Abstract
Glioma stem-cells are associated with the brain vasculature. However, the way in which this vascular niche regulates stem-cell renewal and fate remains unclear. Here, we show that factors emanating from brain endothelial cells positively control the expansion of long-term glioblastoma stem-like cells. We find that both pharmacological inhibition of and RNA interference with the mammalian target of rapamycin (mTOR) pathway reduce their spheroid growth. Conversely, the endothelial secretome is sufficient to promote this mTOR-dependent survival. Thus, interfering with endothelial signals might present opportunities to identify treatments that selectively target malignant stem-cell niches.
Keywords: brain tumour, endothelial, mTOR, secretome, stem cell
Introduction
Glioblastoma multiforme (GBM) is the most frequent and malignant primary tumour of the central nervous system in adults. Despite the availability of aggressive therapies, median survival rates from the time of diagnosis range between 12 and 15 months, and fewer than 3% of patients survive more than 5 years (Fisher et al, 2007). The cancer-stem-cell hypothesis proposes that GBM tumours derive from a small fraction of cells that constitute a reservoir of self-sustaining cells with the exclusive ability to self-renew and maintain the tumour (Singh et al, 2003, 2004; Yuan et al, 2004; Vescovi et al, 2006; Patru et al, 2010). Regardless of the brain tumour ontogeny, glioblastoma stem-like cells (GSCs) might be involved in resistance to radiotherapies and contribute to tumour recurrence and aggressiveness (Bao et al, 2006). Furthermore, alterations in GSC levels affect tumour growth in experimental models of glioma (Androutsellis-Theotokis et al, 2006; Piccirillo et al, 2006; Clement et al, 2007). Recently, the Akt/mammalian target of rapamycin (mTOR) signalling nexus has emerged as a regulator of cancer-stem-cell properties (Hambardzumyan et al, 2008; Bleau et al, 2009). However, the way in which this pathway might protect the immature state in GSCs within the tumour is still poorly understood.
It is generally agreed that GSCs are sheltered within a vascular structure that provides a specific and confined microenvironment, that is probably involved in the regulation of stem-cell renewal and fate (Gilbertson & Rich, 2007; Sneddon & Werb, 2007). In support of this, GSCs were identified in close proximity to endothelial cells, and targeting the tumour vasculature eradicated the self-renewing subpopulation (Batchelor et al, 2007; Calabrese et al, 2007; Folkins et al, 2007). Moreover, irradiation of GSC niches increases survival of patients with malignant glioma (Evers et al, 2010). Thus, the vascular niche might provide essential, selective signals to maintain GSC integrity (Becher et al, 2008; Charles et al, 2010). Here, we show that the two mTOR complexes mTORC1 and mTORC2 function as a gateway to maintain GSC expansion. Furthermore, the endothelial secretome sustains mTOR activity and GSC survival, and therefore targeting the communication between endothelial cells and GSCs might be an effective therapeutic strategy for glioma.
Results And Discussion
The mTOR pathway is active in growing GSC
Adult GBM tumours contains a subpopulation of cells which retain the ability to expand ex vivo as neurospheres in mitogen-defined medium, and share many characteristics with normal stem cells including multipotent differentiation and the ability to self-renew (Singh et al, 2004). We have established four patient-derived long-term GSC lines (Patru et al, 2010) that can efficiently form neurospheres while expressing neural-stem-cell markers: the transcription factor Sox2 (SRY (sex determining region Y)-box 2) and the intermediate filament protein Nestin (Fig 1A). To assess their multipotency ability further, single-cell GSC suspensions were subjected to differentiation assay by induction of cell adhesion and mitogen withdrawal. In such conditions, most cells express Tubulin βIII, a marker for neural lineage. Meanwhile, Nestin is barely detectable after 72 h (Fig 1A). As proposed previously, this specific subpopulation of glioma cells might represent a validated system in which to generate long-term, human, adult tumour stable cell lines that can copy parental tumour behaviour with the unique ability to self-renew and initiate tumours (Lee et al, 2006; De Witt Hamer et al, 2008; Patru et al, 2010).
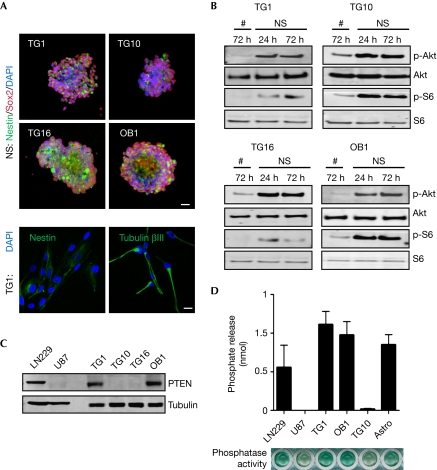
Figure 1.
The mTOR pathway is active in growing, but not differentiated glioblastoma stem-like cells. (A) Upper panel: confocal analysis of four GSCs (TG1, TG10, TG16 and OB1) grown as neurosphere (NS) and labelled with Nestin (green), Sox2 (red) and nucleus (blue). Lower panel: confocal analysis of TG1 engaged in differentiation for 72 h and processed for immunostaining with Nestin and Tubulin βIII (green) together with nuclear counterstaining (blue). Scale bars, 10 μm. (B) Akt and S6 phosphorylations (p) were tested on total cell lysates from differentiated cells (#) and growing NS. (C) Total cell lysates from LN229 and U87 glioma cells were analysed for PTEN expression together with the four GSCs. Tubulin was used as a loading control. (D) Phosphatase activity was measured from PTEN immunoprecipitate fractions by colorimetric assay. LN229 and primary rat astrocytes (astro) were used as positive controls and U87 was used as a negative control. Graph represents mean+s.e.m. of three independent experiments. DAPI, 4,6-diamidino-2-phenylindole; GSC, glioblastoma stem-like cell; mTOR, mammalian target of rapamycin; NS, neurosphere.
We investigated whether intrinsic signalling pathways are regulated differently in growing neurospheres and differentiated cultures. As mTOR is aberrantly active in glioma (Eyler et al, 2008; Akhavan et al, 2010), phosphorylation of its effectors Akt and S6 was examined. Interestingly, only sphere-forming cells show basal activation of Akt and S6, whereas no signal is detected in differentiated cells (Fig 1B). Thus, our data indicate that mTOR activation is lost on differentiation. Expression of phosphatase and tensin homologue (PTEN)—an upstream regulator of mTOR frequently altered in GBM (Koul, 2008)—was monitored. Although a full-length form of PTEN is expressed in the patient cell lines TG1 and OB1, it is almost undetectable in TG10 and TG16 cells (Fig 1C). Consistent with this, TG1 and OB1 show functional lipid phosphatase activity in vitro (Fig 1D). PTEN status does not correlate with the mTOR basal activation observed in the four GSCs. Hence, mTOR activation seems to rely on environmental modifiers such as non-adhesive cultures and presence of mitogens, rather than intrinsic properties.
Inhibition of the mTOR pathway prevents GSC expansion
To evaluate its contribution to GSC expansion, mTOR was pharmacologically inhibited at three levels: mTOR complex 1 (rapamycin), mTOR kinase activity (PP242) and dual inhibition of phosphatidylinositol 3-kinase and mTOR (PI103). A 24-h treatment on growing neurospheres significantly alters mTOR phosphorylation and downstream signalling in GSCs (Fig 2A). Remarkably, all three mTOR inhibitors decrease the number and size of Sox2-expressing neurospheres, as well as cell viability (Fig 2B–E; supplementary Fig S1-2 online). Hence, pharmacological inhibition of the mTOR pathway leads to a reduction in the number of GSCs. This suggests that both phosphatidylinositol 3-kinase and the two mTOR complexes might participate in the maintenance of GSC integrity, but have no effect on differentiated cultures (Fig 2C). Our data extend previous reports on the proposed role of the Akt and mTOR pathway in GSC self-renewal (Eyler et al, 2008; Gallia et al, 2009).
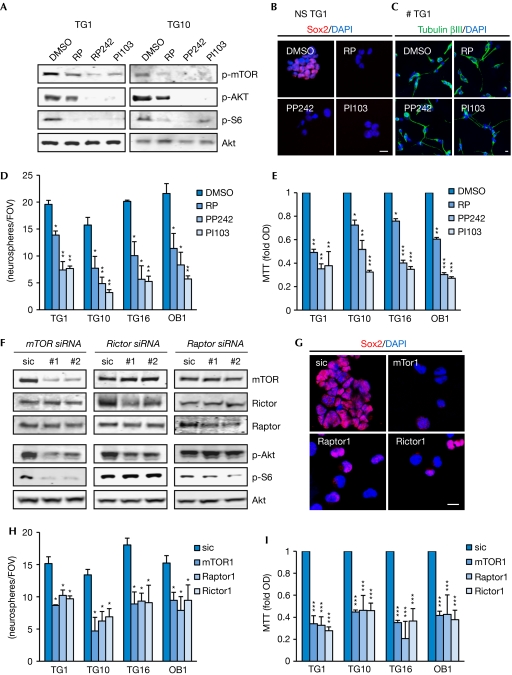
Figure 2.
Inhibition of the mTOR pathway prevents glioblastoma stem-like cell expansion. (A–E) GSCs were treated with vehicle (DMSO), rapamycin (RP 50 nM), PP242 (1 μM) and PI103 (10 μM). (F–I) GSCs were transfected with siRNA against mTor, Rictor and Raptor or a control siRNA (sic) for 72 h. (A,F) Protein extracts were tested by western blot analysis with the indicated antibodies. (B,C,G) Confocal analysis on growing TG1 neurospheres (NS) stained with Sox2 (red) and nucleus (blue) in B and G, and on differentiated TG1 (≠) stained with Tubulin βIII (green) in C. Scale bars, 10 μm. (D,H) The number of secondary neurospheres per field of view (FOV) was counted. (E,I) Cell viability was analysed by MTT assay and normalized to the optical density (OD) obtained with DMSO and sic controls. All graphs represent mean+s.e.m. of three independent experiments. Analysis of variance test: ***P<0.001, **P<0.01, *P<0.05. DAPI, 4,6-diamidino-2-phenylindole; GSC, glioblastoma stem-like cell; mTOR, mammalian target of rapamycin; p, phosphorylated; siRNA, small-interfering RNA.
To confirm the role of the mTOR machinery in GSC maintenance, we knocked down mTOR expression by using RNA interference, as well as that of its partners Rictor and Raptor (Fig 2F; supplementary Fig S5 online). After 72 h, Akt and S6 activation are severely impaired in the absence of mTOR, whereas Rictor and Raptor silencing only hamper either Akt serine phosphorylation or S6 activation, respectively (Fig 2F). Thus, signalling through Raptor and Rictor could still be independently blocked, indicating that despite its constitutive activation, the mTOR axis is not aberrantly regulated in GSCs. Interestingly, Sox2 expression is weakened when either mTOR, Rictor or Raptor are silenced (Fig 2G). In addition, GSC expansion is severely diminished as monitored by secondary neurosphere formation and MTT assay (Fig 2H–I). Taken together, our results indicate that the two mTOR complexes cooperate to maintain GSC properties and that their individual loss-of-function interferes with GSC survival. The Akt/mTOR axis has been proposed to be a regulator of GSC growth and survival, and, in some cases, of differentiation (Eyler et al, 2008; Gallia et al, 2009; Sunayama et al, 2010). Our results support these findings by demonstrating a dual requirement for Raptor and Rictor. However, we found that blocking the mTOR pathway was not sufficient to enhance cell differentiation; instead, cell viability was severely affected.
Endothelial cells preserve GSC integrity through mTOR
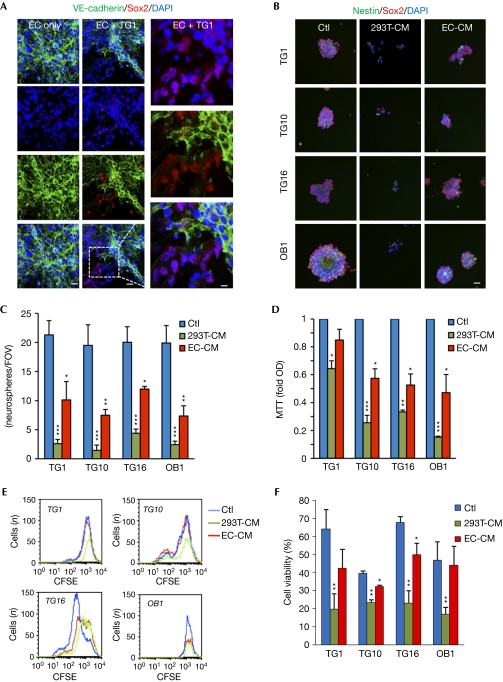
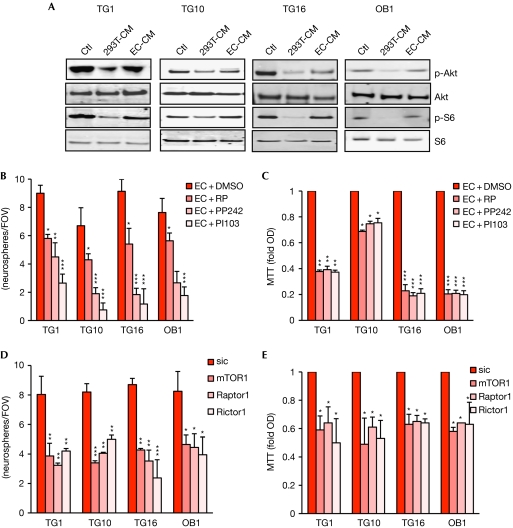
Similarly to embryonic and adult stem cells, cancer stem cells are found within a specific and confined microenvironment that is orchestrated by a vascular unit (Gilbertson & Rich, 2007). For example, GSCs are observed in close proximity to tumour blood vessels. Anti-angiogenic drugs eliminate most GSCs from the tumour mass (Calabrese et al, 2007), indicating that brain endothelial cells could protect GSCs. To investigate this, GSCs were cultured on brain endothelial-cell monolayer, using an in vitro model for the human blood–brain barrier (Weksler et al, 2005). Surprisingly, even after 24 h of coculture without mitogens, GSCs retain their identity, as shown by Sox2 expression and the lack of morphological signs of differentiation (Fig 3A; supplementary Fig S3 online). Similar effects were observed when using macrovascular endothelial cell monolayers (data not shown). This suggests that endothelial cells could act as a feeding bed for GSCs by providing the essential microenvironment that might be similar to the vascular niche, at least at the level of GSC protection. To challenge the effects of the endothelial secretome, endothelial cell-conditioned medium (CM) was used to culture GSCs (supplementary Fig S1,S4 online). Epithelial CM provokes a decrease in GSC expansion, by contrast, growth of Sox2/Nestin-expressing neurospheres persists in the presence of the endothelial-cell secretome (Fig 3B–D). The secreted protein profile, rather than protein concentration in CM, seems to mediate the functional effects (supplementary Fig S4 online). Interestingly, the ratio of proliferating to quiescent cells, estimated by carboxyfluorescein succinimidyl ester dilution, was similar in both culture conditions, although the cell number appears to be lower when cultured in 293T-CM, especially for TG1 and TG10 (Fig 3E). As cycling rates were monitored exclusively on viable cells, on the basis of their size and granulosity, we inferred that endothelial cells might have a prosurvival effect. This was further investigated by propidium-iodide incorporation experiments. Indeed, endothelial cell-CM, but not epithelial CM was able to maintain a high proportion of living cells (Fig 3F). Overall, our results support the idea of an informative transference between endothelial cells and GSCs, whereby endothelial-secreted factors could retain GSC properties. Our findings are similar to angiocrine factors that are shown to regulate hematopoietic stem-cell fate (Kobayashi et al, 2010). We next explored whether endothelial cell secretome might modulate the mTOR signalling nexus in GSCs. Activation of mTOR downstream effectors Akt and S6 is maintained with endothelial but not epithelial soluble factors (Fig 4A). Supporting this, mTOR inhibitors halt endothelial-promoted secondary neurosphere formation and reduce cell viability (Fig 4B,C). Importantly, reducing mTOR, Raptor and Rictor expression by using short-interfering RNA (siRNA) hinders endothelial CM-mediated GSC expansion. Thus, the endothelial secretome might act as a substitute for the additive mitogens contained in GSC-culture media, and sustain neurosphere integrity and mTOR activity.
Figure 3.
Brain endothelial cells preserve glioblastoma stem-like cell properties. (A) Human brain endothelial cells (EC) were cultured for 24 h without mitogens, alone or cocultured with TG1. Confocal analysis of VE-cadherin (green), Sox2 (red) and nucleus (blue) staining. Scale bars, 10 μm. (B–F) GSCs were treated for 72 h with EC-conditioned medium (EC-CM) and compared with control medium (Ctl) and epithelial CM (293T-CM). (B) Confocal analysis of TG1 stained with Nestin (green), Sox2 (red) and nucleus (blue). Scale bars, 10 μm. (C) The number of secondary neurospheres per field of view (FOV) was counted. (D) Cell viability was analysed by MTT and normalized to the optical density (OD) obtained with control (Ctl). (E) Cell-cycle rate was assessed by carboxyfluorescein succinimidyl ester dilution and analysed by flow cytometry. (F) Cell death was measured on propidium iodide-stained cells by flow cytometry. All graphs represent mean+s.e.m. of three independent experiments. Analysis of variance test: ***P<0.001, **P<0.01, *P<0.05. CFSE, carboxyfluorescein succinimidyl ester; DAPI, 4,6-diamidino-2-phenylindole; GSC, glioblastoma stem-like cell; mTOR, mammalian target of rapamycin.
Figure 4.
Brain endothelial cell secretome can restore mTOR activation in glioblastoma stem-like cells. (A) GSCs were treated with human brain endothelial cell-conditioned medium (EC-CM) for 72 h and compared with control medium (Ctl) and 293T-CM. Protein extracts were tested by western blot with the indicated antibodies. (B,C) GSCs were treated with EC-CM alone or in the presence of rapamycin (RP 50 nM), PP242 (1 μM) and PI103 (10 μM) for 24 h. (D,E) GSCs were transfected with siRNA against mTor, Rictor and Raptor or a control siRNA (sic) for 72 h. (B,D) The number of secondary neurospheres per field of view (FOV) was counted. (C,E) Cell viability was analysed by MTT and normalized to optical density (OD) obtained with DMSO and sic, respectively. All graphs represent mean+s.e.m. of three independent experiments. Analysis of variance test: ***P<0.001, **P<0.01, *P<0.05. DAPI, 4,6-diamidino-2-phenylindole; GSC, glioblastoma stem-like cell; mTOR, mammalian target of rapamycin; siRNA, small-interfering RNA.
In summary, our study reinforces the emerging concept that brain vasculature supplies essential signals to maintain GSC identity (supplementary Fig S5 online). Altogether, our results reveal an active communication between endothelial cells and GSCs, and support a model in which endothelial-soluble factors maintain stemness. In this dialogue, the mTOR signalling nexus emerges as a key player. Therefore, altering the molecular transmission between endothelial cells and cancer stem cells might be an effective therapeutic strategy for tumour treatment.
Methods
Cell culture and siRNA transfection. Four human GSCs (TG1, TG10, TG16 and OB1) were obtained as described previously (Patru et al, 2010), and maintained in DMEM/F12 plus N2, G5 and B27 (Invitrogen). To induce differentiation, cells were placed in 10% fetal bovine serum and attached cells were cultured in serum-free DMEM/F12 for a further 72 h. Immortalized human brain microvascular endothelial cells (hCMEC/D3) were cultured as described previously (Weksler et al, 2005). HEK293T, LN229 and U87 (ATCC) were maintained in DMEM-10% fetal bovine serum. Conditioned media from HEK293T and hCMEC/D3 were obtained from 72 h-old cultures in serum-free EBM2 (Lonza). Stealth non-silencing control and selected siRNA for human mTOR, Rictor and Raptor (Invitrogen) were transfected using RNAiMAX lipofectamine (Invitrogen).
Reagents and antibodies. Rapamycin and PI103 were purchased from MERCK, and PP242 was purchased from Sigma. The following antibodies were used: phospho mTOR, mTOR, phospho Akt, Akt, S6, Rictor, Raptor and PTEN (Cell Signaling), phospho S6, Nestin, Sox2 and Tubulin βIII (Millipore), Tubulin and VE-cadherin (Santa Cruz).
Immunofluorescence. Cells grown as neurospheres were dropped on polylysine-coated slides and fixed in PBS-paraformaldehyde 4%. For coculture experiment, endothelial cells were seeded on collagen-treated coverslips 72 h before adding GSCs. GSCs were left for a further 24 h in serum-free EBM2 and fixed. Immunofluorescence was performed as described previously (Gavard & Gutkind, 2006) using Alexa488- and Alexa568-conjugated antibodies (Invitrogen). Samples were mounted in DAPI-containing medium (Vector Labs). Confocal acquisitions were performed on TCS/SP2 Leica (Cochin Imaging facility).
Western blot. Proteins were collected in TNT buffer (10 mM Tris–HCl, pH 7.5, 150 mM NaCl, 1% Triton X-100, 2 mM EDTA) plus protease inhibitors (Sigma), 200 mM NaF and 0.1 mM Na3VO4. Equal amounts of proteins (microBCA kit, Pierce) were separated with 4–12% Nupage gels (Invitrogen) and transferred onto polyvinylidene difluoride membranes (Thermo Scientific). Alexa680-conjugated secondary antibodies (Invitrogen) were used and membranes were scanned using the Odyssey infrared imaging system (Licor).
PTEN functional assay. Phosphatase assays were performed as described previously (Georgescu et al, 1999). Briefly, phosphate released from 100 μM water-soluble di-C8-PIP3 (Echelon Biosciences) was measured at 620 nm following incubation with Biomol Green reagent on anti-PTEN (Cascade bioscience) and anti-GFP (Roche) immunoprecipitate fractions. Background values from GFP were subtracted from the PTEN immunoprecipitate ones.
Secondary neurosphere formation and MTT assays. GSCs were dissociated by up-and-down pipetting and cultured in a 48-well plate format. After 1 and 2 days of drug treatment and siRNA transfection, respectively, cells were dissociated again and single cells were maintained for 3 more days. Counts were blindly performed on five random fields of view, and the mean number of neurospheres per field of view was calculated from three independent experiments. Viability was evaluated by MTT (1-(4,5-dimethylthiazol-2-yl)-3,5-diphenylformazan, thiazolyl blue formazan) assay according to the manufacturer's instruction (Sigma), and absorbance values were read at 590 nm. Statistical analyses were performed using a two-way analysis of variance test (Prism software).
Flow cytometry. Cell proliferation was evaluated using the Cell Trace carboxyfluorescein succinimidyl ester proliferation kit according to the manufacturer's instructions (5 μM, Invitrogen). Cell death was determined by propidium iodide staining (50 μg/ml, Calbiochem). Flow cytometry analyses were performed on FACSCalibur and processed using Flowjo software.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
This research was funded by the Ligue Nationale contre le Cancer, Association pour la Recherche sur le Cancer, Fondation de France, ANR JCJC and by a Marie Curie International Reintegration Grant. E.M.G.M. is supported by the Association pour la Recherche sur le Cancer, A.L.G. by the Université Paris Descartes and J.D. by the Fondation pour la Recherche Medicale.
Footnotes
The authors declare that they have no conflict of interest.
References
- Akhavan D, Cloughesy TF, Mischel PS (2010) mTOR signaling in glioblastoma: lessons learned from bench to bedside. Neuro Oncol 12: 882–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Androutsellis-Theotokis A, Leker RR, Soldner F, Hoeppner DJ, Ravin R, Poser SW, Rueger MA, Bae SK, Kittappa R, McKay RD (2006) Notch signalling regulates stem cell numbers in vitro and in vivo. Nature 442: 823–826 [DOI] [PubMed] [Google Scholar]
- Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN (2006) Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444: 756–760 [DOI] [PubMed] [Google Scholar]
- Batchelor TT et al. (2007) AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 11: 83–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher OJ et al. (2008) Gli activity correlates with tumor grade in platelet-derived growth factor-induced gliomas. Cancer Res 68: 2241–2249 [DOI] [PubMed] [Google Scholar]
- Bleau AM, Hambardzumyan D, Ozawa T, Fomchenko EI, Huse JT, Brennan CW, Holland EC (2009) PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell Stem Cell 4: 226–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese C et al. (2007) A perivascular niche for brain tumor stem cells. Cancer Cell 11: 69–82 [DOI] [PubMed] [Google Scholar]
- Charles N, Ozawa T, Squatrito M, Bleau A-M, Brennan CW, Hambardzumyan D, Holland EC (2010) Perivascular nitric oxide activates notch signaling and promotes stem-like character in PDGF-induced glioma cells. Cell Stem Cell 6: 141–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement V, Sanchez P, de Tribolet N, Radovanovic I, Ruiz i Altaba A (2007) HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol 17: 165–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Witt Hamer PC, Van Tilborg AA, Eijk PP, Sminia P, Troost D, Van Noorden CJ, Ylstra B, Leenstra S (2008) The genomic profile of human malignant glioma is altered early in primary cell culture and preserved in spheroids. Oncogene 27: 2091–2096 [DOI] [PubMed] [Google Scholar]
- Evers P, Lee PP, DeMarco J, Agazaryan N, Sayre JW, Selch M, Pajonk F (2010) Irradiation of the potential cancer stem cell niches in the adult brain improves progression-free survival of patients with malignant glioma. BMC Cancer 10: 384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyler CE, Foo WC, LaFiura KM, McLendon RE, Hjelmeland AB, Rich JN (2008) Brain cancer stem cells display preferential sensitivity to Akt inhibition. Stem Cells 26: 3027–3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JL, Schwartzbaum JA, Wrensch M, Wiemels JL (2007) Epidemiology of brain tumors. Neurologic Clinics 25: 867–890 [DOI] [PubMed] [Google Scholar]
- Folkins C, Man S, Xu P, Shaked Y, Hicklin DJ, Kerbel RS (2007) Anticancer therapies combining antiangiogenic and tumor cell cytotoxic effects reduce the tumor stem-like cell fraction in glioma xenograft tumors. Cancer Res 67: 3560–3564 [DOI] [PubMed] [Google Scholar]
- Gallia GL, Tyler BM, Hann CL, Siu IM, Giranda VL, Vescovi AL, Brem H, Riggins GJ (2009) Inhibition of Akt inhibits growth of glioblastoma and glioblastoma stem-like cells. Mol Cancer Ther 8: 386–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavard J, Gutkind JS (2006) VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol 8: 1223–1234 [DOI] [PubMed] [Google Scholar]
- Georgescu MM, Kirsch KH, Akagi T, Shishido T, Hanafusa H (1999) The tumor-suppressor activity of PTEN is regulated by its carboxyl-terminal region. Proc Natl Acad Sci USA 96: 10182–10187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson RJ, Rich JN (2007) Making a tumour's bed: glioblastoma stem cells and the vascular niche. Nat Rev Cancer 7: 733–736 [DOI] [PubMed] [Google Scholar]
- Hambardzumyan D, Becher OJ, Rosenblum MK, Pandolfi PP, Manova-Todorova K, Holland EC (2008) PI3K pathway regulates survival of cancer stem cells residing in the perivascular niche following radiation in medulloblastoma in vivo. Genes Dev 22: 436–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H et al. (2010) Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nat Cell Biol 12: 1046–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koul D (2008) PTEN signaling pathways in glioblastoma. Cancer Biol Ther 7: 1321–1325 [DOI] [PubMed] [Google Scholar]
- Lee J et al. (2006) Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell 9: 391–403 [DOI] [PubMed] [Google Scholar]
- Patru C et al. (2010) CD133, CD15/SSEA-1, CD34 or side populations do not resume tumor-initiating properties of long-term cultured cancer stem cells from human malignant glio-neuronal tumors. BMC Cancer 10: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccirillo SG, Reynolds BA, Zanetti N, Lamorte G, Binda E, Broggi G, Brem H, Olivi A, Dimeco F, Vescovi AL (2006) Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature 444: 761–765 [DOI] [PubMed] [Google Scholar]
- Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB (2003) Identification of a cancer stem cell in human brain tumors. Cancer Res 63: 5821–5828 [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB (2004) Identification of human brain tumour initiating cells. Nature 432: 396–401 [DOI] [PubMed] [Google Scholar]
- Sneddon JB, Werb Z (2007) Location, location, location: the cancer stem cell niche. Cell Stem Cell 1: 607–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunayama J et al. (2010) Dual blocking of mTor and PI3K elicits a prodifferentiation effect on glioblastoma stem-like cells. Neuro Oncol 12: 1205–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vescovi AL, Galli R, Reynolds BA (2006) Brain tumour stem cells. Nat Rev Cancer 6: 425–436 [DOI] [PubMed] [Google Scholar]
- Weksler BB et al. (2005) Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J 19: 1872–1874 [DOI] [PubMed] [Google Scholar]
- Yuan X, Curtin J, Xiong Y, Liu G, Waschsmann-Hogiu S, Farkas DL, Black KL, Yu JS (2004) Isolation of cancer stem cells from adult glioblastoma multiforme. Oncogene 23: 9392–9400 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.