Abstract
We report the detection and molecular characterization of a rotavirus strain, 10733, isolated from the feces of a buffalo calf affected with diarrhea in Italy. Strain 10733 was classified as a P[3] rotavirus, as the VP8* trypsin cleavage product of the VP4 protein revealed a high amino acid identity (96.2%) with that of rhesus rotavirus strain RRV (P5B[3]), used as the recipient virus in the human-simian reassortant vaccine. Analysis of the VP7 gene product revealed that strain 10733 possessed G6 serotype specificity, a type common in ruminants, with an amino acid identity to G6 rotavirus strains ranging from 88 to 98%, to Venezuelan bovine strain BRV033, and Hungarian human strain Hun4. Phylogenetic analysis based on the VP7 gene of G6 rotaviruses identified at least four lineages and an apparent linkage between each lineage and the VP4 specificity, suggesting the occurrence of repeated interspecies transmissions and genetic reassortment events between ruminant and human rotaviruses. Moreover, strain 10733 displayed a bovine-like NSP4 and NSP5/6 and a subgroup I VP6 specificity, as well as a long electropherotype pattern. The detection of the rare P[3] genotype in ruminants provides additional evidence for the wide genetic and antigenic diversity of group A rotaviruses.
Group A rotaviruses are the main cause of acute viral gastroenteritis in humans and animals throughout the world. Rotaviruses belong to the Reoviridae family and are characterized by a genome consisting of 11 segments of double-stranded RNA (dsRNA) enclosed in a triple-layered capsid (19). The inner capsid protein VP6 bears the subgroup (SG) specificities that allow the classification of group A rotaviruses into SGI, SGII, both SGI and SGII, or neither SG based on the reactivity with SG-specific monoclonal antibodies (19). Rotavirus strains generally possess either the short or long RNA electropherotype (e-type), based on the migration of the 11th dsRNA segment on gels. The majority of animal and human rotavirus strains possess long RNA e-types and belong to SGI and SGII, respectively (36).
Serotype designations are based on independent neutralization determinants on the two outer capsid proteins, VP7 (G serotype) and VP4 (P serotype) (19). On the basis of antigenic and genetic characterization, 15 G serotypes have been identified (19, 36). VP4-specific antisera or monoclonal antibodies raised to VP4 have identified 14 P serotypes, while sequence analysis of VP4 or the VP8* trypsin cleavage product of VP4 have recognized 23 P genotypes (30, 36, 42; Liprandi et al., unpublished data). With a few exceptions (10), strains sharing more than 89% amino acid identity are considered to belong to the same P genotype. Following the guidelines of this typing system, the P serotype is indicated with a number immediately after the letter P, while the genotype is represented by a number in square brackets (19, 30, 36).
One nonstructural protein of relevance is NSP4, which has been studied extensively because of its role in viral morphogenesis and its enterotoxic activity (3, 19). The NSP4 gene of group A rotaviruses may be genetically classified into at least five genogroups, KUN (A)-, Wa (B)-, Au-1 (C)-, EW (D)-, and avian (E)-like (12, 29, 31, 47). Within NSP4 genotypes A and B, rotavirus strains isolated from rabbits, horses, cows, and pigs generally cluster according to the species of origin, suggesting a constant pattern of evolution within species (12). In addition, the 11th dsRNA segment of rotaviruses encodes a phosphoprotein with kinase activity, NSP5, and, via an out-of-phase open reading frame, a smaller protein, NSP6; the NSP5/6 gene has been demonstrated to be suitable to trace the origin of rotaviruses (19, 37).
Rotavirus strains belonging to serotypes G6, G8, and G10, in association with VP4 types P6[1], P7[5], and P8[11], are commonly found in cattle, though strains belonging to G1, G2, G3, and G11 have been detected sporadically (18, 36, 57). An unusual P[17]- and G7-like bovine rotavirus has also been isolated from a calf, presumably the result of interspecies transmission from birds to cows (55). In addition, bovine rotaviruses with novel P[21] and G15 types have recently been identified in India (53). Bovine strains usually segregate in the NSP4 B genogroup, KUN-like (12).
In the present study, we report the isolation and molecular characterization of the VP8*, VP7, VP6, NSP4, and NSP5/6 of an unusual rotavirus strain, 10733, from a 7-day-old buffalo calf affected with severe gastroenteritis in southern Italy. Our results show that buffalo rotavirus strain 10733 possesses typical bovine-like VP6 (SGI), VP7 (G6), NSP4 (KUN-like), and NSP5/6 gene products. However, we report for the first time the identification of a rotavirus strain with an unusually high amino acid identity (96%) to the VP8* trypsin cleavage fragment of VP4, predictive of the P type, of rhesus rotavirus (RRV).
MATERIALS AND METHODS
Virus isolation and evaluation of plaque phenotype.
Rotavirus strain 10733 was isolated in 2001 in southern Italy in a buffalo herd from the feces of a 1-week-old buffalo (Bubalus bubalis) calf with severe diarrhea. Neonatal enteritis, affecting about 20% of the calves, was reported for the herd. Three samples were collected from the herd, and two were found to contain rotavirus-like particles by electron microscopy. An isolate was propagated on African green monkey kidney (MA-104) cells, grown in Dulbecco's modified Eagle's medium (DMEM) (Gibco, Invitrogen Corporation, Grand Island, N.Y.) supplemented with 5 μg of trypsin (Sigma-Aldrich, Milan, Italy) per ml. Evidence of viral replication was checked by both the appearance of cytopathic effect and indirect immunofluorescence (IF) test with a rabbit hyperimmune serum raised against a group A rotavirus (strain Bo/A-125/96 P7[5],G6). After 12 serial passages, the virus was plaque purified three times, and the virus titer was determined by plaque assay with DMEM-0.6% agarose in the presence of 5 μg of trypsin per ml and 2.5% fetal bovine serum and expressed as PFU per ml. Strain 10733 adapted well to growth in MA-104 cells and produced plaques of 0.1 to 1 mm under DMEM-0.6% agarose, reaching titers of 1.5 × 107 PFU/ml by the ninth passage.
Electropherotype determination.
Viral dsRNA was extracted from infected cells showing 50% cytopathic effect. Cryolysates were extracted with Vertrel XF (Du Pont, Wilmington, Del.) as previously described (44) and clarified twice by centrifugation for 30 min at 4,800 × g. Subsequently, the supernatants were centrifuged for 8 h at 85,000 × g on a 30% sucrose layer in an SW28 rotor (Beckman Coulter, Inc., Fullerton, Calif.). After digestion of the viral pellet with 1% sodium dodecyl sulfate and 1 μg of proteinase K (Sigma-Aldrich) per ml, viral dsRNA was extracted with phenol-chloroform, precipitated with ethanol and 0.3 M sodium acetate, and suspended in TE (Tris-EDTA, pH 8.0). The dsRNA e-type was visualized in a TAE (Tris-EDTA-acetate buffer, pH 8.0) gel with 0.5% agarose for resolving and 1.5% agarose for stacking, after electrophoresis at 15 V for 18 h and ethidium bromide staining.
RNA extraction and PCR amplification of the VP7, VP4, VP6, and NSP4.
Viral dsRNA from both the fecal specimen and the 3rd viral passage on MA-104 cells was extracted by adsorption on cellulose CF11 as described previously (59). Viral dsRNA was denatured in dimethyl sulfoxide (Sigma-Aldrich) at 97°C for 5 min. Reverse transcription of dsRNA was carried out with murine leukemia virus reverse transcriptase (Applied Biosystems Italy), while PCR amplification was carried out with AmpliTaq Gold DNA polymerase (Applied Biosystems Italy).
The full-length VP7 gene (1,062 bp) was reverse transcribed and amplified with primer Beg9 (24) and primer End9deg, degenerated on the basis of the 3′ end of the gene of 13 VP7 alleles (GGTCACATCDWMCARYTCTAAYYHM). The VP8* subunit of the VP4, the connecting peptide, and the N terminus of the VP5* subunit of VP4 (876 bp) were reverse transcribed and amplified with primer pair Con2-Con3 (20). The nearly full-length gene (725 bp) encoding the NSP4 protein was amplified with primer pair 10Beg16-10End722 (39), while the NSP5/NSP6 full-length gene (667 bp) was amplified with a primer pair described by Krishna Mohan and Atreya (37). The VP6 genogroup, predictive of the VP6 subgroup specificity, was determined by amplification of a 379-bp fragment, spanning from amino acids 241 to 367 of the VP6, with primer pair VP6F-VP6R (32).
Sequence analysis.
For direct sequencing of PCR amplicons, three distinct amplicons were analyzed and a consensus sequence was determined. Moreover, the VP7- and VP8*-encoding genes of buffalo strain 10733 were cloned into the vector pCRT7/NT-TOPO (Invitrogen BV, Groningen, The Netherlands), and the sequence was determined with three plasmid clones. Sequences were assembled and analyzed with the Bioedited software package (Department of Microbiology, North Carolina State University) (28).
Phylogenetic and molecular evolutionary analyses were conducted with MEGA version 2.1 (Arizona State University) (38). Phylogenetic trees based on the VP7, VP8*, NSP4, and NSP5/6 gene products were elaborated with both parsimony and distance methods, supplying statistical support with bootstrapping over 100 replicates.
Nucleotide sequence accession numbers.
GenBank accession numbers AY281360, AY281359, AY293829, and AY293830 were assigned to VP7, VP8*, NSP4, and NSP5, respectively.
RESULTS
dsRNA electrophoretic pattern.
The e-type of buffalo rotavirus strain 10733 displayed a typical long electrophoretic pattern. When compared to the electropherotypes of reference rotavirus strains in the laboratory, strain 10733 displayed a slightly different pattern in the mobility of segments 4, 7, 8, and 9 with respect to those of canine RV198/95, RV52/96 and CU-1 (P5A[3],G3), porcine S80 (P9[7],G1), and human YO (P1A[8],G3) rotavirus strains (data not shown).
Sequence analyses.
To molecularly analyze buffalo rotavirus strain 10733, the genes encoding the VP7, VP8* trypsin cleavage product of VP4, VP6 SG-specific fragment, NSP4, and NSP5/6 were determined. The basic structure of the VP7-encoding cognate gene of the Italian buffalo strain 10733 was 1,062 nucleotides long, with two in-phase open reading frames (ORFs) beginning at nucleotides 49 and 136, and a single TAG codon at nucleotide 1027, coding for a VP7 protein of 297 or 326 amino acids, respectively.
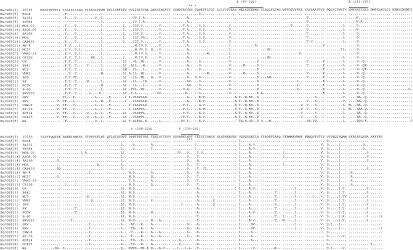
The complete deduced amino acid sequence of the VP7 gene from buffalo strain 10733 was determined and compared to those of reference rotavirus strains belonging to all known G serotypes (Table 1). The VP7 amino acid sequence of strain 10733 was 98.7 to 87.4% identical to those of rotavirus strains exhibiting G6 serotype specificity, and the highest amino acid identity (98.7 and 93.5%) was found to P3[9],G6 human rotavirus strains Hun4 (Hungary) and PA151 (Italy), respectively. The VP7 protein of strain 10733 had a potential N-linked glycosylation site located at amino acid 69 (Asn), which tends to be conserved among rotavirus strains with the exception of the G6 bovine rotavirus strains RF and NCDV (10, 13, 48). In addition, buffalo strain 10733 had a second potential glycosylation site at amino acid 238, like most bovine and the human P[9],G6 rotavirus strains (Fig. 1).
TABLE 1.
Amino acid comparison of VP7 of buffalo 10733, bovine VMRI-29 and NCDV, and human PA169 rotavirus strains with those of well-established G serotypes and with a selection of G6 rotavirusesa
| Strain (origin) | VP7 serotype | VP4 genotype | % Amino acid identity with:
|
|||
|---|---|---|---|---|---|---|
| 10733 | VMRI-29 | PA169 | NCDV | |||
| WA (human) | 1 | 82.40 | 80.55 | 82.40 | 80.86 | |
| S2 (human) | 2 | 76.85 | 75.92 | 77.46 | 75.30 | |
| RRV (simian) | 3 | 3 | 86.42 | 85.80 | 87.65 | 86.11 |
| ST3 (human) | 4 | 78.89 | 77.77 | 79.01 | 76.85 | |
| OSU (porcine) | 5 | 83.95 | 82.71 | 83.33 | 81.48 | |
| 10733 (buffalo)b | 6 | 3 | 92.59 | 92.28 | 88.88 | |
| Hun4 (human) | 6 | 9 | 98.77 | 92.63 | 92.63 | 89.59 |
| PA151 (human) | 6 | 9 | 93.51 | 93.20 | 92.9 | 90.12 |
| Se584 | 6 | 9 | 93.32 | 92.31 | 98.7 | 89.64 |
| RN-4 (bovine) | 6 | 11 | 92.59 | 97.53 | 92.28 | 90.45 |
| VMRI-29 (bovine) | 6 | 11 | 92.59 | 92.59 | 89.81 | |
| MC27 (bovine) | 6 | 11 | 91.36 | 96.80 | 92.59 | 89.50 |
| C8336 (bovine) | 6 | 11 | 90.74 | 90.26 | 91.66 | 89.50 |
| MG6 (human) | 6 | 14 | 90.12 | 90.74 | 96.91 | 90.74 |
| MG6.01 (human) | 6 | 14 | 91.97 | 92.59 | 98.14 | 91.66 |
| ASG6.02 (human) | 6 | 14 | 92.28 | 92.59 | 98.76 | 91.97 |
| PA169 (human) | 6 | 14 | 92.28 | 92.59 | 92.49 | |
| CAP455 (caprine) | 6 | 14 | 91.04 | 91.66 | 98.45 | 91.05 |
| UK (bovine) | 6 | 5 | 89.81 | 90.74 | 94.13 | 97.22 |
| B641 (bovine) | 6 | 5 | 88.88 | 89.81 | 93.51 | 96.29 |
| WC3 (bovine) | 6 | 5 | 89.19 | 90.12 | 93.20 | 96.60 |
| IND (bovine) | 6 | 5 | 88.27 | 88.58 | 92.28 | 95.37 |
| VMRI (bovine) | 6 | 5 | 88.58 | 90.12 | 92.28 | 96.29 |
| RF (bovine) | 6 | 1 | 88.88 | 90.12 | 92.90 | 98.45 |
| NCDV (bovine) | 6 | 1 | 88.88 | 89.81 | 92.59 | |
| BRV033 (bovine) | 6 | 1 | 87.96 | 88.27 | 89.81 | 95.37 |
| B-60 (bovine) | 6 | NDc | 88.58 | 89.81 | 92.28 | 95.06 |
| Ch2 (avian) | 7 | 17 | 59.56 | 60.49 | 61.11 | 60.49 |
| B37 (human) | 8 | 79.62 | 78.70 | 81.17 | 79.93 | |
| 116E (human) | 9 | 81.79 | 80.24 | 81.48 | 80.55 | |
| 61A (bovine) | 10 | 84.56 | 83.02 | 84.25 | 82.09 | |
| YM (porcine) | 11 | 85.18 | 83.95 | 84.87 | 82.40 | |
| L26 (human) | 12 | 80.55 | 78.7 | 79.32 | 78.39 | |
| L338 (equine) | 13 | 78.70 | 77.46 | 78.70 | 78.08 | |
| CH3 (equine) | 14 | 87.89 | 78.39 | 79.30 | 78.70 | |
| Hg18 (bovine) | 15 | 82.71 | 80.24 | 80.55 | 77.46 | |
GenBank accession nos. of the VP7 genes of strains follow (in parentheses after the strain): WA (M21843), S2 (M11164), RRV (AF295303), ST3 (P10501), OSU (X04613), Hun4 (AJ487833), PA151 (Q86271), Se584 (AJ311740), RN-4 (P31632), VMRI-29 (Q96641), MC27 (AF162435), C8336 (Q65698), MG6 (U22011), MG6.01 (AF207062), ASG6.02 (AF421183), PA169 (Q86270), CAP455 (AY128708), UK (X00896), B641 (P25175), WC3 (AY050272), IND (Q65692), VMRI (Q96643), RF (Q86515), NCDV (M63266), BRV033 (Q96644), B-60 (Q86193), Ch2 (X56784), B37 (J04334), 116E (L14072), 61A (X53403), YM (M23194), L26 (M58290), L338 (D13549), CH3 (D25229), and Hg18 (AF237666).
Predicted G serotype on the basis of sequence identity.
ND, not determined.
FIG. 1.
Deduced amino acid sequence of the VP7 protein of buffalo strain 10733, of a selection of G6 and G3 rotaviruses, and of strain Wa, P1A[8],G1. The VP7 antigenic regions A, B, C, and F are indicated. The glycosylation site NST (amino acids 69 to 71) is indicated by asterisks. The accession numbers of the VP7 sequences are listed in Table 1, footnote a. Abbreviations: Bo, bovine; Bu, buffalo; Go, goat; Hu, human; Po, porcine; Si, simian.
The VP7 antigenic regions A (amino acids 87 to 101), B (amino acids 143 to 152), C (amino acids 208 to 223), and F (amino acids 235 to 242) (11, 16, 48) of buffalo strain 10733 clearly support its classification as serotype G6. Within the VP7 hypervariable regions A, B, C, and F, the VP7 of buffalo strain 10733 was completely identical only to the Hungarian human P(9),G6 strain, while it showed some amino acid substitutions with respect to those of G6 rotavirus strains isolated from different species and geographic origins (Fig. 1). VP7 hypervariable region A of strain 10733 was also identical to that of the human rotavirus strain PA151, while VP7 region B of strain 10733 had an Ala at position 145 instead of Asn or Asp. In VP7 region C, buffalo strain 10733 differed from the serotype G6 human, bovine, or caprine rotavirus strains in at least position 208 (Leu to Ser), but amino acid substitutions were also observed at positions 209, 211, 213, and 221. Finally in VP7 region F, buffalo strain 10733 differed from other G6 rotavirus strains at positions 238, 241, and 242.
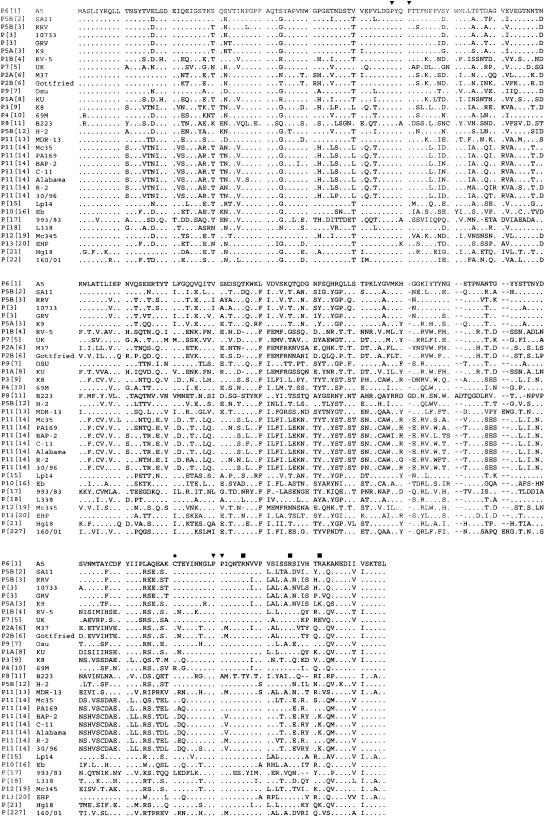
When compared to published VP8* trypsin cleavage product sequences, the VP8* of buffalo rotavirus strain 10733 showed the highest nucleotide sequence and amino acid sequence identity, 95.6% and 96.2%, respectively, with those of the P5B[3] simian RRV strain (data not shown and Table 2). Of note, the second best match was to a goat G3 rotavirus, strain GRV, recently identified in Japan (40), with about 83% identity at the nucleotide and 90.8% at the amino acid level. Since it has been established that only rotaviruses that exhibit a VP4 amino acid identity of 89% or greater belong to the same P serotype or subtype (22), strain 10733 can be assigned to the P[3] genotype. The few amino acid differences (less than 3.8%) between the VP8* of strain 10733 and RRV are predictive of a serotype P5B specificity, even if this should be confirmed serologically.
TABLE 2.
Amino acid comparison of VP8* of buffalo 10733, caprine GRV, and simian RRV rotavirus strains with well-established P genotypesa
| Strain (origin) | P genotype | P serotype | % Amino acid identity (1 to 247) with:
|
||
|---|---|---|---|---|---|
| 10733 | GRV | RRV | |||
| A5 (bovine) | 1 | 6 | 80.9 | 81.2 | 79.8 |
| SA11 (simian) | 2 | 5B | 85.4 | 84.5 | 84.0 |
| RRV (simian) | 3 | 5B | 96.2 | 91.0 | |
| 10733 (buffalo) | 3 | ND | 91.3 | 96.2 | |
| GRV (caprine) | 3 | ND | 91.3 | 90.9 | |
| K9 (canine) | 3 | 5A | 88.2 | 87.8 | 85.7 |
| CU-1 (canine) | 3 | 5A | 87.1 | 86.5 | 85.7 |
| L26 (human) | 4 | 1B | 61.8 | 62.8 | 61.1 |
| UK (bovine) | 5 | 7 | 66.3 | 69.1 | 66.3 |
| M37 (human) | 6 | 2A | 64.9 | 63.9 | 64.2 |
| Gottfried (porcine) | 6 | 2B | 64.2 | 63.5 | 64.2 |
| OSU (porcine) | 7 | 9 | 79.2 | 78.1 | 77.1 |
| WA (human) | 8 | 1A | 61.4 | 63.5 | 60.7 |
| K8 (human) | 9 | 3 | 62.1 | 63.2 | 62.1 |
| 69M (human) | 10 | 4 | 77.7 | 77.2 | 78.3 |
| B223 (bovine) | 11 | 8 | 45.2 | 46.9 | 45.5 |
| H-2 (equine) | 12 | 72.2 | 73.6 | 72.57 | |
| MDR-13 (porcine) | 13 | 67.7 | 70.5 | 67.0 | |
| ALA (lapine) | 14 | 11 | 61.8 | 63.2 | 61.5 |
| Lp14 (ovine) | 15 | 78.8 | 80.5 | 78.8 | |
| EW (murine) | 16 | 10 | 68.7 | 68.2 | 67.7 |
| 993/83 (bovine) | 17 | 48.2 | 49.3 | 47.2 | |
| L338 (equine) | 18 | 73.3 | 75.7 | 74.6 | |
| Mc323 (human) | 19 | 12 | 64.2 | 65.6 | 63.5 |
| EHP (murine) | 20 | 13 | 73.3 | 78.1 | 75.7 |
| Hg18 (bovine) | 21 | 68.7 | 71.2 | 67.7 | |
| 160/01 (lapine) | 22 | 65.9 | 68.8 | 64.9 | |
GenBank accession nos. for the VP4 genes of strains follow (in parentheses after the strain): A5 (D13395), SA11 (M23188), RRV (M18736), GRV (AB055967), K9 (D14725), CU-1 (D13401), L26 (M58292), UK (M22306), M37 (L20887), Gottfried (M33516), OSU (X13190), WA (P11193), K8 (D90260), 69M (M60600), B223 (D13394), H-2 (L04638), MDR-13 (L07886), ALA (U62149), Lp14 (L11599), EW (U08429), 993/83 (D16352), L338 (D13399), Mc323 (D38052), EHP (U08424), Hg18 (AF237665), and 160/01 (AF526376). ND, not determined.
Figure 2 shows the deduced amino acid sequence of the VP8* of buffalo strain 10733 and representative VP8* amino acid sequences of rotavirus strains representative of all P genotypes described to date. The potential cleavage sites (arginines 231, 241, and 247) and the highly conserved prolines at residues 68, 71, 225, and 226 were maintained (2, 19).
FIG. 2.
Deduced amino acid sequence of the VP8* trypsin cleavage product of the VP4 protein of Italian buffalo strain 10733 and of a selection of strains representing the remaining P genotypes. The highly conserved cysteine (•), prolines (▾), and arginines (▪) are indicated. For optimal alignment, gaps were introduced in the sequences. The first eight residues in the sequence of strain 10733 are inferred from the sequence of primer Con3 (nucleotides 10 to 32).
Comparative analysis of the deduced amino acid sequences of the fragment of VP6 (amino acids 281 to 350) known to correlate with SG specificity (32) allowed characterization of strain 10733 as genogroup I, indicating that buffalo rotavirus strain 10733 belongs to SGI (data not shown). Specific amino acids at positions 305 (Ala), 310 (Asn), 315 (Glu), 339 (Ser), and 342 (Met), all predictive of SGI specificity (32), were all conserved (data not shown).
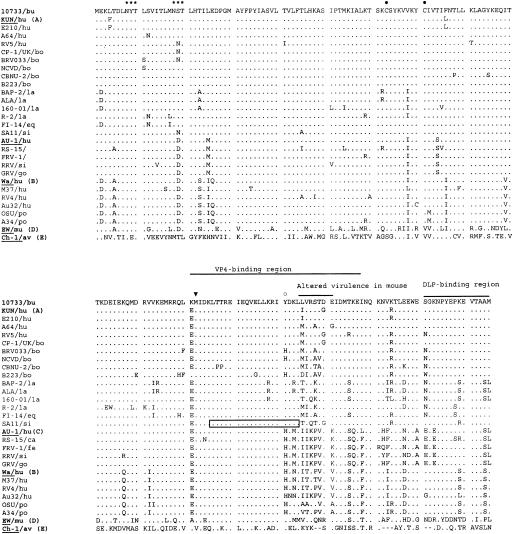
In addition, the 175-amino-acid-long NSP4 protein was deduced from genome segment 10 of buffalo rotavirus strain 10733 and compared with bovine strains of the NSP4 A (KUN-like) genotype and other representative NSP4 sequences of genogroups A, B, C, D, and E (Fig. 3). The NSP4 of strain 10733 showed the highest amino acid identity (97.1%) to the unusual G10 human strain A64 and to the bovine strain UK, P7[5],G6. Common structural features of the NSP4 protein, such as N-linked glycosylation sites at amino acids 8 and 18, the cysteine residues at amino acids 63 and 71, and tyrosine-131 (a key residue for enterotoxic activity) were conserved. Also, the sequence of the enterotoxic peptide was highly conserved (19). There were scattered amino acid variations throughout the protein with respect to the KUN-like strains. In the hypervariable region (amino acids 135 to 141) associated with altered virulence in mice (12), there were at least two amino acid changes for all the other NSP4s, except for human strain E210, which differs in a single residue, Iso-135 to Leu.
FIG. 3.
Deduced amino acid sequence of the NSP4 protein of Italian buffalo strain 10733 and of a selection of strains representing the five distinct NSP4 genotypes (12, 47). The potential glycosylation sites are indicated by asterisks. The highly conserved cysteine (•) and methionine (▾) residues and the putative VP4 and double-layered particle (DLP)-binding regions are shown. The enterotoxic peptide of simian rotavirus SA-11 strain at amino acids 114 to 135 and tyrosine-131 (○), considered a key residue for enterotoxigenic activity, are shown (19). Also, the hypervariable region (amino acids 135 to 141) associated with altered virulence in mouse is shown (12). Abbreviations: av, avian; bo, bovine; bu, buffalo; ca, canine; eq, equine; fe, feline; go, goat; hu, human; la, lapine; mu, murine; po, porcine; si, simian.
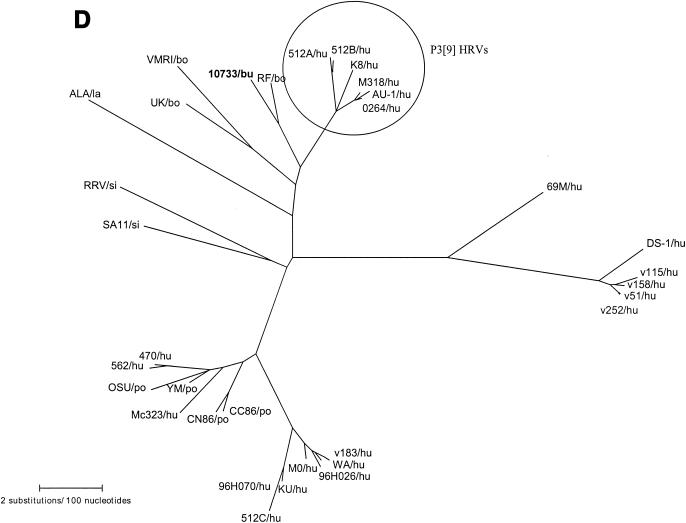
The complete sequence of the NSP5/6 genome segment 11 was 667 nucleotides long, with the sequence coding for NSP5 located between nucleotides 22 and 618, and a second out-of-phase ORF (coding for NSP6) conserved between nucleotides 80 and 358. The predicted NSP5 and NSP6 proteins were 198 and 92 amino acids in length, respectively (data not shown). The serine residues (at positions 153, 155, 163, and 165) involved in phosphorylation (17), the cysteine residues at positions 171 and 174, and the highly conserved COOH terminus in the NSP5 protein were maintained. By comparison to sequences in GenBank, the amino acid identity of the NSP5 of strain 10733 with those of group A rotaviruses ranged from 96.7% (bovine strain RF) to 49.5% (avian strain PO-13). High amino acid identity was also found to P3[9] human strains, 512B (93.9%) and K8 and AU-1 (93.4%), as well as to the P7[5] bovine strain UK (92.9%).
Phylogenetic analyses of VP7, VP8* trypsin cleavage product, NSP4, and NSP5/6 of buffalo rotavirus strain 10733.
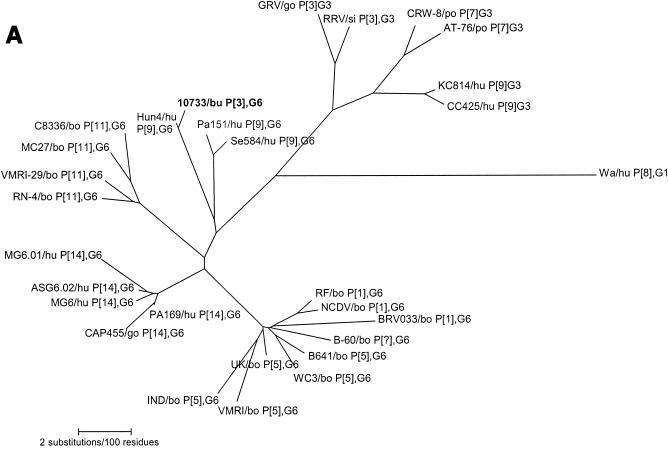
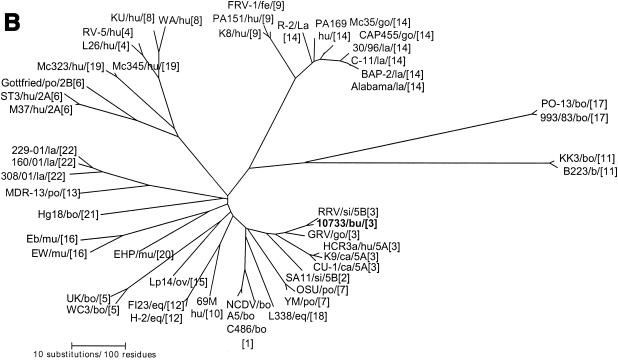
Among rotavirus strains with serotype G6 specificity, four distinct VP7 branches or groups were observed (Fig. 4A). One group comprised most of the P6[1] and P7[5] serotype G6 bovine rotaviruses from various parts of the world, including the reference strains RF (France), UK (Britain), and NCDV and WC3 (United States). A second group included exclusively P[14],G6 human strains PA169 (Italy), MG6.01, ASG6.02, and MG6 (Australia), and the P[14] caprine strain CAP455 (South Africa), while the third group was formed by only P8[11],G6 bovine strains C8336, MC27, and VMRI-29 (United States), and KN-4 (Japan). The fourth group included buffalo rotavirus strain 10733 and the P3[9],G6 human rotavirus strains Hun4 (Hungary), PA151 (Italy), and Se584 (United States).
FIG. 4.
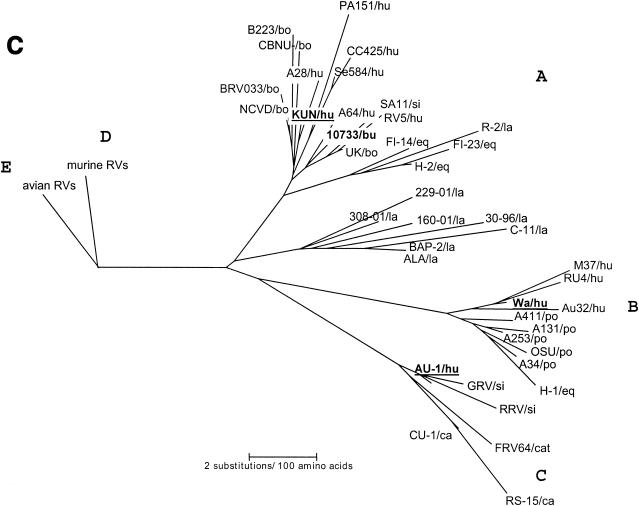
Phylogenetic trees of the VP7, VP8*, and NSP4 amino acid sequences of Italian buffalo strain 10733. (A) VP7 tree, displaying the relationships among a selection of serotype G6 and G3 animal and human rotavirus strains. The dendrogram is drawn to scale and rooted with the human strain Wa (P1A[8],G1). (B) VP8* tree, displaying the relationships among strains representative of all the VP4 genotypes recognized to date. The dendrogram is drawn to scale and rooted with the avian-like bovine strain Bo/993/83 (P[17],G7). (C) NSP4 tree, displaying the relationships among a selection of animal and human rotavirus strains representative of the five NSP4 genetic groups. The branches of the murine (genogroup D) and avian (genogroup E) strains are not to scale. The prototypes of the NSP4 genotypes A, B, and C (strains KUN, Wa, and Au-1, respectively) are boldfaced and underlined. Buffalo 10733 strain is shown in boldface type. (D) NSP5 tree, displaying the relationships among animal and human rotavirus strains. The tree is unrooted and is inferred from the nucleotide alignment. The P3[9] human rotaviruses (HRVs) are enclosed in a circle. Abbreviations: bo, bovine; bu, buffalo; ca, canine; eq, equine; fe, feline; go, goat; hu, human; la, lapine; mu, murine; po, porcine; si, simian.
Phylogenetic analysis of the VP8* of buffalo strain 10733 revealed a closer genetic relationship to the P5B[3] simian RRV and to the caprine GRV strains than to the P5A[3] canine (CU-1 and K9) and human (HCR3a) strains, as evidenced in Fig. 4B. Phylogenetically, the NSP4 protein of buffalo strain 10733 clustered with those of bovine rotaviruses that belong to the A (KUN-like) genogroup (Fig. 4C), and the NSP5 protein of strain 10733 clustered with those of bovine rotavirus strains, which in turn are closely related to those of P3[9] human rotavirus strains (Fig. 4D).
DISCUSSION
The epidemiology of buffalo rotaviruses is still widely unknown. Herd management as well the strict species affinity between cows and buffalo might explain the detection of buffalo rotaviruses with G6, G8, or G10 associated with P6[1], P7[5], or P8[11] specificities in Italy (43, 52). Similarly, buffalo rotaviruses displaying bovine-like VP7 and VP4 types have been detected in India, with P8[11],G10 being the prevalent combination (27).
In this study, we isolated a buffalo strain, 10733, displaying a long e-type and SGI specificity. Such a pattern is commonly described for animal rotaviruses (36). Sequence analysis of the VP7, VP8*, NSP4, and NSP5/6 genes revealed G6 and P[3] specificities, as well a bovine-like NSP5 gene and an NSP4 A (KUN-like) genogroup.
High amino acid and nucleotide sequence identities of the VP8* of strain 10733 to that of strain RRV, as well as the close phylogenetic relationship, strongly suggest that strains 10733 and RRV have analogous VP4s and may allow us to predict the specificity of buffalo strain as P5B[3]. While analyzing the sequences in the databases, we found that another strain, goat rotavirus GRV, shared high genetic relatedness with strains 10733 and RRV in the VP4 gene (40). The three strains were clustered together in the VP8* tree, constituting a lineage distinct from the P5A[3] rotavirus strains of canine, feline, and human origin (Fig. 4B). In addition, strain GRV displayed the same NSP4 C (AU-1-like) genogroup and G3 specificity as strain RRV (40), while strain 10733 exhibited an NSP4 A (KUN-like) genogroup, where all the bovine strains are grouped (12).
Analysis of VP7 provided contradictory data on the origin of strain 10733, as its VP7 exhibited the highest similarity (98.7%) to the Hungarian human strain Hun4, P3[9],G6. With a few exceptions, the G6 VP7 allele is one of the most common VP7 antigenic specificities of bovine rotaviruses (19), though it has also been detected in rotaviruses from humans (4, 14, 21). A significant intrahomotypic divergence within G6 rotaviruses from humans and cows has been identified, suggesting the existence of antigenically distinguishable G6 subtypes, as well as a correlation of subtypes with species of origin and P type (6, 14). By phylogenetic analysis of VP7, it was possible to observe that, while there is little or no difference between P[5],G6 and P[1],G6 rotaviruses identified in different parts of the world, the P[14],G6 and P8[11],G6 strains clearly form two distinct groups. It has been speculated previously (10) that the P[14] allele crossed the species barrier via sequential reassortments from rabbits to cows and from cows to humans. The existence of a G6 lineage restricted to human and caprine P[14] rotaviruses strongly supports this hypothesis. Interestingly, there was an additional cluster including the Italian strains 10733, P[3], and PA151, P[9], the American strain Se584, P[9], and the Hungarian strain Hun4, P[9] (4, 21, 26).
The linkage observed between G6 lineage and P type is indicative of multiple reassortment events occurring in the context of a constant pattern of linear evolution. Accordingly, introduction of bovine alleles into the human set of rotavirus gene alleles may have occurred on different occasions, and this would be confirmed by several pieces of evidence: (i) the existence of P3[9],G6 human strains genetically related to bovine strains (21, 26); (ii) the sporadic detection of unusual human strains bearing bovine-like specificities (1, 4, 5, 15); (iii) the high genetic relatedness between bovine and human strains in the NSP4 (12) and P3[9] human rotaviruses in the NSP5 gene (60); and (iv) genetic relatedness of strain 10733 to the unusual G10 human strain A64 (5) in NSP4 and to the unusual human strains Hun4, PA151, and Se584 (P3[9],G6) in the VP7 gene.
Phylogenetic analysis of multiple dsRNA segments has revealed that reassortment between homologous viruses (i.e., viruses of the same species) occurs frequently (33, 41, 58). While the exchange of genes between homologous strains is frequent but difficult to identify, heterologous exchanges are presumably much rarer but lead to the onset of “unusual” strains that may be readily identified because of uncommon G and/or P types. Cosegregation of G6 lineage and P type may be explained as preferential gene selection (49), as development of stable and advantageous conformational alterations in the outer viral capsid of some VP4-VP7 reassortants (51), or as ecological obstacles to free reassortment.
The detection of the rare P[3] RRV-like VP4 allele in ruminants raises questions on the origin and the possible source of introduction of this new VP4 type in the set of alleles of ruminant rotaviruses. The simian rhesus strain RRV was originally isolated from the feces of a 3- to 5-month-old rhesus monkey with diarrhea (56). Strain RRV has been classified both serologically and genetically as P5B[3],G3 (36), with an SGI VP6 and AU1-like NSP4 genotype (9). While the G3 specificity has been reported in a wide range of mammalian species (19), so far, the prototype strain RRV is the only rotavirus possessing the P5B[3] VP4 allele (30). Although it is important as a reference strain in laboratories and as the recipient virus in the human-rhesus vaccine (35, 44, 45), to our knowledge, viruses with RRV-like P type have never been detected, and the P5B[3] VP4 allele is not considered of epidemiological relevance in either humans or animals.
Thus, the finding that ruminant rotaviruses (strains 10733 and GRV) are highly similar in the VP4 allele to strain RRV has only two possible explanations: (i) the RRV-like VP4 type is an allele also present in rotaviruses circulating in ruminants but has not been detected previously because of its rare distribution and/or the typing assays used; and (ii) the RRV-like gene allele has been introduced recently into the genome of ruminant rotaviruses as the result of a reassortment event that occurred with a wild simian rotavirus or during the vaccine trials. For instance, horizontal transmission of a vaccine virus has been demonstrated both in vaccinees and in placebo recipients during vaccine trials in Venezuela (50). As the rhesus rotavirus vaccine has been administered to infants in several countries throughout the world since the mid-1980s (7, 19, 23, 36, 54), environmental contamination with rhesus strain RRV may have occurred several times and in different geographic locations in the last 20 years. The latter hypothesis is extremely intriguing, evocative of the potential biological risks coming from manipulation of live viruses that possess genetic and antigenic plasticity such as RNA segmented viruses. However, divergence within the VP8* sequence of the RRV-like P[3] strains (up to 16 to 17% at the nucleotide level and up to 9% at the amino acid level) seems to be consistent with analogous variation described within strains of the same P type (10, 25, 34, 42) and suggests that a VP4 RRV-like allele is circulating in ruminants, presumably at very low frequency. Furthermore, the chances of environmental contamination would be expected to be far greater in countries where the vaccine trials were made, but the surveillance systems active in those countries have not reported, to date, the existence of either human or animal RRV-like rotaviruses, and therefore, it is highly unlikely that reassortment between strain RRV and other rotaviruses may have occurred in the field.
The detection of a buffalo P5B[3],G6 rotavirus, while providing additional evidence for the genetic and antigenic diversity of group A rotaviruses, is also important from the perspective of understanding the basis of rotavirus host specificity. Although rotaviruses have low host specificity, heterologous infections usually have no clinical signs and are not efficiently propagated (8, 9, 19). Strain 10733 was isolated from a diarrheic buffalo calf, so the presence of this VP4 allele in the background of one bovine-like rotavirus is presumably still permissive for symptomatic infection. Interestingly, the P5B[3],G3 RRV strain is also the only nonlapine rotavirus able to determine productive infection in the rabbit model (8, 9).
Comparative sequence analysis of other genes of strain 10733, as well as experimental infection of buffalo and/or bovine calves, could provide important information on the molecular mechanisms of rotavirus host range restriction as well as help to elucidate the origin of this strain.
Acknowledgments
This work was partially supported by grants from CEGBA (Centro di Eccellenza di Genomica in campo Biomedico ed Agrario), from MURST (Ministero per l'Università e la Ricerca Scientifica e Tecnologica), and from Ministero della Salute (Ricerca Corrente 2000).
We thank Donato Narcisi, Carlo Armenise, Paola Fiorente, and Filomena Cariola for expert technical assistance. We are extremely grateful to Leland Eugene Carmichael for continued encouragement throughout the study.
REFERENCES
- 1.Adah, M. I., A. Wade, and K. Taniguchi. 2001. Molecular epidemiology of rotaviruses in Nigeria: detection of unusual strains with G2P[6] and G8P[1] specificities. J. Clin. Microbiol. 39:3969-3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arias, C. F., P. Romero, V. Alvarez, and S. Lopez. 1996. Trypsin activation pathway of rotavirus infectivity. J. Virol. 70:5832-5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ball, J. M., P. Tian, C. Q.-Y. Zeng, A. P. Morris, and M. K. Estes. 1996. Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science 272:101-104. [DOI] [PubMed] [Google Scholar]
- 4.Banyai, K., J. R. Gentsch, R. I. Glass, and G. Szucs. 2003. Detection of human rotavirus serotype G6 in Hungary. Epidemiol. Infect. 130:107-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beards, G., L. Xu, A. Ballard, U. Desselberger, and M. A. McRae. 1992. A serotype 10 human rotavirus. J. Clin. Microbiol. 30:1432-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang, K. O., A. V. Parwan, and L. J. Saif. 2000. Comparative sequence analysis of the VP7 genes G6, G8 and G10 bovine group A rotaviruses and further characterisation of G6 subtypes. Arch. Virol. 145:725-737. [DOI] [PubMed] [Google Scholar]
- 7.Christy, C., H. P. Madore, M. E. Pichichero, C. Gala, P. Pincus, D. Vosefski, H. Hoshino, A. Kapikian, and R. Dolin. 1988. Field trial of rhesus rotavirus vaccine in infants. Pediatr. Infect. Dis. J. 7:645-650. [DOI] [PubMed] [Google Scholar]
- 8.Ciarlet, M., M. K. Estes, C. Barone, R. F. Ramig, and M. E. Conner. 1998. Analysis of host range restriction determinants in the rabbit model: comparison of homologous and heterologous rotavirus infections. J. Virol. 72:2341-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciarlet, M., M. K. Estes, and M. E. Conner. 2000. Simian rhesus rotavirus is a unique heterologous (non-lapine) rotavirus strain capable of reproductive replication and horizontal transmission in rabbits. J. Gen. Virol. 81:1237-1249. [DOI] [PubMed] [Google Scholar]
- 10.Ciarlet, M., M. K. Estes, and M. E. Conner. 1997. Comparative amino acid sequence analysis of the outer capsid protein VP4 from four lapine rotavirus strains reveal identity with genotype P[14] human rotavirus. Arch. Virol. 142:1059-1069. [DOI] [PubMed] [Google Scholar]
- 11.Ciarlet, M., Y. Hoshino, and F. Liprandi. 1997. Single point mutation may affect the serotype reactivity of G11 porcine rotavirus strains: a widening spectrum? J. Virol. 71:8213-8220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciarlet, M., F. Liprandi, M. E. Conner, and M. K. Estes. 2000. Species specificity and interspecies relatedness of NSP4 genetic groups by comparative NSP4 analyses of animal rotaviruses. Arch. Virol. 145:371-383. [DOI] [PubMed] [Google Scholar]
- 13.Ciarlet, M., J. E. Ludert, and F. Liprandi. 1995. Comparative amino acid sequence analysis of the major outer capsid protein (VP7) of porcine rotaviruses with G3 and G5 serotype specificities isolated in Venezuela and Argentina. Arch. Virol. 140:437-451. [DOI] [PubMed] [Google Scholar]
- 14.Cooney, M. A., R. J. Gorrell, and E. A. Palombo. 2001. Characterisation and phylogenetic analysis of the VP7 protein of serotype G6 and G8 human rotaviruses. J. Med. Microbiol. 50:462-467. [DOI] [PubMed] [Google Scholar]
- 15.Das, M., S. J. Dunn, G. N. Woode, H. B. Greenberg, and C. D. Rao. 1993. Both surface proteins (VP4 and VP7) of an asymptomatic neonatal rotavirus strain (I321) have high levels of sequence identity with the homologous proteins of a serotype 10 bovine rotavirus. Virology 194:374-379. [DOI] [PubMed] [Google Scholar]
- 16.Dyall-Smith, M. L., I. Lazdins, G. W. Tregear, and I. H. Holmes. 1986. Location of the major antigenic sites involved in rotavirus serotype-specific neutralization. Proc. Natl. Acad. Sci. 83:3465-3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eichwald, C., F. Vascotto, E. Fabbretti, and O. R. Burrone. 2002. Rotavirus NSP5: mapping phosphorylation sites and kinase activation and viroplasm localization domains. J. Virol. 76:3461-3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Attar, L., W. Dhaliwal, M. Iturriza-Gomara, and J. C. Bridger. 2002. Identification and molecular characterization of a bovine G3 rotavirus which causes age-independent diarrhea in cattle. J. Clin. Microbiol. 40:937-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Estes, M. K. 2001. Rotaviruses and their replication, p. 1747-1785. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Strais (ed.), Fields virology, 4th ed. Lippincott Williams and Wilkins, Philadelphia, Pa.
- 20.Gentsch, J. R., R. I. Glass, P. Woods, V. Gouvea, M. Gorziglia, J. Flores, B. K. Das, and M. K. Bhan. 1992. Identification of group rotavirus gene 4 types by polymerase chain reaction. J. Clin. Microbiol. 30:1365-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerna, G., A. Sarasini, M. Parea, S. Arista, P. Miranda, H. Brussow, Y. Hoshino, and J. Flores. 1992. Isolation and characterization of two distinct human rotavirus strains with G6 specificity. J. Clin. Microbiol. 30:9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorziglia, M., G. Larralde, A. Z. Kapikian, and R. M. Chanock. 1990. Antigenic relationships among human rotaviruses as determined by outer capsid protein VP4. Proc. Natl. Acad. Sci. 87:7155-7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gothefors, L., G. Wadell, P. Juto, K. Taniguchi, A. Z. Kapikian, and R. I. Glass. 1989. Prolonged efficacy of rhesus rotavirus vaccine in Swedish children. J. Infect. Dis. 159:753-757. [DOI] [PubMed] [Google Scholar]
- 24.Gouvea, V., R. I. Glass, P. Woods, K. Taniguchi, H. F. Clark, B. Forrester, and Z.-Y. Fang. 1990. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J. Clin. Microbiol. 28:276-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gouvea, V., R. C. Lima, R. E. Linhares, H. F. Clark, C. M. Nosawa, and N. Santos. 1999. Identification of two lineages (WA-like and F45-like) within the major rotavirus genotype P[8]. Virus Res. 59:141-147. [DOI] [PubMed] [Google Scholar]
- 26.Griffin, D. D., T. Nakagomi, Y. Hoshino, O. Nakagomi, C. D. Kirkwood, U. D. Parashar, R. I. Glass, J. R. Gentsch, and the National Rotavirus Strain Surveillance System. 2002. Characterization of nontypeable rotavirus strains from the United States: identification of a new rotavirus reassortant (P2A[6], G12) and rare P3[9] strains related to bovine rotaviruses. Virology 294:256-269. [DOI] [PubMed] [Google Scholar]
- 27.Gulati, B. R., O. Nakagomi, Y. Koshimura, T. Nakagomi, and R. Pandey. 1999. Relative frequencies of G and P types among rotaviruses from India diarrheic cow and buffalo calves. J. Clin. Microbiol. 37:2074-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall, T. A. 1999. BioEdit: a user-friendly biological sequence alignment and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41:95-98. [Google Scholar]
- 29.Horie, Y., O. Masamune, and O. Nakagomi. 1997. Three major alleles of rotavirus NSP4 proteins identified by sequence analysis. J. Gen. Virol. 78:2341-2346. [DOI] [PubMed] [Google Scholar]
- 30.Hoshino, Y., R. W. Jones, and A. Z. Kapikian. 2002. Characterization of neutralization specificities of outer capsid protein VP4 of selected murine, lapine and human rotavirus strains. Virology 299:64-71. [DOI] [PubMed] [Google Scholar]
- 31.Ito, H., M. Sugiyama, Y. Masubuchi, Y. Mori, and N. Minamoto. 2001. Complete nucleotide sequence of a group A avian rotavirus genome and a comparison with counterparts of mammalian rotaviruses. Virus Res. 75:123-138. [DOI] [PubMed] [Google Scholar]
- 32.Iturriza-Gómara, M., C. Wong, S. Blome, U. Desselberger, and J. J. Gray. 2002. Molecular characterization of VP6 genes of human rotavirus isolates: correlation of genogroups with subgroups and evidence for independent segregation. J. Virol. 76:6596-6601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iturriza-Gómara, M., B. Isherwood, U. Desselberger, and J. J. Gray. 2001. Reassortment in vivo: driving force for diversity of human rotavirus strains isolated in the United Kingdom between 1995 and 1999. J. Virol. 75:3696-3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iturriza-Gómara, M., J. Green, D. W. G. Brown, U. Desselberger, and J. J. Gray. 2000. Diversity within the VP4 gene of rotavirus P[8] strains: implications for reverse transcription-PCR genotyping. J. Clin. Microbiol. 38:898-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kapikian, A. Z. 2002. Ecological studies, rotavirus vaccination and intussusception. Lancet 359:1065-1066. [DOI] [PubMed] [Google Scholar]
- 36.Kapikian, A. Z., Y. Hoshino, and R. M. Chanock. 2001. Rotaviruses, p. 1787-1833. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Strais (ed.), Fields virology, 4th ed. Lippincott Williams and Wilkins, Philadelphia, Pa.
- 37.Krishna Mohan, K. V., and C. D. Atreya. 2001. Nucleotide sequence analysis of rotavirus gene 11 from two tissue culture-adapted ATCC strains, RRV and Wa. Virus Genes 23:321-329. [DOI] [PubMed] [Google Scholar]
- 38.Kumar, S., K. Tamura, I. B. Jakobsen, and M. Nei. 2001. MEGA2: Molecular Evolutionary Genetics Analysis software. Bioinformatics 17:1244-1245. [DOI] [PubMed] [Google Scholar]
- 39.Lee, C.-N., Y.-L. Wang, C.-L. Kao, C.-L. Zao, C.-Y. Lee, and H.-N. Chen. 2000. NSP4 gene analysis of rotaviruses recovered from infected children with and without diarrhea. J. Clin. Microbiol. 38:4471-4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee, J.-B., S.-J. Youn, T. Nakagomi, S.-Y. Park, T.-J. Kim, C.-S. Song, H.-K. Jang, B.-S. Kim, and O. Nakagomi. 2003. Isolation, serologic and molecular characterization of the first G3 caprine rotavirus. Arch. Virol. 148:643-657. [DOI] [PubMed] [Google Scholar]
- 41.Manula, L., and C. H. Von Bonsdorff. 2002. Frequent reassortments may explain the genetic heterogeneity of rotaviruses: analysis of Finnish rotavirus strains. J. Virol. 76:11793-11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martella, V., M. Ciarlet, A. Camarda, A. Pratelli, M. Tempesta, G. Greco, A. Cavalli, G. Elia, N. Decaro, V. Terio, G. Bozzo, M. Camero, and C. Buonavoglia. 2003. Molecular characterization of the VP4, VP6, VP7, and NSP4 genes of lapine rotaviruses identified in Italy: emergence of a novel VP4 genotype. Virology 314:358-370. [DOI] [PubMed] [Google Scholar]
- 43.Martella, V., A. Pratelli, O. Pinto, G. Ferrara, M. Tempesta, and D. Buonavoglia. 1999. Typing by polymerase chain reaction of buffalo rotaviruses isolated in Italy. J. Vet. Med. B 19:871-876. [DOI] [PubMed] [Google Scholar]
- 44.Mendez, I. I., L. L. Hermann, P. R. Hazelton, and K. M. Coombs. 2000. A comparative analysis of Freon substitutes in the purification of reovirus and calicivirus. J. Virol. Methods 90:59-67. [DOI] [PubMed] [Google Scholar]
- 45.Midthun, K., H. B. Greenberg, Y. Hoshino, A. Z. Kapikian, R. G. Wyatt, and R. M. Chanock. 1985. Reassortant rotaviruses as potential live rotavirus vaccine candidates. J. Virol. 53:949-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Midthun, K., Y. Hoshino, A. Z. Kapikian, and R. M. Canock. 1986. Single gene substitution rotavirus reassortants containing the major neutralization protein (VP7) of human rotavirus serotype 4. J. Clin. Microbiol. 24:822-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mori, Y., M. A. Borgan, N. Ito, M. Sugiyama, and N. Minamoto. 2002. Diarrhea-inducing activity of avian rotavirus NSP4 glycoproteins, which differ greatly from mammalian rotavirus NSP4 glycoproteins in deduced amino acid sequence, in suckling mice. J. Virol. 76:5829-5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nishikawa, K., Y. Hoshino, K. Taniguchi, K. Y. Green, H. B. Greenberg, A. Z. Kapikian, R. M. Chanock, and M. Gorziglia. 1989. Rotavirus VP7 neutralization epitopes of serotype 3 strains. Virology 171:503-515. [DOI] [PubMed] [Google Scholar]
- 49.Okada, J., N. Kobayashi, K. Taniguchi, and S. Urasawa. 1998. Preferential selection of heterologous G3-VP7 gene in the genetic background of simian rotavirus SA-11 detected by using a homotypic single-VP7 gene-substitution reassortant. Antiviral Res. 38:15-24. [DOI] [PubMed] [Google Scholar]
- 50.Perez-Schael, I., M. J. Guntinas, M. Perez, V. Pagone, A. M. Rojas, R. Gonzales, W. Cunto, Y. Hoshino, and A. Z. Kapikian. 1997. Efficacy of the rhesus-rotavirus based quadrivalent vaccine in infants and young children in Venezuela. N. Engl. J. Med. 337:1181-1187. [DOI] [PubMed] [Google Scholar]
- 51.Pesavento, J. B., A. M. Billingsley, E. J. Roberts, R. F. Ramig, and B. V. V. Prasad. 2003. Structures of rotavirus reassortants demonstrate correlation of altered conformation of the VP4 spike and expression of unexpected VP4-associated phenotypes. J. Virol. 77:3291-3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pratelli, A., V. Martella, M. Tempesta, and C. Buonavoglia. 1999. Characterization by polymerase chain reaction of ruminant rotaviruses isolated in Italy. Microbiologica 22:105-109. [PubMed] [Google Scholar]
- 53.Rao, C. D., K. Gowda, and B. S. Y. Reddy. 2000. Sequence analysis of VP4 and VP7 genes of nontypeable strains identifies a new pair of outer capsid proteins representing novel P and G genotypes in bovine rotaviruses. Virology 276:104-113. [DOI] [PubMed] [Google Scholar]
- 54.Rennels, M. B., G. A. Losonsky, A. E. Young, C. L. Shindledecker, A. Z. Kapikian, and M. M. Levine. 1990. An efficacy trial of rhesus rotavirus vaccine in Maryland. Am. J. Dis. Child. 144:601-604. [DOI] [PubMed] [Google Scholar]
- 55.Rohwedder, A., K. I. Schutz, N. Minamoto, and H. Brussow. 1995. Sequence analysis of pigeon, turkey, and chicken rotavirus VP8* identifies rotavirus 993/83, isolated from calf feces, as a pigeon rotavirus. Virology 210:231-235. [DOI] [PubMed] [Google Scholar]
- 56.Stuker, G., L. S. Oshiro, and N. J. Schmidt. 1980. Antigenic comparisons of two new rotaviruses from rhesus monkeys. J. Clin. Microbiol. 11:202-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Varshney, B., M. R. Jagannath, R. R. Vethanayagam, S. Kodhandharaman, H. V. Jagannath, K. Gowda, D. K. Singh, and D. Rao. 2002. Prevalence of, and antigenic variation in, serotype G10 rotaviruses and detection of serotype G3 strains in diarrheic calves: implications for the origin of G10P11 or P11 type reassortant asymptomatic strains in newborn children in India. Arch. Virol. 147:143-165. [DOI] [PubMed] [Google Scholar]
- 58.Watanabe, M., T. Nakagomi, Y. Koshimura, and O. Nakagomi. 2001. Direct evidence for genome segment reassortment between concurrently circulating human rotavirus strains. Arch. Virol. 146:557-570. [DOI] [PubMed] [Google Scholar]
- 59.Wilde, J., J. Eiden, and R. Yolken. 1990. Removal of inhibitory substances from human fecal specimens for detection of group A rotaviruses by reverse transcriptase and polymerase chain reactions. J. Clin. Microbiol. 28:1300-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu, H, K. Taniguchi, T. Urasawa, and S. Urasawa. 1998. Serological and genomic characterization of human rotaviruses detected in China. J. Med. Virol. 55:168-176. [DOI] [PubMed] [Google Scholar]