP. Introduction of unsaturation into the N-n-alkyl chain of the nicotinic receptor antagonists, NONI and NDNI: effect on affinity and selectivity. American Association of Pharmaceutical Scientists Journal. 2005;7(1):E201–217. doi: 10.1208/aapsj070119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, Whiteaker P, Marks MJ, Collins AC, Lester HA. Nicotine activation of alpha4* receptors: sufficient for reward, tolerance, and sensitization. Science. 2004;306(5698):983–985. doi: 10.1126/science.1099420. [DOI] [PubMed] [Google Scholar]
- Teng L, Crooks PA, Buxton ST, Dwoskin LP. Nicotinic-receptor mediation of S(-)nornicotine-evoked [3H]overflow from rat striatal slices preloaded with [3H]dopamine: differential inhibition of synaptosomal and vesicular [3H]dopamine uptake. The Journal of Pharmacology and Experimental Therapeutics. 1997;280(3):1432–1444. [PubMed] [Google Scholar]
- Vann RE, Rosecrans JA, James JR, Philibin SD, Robinson SE. Neurochemical and behavioral effects of bupropion and mecamylamine in the presence of nicotine. Brain Research. 2006;1117:18–24. doi: 10.1016/j.brainres.2006.07.110. [DOI] [PubMed] [Google Scholar]
- Wada E, McKinnon D, Heinemann S, Patrick J, Swanson LW. The distribution of mRNA encoded by a new member of the neuronal nicotinic acetylcholine receptor family (alpha 5) in the rat central nervous system. Brain Research. 1990;526(1):45–53. doi: 10.1016/0006-8993(90)90248-a. [DOI] [PubMed] [Google Scholar]
- Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, Swanson LW. Distribution of alpha 2, alpha 3, alpha4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. The Journal Comparative Neurology. 1989;284(2):314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- Walters CL, Brown S, Changeux JP, Martin B, Damaj MI. The beta2 but not alpha7 subunit of the nicotinic acetylcholine receptor is required for nicotine-conditioned place preference in mice. Psychopharmacology. 2006;184(3-4):339–344. doi: 10.1007/s00213-005-0295-x. [DOI] [PubMed] [Google Scholar]
- Wei X, Sumithran SP, Deaciuc AG, Burton HR, Bush LP, Dwoskin LP, Crooks PA. Identification and synthesis of novel alkaloids from the root system of Nicotiana tabacum: Affinity for neuronal nicotinic acetylcholine receptors. Life Sciences. 2005;78:495–505. doi: 10.1016/j.lfs.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Whiteaker P, Marks MJ, Grady SR, Lu Y, Picciotto MR, Changeux JP, Collins AC. Pharmacological and null mutation approaches reveal nicotinic receptor diversity. European Journal of Pharmacology. 2000;393(1-3):123–135. doi: 10.1016/s0014-2999(00)00052-2. [DOI] [PubMed] [Google Scholar]
- Wilkins LH, JR., Grinevich VP, Ayers JT, Crooks PA, Dwoskin LP. N-n-alkylnicotinium analogs, a novel class of nicotinic receptor antagonists: interaction with α4β2* and α7* neuronal nicotinic receptors. The Journal of Pharmacology and Experimental Therapeutics. 2003;304(1):400–410. doi: 10.1124/jpet.102.043349. [DOI] [PubMed] [Google Scholar]
- Wilkins LH, Haubner A, Ayers JT, Crooks PA, Dwoskin LP. N-n-Alkylnicotinium analogs, a novel class of nicotinic receptor antagonist: inhibition of S(-)-nicotine-evoked [3H]dopamine overflow from superfused rat striatal slices. The Journal of Pharmacology and Experimental Therapeutics. 2002;301(3):1088–1096. doi: 10.1124/jpet.301.3.1088. [DOI] [PubMed] [Google Scholar]
- Wilkins LH, Miller DK, Ayers JT, Crooks PA, Dwoskin LP. N-n-Alkylnicotinium analogs, a novel class of antagonists at alpha 4 beta 2* nicotinic receptors: inhibition of S(-)-nicotine-evoked 86Rb+ efflux from rat thalamic synaptosomes. American Association of Pharmaceutical Scientists Journal. 2006;7(4):E922–930. doi: 10.1208/aapsj070490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Neurobiology of addiction. Current Opinion in Neurobiology. 1996;6(2):243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- Wise RA. Addiction becomes a brain disease. Neuron. 2000;26:27–33. doi: 10.1016/s0896-6273(00)81134-4. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychological Review. 1987;94(4):469–492. [PubMed] [Google Scholar]
- Wonnacott S. Presynaptic nicotinic Ach receptors. Trends in Neuroscience. 1997;20(2):92–98. doi: 10.1016/s0166-2236(96)10073-4. [DOI] [PubMed] [Google Scholar]
- Wonnacott S, Kaiser S, Mogg A, Soliakov L, Jones IW. Presynaptic nicotinic receptors modulating dopamine release in the rat striatum. European Journal of Pharmacology. 2000;393(1-3):51–58. doi: 10.1016/s0014-2999(00)00005-4. [DOI] [PubMed] [Google Scholar]
- Wonnacott S, Sidhpura N, Balfour DJ. Nicotine: from molecular mechanisms to behaviour. Current Opinion in Pharmacology. 2005;5(1):53–59. doi: 10.1016/j.coph.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Wooltorton JRA, Pidoplichko VI, Broide RS, Dani JA. Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. Journal of Neuroscience. 2003;23(8):3176–3185. doi: 10.1523/JNEUROSCI.23-08-03176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F, Bayram E, Sumithran SP, Ayers JT, Zhan CG, Schmitt JD, Dwoskin LP, Crooks PA. QSAR modeling of mono- and bis-quaternary ammonium salts that act as antagonists at neuronal nicotinic acetylcholine receptors mediating dopamine release. Bioorganic & Medicinal Chemistry. 2006;14(9):3017–3037. doi: 10.1016/j.bmc.2005.12.036. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Lockman PR, Geldenhuys WJ, Allen DD, Dwoskin LP, Crooks PA. Synthesis of bis-pyridinium analogs and structurally related cyclophanes with high affinity for the blood brain barrier choline transporter. American Association Pharmaceutical Scientists. 2005;7(S2):W4079. PharmSci (Supplement) Abstract. [Google Scholar]
- Zhang Z, Pivavarchyk M, Deaciuc AG, Dwoskin LP, Crooks PA. Tetrakis-quaternary ammonium salts: novel bPiDDB analogs as selective neuronal nicotinic receptor antagonists for subtypes mediating nicotine-evoked dopamine release. American Association Pharmaceutical Scientists. 2007 Abstract. [Google Scholar]
- Zhou FM, Liang Y, Dani JA. Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nature Neuroscience. 2001;4(12):1224–1229. doi: 10.1038/nn769. [DOI] [PubMed] [Google Scholar]
- Zoli M, Moretti M, Zanardi A, McIntosh JM, Clementi F, Gotti C. Identification of the nicotinic receptor subtypes expressed on dopaminergic terminals in the rat striatum. Journal of Neuroscience. 2002;22(20):8785–8789. doi: 10.1523/JNEUROSCI.22-20-08785.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwart R, Vijverberg HP. Four pharmacologically distinct subtypes of alpha4beta2 nicotinic acetylcholine receptor expressed in Xenopus laevis oocytes. Molecular Pharmacology. 1998;54(6):1124–1131. [PubMed] [Google Scholar]