Abstract
Mature Xenopus oocytes are arrested in meiosis by the activity of XErp1/Emi2, an inhibitor of the ubiquitin-ligase anaphase-promoting complex/cyclosome (APC/C). On fertilization, XErp1 is degraded, resulting in APC/C activation and the consequent degradation of cell-cycle regulators and exit from meiosis. In this study, we show that a modest increase in the activity of the ubiquitin-conjugating enzyme UbcX overrides the meiotic arrest in an APC/C-dependent reaction. Intriguingly, XErp1 remains stable in these conditions. We found that UbcX causes the ubiquitylation of XErp1, followed by its dissociation from the APC/C. Our data support the idea that ubiquitylation regulates the APC/C-inhibitory activity of XErp1.
Keywords: APC/C, CSF, XErp1/Emi2, UbcX/UbcH10
Introduction
In vertebrates, mature oocytes await fertilization when they are arrested at metaphase of meiosis II. This resting state depends on the cytostatic factor (CSF), which maintains the anaphase-promoting complex/cyclosome (APC/C)—a ubiquitin E3 ligase—in an inactive state (Maller et al, 2002). Active APC/C together with its cofactor cell division cycle 20 (Cdc20; APC/CCdc20) targets the anaphase inhibitor securin and the Cdk1 (cyclin-dependent kinase 1)-activating cyclin B for proteasome-dependent destruction resulting in exit from meiosis. Recently, XErp1/Emi2 was identified as the molecular nature of CSF in Xenopus eggs that directly inhibits the APC/C (Schmidt et al, 2005; Tung et al, 2005). Fertilization of the egg causes the activation of calmodulin/calcium-dependent kinase II (CaMKII). CaMKII, together with Polo-like kinase 1 (Plx1), then targets XErp1 for degradation, resulting in APC/C activation and the consequent completion of meiosis II (Liu & Maller, 2005; Rauh et al, 2005). The activity of XErp1 during CSF arrest is negatively regulated by Cdk1 and positively controlled by p90RSK kinase (Nishiyama et al, 2007; Wu et al, 2007a, 2007b). Specifically, phosphorylation of XErp1 by Cdk1 decreases both its half-life and its affinity for the APC/C. These phosphorylations are removed by the protein phosphatase 2A, which binds to XErp1 upon phosphorylation of XErp1 by p90RSK. Thus, by alternate activation of APC/C or CSF (XErp1), these cybernetic mechanisms ensure constant Cdk1 activity at metaphase II, despite continuous cyclin B synthesis.
A similar mechanism controls the association between Cdc20 and components of the spindle assembly checkpoint (SAC) by a dynamic balance of ubiquitylation and deubiquitylation. Activation of the SAC by unattached kinetochores leads to the association of Cdc20 with the SAC proteins Mad2 and BubR1, which prevent Cdc20 from activating the APC/C. Addition of the E2 enzyme UbcH10 to SAC-arrested cell extract triggers the APC/CCdc20-dependent multi-ubiquitylation of Cdc20, and possibly other components of the APC/C–Cdc20–SAC complex, resulting in the release of Mad2 and BubR1 from Cdc20 (Reddy et al, 2007; Ge et al, 2009). Although in checkpoint arrest conditions this ubiquitylation reaction is antagonized by the activity of the ubiquitin hydrolase USP44 (Stegmeier et al, 2007), it rapidly activates APC/C in a switch-like manner as soon as the last kinetochore is properly attached. In this study, we show that ectopic UbcX—the Xenopus orthologue of UbcH10 (Yu et al, 1996)—induces release from CSF arrest. In the presence of elevated levels of active UbcX, XErp1 is ubiquitylated and dissociated from the APC/C, suggesting that the APC/C inhibitory activity of XErp1 in CSF arrest can be modulated in an UbcX-dependent manner.
Results And Discussion
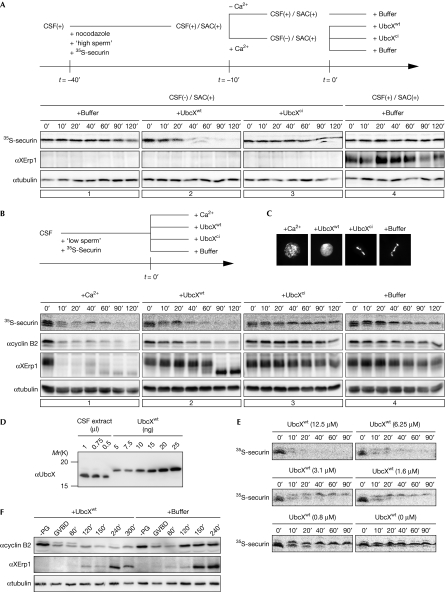
The finding that the APC/C can liberate itself from inhibition by the SAC prompted us to test whether a similar mechanism controls CSF activity. First, we analysed whether UbcX can suppress SAC activity in Xenopus egg extract. Reportedly, egg extract with an artificially high ratio of nucleus to cytoplasm activates the SAC on treatment with spindle poisons (Minshull et al, 1994). We consistently observed that in vitro translated 35S-labelled securin (35S-securin) remained stable when calcium was added to the extract containing high concentrations of sperm nuclei and nocodazole (Fig 1A, panel 1). Western blot analyses showed that calcium triggered XErp1 destruction, demonstrating that APC/C inhibition was maintained by SAC activity, but not by CSF activity (Fig 1A, panel 1). Importantly, addition of wild-type UbcX (UbcXwt), but not the catalytically inactive form (UbcXci), to SAC-arrested extract induced the destruction of 35S-securin (Fig 1A), suggesting that the mechanism of UbcH10-mediated SAC inactivation is conserved between humans and Xenopus.
Figure 1.
Ectopic UbcXwt overrides spindle assembly checkpoint and cytostatic factor arrest in Xenopus egg extract. (A) CSF extract containing 35S-securin was supplemented with nocodazole and high concentrations of sperm to activate the SAC in the CSF arrest. CSF arrest was released by the addition of calcium. Samples were taken at the indicated time points after the addition of the specified reagents, 35S-securin was detected by autoradiography and XErp1 and α-tubulin by WB. (B) The indicated reagents were added to CSF extract, and the stabilities of 35S-securin, XErp1 and cyclin B2 were analysed as before. (C) The extract was treated as in (B) and chromatin structures were analysed after 90 mins. (D) The amount of UbcX in CSF extract was determined by WB, using recombinant UbcXwt as standard. (E) CSF extract was supplemented with the indicated concentrations of UbcXwt and CSF release was monitored by autoradiographic analysis of 35S-securin. (F) Stage VI oocytes were injected with buffer or with 80 ng UbcXwt, resulting in 8.9 μM exogenous UbcX, which is approximately 11-fold more than levels of the endogenous protein. Maturation was induced by progesterone treatment and samples were taken for WB analysis at the indicated time points after GVBD. ci, catalytically inactive; CSF, cytostatic factor; GVBD, germinal vesicle breakdown; PG, progesterone; SAC, spindle assembly checkpoint; 35S-securin, in vitro translated 35S-labelled securin; WB, western blotting; wt, wild type.
Next, we analysed whether recombinant UbcXwt is able to release CSF arrest. Addition of calcium to CSF extract caused the degradation of XErp1, 35S-securin and cyclin B2 and the consequent decondensation of chromatin (Fig 1B,C). Intriguingly, UbcXwt, but not UbcXci, triggered calcium-independent exit from meiosis, as indicated by UbcXwt-mediated destruction of APC/C substrates and chromatin decondensation (Fig 1B,C). Notably, in these conditions XErp1 was dephosphorylated but not degraded on exit from meiosis, as indicated by the shift in its electrophoretic mobility, suggesting that UbcXwt neutralizes CSF activity by a different means than XErp1 degradation. Like UbcX, catalytically active UbcH10 triggered premature CSF release (supplementary Fig S1A online), demonstrating that UbcX and UbcH10 are equivalent in their ability to override the CSF arrest.
In conditions in which the SAC is active, ubiquitylation of Cdc20 is antagonized by the ubiquitin hydrolase USP44 (Stegmeier et al, 2007). However, we could not deplete USP44 from CSF extract using any of the three antibodies we raised against Xenopus USP44, or efficiently inhibit hydrolases in CSF extract using ubiquitin aldehyde, a competitive pan-ubiquitin hydrolase inhibitor (Hershko & Rose, 1987; data not shown). Therefore, for technical reasons, we were not able to determine whether USP44 is involved in the regulation of CSF arrest. To exclude the possibility that the UbcXwt-induced CSF release was due to an unspecified overload of the system, we analysed the amount of UbcXwt required for this release. Quantitative western blot analyses determined the concentration of endogenous UbcX in CSF extract to be approximately 0.8 μM (Fig 1D). Titration experiments showed that a twofold excess of UbcXwt (approximately 1.6 μM) was sufficient to trigger partial destabilization of 35S-securin, whereas efficient APC/C activation was mediated by an eightfold excess of UbcXwt (Fig 1E). To further analyse whether elevated levels of UbcX interfere with the function of XErp1 during meiotic progression of Xenopus oocytes, we injected recombinant UbcXwt into stage VI oocytes and induced meiotic maturation by progesterone treatment. Consistent with the idea that the APC/C-inhibitory function of XErp1 is essential for the transition from meiosis I to meiosis II (Ohe et al, 2007), we observed that injection of UbcXwt into oocytes prevented entry into meiosis II, as indicated by the failure to reaccumulate cyclin B2 after meiosis I (Fig 1F). In summary, these data—together with the observation that UbcXci did not affect the CSF arrest (Fig 2B)—demonstrate that the APC/C-inhibitory activity of XErp1 is sensitive to subtle changes in E2 activity.
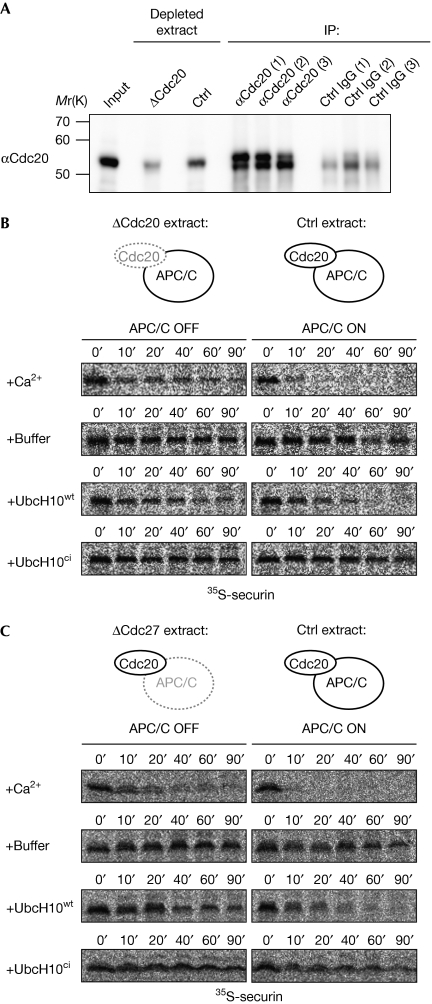
Figure 2.
UbcH10wt-induced cytostatic factor release requires anaphase-promoting complex/cyclosome activity. (A) Cdc20 was depleted from CSF extracts by three rounds of immunodepletion. Cdc20 was detected by WB analysis in the input fraction, in the extract after the third round of Cdc20 depletion (ΔCdc20) or control depletion, as well as on the α-Cdc20 and control beads (IP) after the indicated rounds of depletion. (B) Cdc20- or control-depleted extract was supplemented with calcium, buffer, UbcH10wt or UbcH10ci and the stability of 35S-securin was analysed by autoradiography. (C) CSF extract that was depleted of Cdc27 or control-depleted extract was supplemented with the indicated reagents and 35S-securin was detected at the indicated time points. APC/C, anaphase-promoting complex/cyclosome; Cdc, cell division cycle; ci, catalytically inactive; CSF, cytostatic factor; Ctrl, control; IgG, immunoglobulin G; IP, immunoprecipitation; 35S-securin, in vitro translated 35S-labelled securin; WB, western blotting; wt, wild type.
As UbcXwt/UbcH10wt triggered CSF release but not XErp1 destruction, we investigated whether APC/C activity is required for the exit from meiosis, by Cdc20 immunodepletion (Fig 2A). As expected, addition of calcium to Cdc20-depleted extract did not induce 35S-securin destruction (Fig 2B). Similarly, addition of UbcH10 to the extract depleted of Cdc20 or the core APC/C-subunit Cdc27 did not mediate 35S-securin degradation (Fig 2B,C). Add-back of recombinant Cdc20 confirmed the specificity of the Cdc20 depletion (supplementary Fig S1C,D online). Thus, although ectopic E2 enzyme does not destabilize XErp1, APC/CCdc20-dependent ubiquitylation is still essential for this calcium-independent abrogation of the meiosis II arrest.
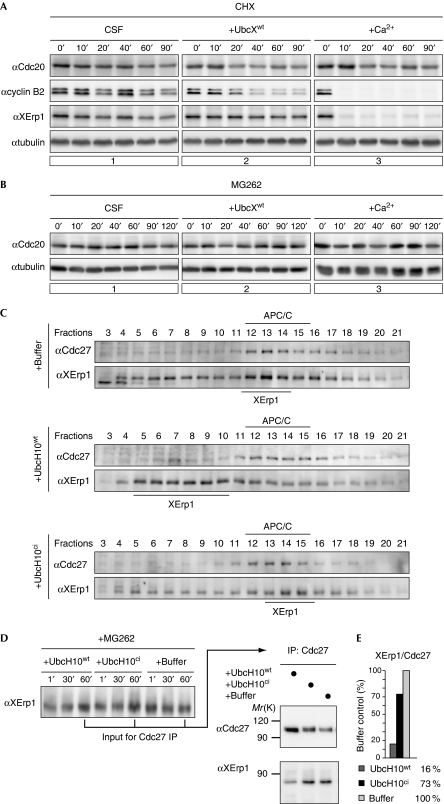
Recently, the model that suggests that the APC/C can liberate itself from SAC inhibition has been challenged. Specifically, it has been postulated that in the presence of Mad2/BubR1/Bub3, Cdc20 is turned into an APC/C substrate resulting in its ubiquitylation and degradation (Nilsson et al, 2008). Thus, although the ‘activation model’ proposes that ubiquitylation of Cdc20 results in APC/C activation, the ‘inactivation model’ proposes that it induces Cdc20 degradation, that is, APC/C inactivation. In support of the latter, it has been reported that budding yeast Cdc20 is destabilized in response to SAC activation (Pan & Chen, 2004; King et al, 2007). The controversy about these models prompted us to determine whether CSF arrest is mediated by the destabilization of Cdc20. As shown in Fig 3A, inhibition of protein synthesis by cycloheximide (CHX) did not significantly reduce Cdc20 levels in CSF extract. To corroborate this finding, we analysed the levels of Cdc20 when protein destruction was blocked. Addition of MG262 to CSF extract did not affect Cdc20 levels (Fig 3B), but it completely inhibited calcium-induced degradation of cyclin B (data not shown) confirming efficient inhibition of the proteasome by MG262. Thus, Xenopus Cdc20 is not a short-lived protein in egg extract. Moreover, as CHX-treated extract was able to trigger calcium-induced degradation of cyclin B (Fig 3A), our data demonstrate that Cdc20 levels are sufficient to effectively target APC/C substrates for destruction even when its resynthesis is inhibited. These results—together with our observation that exogenous UbcX induces premature CSF release, rather than an enhancement of the arrest—indicate that a stable CSF state is not aided by the destabilization of Cdc20.
Figure 3.
Cell division cycle 20 is stable in cytostatic factor arrest and UbcH10wt causes the dissociation of the anaphase-promoting complex/cyclosome–XErp1 complex. (A) CSF extract treated with CHX was supplemented with buffer control, UbcXwt or calcium. Samples were taken at the indicated time points, treated with phosphatase and analysed by WB for Cdc20, XErp1, cyclin B2 and α-tubulin. (B) CSF extract treated with MG262 was supplemented with buffer control, UbcXwt, or calcium and samples were taken. After phosphatase treatment, Cdc20 and tubulin were analysed by WB. (C) CSF extracts were incubated with buffer, UbcH10wt or UbcH10ci and centrifuged through glycerol gradients. Fractions were taken from high (fraction 3) to low (fraction 21) glycerol concentrations. Cdc27 and XErp1 were detected by WB. (D) CSF extracts containing MG262 were treated as described in (C) and Cdc27 was immunoprecipitated. The immunoprecipitates were subjected to CIP phosphatase treatment and Cdc27 and XErp1 were detected by WB analysis. (E) Quantification of (D). The integrated intensities of Cdc27 and XErp1 signals were measured and the ratio of the signals of XErp1 to Cdc27 was calculated. Buffer control was arbitrarily set to 100. APC/C, anaphase-promoting complex/cyclosome; Cdc, cell division cycle; CHX, cycloheximide; ci, catalytically inactive; CIP, calf intestinal alkaline phosphatase; CSF, cytostatic factor; IP, immunoprecipitation; WB, western blotting; wt, wild type.
Next, we addressed the mechanism of UbcX/UbcH10-mediated CSF release. As addition of these E2 enzymes did not induce XErp1 destruction, we investigated whether UbcH10wt interferes with the APC/C-inhibitory function of XErp1. First, we used sedimentation velocity centrifugation to analyse the interaction between XErp1 and the APC/C in extract. Consistent with their effect on the CSF arrest, UbcH10wt but not UbcH10ci caused the dissociation of XErp1 from the APC/C (Fig 3C). To investigate more directly whether UbcH10wt affects the interaction between XErp1 and the APC/C, we analysed the amount of XErp1 coprecipitating with Cdc27 from the extract treated with UbcH10 and MG262 to prevent exit from meiosis. Intriguingly, a significantly reduced amount of XErp1 was detected when Cdc27 was purified from the extract supplemented with UbcH10wt than from the extract containing control buffer or UbcH10ci (Fig 3E). Equal amounts of XErp1 in the input samples confirmed that this effect was due to reduced binding of XErp1 to the APC/C (Fig 3D). In summary, addition of UbcH10wt causes premature CSF release, by interfering with the ability of XErp1 to bind to the APC/C.
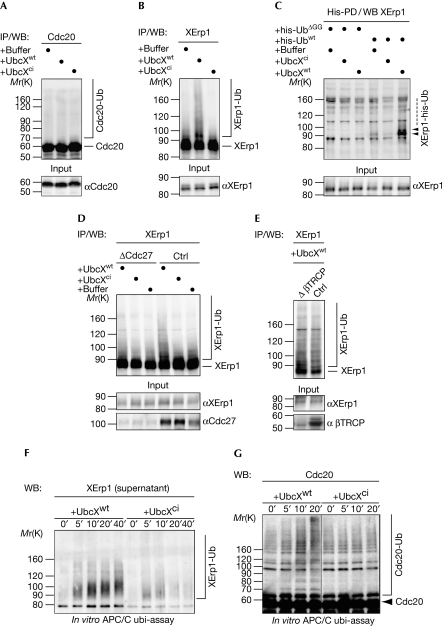
Elevated levels of UbcH10 drive SAC inactivation by APC/C-dependent ubiquitylation of Cdc20 or other components of the APC/C–Cdc20 checkpoint complex (Reddy et al, 2007). However, when we affinity-purified Cdc20 from CSF extract supplemented with UbcXwt, no slower-migrating forms of Cdc20, indicative of ubiquitylated Cdc20, could be detected (Fig 4A). By contrast, a smear characteristic of ubiquitylation was detected when XErp1 was immunopurified from UbcXwt-containing extract (Fig 4B). Consistent with its inability to cause premature CSF release, UbcXci did not change the electrophoretic mobility of XErp1 (Fig 4B). To confirm that ubiquitylation accounts for the high-molecular smear, we used his-tagged ubiquitin (his-Ub) to affinity-purify ubiquitylated proteins from CSF extract. Denaturing conditions during purification ensured that only proteins that were covalently attached to his-Ub were purified. As a control, CSF extract was supplemented with a truncated version of his-Ub (his-UbΔGG) that was unable to form isopeptide bonds with substrates. As shown in Fig 4C, XErp1 was ubiquitylated in a reaction dependent on active UbcX and conjugatable His-Ubwt. As shown in Fig 4D,E, depletion of Cdc27 but not β-TRCP (transduction repeat-containing protein) abrogated the UbcXwt-mediated ubiquitylation of XErp1, demonstrating that this reaction required APC/C activity but not SCFβ-TRCP activity. To test this directly, we performed in vitro ubiquitylation assays using immunopurified APC/C. Addition of UbcXwt but not UbcXci to immunopurified APC/C caused significant ubiquitylation of XErp1 (Fig 4F). Notably, we observed a small UbcXwt-dependent increase in slower-migrating forms of Cdc20, indicating that the APC/C-associated fraction of Cdc20 is ubiquitylated by UbcX (Fig 4G). Importantly, when we performed in vitro APC/C-ubiquitylation assays using active UbcXwt, and then separated the APC/C-bound fraction from the supernatant, we observed the dissociation of ubiquitylated forms of XErp1 from the APC/C (Fig 4F). Efficient dissociation of XErp1 was not detected when immunopurified APC/C was incubated with UbcXci (Fig 4F). Collectively, these data suggest that UbcX triggers the ubiquitylation of XErp1 and, to a lesser extent, the ubiquitylation of Cdc20, resulting in the dissociation of the APC/C inhibitor XErp1 from the APC/C.
Figure 4.
UbcXwt promotes XErp1 ubiquitylation. (A) CSF extract incubated with buffer, UbcXwt or UbcXci for 30 min was processed for WB analysis (input) or used to IP Cdc20, followed by WB analysis. (B) CSF extract was treated as in (A) and XErp1 was immunoprecipitated and analysed by WB. (C) CSF extracts containing his-Ubwt or his-UbΔGG were treated as in (A) and his-ubiquitin-modified proteins were purified with Ni2+NTA beads under denaturating conditions (his-PD). XErp1 was detected by WB. (D) CSF extract that was depleted of Cdc27 or control-depleted extract was supplemented with the indicated reagent. Samples were taken and Cdc27 and XErp1 were detected by WB analysis (input), or XErp1 was immunoprecipitated and analysed by WB. (E) β-TRCP or control-depleted CSF extract was treated with UbcXwt and processed for WB analysis (input) or used to IP XErp1, followed by WB for XErp1. (F) APC/C was immunoprecipitated from CSF extract using magnetic beads coated with Cdc27 antibodies and treated with UbcXwt or UbcXci for the indicated times. Then, the APC/C was removed from the reaction by the recapture of the beads and XErp1 in the supernatant was detected by WB. (G) APC/C was immunoprecipitated and treated as in (F) and the reactions were analysed by WB for Cdc20 at the indicated timepoints. Note: Samples in A, B, D and E were subjected to CIP phosphatase treatment before WB analysis. Arrowheads indicate modified XErp1. APC/C, anaphase-promoting complex/cyclosome; Cdc, cell division cycle; ci, catalytically inactive; CIP, calf intestinal alkaline phosphatase; CSF, cytostatic factor; his-UbΔGG, his-ubiquitinΔGG; his-Ubwt, his-ubiquitin; IP, immunoprecipitation; PD, pull-down; Ub, ubiquitin; WB, western blotting; wt, wild type; XErp1-Ub, ubiquitin-modified XErp1.
Thus, our studies identify a new mechanism for regulation of XErp1 activity in Xenopus egg extract. On fertilization, CaMKII and Plx1 cooperate to neutralize XErp1 activity by targeting it for rapid, SCFβ-TRCP-dependent destruction resulting in the irreversible exit from meiosis. During CSF arrest, continuous cyclin B synthesis (Yamamoto et al, 2005) is compensated for by transient Cdk1-dependent inactivation of XErp1. Here, we show that the APC/C can liberate itself from inhibition by CSF. Specifically, we observed that increasing the activity of the APC/C-cooperating E2 enzyme UbcX causes both the ubiquitylation of XErp1 and the dissociation of XErp1 from the APC/C, suggesting that the APC/C-mediated attachment of ubiquitin to XErp1 reduces its affinity for the APC/C. Although future studies are required to identify the acceptor lysine residue, the different consequences of APC/CCdc 20- and SCFβ-TRCP-mediated ubiquitylation of XErp1 suggest that the two processes must differ in either the site of ubiquitin attachment or the type of ubiquitin linkages. Intriguingly, although we were unable to detect ubiquitylated Cdc20 in UbcXwt-supplemented CSF extract, our in vitro APC/C assays showed that UbcXwt is able to ubiquitylate Cdc20, albeit inefficiently, indicating that only a small fraction of Cdc20 is modified and/or ubiquitylated Cdc20 is an efficient substrate of ubiquitin hydrolases. Thus, although we cannot rule out that the ubiquitylation of Cdc20 in addition to the one of XErp1 contributes to the reduced affinity of XErp1 for the APC/CCdc20, our data exclude the possibility that CSF-arrest is mediated by the destabilization of Cdc20. This conclusion is made on the basis of the observations that neither inhibition of protein translation or protein degradation detectably affected Cdc20 levels in CSF extract and, in conditions in which Cdc20 resynthesis is inhibited, the amount of Cdc20 in the extract is sufficient to mediate the destruction of APC/C substrates on calcium stimulus. As the second APC/C activator, Cdh1, is not expressed in Xenopus egg extract (Lorca et al, 1998), the proteolysis of APC/C substrates must be mediated by Cdc20.
Our studies show that UbcX levels increase at the transition from meiosis I to meiosis II (supplementary Fig S1B online), but remain constant during meiosis II. Thus, it is tempting to speculate that the sudden increase in UbcX activity results in partial APC/C activation, resulting in exit from meiosis I. According to this hypothesis, the delayed accumulation of XErp1 (supplementary Fig S1B online) would reestablish APC/C inhibition, thus preventing entry into interphase and, finally, resulting in metaphase II-arrested mature eggs. As we have no evidence that UbcX is activated on fertilization, we favour the model that calcium-induced degradation of XErp1 is the initial trigger that partly activates the APC/C, which enables UbcX to facilitate the dissociation of XErp1 from the APC/C. This model implies that the robust, yet, rapidly switchable CSF arrest is mediated by a dynamic association of XErp1 with the APC/C, and that UbcX-mediated ubiquitylation helps to shift the balance towards the APC/C-unbound form of XErp1. Although under CSF arrest this mechanism would not be sufficient to result in APC/C activation, it could support the activation of the APC/C once fertilization induces the destruction of XErp1. Thus, the UbcX-mediated dissociation of XErp1 from the APC/C could provide a positive-feedback loop that contributes to an abrupt switch-like onset of anaphase on fertilization. In somatic cells, attempts of the APC/C to escape checkpoint inhibition in the presence of malattached chromosomes are constantly antagonized by the deubiquitylation of Cdc20 (Stegmeier et al, 2007). To fully understand how APC/C activity is regulated in CSF arrest, a future aim will be to identify the pathway that antagonizes UbcX-mediated ubiquitylation of XErp1.
Methods
Plasmids. UbcH10 and UbcX were amplified from complementary DNA. Catalytically inactive forms were generated by mutating Cys 114.
Cytostatic factor extracts were prepared as described previously (Murray, 1991). When indicated, CaCl2 was added to a concentration of 600 μM, CHX to 350 μM, MG262 to 100 μM and nocodazole to 33 μM. ‘High’ and ‘low’ sperm extracts contained 1.2 × 107 and 1 × 106 sperm nuclei per ml extract, respectively. His-tagged UbcH10wt/ci and UbcXwt/ci were added to a final concentration of 12 and 6 μM, respectively. Proteins were dephosphorylated in 1 × calf intestinal alkaline phosphatase (CIP) buffer with 10 U of CIP (NEB).
Xenopus oocytes were obtained, cultured and injected as described previously (Ohe et al, 2007). Oocytes were homogenized in 1 × CIP buffer with 10 U of CIP (New England Biolabs) and complete protease inhibitors (Roche).
Immunodepletion experiments and purification of ubiquitin conjugates were performed as described (Glockzin et al, 2003; Schmidt et al, 2005).
In vitro ubiquitylation assays were carried out as described previously (Schmidt et al, 2005). To assay the ubiquitylation and release of XErp1 from the APC/C, magnetic beads were separated from the supernatant.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (grant SPP1384) to O.S. and T.U.M. E.H. is a recipient of a DocfForte fellowship from the Austrian Academy of Sciences. We are grateful to T. Lorca and M. Scheffner for reagents. We thank members of the Mayer laboratory, especially Tobias Oelschlägel for helpful discussions and Lucia Sironi for critical reading of the manuscript. We also acknowledge the members of the Tierforschungsanstalt (TFA) and Alejandro Rojas, Falk Hildebrandt and Andreas Heim for technical help during preparation of the paper.
Footnotes
The authors declare that they have no conflict of interest.
References
- Ge S, Skaar JR, Pagano M (2009) APC/C- and Mad2-mediated degradation of Cdc20 during spindle checkpoint activation. Cell Cycle 8: 167–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glockzin S, Ogi FX, Hengstermann A, Scheffner M, Blattner C (2003) Involvement of the DNA repair protein hHR23 in p53 degradation. Mol Cell Biol 23: 8960–8969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko A, Rose IA (1987) Ubiquitin-aldehyde: a general inhibitor of ubiquitin-recycling processes. Proc Natl Acad Sci USA 84: 1829–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King EM, van der Sar SJ, Hardwick KG (2007) Mad3 KEN boxes mediate both Cdc20 and Mad3 turnover, and are critical for the spindle checkpoint. PLoS ONE 2: e342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Maller JL (2005) Calcium elevation at fertilization coordinates phosphorylation of XErp1/Emi2 by Plx1 and CaMK II to release metaphase arrest by cytostatic factor. Curr Biol 15: 1458–1468 [DOI] [PubMed] [Google Scholar]
- Lorca T, Castro A, Martinez AM, Vigneron S, Morin N, Sigrist S, Lehner C, Doree M, Labbe JC (1998) Fizzy is required for activation of the APC/cyclosome in Xenopus egg extracts. EMBO J 17: 3565–3575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maller JL, Schwab MS, Gross SD, Taieb FE, Roberts BT, Tunquist BJ (2002) The mechanism of CSF arrest in vertebrate oocytes. Mol Cell Endocrinol 187: 173–178 [DOI] [PubMed] [Google Scholar]
- Minshull J, Sun H, Tonks NK, Murray AW (1994) A MAP kinase-dependent spindle assembly checkpoint in Xenopus egg extracts. Cell 79: 475–486 [DOI] [PubMed] [Google Scholar]
- Murray AW (1991) Cell cycle extracts. Methods Cell Biol 36: 581–605 [PubMed] [Google Scholar]
- Nilsson J, Yekezare M, Minshull J, Pines J (2008) The APC/C maintains the spindle assembly checkpoint by targeting Cdc20 for destruction. Nat Cell Biol 10: 1411–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama T, Ohsumi K, Kishimoto T (2007) Phosphorylation of Erp1 by p90rsk is required for cytostatic factor arrest in Xenopus laevis eggs. Nature 446: 1096–1099 [DOI] [PubMed] [Google Scholar]
- Ohe M, Inoue D, Kanemori Y, Sagata N (2007) Erp1/Emi2 is essential for the meiosis I to meiosis II transition in Xenopus oocytes. Dev Biol 303: 157–164 [DOI] [PubMed] [Google Scholar]
- Pan J, Chen RH (2004) Spindle checkpoint regulates Cdc20p stability in Saccharomyces cerevisiae. Genes Dev 18: 1439–1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh NR, Schmidt A, Bormann J, Nigg EA, Mayer TU (2005) Calcium triggers exit from meiosis II by targeting the APC/C inhibitor XErp1 for degradation. Nature 437: 1048–1052 [DOI] [PubMed] [Google Scholar]
- Reddy SK, Rape M, Margansky WA, Kirschner MW (2007) Ubiquitination by the anaphase-promoting complex drives spindle checkpoint inactivation. Nature 446: 921–925 [DOI] [PubMed] [Google Scholar]
- Schmidt A, Duncan PI, Rauh NR, Sauer G, Fry AM, Nigg EA, Mayer TU (2005) Xenopus polo-like kinase Plx1 regulates XErp1, a novel inhibitor of APC/C activity. Genes Dev 19: 502–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmeier F et al. (2007) Anaphase initiation is regulated by antagonistic ubiquitination and deubiquitination activities. Nature 446: 876–881 [DOI] [PubMed] [Google Scholar]
- Tung JJ, Hansen DV, Ban KH, Loktev AV, Summers MK, Adler JR III, Jackson PK (2005) A role for the anaphase-promoting complex inhibitor Emi2/XErp1, a homolog of early mitotic inhibitor 1, in cytostatic factor arrest of Xenopus eggs. Proc Natl Acad Sci USA 102: 4318–4323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JQ, Hansen DV, Guo Y, Wang MZ, Tang W, Freel CD, Tung JJ, Jackson PK, Kornbluth S (2007a) Control of Emi2 activity and stability through Mos-mediated recruitment of PP2A. Proc Natl Acad Sci USA 104: 16564–16569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q et al. (2007b) A role for Cdc2- and PP2A-mediated regulation of Emi2 in the maintenance of CSF arrest. Curr Biol 17: 213–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto TM, Iwabuchi M, Ohsumi K, Kishimoto T (2005) APC/C-Cdc20-mediated degradation of cyclin B participates in CSF arrest in unfertilized Xenopus eggs. Dev Biol 279: 345–355 [DOI] [PubMed] [Google Scholar]
- Yu H, King RW, Peters JM, Kirschner MW (1996) Identification of a novel ubiquitin-conjugating enzyme involved in mitotic cyclin degradation. Curr Biol 6: 455–466 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.