Abstract
Thrombospondin (TSP)-1 is a matricellular glycoprotein with immunoregulatory properties, which include inhibition of APC function. We show in transplantation that TSP-1 inhibits T cell allosensitization and consequently suppresses immune rejection. This was revealed by comparing wild-type (WT) versus TSP-1 null allografts in corneal transplantation, as the cornea is a rich source of TSP-1. Compared with only 50% of rejected WT allografts, nearly all TSP-1 null allografts succumbed to rejection. This effect was reflected by donor-derived APCs, which exhibited a distinctively greater capacity for allosensitization in transplanted hosts. Corroborated in MLRs, greater proliferation levels and robust IFN-γ (but not IL-10)–positive T cells resulted from stimulation by TSP-1 null APCs relative to WT ones. Moreover, enhanced expression of MHC class II and B7 maturation markers were detected on TSP-1 null APCs during inflammation. Increased expression of CCR7 was further matched by enhanced lymph node migration of TSP-1 null APCs posttransplantation. We therefore conclude that APC-derived TSP-1 suppresses their capacity to allosensitize T cells, and this regulation stems from their resistance to taking on a mature form. Future strategies targeting APCs for TSP-1 upregulation may thus be effective in promoting allograft survival.
Mounting evidence has qualified the matricellular glycoprotein (1), thrombospondin (TSP)-1, as a key immunoregulatory factor (2). Appreciation of this important role stems from Crawford et al. (3), who elegantly demonstrated that TSP-1 is an important activator of latent TGF-β, and thus deletion of TSP-1 in mice invariably leads to spontaneous multi-organ inflammation. Furthermore, the significance of APCs in orchestrating such events was later identified, because tethering of TSP-1 to APCs via CD36 is paramount for TGF-β activation and function (4, 5). Other reports further established that ligation of TSP-1 by APCs (via CD36 and/or CD47) regulates their function (6–10). This level of regulation was found to include downregulation of TNF-α and IL-12, with concomitant upregulation of IL-10 expression (6, 8), as well as a consequent reduced capacity for such APCs to sensitize and mount a T cell (Th1) reaction (6, 7).
Little is known, however, about the role of TSP-1 in transplantation, and this is particularly the case regarding whether it has any influence on the allostimulatory capacity of APCs and consequent immune rejection. To address this, we compared alloimmunity with TSP-1 null versus wild-type (WT) allografts by utilizing the corneal transplantation model, because TSP-1 is constitutively expressed by the cornea (11, 12). Apart from this, donor corneas placed on inflamed graft beds lead to allosensitization via the “indirect” pathway (i.e., mediated by host-derived APCs), as well significant allosensitization via the “direct” pathway (mediated by donor-derived APCs carried over in the graft) (13), thus making this an excellent model for the purposes of this study.
We found that TSP-1 is a potent suppressor of immune rejection and does so via downregulating the capacity of APCs to allosensitize T cells. Although TSP-1 is known to be expressed by various cell types [e.g., platelets, fibroblasts, endothelial cells, and immune cells (1, 8)], our data point toward the contribution of APC-derived TSP-1 in mediating this suppressive effect. Furthermore, this level of regulation appeared to stem from a resistance (conferred by their TSP-1 expression) of APCs to take on a phenotypically and functionally mature form. Thus, we have identified an important function for TSP-1 in the transplant setting, and future strategies targeting upregulation of TSP-1 in APCs could be an effective means to promote allograft survival.
Materials and Methods
Animals and anesthesia
C57BL/6 and BALB/c male mice 8–12 wks of age were purchased from Taconic Farms (Hudson, NY). Male TSP-1 null mice (C57BL/6 background), originally received from Dr. J. Lawler (Beth Israel Deaconess Medical Center, Boston, MA), were bred in-house at Schepens Eye Research Institute (Boston, MA). All mice were housed in a specific pathogen-free environment. Moreover, all animals were treated according to guidelines established by the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research, and the Public Health Policy on Humane Care and Use of Laboratory Animals (U.S. Public Health Review). All procedures were approved by the Institutional Animal Care and Use Committee. Anesthesia was used for all surgical procedures with i.p. administered ketamine/xylazine suspensions (120 and 20 mg/kg, respectively).
Corneal transplantation
The corneal transplantation procedure has been detailed elsewhere (14). In brief, inflamed graft beds were used in this study, because they lead to maximal levels of allosensitization. This was accomplished by placement of three figure-of-eight suture knots with 11–0 nylon sutures (Sharpoint; Vanguard, Houston, TX) along the circumference of a 1.5-mm central trephine demarcation. Each knot consisted of two intrastromal incursions ~120° apart, extending apically from slightly above the limbus to the trephine demarcation. After 14 d, sutures were removed and penetrating corneal transplantation was performed. To accomplish this, we excised the central cornea (2-mm diameter) from a healthy donor mouse and placed it on ice in Optisol-GS (Bausch & Lomb Surgical, Irvine, CA). A 1.5-mm trephine was used to mark the central cornea of recipient BALB/c mice, which were subsequently excised to create the host bed. The donor button was then placed onto the host bed and secured with eight interrupted 11–0 nylon sutures. Graft survival was evaluated in nonmasked fashion using a slit-lamp biomicroscope and was performed twice a week over the course of 8 wk (15).We used a standardized opacity-grading scheme (range, 0–5+) to identify rejection. This was defined as two consecutive time points with a score of ≥2+ (i.e., a level of graft opacity that occludes clear recognition of iris detail).
Mixed leukocyte reaction
BALB/c responder T cells were harvested 2 wk posttransplantation from cervical and submandibular lymph nodes (LNs), as previously described (15). T cells were magnetically sorted with CD90 Ab (Miltenyi Biotec, Auburn, CA) according to the manufacturer’s instructions. APCs (or stimulators) were isolated from the spleen of C57BL/6 mice and enriched by depletion of splenic T cells via magnetic sorting with anti-CD90 Abs according to manufacturer’s protocol. APCs were then incubated overnight (37°C and 5.0% CO2) with 50 ng/ml TNF-α (Millipore, Billerica, MA) in 10% FBS supplemented RPMI 1640 (Bio Whittaker, Walkersville, MD). Stimulators were then thoroughly washed and subsequently cocultured with responder T cells at a 1:1 ratio for 4 d in triplicate using U-bottom 96-well plates. Approximately 16 h before termination of cultures, BrdU reagent (Sigma-Aldrich, St. Louis, MO) was added according to manufacturer’s protocol. Alternatively, other samples after 4 d of incubation were stimulated with 50 ng/ml PMA and 1 µg/ml ionomycin (Sigma-Aldrich) for ~10 h with the addition of 0.6 µl/ml Golgi Plug (BD Pharmingen, Franklin Lakes, NJ). Responder cells were collected for subsequent flow cytometry analyses of T cell proliferation or intracellular cytokine expression.
ELISPOT assay
ELISPOT assay was used to delineate the contribution of allosensitization by donor-derived APCs (i.e., direct pathway) and host-derived APCs (i.e., indirect pathway), as described previously (13, 16, 17). In brief, 96-well ELISPOT plates (Whatman Polyfiltronics, Rockland, MA) were coated with 4 µg/ml purified α-IFN-γ Ab (BD Pharmingen) and washed thoroughly before blocking for 1.5 h using 1% BSA. Magnetically sorted T cells were added to wells in a final volume of 200 µl serum-free RPMI 1640 medium loaded with allogenic APCs for 48 h to enumerate direct allosensitization; or, syngeneic APCs together with donor sonicate to enumerate indirect allosensitization. The plates were washed thoroughly with 0.025% Tween 20 and incubated with biotinylated α-IFN-γ Ab (2 µg/ml) for 2 h. Plates were washed thoroughly and incubated for 1 h with avidin-HRP. Lastly, plates were washed with PBS, and spots were developed using the AEC Substrate kit (BD Pharmingen). The resulting spots were counted using a computer-assisted ELISPOT image analyzer (Cellular Technology, Shaker Heights, OH).
Thermal cautery
Application of thermal cautery to the cornea is a standard experimental method of inducing corneal inflammation (16, 18). In brief, using the tip of a handheld thermal cautery (Aaron Medical Industries, St. Petersburg, FL), we applied five superficial burns to the central 50% of the cornea. Corneas were excised 7 d after cautery and digested into single-cell suspensions for subsequent flow cytometric analyses.
Cornea digestion
Single-cell suspensions were prepared from the corneal samples using collagenase digestion, as previously described (15). In brief, corneal buttons were removed and minced into small fragments, followed by digestion with 2 mg/ml collagenase type IV (Sigma-Aldrich) and 0.05 mg/ml DNase I (Roche, Basel, Switzerland) for 1 h at 37°C with agitation. The suspension was then triturated through a 10-ml syringe to homogenize the remaining tissue and filtered through a 70-µm cell strainer. Cells were then used for flow cytometry analysis.
Flow cytometry
All samples underwent Fc receptor blockade via incubation with α-CD16/CD32 (BD Pharmingen) at 4°C in 0.5% BSA (Sigma-Aldrich). Subsequent Ab labeling included α-CD4 (BD Pharmingen), α-IAb (BD Pharmingen), α-CD80 (BioLegend, San Diego, CA), α-CD86 (BioLegend), α-CD80 (BioLegend), and α-CCR7 (BioLegend). For intracellular staining, cells underwent a fixation/permeabilization step (eBioscience, San Diego, CA), followed by a thorough wash and Fc-receptor blockade. Subsequent Ab labeling included α-BrdU (Fast Immune; BD Pharmingen), α-IFN-γ, α-IL-4, α-IL-10, and α-IL-17 (BD Pharmingen). All Abs were analyzed with the appropriate isotype controls.
Ex vivo Hoechst staining of corneal grafts
Grafts were stained for 60 min at 37°C with 5 µM Hoechst 33324 (Invitrogen, Carlsbad, CA) dissolved in culture medium (Biochrom AG, Berlin, Germany) supplemented with 2% FBS. Grafts were subsequently washed three times for 5 min each with sterile PBS. Further removal of excess dye was achieved with alternating short incubations in two separate media. This included a 10-min incubation at 4°C with Optisol-GS, which is a corneal preservation media containing ingredients that reverse tissue swelling (e.g., adenosine triphosphate precursors, chondroitin sulfate, and dextran). After this, grafts were placed in fresh PBS for 20 min at room temperature, which, in contrast, leads to graft swelling (water binds to stromal glycosaminoglycans). Hence alternating washes between Optisol-GS and PBS results in a “pumping” or stromal fluid exchange that effectively rinses excess dye from tissue. This was repeated three times and does not alter graft function, as measured by absence of propidium iodide staining and assessment of corneal endothelial cell morphology via ZO-1 staining (BD Pharmingen).
Statistical analyses
Student t test and ANOVA were calculated for ELISPOT assays. Error bars displayed in figures were calculated from the ±SEM, and p < 0.05 was considered statistically significant. In addition, Kaplan-Meier survival curves and respective log-rank tests were used to compare corneal graft survival.
Results
Graft-derived TSP-1 inhibits immune rejection
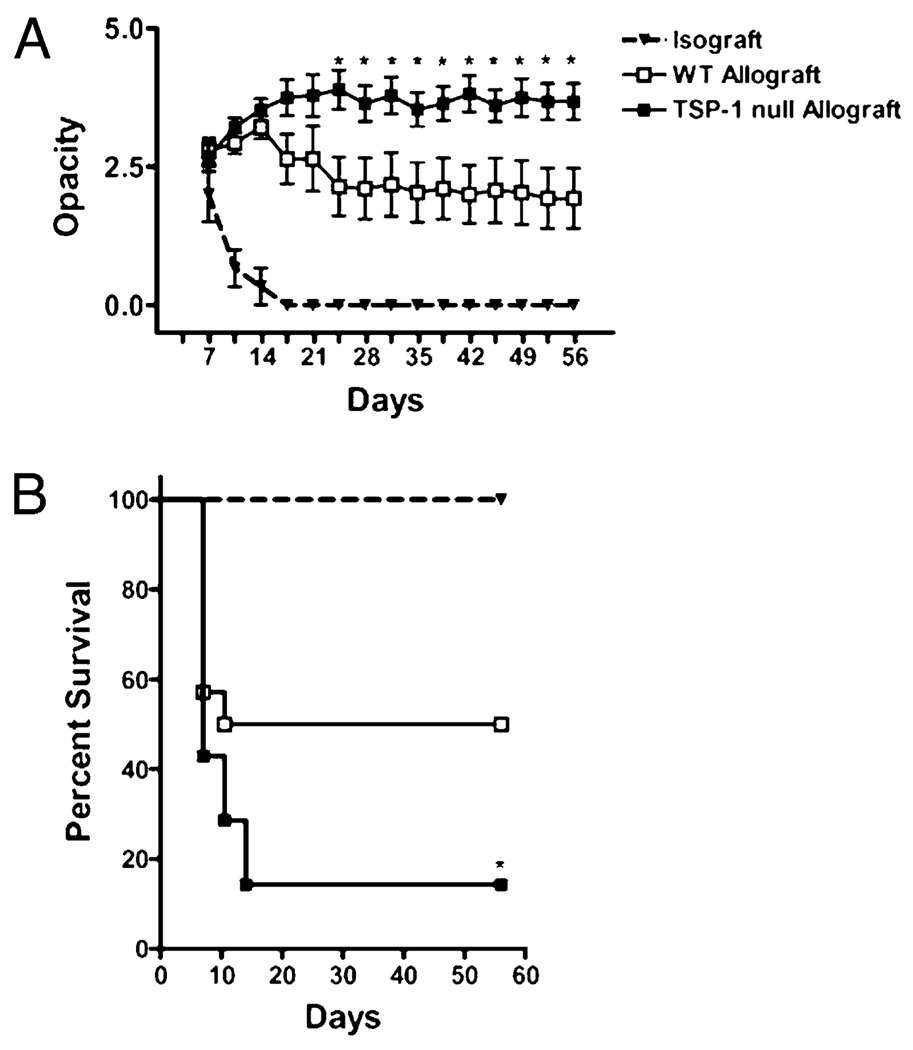
Our initial focus was to determine whether graft-derived TSP-1 plays a role in promoting transplant survival; therefore, we used the corneal transplant model because corneal tissue constitutively expresses TSP-1 (11, 12). BALB/c recipients were transplanted with allografts harvested from WT versus TSP-1 null mice (both strains originating from a C57BL/6 background). Immune rejection was then evaluated on the basis of donor corneal opacity using the standardized scoring scheme, whereby a score of ≥2+ is indicative of immune rejection (15). We found that although the transplantation of WT allografts resulted in an average score of ~2+ from day 24 posttransplantation and onward, TSP-1 null allografts had a significantly greater average score of ~4+ during this period (Fig. 1A). Kaplan-Meier survival curves indicated that only 50% of WT allografts succumbed to rejection compared with more than 90% rejection of TSP-1 null allografts (p = 0.037; Fig. 1B), thus suggesting that graft-derived TSP-1 is a potent inhibitor of allograft rejection.
FIGURE 1.
Graft-derived TSP-1 protects transplants from immune rejection. BALB/c mice were transplanted with corneal isografts (BALB/c; triangles), WT allografts (C57BL/6; open squares), or TSP-1 null allografts (C57BL/6; filled squares), and graft survival was followed biomicroscopically for 8 wk (n = 14 per group). A, Immune rejection was evaluated on the basis of donor corneal opacity using the standardized scoring scheme of 0 to 5+ (0 indicates a clear cornea; 5+ indicates an opaque cornea), whereby a score of ≥2+ is considered rejected. *p < 0.05 WT versus TSP-1 null allografted groups. B, Kaplan-Meier survival curves indicate that WT allografts enjoy a marked enhancement in allograft survival. *p = 0.037.
Graft-derived TSP-1 negatively regulates direct, but not indirect type, allosensitization
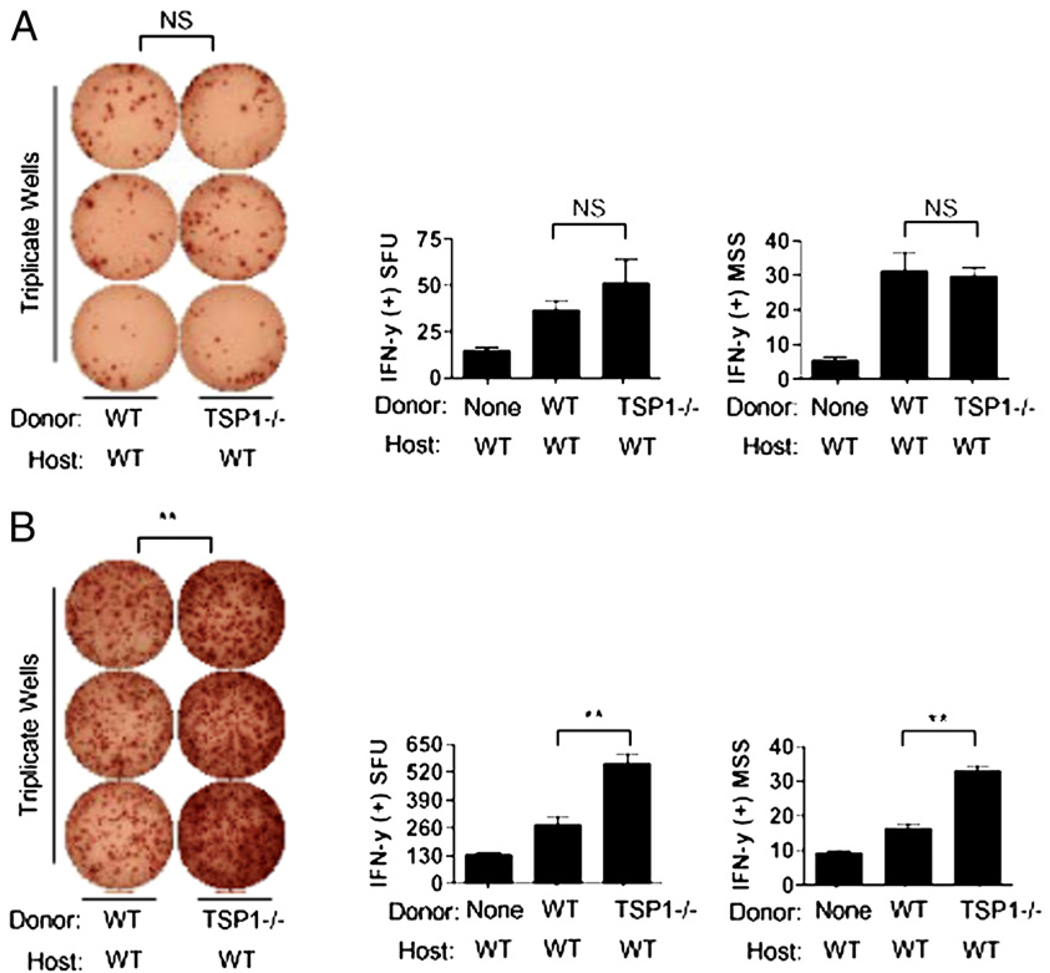
We next evaluated whether the protection afforded to transplants by graft-derived TSP-1 is reflected by decreased levels of allosensitization. The ELISPOT assay (IFN-γ) was used for measurement of alloreactive T cell frequencies posttransplantation of WT versus TSP-1 null allografts. Recipient T cells were harvested posttransplantation from LNs, as previously published (15). We enumerated indirectly allosensitized T cells, because this is the dominant pathway of allorecognition in corneal transplantation (13, 16, 19). Recipient T cells were thus stimulated in culture using host-matching APCs (BALB/c) pulsed with donor allopeptides (C57BL/6), as previously described (16, 17, 19). In using this system, somewhat to our surprise, we observed no significant differences between the groups (Fig. 2A). Transplantation of WT or TSP-1 null allografts led to similar frequencies of indirectly allosensitized T cells (i.e., spot-forming units; p > 0.05) and levels of IFN-γ secretion from these T cells (i.e., mean spot size; p > 0.05), thus suggesting that graft-derived TSP-1 has no significant effect on allosensitization mediated by host derived-APCs.
FIGURE 2.
Downregulation of direct, but not indirect, type allosensitization conferred by graft-derived TSP-1. A, Absence of graft-derived TSP-1 has no significant effect on indirect allosensitization. Magnetically sorted T cells from allografted hosts (n = 10 per group) 2 wk posttransplantation were cocultured with host-matching APCs loaded with donor allopeptides to measure the frequencies of indirectly allosensitized T cells. Both the SFUs and MSS were enumerated after 48-h incubation. B, Absence of graft derived TSP-1 markedly enhances direct allosensitization. T cells collected identically from allografted hosts (n = 10 per group) were cocultured with donor-matching APCs for 48 h to measure frequencies of directly allosensitization. Data are representative of two experiments. **p < 0.05. MSS, mean spot size; SFU, spot-forming units.
We next measured the direct pathway of allosensitization, which is mediated by donor-derived APCs. We again used the ELISPOT assay (IFN-γ), but stimulated recipient T cells in culture with donor-matching APCs (16, 17, 19). This allows specific enumeration of directly allosensitized T cells after the transplantation of WT versus TSP-1 null allografts. Using this system, we found a marked and significant increase in direct allosensitization in TSP-1 null allografted recipients compared with WT allografted ones (Fig. 2B). This was indicated both by increased alloreactive T cell frequencies (p < 0.05) and levels of IFN-γ secreted by such T cells (p < 0.05). Thus, taken together, these data suggest that graft-derived TSP-1 has a distinct regulatory effect on allosensitization mediated by donor-derived APCs.
Important contribution of TSP-1 derived from APCs in regulating allosensitization
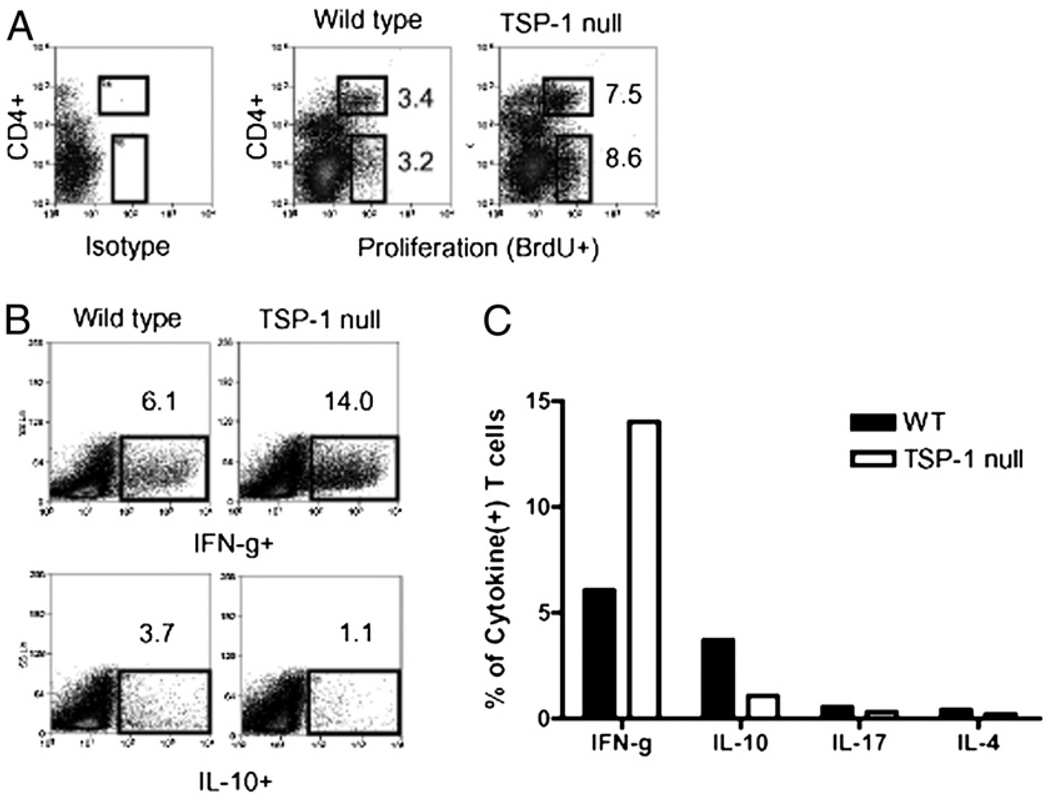
We next examined the specific contribution of TSP-1 derived from APCs in regulating allosensitization, because APCs, as well as nonleukocyte populations, are significant sources of TSP-1 (2, 12, 20). To accomplish this, we set up primary MLRs, whereby WT versus TSP-1–deficient APCs (C57BL/6) were used to stimulate naive allogeneic T cells (WT BALB/c). Furthermore, APC stimulators were pretreated with TNF-α (and subsequently washed thoroughly) to model the proinflammatory environment seen in graft beds during transplantation (21). Using this assay, we found that TSP-1–deficient APCs led to a 2.5-fold increase in T cell proliferation. This consisted of a 2.2- and 2.7-fold increase in the CD4+ and CD4− T cell compartments, respectively (Fig. 3A). Furthermore, we evaluated the cytokine expression of expanded T cells via intracellular flow cytometry and found that TSP-1 null APCs led to a 2.3-fold increase in IFN-γ+ T cell (i.e., Th1) frequencies compared with WT APC stimulation. Moreover, TSP-1 null APCs concomitantly downregulated IL-10+ T cell frequencies by 3.4-fold compared with WT APC stimulation (Fig. 3B). We also examined comparative levels of IL-17+ and IL-4+ T cells, but frequencies were less than 0.5% in both groups and thus considered marginal (Fig. 3C). Hence, taken together, these data suggest that TSP-1 derived from APCs regulates their capacity to sensitize alloreactive Th1 cells.
FIGURE 3.
Contribution of APC-derived TSP-1 in regulating their capacity to allosensitize T cells. A, TSP-1–deficient APCs stimulate increased allogeneic T cell proliferation in primary MLRs. Allogeneic (BALB/c) T cells were cocultured with WT versus TSP-1 null APCs (C57BL/6), and T cell proliferation (CD4+ and CD4− fractions) was subsequently measured by flow cytometry for BrdU incorporation. B, APCs deficient of TSP-1 stimulate hyperresponsive Th1 alloimmunity. WT versus TSP-1 null APCs were cocultured with naive allogeneic T cells, and Th subsets were enumerated via flow cytometric analysis of intracellular cytokines. C, Stimulation of IL-17(+) and IL-4(+) T cells in primary MLRs were marginal in both groups. Data are representative of three experiments, and APC stimulators for each experiment were harvested from a pool of at least three individual mice.
TSP-1 derived from APCs confers their resistance to phenotypic maturation during inflammation
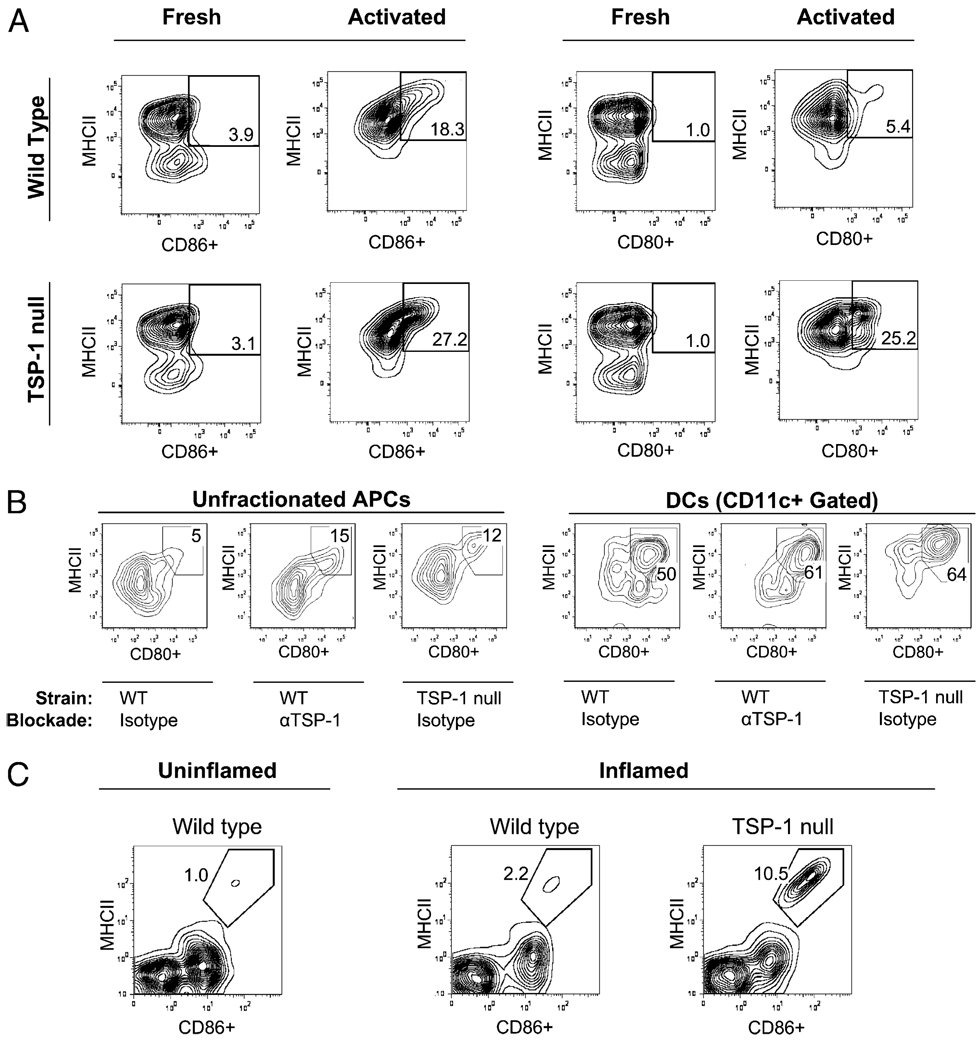
It is widely recognized that immature or maturation “resistant” APCs are poor at T cell sensitization (22, 23), and we thus wondered whether reduced T cell stimulation conferred by APC-derived TSP-1 is contributed by their resistance to phenotypic maturation. To assess this, we used flow cytometry for measurement of WT versus TSP-1–deficient APC expression of maturation markers, including MHC class II (MHC II), CD80, and CD86. We found that the levels of TSP-1–deficient APCs exhibiting a fully mature phenotype (i.e., MHC II+ CD80+ or MHC II+ CD86+) were comparable with WT APCs in the steady state. However, after in vitro exposure to TNF-α, these levels for TSP-1–deficient APCs were markedly greater than those detected in identically treated WT APCs (Fig. 4A). Furthermore, enhanced expression of these markers was also detected on TSP-1 neutralization in WT APCs using an anti–TSP-1 Ab (clone A4.1; 10 µg/ml) (8) (Fig. 4B), thus suggesting that TSP-1 derived from APCs inhibits their maturation during in vitro inflammatory stimuli.
FIGURE 4.
TSP-1 expressed by APCs confers resistance to phenotypic maturation in inflammation. A, In vitro activation of TSP-1 null APCs results in enhanced frequencies of fully matured APCs. APCs were collected fresh from WT versus TSP-1 null mice (n = 3 mice per group) or subsequently activated for 72 h in vitro (TNF-α, 50 ng/ml) and evaluated via flow cytometry. B, In vitro neutralization of TSP-1 via Ab blockade similarly leads to enhanced maturation of APCs, including DCs. Activated APCs for 24 h were treated with TSP-1 neutralizing Ab (10 µg/ml) or isotype control Ab (10 µg/ml) and evaluated via flow cytometry. C, In vivo inflammation in TSP-1 null mice also leads to enhanced frequencies of fully mature APCs. Thermal cautery was administered to the corneas of WT versus TSP-1 null mice (n = 8 corneas pooled per group). Corneas were subsequently collected, digested enzymatically, and evaluated by flow cytometry. Data are representative of at least two experiments.
To address whether APC-derived TSP-1 has a similar inhibitory effect on APC maturation in vivo, we administered an inflammatory challenge to corneas of WT versus TSP-1 null mice and subsequently assessed APC maturation via flow cytometry. We used a standardized method involving thermal cautery to induce corneal inflammation (18) [where TNF-α plays a significant role (18, 21)]. One week post-cautery, corneas were collected and digested enzymatically into single-cell suspensions for flow cytometry assessment. Because our previous in vitro data indicated that the frequency of fully mature APCs in the steady-state is similar in both WT and TSP-1–deficient APCs, we compared steady-state WT corneas with inflamed WT versus TSP-1 null corneas. Using this approach, we found that relative to uninflamed WT corneas, there was only a 2-fold increase in the number of fully mature APCs from inflamed WT corneas, whereas a 10-fold increase was observed in inflamed TSP-1 null corneas (Fig. 4B). Thus, our results indicate that TSP-1 expression by APCs inhibits their maturation in response to in vivo inflammation.
Impaired CCR7 upregulation and lymph node homing by APC-derived TSP-1
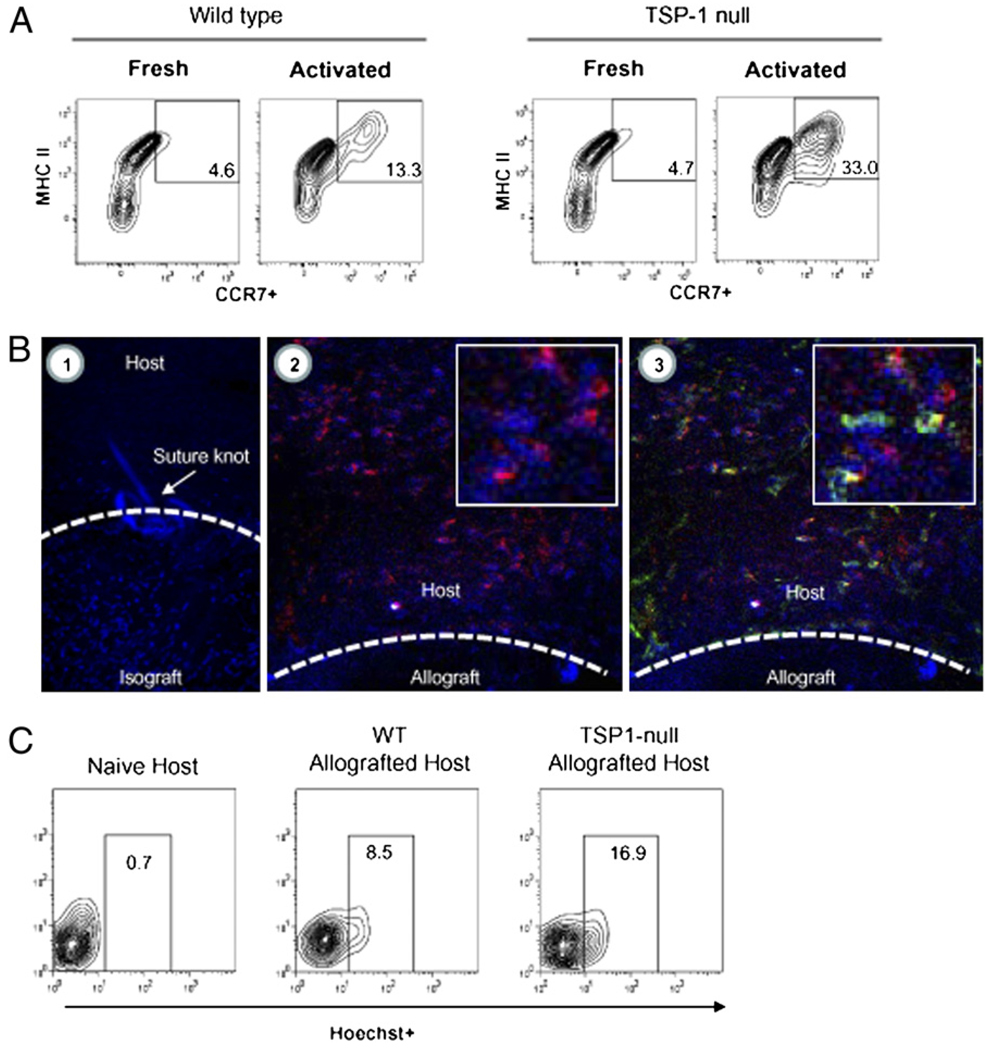
Upregulation of CCR7 facilitates APC homing to LNs via CCL19/21 gradients, and thus maximizes consequent contact with naive T cells for allosensitization (24). Because upregulation of CCR7 coincides with APC maturation, we assessed whether APC-derived TSP-1 plays a role in influencing their CCR7 expression. To accomplish this, we used flow cytometry to enumerate MHC II+ APCs in the steady-state and after in vitro exposure to TNF-α of WT versus TSP-1–deficient APCs. Consistent with our previous in vitro findings, we found that the level of TSP-1–deficient APCs exhibiting a CCR7+ phenotype was comparable with WT APCs in the steady-state. However, after in vitro exposure to TNF-α, these levels for TSP-1–deficient APCs were markedly greater than those detected in identically treated WT APCs (Fig. 5A), thus suggesting that TSP-1 derived from APCs inhibits their CCR7 upregulation to in vitro inflammatory stimuli.
FIGURE 5.
Impaired CCR7 upregulation and LN homing exhibited by APC-derived TSP-1. A, In vitro activation of TSP-1 null APCs leads to high frequency of MHC II+ cells expressing CCR7. APCs were collected fresh from WT versus TSP-1 null mice (n = 3 mice per group) or subsequently activated in vitro (TNF-α, 50 ng/ml) for flow cytometric evaluation. B, Ex vivo staining of corneal grafts with Hoechst vital dye tracks the egress of donor APCs in vivo posttransplantation (original magnification ×400). Stained isografts (BALB/c → BALB/c) evaluated several hours posttransplantation demonstrates that ex vivo staining (blue; B1) is mostly restricted to the graft and not the host bed. In contrast, stained allografts (C57BL/6, IAb → BALB/c, IAd) evaluated at 24 h posttransplantation demonstrate that exiting donor cells in the host bed are largely CD45+ (red; B2) and express donor IAb (green; B3) at variable levels (inlays are respective digitally enlarged portions of host beds). C, TSP-1 null allografts lead to a marked increase of donor-derived APCs in regional LNs. Donor allografts were stained ex vivo with Hoechst and washed thoroughly for subsequent transplantation. LNs of naive or allografted hosts (n = 6 per group) were collected 24 h posttransplantation, and the CD11c+ fraction was enumerated for donor-derived APCs (Hoechst+). Data are representative of multiple experiments.
We next examined whether TSP-1 derived from APCs affects their LN homing function in the transplantation setting. We adopted a method described by Collins et al. (25) to do so, whereby grafts are stained with a vital dye ex vivo before transplantation for subsequent tracking of donor APCs in host LN. We used the vital nuclear dye Hoechst 33342, which is commonly used for labeling and tracking immune cells in vivo; we further determined that it has no adverse effects on corneal transplants (data not shown). To evaluate the efficacy of this technique in tracking donor APCs in corneal transplantation, we first stained syngeneic grafts (i.e., isografts) that were evaluated several hours posttransplantation via confocal microscopy. We found that Hoechst+ cells were largely restricted to the graft, and thus indicated the absence of unintended secondary or “leaky” staining of host tissues (Fig. 5B). We also evaluated ex vivo stained allografts 24 h posttransplantation, and confirmed that exiting donor cells (Hoechst+) are bone marrow derived (CD45+) and express (at least at variable levels) donor-derived MHC II (I-Ab) (Fig. 5B).
Thus, to next determine whether APC-derived TSP-1 affects their LN homing function in the transplantation setting, WT versus TSP-1 null allografts were stained ex vivo, and regional LNs were assessed by 24 h posttransplantation, as previously shown (26).We used flow cytometry to enumerate the percentage donor APCs (Hoechst+) in the dendritic cell (DC) (CD11c+) fraction. By using this approach, we found in WT allografted recipients that 8.6% of LN APCs were donor derived. Strikingly, in TSP-1 null allografted recipients, 16.9% of LN APCs were donor derived (Fig. 5C), thus suggesting that TSP-1 derived from APCs regulates their migration to regional LNs in transplantation.
Discussion
To our knowledge, we show for the first time that APC-derived TSP-1 is an important mechanism by which allosensitization is regulated in transplantation. Our data suggest that this level of regulation by APCs stems from their resistance (conferred by TSP-1 expression) to acquiring a phenotypically and functionally mature form, and this observation was consistent when we assayed the DC population specifically. This is a key finding, because the absence of graft-derived TSP-1 leads to markedly greater rates of immune rejection.
We originally hypothesized, in explaining this significant effect seen on graft survival, that graft-derived TSP-1 would downregulate both direct (i.e., donor APCs) and indirect (i.e., host APCs) pathways of allosensitization. This was a logical hypothesis given that corneal cells (e.g., corneal epithelial and endothelial cells) constitutively express TSP-1 (11, 12, 20)—levels that are further upregulated in corneal injury (20). Hence, absence of corneal TSP-1 exposure of donor APCs (that originate from the graft) and host APCs (that infiltrate the graft) in TSP-1 null allografts was conversely anticipated to increase their respective capacities for consequent allosensitization. However, we found that although TSP-1 null allografts caused increased levels of direct allosensitization as anticipated, there was surprisingly no significant change seen in indirect allosensitization. Hence these data indicate that graft-derived TSP-1 regulates allosensitization mediated by donor APCs only, and thus raises the possibility that the relevant source of TSP-1 in corneal grafts is donor APCs. This is in accord with Doyen et al. (8), who demonstrated that APCs such as DCs are a significant source of TSP-1.
By using primary MLRs, which mimic allosensitization in vitro, we were able to confirm the important contribution of APC-derived TSP-1 in regulating their capacity for allosensitization. When TSP-1 null APCs were cocultured with naive allogeneic T cells, we observed a marked increase in levels of T cell proliferation, and the frequency of IFN-γ+ T cells was 2-fold greater than in those cultures stimulated with WT APCs. We also observed that this increased expansion of IFN-γ+ T cells stimulated by TSP-1 null APCs coincided with a marked decrease in IL-10+ T cell frequencies. This finding may, therefore, indicate a potential mechanism by which APC-derived TSP-1 regulates allosensitization, by expanding IL-10+ T cells, which have been shown to suppress T cell proliferation and promote corneal transplant survival (27). This is in accord with Bouguermouh et al. (28) and Grimbert et al. (29), who respectively showed that ligation of TSP-1 on T cells limits Th1 expansion.
Muted T cell responses are also likely to be explained by our observations that APC-derived TSP-1 confers their resistance to acquiring a phenotypically and functionally mature form. This was initially revealed to us by stimulating TSP-1–deficient APCs with TNF-α in vitro, which led to a very high frequency of fully matured APCs (i.e., MHC II+ CD80+/CD86+; MHC II+ CCR7+), and this observation was consistent in the DC (CD11c+ fraction) as well, which similarly showed increased frequencies of MHC II+ CD80+ profiles. Furthermore, we found importance of this process in vivo, as an inflammatory challenge to cornea of TSP-1 null mice led to increased numbers of fully matured APCs. Also relevant in vivo, enhanced CCR7 expression in TSP-1 null APCs was consistent with the 2-fold increase in regional LN homing of donor APCs found posttransplantation of TSP-1 null allografts. Together, these data support enhanced direct allosensitization observed in such recipients of TSP-1 null allografts.
Also notable is that similar to donor APCs in transplantation, our data suggest that host-derived APCs are likely to express and be regulated by TSP-1 as well. Indeed, APCs that infiltrate tissue in inflammation are largely derived from peripheral blood monocytic cells, and Doyen et al. (8) demonstrated that APCs originating from peripheral blood monocytic cells express TSP-1 and upregulate its expression in response to inflammatory stimuli. Consistent with this, we observed that an in vivo inflammatory challenge to corneas of TSP-1 null mice results in strong increases in the number of fully matured APCs present in the inflamed cornea. These observations suggest not only that maturation of infiltrated APCs in inflammation are regulated by TSP-1, but that this process of TSP-1–mediated regulation of infiltrated APCs is relevant in corneal tissue. Therefore, it is quite likely that, in corneal transplantation, equivalent TSP-1 expression in host APCs is likely to explain the unaltered indirect allosensitization observed in WT recipients receiving WT or TSP-1 null allografts. Furthermore, because corneal endothelial and epithelial cells also express TSP-1, it is likely that such non-APC sources may contribute to this effect as well.
TSP-1 also functions as an inhibitor of angiogenesis, and as such was shown to promote failure of islet and skin grafts via limiting revascularization required for their blood supply (30, 31). However, the process of revascularization is irrelevant in corneal transplantation, because this tissue is normally avascular. Although pathologic corneal angiogenesis can occur, surmounting evidence including a recent report by Dietrich et al. (32), indicates corneal angiogenesis not to be critical in mediating corneal graft rejection. Furthermore, we observed no significant differences in corneal angiogenesis in WT versus TSP-1 null allografts (data not shown), and thus the angiostatic role of TSP-1 appears to be of limited significance in this study.
In summary, TSP-1–derived APCs, which include but may not be limited to DCs, are an important means by which allosensitization and consequent immune rejection are inhibited. Focusing our examinations on donor APCs revealed that this regulation stems from their resistance to become mature, which consequently makes them less likely to migrate to regional LNs and inhibits their capacity to directly allosensitize T cells. Importance of this mechanism is further highlighted by the understanding that the indirect pathway is indeed dominant in corneal transplantation (13, 15–17, 19); however, TSP-1 null allografts led to significant enhancement of direct allosensitization and markedly greater levels of rejection. Thus, future strategies aimed at upregulating TSP-1 expression specifically by APCs may, therefore, be effective in promoting transplant survival.
Acknowledgments
This work was supported by National Institutes of Health/National Eye Institute Grants F32EY018292 (to D.R.S.) and R01EY012963 (to R.D.).
Abbreviations used in this paper
- DC
dendritic cell
- LN
lymph node
- MHC II
MHC class II
- MSS
mean spot size
- SFU
spot-forming units
- TSP
thrombospondin
- WT
wild type
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Adams JC, Lawler J. The thrombospondins. Int. J. Biochem. Cell Biol. 2004;36:961–968. doi: 10.1016/j.biocel.2004.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarfati M, Fortin G, Raymond M, Susin S. CD47 in the immune response: role of thrombospondin and SIRP-alpha reverse signaling. Curr. Drug Targets. 2008;9:842–850. doi: 10.2174/138945008785909310. [DOI] [PubMed] [Google Scholar]
- 3.Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, Boivin GP, Bouck N. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell. 1998;93:1159–1170. doi: 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- 4.Yehualaeshet T, O‘Connor R, Green-Johnson J, Mai S, Silverstein R, Murphy-Ullrich JE, Khalil N. Activation of rat alveolar macrophage-derived latent transforming growth factor beta-1 by plasmin requires interaction with thrombospondin-1 and its cell surface receptor, CD36. Am. J. Pathol. 1999;155:841–851. doi: 10.1016/s0002-9440(10)65183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masli S, Turpie B, Streilein JW. Thrombospondin orchestrates the tolerance-promoting properties of TGFbeta-treated antigen-presenting cells. Int. Immunol. 2006;18:689–699. doi: 10.1093/intimm/dxl006. [DOI] [PubMed] [Google Scholar]
- 6.Demeure CE, Tanaka H, Mateo V, Rubio M, Delespesse G, Sarfati M. CD47 engagement inhibits cytokine production and maturation of human dendritic cells. J. Immunol. 2000;164:2193–2199. doi: 10.4049/jimmunol.164.4.2193. [DOI] [PubMed] [Google Scholar]
- 7.Marteau F, Gonzalez NS, Communi D, Goldman M, Boeynaems JM, Communi D. Thrombospondin-1 and indoleamine 2,3-dioxygenase are major targets of extracellular ATP in human dendritic cells. Blood. 2005;106:3860–3866. doi: 10.1182/blood-2005-05-1843. [DOI] [PubMed] [Google Scholar]
- 8.Doyen V, Rubio M, Braun D, Nakajima T, Abe J, Saito H, Delespesse G, Sarfati M. Thrombospondin 1 is an autocrine negative regulator of human dendritic cell activation. J. Exp. Med. 2003;198:1277–1283. doi: 10.1084/jem.20030705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tabib A, Krispin A, Trahtemberg U, Verbovetski I, Lebendiker M, Danieli T, Mevorach D. Thrombospondin-1-N-terminal domain induces a phagocytic state and thrombospondin-1-C-terminal domain induces a tolerizing phenotype in dendritic cells. PLoS One. 2009;4:e6840. doi: 10.1371/journal.pone.0006840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kukreja A, Radfar S, Sun BH, Insogna K, Dhodapkar MV. Dominant role of CD47-thrombospondin-1 interactions in myeloma-induced fusion of human dendritic cells: implications for bone disease. Blood. 2009;114:3413–3421. doi: 10.1182/blood-2009-03-211920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cursiefen C, Masli S, Ng TF, Dana MR, Bornstein P, Lawler J, Streilein JW. Roles of thrombospondin-1 and -2 in regulating corneal and iris angiogenesis. Invest. Ophthalmol. Vis. Sci. 2004;45:1117–1124. doi: 10.1167/iovs.03-0940. [DOI] [PubMed] [Google Scholar]
- 12.Sekiyama E, Nakamura T, Cooper LJ, Kawasaki S, Hamuro J, Fullwood NJ, Kinoshita S. Unique distribution of thrombospondin-1 in human ocular surface epithelium. Invest. Ophthalmol. Vis. Sci. 2006;47:1352–1358. doi: 10.1167/iovs.05-1305. [DOI] [PubMed] [Google Scholar]
- 13.Huq S, Liu Y, Benichou G, Dana MR. Relevance of the direct pathway of sensitization in corneal transplantation is dictated by the graft bed microenvironment. J. Immunol. 2004;173:4464–4469. doi: 10.4049/jimmunol.173.7.4464. [DOI] [PubMed] [Google Scholar]
- 14.Dastjerdi MH, Saban DR, Okanobo A, Nallasamy N, Sadrai Z, Chauhan SK, Hajrasouliha AR, Dana R. Effects of topical and subconjunctival bevacizumab in high-risk corneal transplant survival. Invest. Ophthalmol. Vis. Sci. 2010;51:2411–2417. doi: 10.1167/iovs.09-3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saban DR, Chauhan SK, Zhang X, El Annan J, Jin Y, Dana R. ‘Chimeric‘ grafts assembled from multiple allodisparate donors enjoy enhanced transplant survival. Am. J. Transplant. 2009;9:473–482. doi: 10.1111/j.1600-6143.2008.02535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen L, Jin Y, Freeman GJ, Sharpe AH, Dana MR. The function of donor versus recipient programmed death-ligand 1 in corneal allograft survival. J. Immunol. 2007;179:3672–3679. doi: 10.4049/jimmunol.179.6.3672. [DOI] [PubMed] [Google Scholar]
- 17.Benichou G, Valujskikh A, Heeger PS. Contributions of direct and indirect T cell alloreactivity during allograft rejection in mice. J. Immunol. 1999;162:352–358. [PubMed] [Google Scholar]
- 18.Dekaris I, Zhu SN, Dana MR. TNF-alpha regulates corneal Langerhans cell migration. J. Immunol. 1999;162:4235–4239. [PubMed] [Google Scholar]
- 19.Boisgérault F, Liu Y, Anosova N, Dana R, Benichou G. Differential roles of direct and indirect allorecognition pathways in the rejection of skin and corneal transplants. Transplantation. 2009;87:16–23. doi: 10.1097/TP.0b013e318191b38b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uno K, Hayashi H, Kuroki M, Uchida H, Yamauchi Y, Kuroki M, Oshima K. Thrombospondin-1 accelerates wound healing of corneal epithelia. Biochem. Biophys. Res. Commun. 2004;315:928–934. doi: 10.1016/j.bbrc.2004.01.146. [DOI] [PubMed] [Google Scholar]
- 21.Zhu S, Dekaris I, Duncker G, Dana MR. Early expression of proinflammatory cytokines interleukin-1 and tumor necrosis factor-alpha after corneal transplantation. J. Interferon Cytokine Res. 1999;19:661–669. doi: 10.1089/107999099313811. [DOI] [PubMed] [Google Scholar]
- 22.Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23:445–449. doi: 10.1016/s1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- 23.Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat. Rev. Immunol. 2007;7:610–621. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- 24.Förster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat. Rev. Immunol. 2008;8:362–371. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- 25.Collins MK, Tay CS, Erlebacher A. Dendritic cell entrapment within the pregnant uterus inhibits immune surveillance of the maternal/fetal interface in mice. J. Clin. Invest. 2009;119:2062–2073. doi: 10.1172/JCI38714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Hamrah P, Zhang Q, Taylor AW, Dana MR. Draining lymph nodes of corneal transplant hosts exhibit evidence for donor major histocompatibility complex (MHC) class II-positive dendritic cells derived from MHC class II-negative grafts. J. Exp. Med. 2002;195:259–268. doi: 10.1084/jem.20010838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chauhan SK, Saban DR, Lee HK, Dana R. Levels of Foxp3 in regulatory T cells reflect their functional status in transplantation. J. Immunol. 2009;182:148–153. doi: 10.4049/jimmunol.182.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bouguermouh S, Van VQ, Martel J, Gautier P, Rubio M, Sarfati M. CD47 expression on T cell is a self-control negative regulator of type 1 immune response. J. Immunol. 2008;180:8073–8082. doi: 10.4049/jimmunol.180.12.8073. [DOI] [PubMed] [Google Scholar]
- 29.Grimbert P, Bouguermouh S, Baba N, Nakajima T, Allakhverdi Z, Braun D, Saito H, Rubio M, Delespesse G, Sarfati M. Thrombospondin/CD47 interaction: a pathway to generate regulatory T cells from human CD4+ CD25-T cells in response to inflammation. J. Immunol. 2006;177:3534–3541. doi: 10.4049/jimmunol.177.6.3534. [DOI] [PubMed] [Google Scholar]
- 30.Olerud J, Johansson M, Lawler J, Welsh N, Carlsson PO. Improved vascular engraftment and graft function after inhibition of the angiostatic factor thrombospondin-1 in mouse pancreatic islets. Diabetes. 2008;57:1870–1877. doi: 10.2337/db07-0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Isenberg JS, Pappan LK, Romeo MJ, Abu-Asab M, Tsokos M, Wink DA, Frazier WA, Roberts DD. Blockade of thrombospondin-1-CD47 interactions prevents necrosis of full thickness skin grafts. Ann. Surg. 2008;247:180–190. doi: 10.1097/SLA.0b013e31815685dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dietrich T, Bock F, Yuen D, Hos D, Bachmann BO, Zahn G, Wiegand S, Chen L, Cursiefen C. Cutting edge: lymphatic vessels, not blood vessels, primarily mediate immune rejections after transplantation. J. Immunol. 2010;184:535–539. doi: 10.4049/jimmunol.0903180. [DOI] [PMC free article] [PubMed] [Google Scholar]