Abstract
Ribosomes are large ribozymes that synthesize all cellular proteins. As protein synthesis is rate-limiting for bacterial growth and ribosomes can comprise a large portion of the cellular mass, elucidation of ribosomal turnover is important to the understanding of cellular physiology. Although ribosomes are widely believed to be stable in growing cells, this has never been rigorously tested, owing to the lack of a suitable experimental system in commonly used bacterial model organisms. Here, we develop an experimental system to directly measure ribosomal stability in Escherichia coli. We show that (i) ribosomes are stable when cells are grown at a constant rate in the exponential phase; (ii) more than half of the ribosomes made during exponential growth are degraded during slowing of culture growth preceding the entry into stationary phase; and (iii) ribosomes are stable for many hours in the stationary phase. Ribosome degradation occurs in growing cultures that contain almost no dead cells and coincides with a reduction of comparable magnitude in the cellular RNA concentration.
Keywords: ribosome, RNA, degradation, stability
Introduction
Ribosomal metabolism is expensive for growing cells as synthesis of ribosomal components comprises most of the transcriptional activity and a large portion of protein translation. Recently, it was shown that translational capacity is rate-limiting for Escherichia coli growth for a range of growth rates (Scott et al, 2010). Indeed, ribosomes form up to 28% of the dry mass of fast-growing E. coli (Bremer & Dennis, 2008). Accordingly, most of the cellular RNA-degradation activity is of processed pre-ribosomal RNA spacers in fast-growing cells or of newly synthesized ribosomal RNA (rRNA) in slowly growing cells (Gausing, 1977; Deutscher, 2009). rRNA fragments were found in degradosomes purified from growing E. coli cells (Bessarab et al, 1998). Although fast degradation of ribosomes has been suggested to occur in some bacteria with highly fragmented rRNAs (Hsu et al, 1994), this is contested in E. coli, as in Saccharomyces cerevisiae, in which translationally active ribosomes are stable under normal growth conditions (LaRiviere et al, 2006; Zundel et al, 2009). Nevertheless, under stress conditions, a portion of cellular ribosomes can be quickly degraded in E. coli (Deutscher, 2003, 2009; El-Sharoud, 2004). Specifically, ribosome degradation in E. coli has been described in non-growing cells in starvation conditions (Jacobson & Gillespie, 1968; Kaplan & Apirion, 1975; Davis et al, 1986; Zundel et al, 2009), with increased membrane permeability (Jackson & DeMoss, 1965; Yuan & Shen, 1975), during antibiotics treatment (Suzuki & Kilgore, 1967; Shen & Bremer, 1977; Silvers & Champney, 2005) and in response to extensive protein overexpression (Dong et al, 1995). Recently, degradation of translationally incompetent mutant ribosomes was directly demonstrated in yeast and degradation control pathways were elucidated (LaRiviere et al, 2006; Cole et al, 2009; Fujii et al, 2009).
In this work, we develop a test system that allows direct measurement of ribosomal stability in growing E. coli cultures. We show that whereas ribosomes are stable during constant exponential growth and in the stationary phase, they are degraded before entry of the culture into stationary phase.
Results And Discussion
Experimental setup
The experimental system developed here is a modification of pulse-chase protocols that are commonly used to study macromolecular degradation. We cloned an rRNA operon under repressible arabinose BAD (araBAD) or rhamnose BAD (rhaBAD) promoters and added streptavidin-binding (Leonov et al, 2003) and MS2 RNA aptamers (Youngman & Green, 2005) to 23S rRNA and 16S rRNA, respectively. These enabled us to purify plasmid-borne ribosomes by affinity chromatography (Fig 1A; supplementary methods online). Transcription of rRNA from these constructs can be repressed by glucose. Thus, we can study the fates of tagged ribosome populations by following tagged rRNA synthesis using two promoter and purification systems. As rRNA is the main component of the ribosome by mass and functionality, and constitutes the binding sites for nearly all ribosomal proteins, degradation of the 23S or 16S rRNA effectively corresponds to degradation of the ribosomal particle. The fate of ribosomal proteins that might be released from degraded ribosomal particles is not studied here, but could include degradation, storage in a free cellular pool and reincorporation into existing or newly synthesized ribosomes (Pulk et al, 2010).
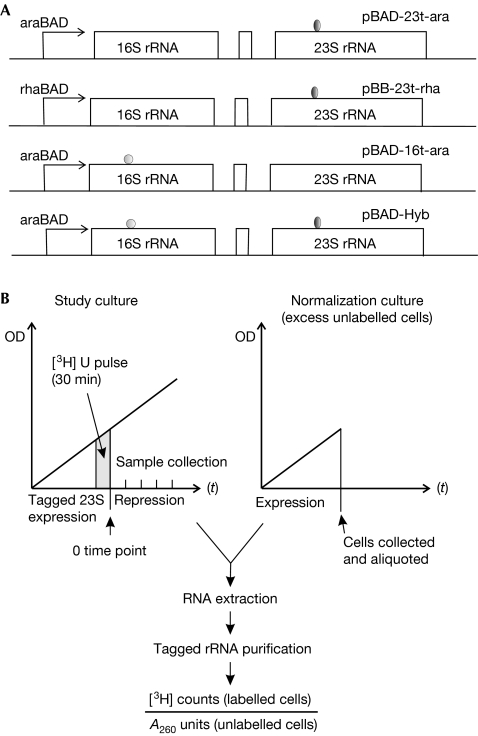
Figure 1.
Experimental set-up. (A) Constructs used. Plasmid names, promoters and purification tags are indicated. (B) A scheme of the experimental setup. A sample of the study culture expressing tagged ribosomes is pulse-labelled with [3H]-uridine, tagged ribosome expression is subsequently repressed and equal sample volumes are collected at defined time points after repression. Each 200-μl study-culture sample is mixed with 20 ml of unlabelled normalization-culture cells expressing tagged ribosomes. A260, absorbance at 260 nm; ara, arabinose; Hyb, hybrid; OD, optical density; p, plasmid; rRNA, ribosomal RNA; rha, rhamnose; [3H]-U, [3H]-uridine.
Cells expressing tagged rRNAs were grown in exponential phase and labelled with a radioactive uridine pulse for 30 min and then tagged rRNA expression was repressed. Samples of 200 μl volume were collected at the indicated time points (Fig 1B). To minimize inter-sample variability in the lysis of bacteria and purification of tagged RNA, each 200-μl study-culture sample was mixed with 20 ml of unlabelled normalization culture expressing the appropriately tagged rRNA before cell lysis (Fig 1B). Labelling of the study culture allowed us to normalize the radioactive signal from the affinity-purified tagged rRNAs against absorbance at 260 nm (A260), which was mostly obtained from the excess unlabelled tagged rRNA derived from the normalization culture. The normalized radioactivity is a direct measure of the total number of surviving radioactive-tagged ribosomes, regardless of the number of cell divisions and resulting dilution effects that might occur after repression of the tagged ribosome synthesis.
Although identical amounts of normalization-culture cells were added at each time point, during the course of the experiment the proportion of study culture increased from approximately 0.3% to 10% of the normalization culture. This is due to study-culture growth, wherein successive 200-μl samples contain increasing numbers of cells. However, because the experiments were conducted under repressing conditions, the increase is mostly of chromosomally encoded ribosomes that do not bind to the affinity matrixes used, and is therefore unlikely to affect the experimental outcome.
As a control, radioactive labelling was carried out after repression of plasmid-borne rRNA synthesis. Little radioactivity was incorporated into tagged ribosomes if [3H]-uridine label was added 10 min after repression (Figs 2, 3).
Figure 2.
Ribosomes are stable in cells growing at a constant rate in turbidostat. (A) Cells harbouring plasmid pBAD-23t-ara were grown under inducing conditions until OD600 reached 0.2 U/ml, labelled for 30 min with [3H]-uridine and transferred in repressing growth medium into the turbidostat. Samples were collected every 30 min and tagged 23S rRNAs were purified by affinity selection. Radioactive signal was counted and normalized to the A260 units measured from the large excess of unlabelled cells added to each sample before cell lysis. Standard errors were calculated from three experiments. The square denotes the result of a control experiment in which radioactive labelling was performed 10 min after repression of tagged rRNA synthesis. (B) Cells harbouring plasmid pBAD-16t-ara were grown and the experiment was carried out as in (A). Y-axis: arbitrary units. A260, absorbance at 260 nm; rRNA, ribosomal RNA.
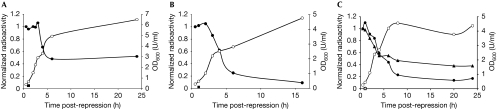
Figure 3.
Degradation of tagged ribosomes in batch cultures. (A) Cells harbouring plasmid pBAD-23t-ara were grown under inducing conditions until OD600 reached 0.2 U/ml, labelled for 30 min with [3H]-uridine and transferred into repressing growth medium. Samples were collected 0.5, 1, 2, 2.5, 3, 4, 6 and 24 h post-repression, and treated as in Fig 2. Filled circles denote normalized radioactivity of tagged 23S rRNAs. Open circles denote OD600 values of the culture. The square denotes the result of a control experiment in which radioactive labelling was performed 10 min after repression of tagged rRNA synthesis. (B) Cells harbouring plasmid pBAD-16t-ara were grown and the experiment was carried out as in (A). (C) Cells harbouring plasmid pBAD-hybrid containing both the 23S rRNA (filled triangles) and 16S rRNA (filled circles) tags were grown and the experiment was carried out as in (A). The square denotes the result of a control experiment performed with tagged 16S rRNA. The open triangle denotes a control experiment performed with tagged 23S rRNA. Experiments were repeated at least five times for (A) and (B), and three times for (C). Y-axis: arbitrary units. ara, arabinose; OD600, optical density at 600 nm; rRNA, ribosomal RNA.
Ribosomes are stable during constant cell growth
First, we measured ribosomal stability in cultures growing with a constant rate using a turbidostat system. Experiments were performed using MG1655 cells harbouring plasmids pBAD-23t-ara or pBAD-16t-ara growing in MOPS minimal medium. Cells were grown in batch culture with arabinose added in mid-exponential growth phase (optical density at 600 nm (OD600)=0.2 U/ml), pulse-labelled with [3H]-uridine for 30 min and transferred in fresh repressing medium into the turbidostat. In the turbidostat, the optical density was maintained between 0.4 and 0.45 U/ml by adding fresh medium. Under these conditions, the culture-doubling time was consistently 50 min during the 5-h experiments. The cells were grown for 30 min after transfer into glucose-containing medium to allow for the completion of tagged ribosomal assembly before taking the first time point. We thereby excluded from the analysis rRNA degradation that might occur concomitantly with pre-rRNA transcription, processing or assembly into ribosomes. As shown in Fig 2, both 23S rRNA and 16S rRNA were stable during the course of the experiment. This indicates that ribosomes are stable in exponentially growing cells.
Degradation of ribosomes in batch culture
For ribosomal stability measurements in batch culture, pBAD-23t-ara, pBB-23t-rha and pBAD-16t-ara were used. Cells were pulse-labelled with [3H]-uridine in mid-exponential growth phase (OD600=0.2 U/ml), and then expression of tagged rRNA was repressed. With both the arabinose and rhamnose inducible expression systems, tagged ribosomes showed a stable phase, lasting up to 3 h post-repression, followed by a degradation phase in which 50–80% of the tagged ribosomes were degraded (Fig 3; supplementary Fig S1 online). Degradation also occurred when labelling was done in the late-exponential growth phase (OD600=1.0; supplementary Fig S2 online). To directly compare the degradation of both ribosomal subunits, we cloned the 23S rRNA and 16S rRNA purification tags into the same vector under the araBAD promoter. This enabled us to measure degradation of both 23S rRNA and 16S rRNA in the same culture. The results shown in Fig 3C indicate that both ribosomal subunits are degraded concomitantly and with similar rates. Although there is variation between experiments in the duration of the early stable phase and the extent of the ensuing ribosome degradation, the degradation always occurs in the transition period between exponential and stationary culture growth. By contrast, tagged ribosomes were mostly stable in the early stationary phase up to 48 h in batch culture (Fig 3; supplementary Figs S1,S2 online). This result is further corroborated by our findings that both cellular RNA and large macromolecular RNA levels were stable in the stationary phase (Fig 4; A.P., unpublished results).
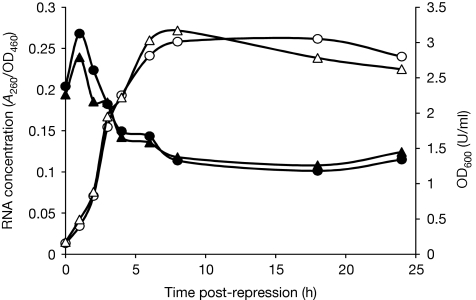
Figure 4.
The dynamics of RNA concentration in growing cells. Cells transformed with pBAD-t-ara were grown under inducing or non-inducing conditions until OD600 reached 0.2 U/ml, after which the cells were transferred to repressing media. At the designated time points, samples were collected, total RNA was extracted, cells were DNase I-treated and absorbance at 260 nm was measured. Optical densities of the cultures at each time point were measured at 460 and 600 nm. Zero time point denotes cultures immediately after transfer into glucose-containing media. Filled circles represent RNA concentrations from cells that were grown in arabinose-containing medium before shifting to glucose. Filled triangles represent RNA concentrations from cells that were grown without inductor before shifting to glucose. Open circles represent OD600 of the culture grown in the presence of arabinose before shifting to glucose. Open triangles represent OD600 of the culture grown without inductor before shifting to glucose. Experiments were repeated at least four times. A260, absorbance at 260 nm; ara, arabinose; OD460, optical density at 460 nm; OD600, optical density at 600 nm.
To control for the possibility that by extracting 23S rRNA from cellular extracts we measured the degradation of a population of poorly assembled particles, we purified tagged 50S and 30S ribosomal subunits from 70S ribosomes. Both showed similar degradation to that measured in the material obtained from total cellular RNA (supplementary Fig S3 online).
Plasmid-borne ribosomes are functional
The in vivo equivalence of ribosomes containing tagged 23S rRNAs to wild-type, chromosomally encoded ribosomes was tested by measuring the relative tagged 23S rRNA content in the trisome, disome, 70S and 50S fractions. Defective protein synthesis by the tagged ribosomes would change the ratio of tagged ribosomes to wild-type ribosomes in the polysome, 70S and 50S fractions, as defective ribosomes would be excluded from actively translating polysome fractions. The tagged 23S rRNAs, expressed from the araBAD promoter, were evenly incorporated into the 50S, 70S and polysomal fractions (supplementary Table S1 online). The slight under-representation of tagged ribosomes in the 50S and 70S fractions was probably caused by the continuing assembly of chromosomally encoded ribosomes while plasmid-derived rRNA synthesis was repressed. Therefore, the plasmid-encoded tagged ribosomes are fully functional in cellular translation.
To address the possibility that ectopic rRNA expression harms host cells and thus causes ribosome degradation, we compared the growth curves of MG1655 cells expressing tagged 23S rRNA from the araBAD and rhaBAD promoters with the cells grown without the inductor. Continuous expression of tagged rRNA had no effect on culture growth (supplementary Fig S4 online).
To test whether the observed ribosome degradation might occur in a subpopulation of dead or dying cells, we directly measured cell viability using propidium iodide to label dead cells. Bacteria expressing tagged 23S rRNA were stained using the LIVE/DEAD BacLight kit (Invitrogen) and compared with cultures grown in repressing conditions (see supplementary methods online). We found that less than 2% of the cultures consisted of dead cells, even after prolonged expression of tagged rRNA (supplementary Fig S5 online). Indeed, there were no systematic differences in proportions of dead cells between tagged rRNA-expressing and non-expressing cultures. This is consistent with the finding that in exponentially growing E. coli cultures, cells are dividing at uniform rates (Roostalu et al, 2008). We conclude that ribosome degradation occurs in viable cells and is not associated with cell damage or death.
Degradation correlates with ribosome concentration
After we determined the stabilities of defined ribosome populations synthesized during 30-min time-frames, we assayed total RNA concentrations across the bacterial growth curve under the same conditions as the ribosome-degradation experiment, shown in Fig 3. Total RNA is a useful proxy for rRNA as it comprises 98% of rRNA and transfer RNA, the relative proportions of which are comparatively constant at different growth rates (Bremer & Dennis, 2008). Cells harbouring pBAD-23t-ara were grown in parallel in the presence or absence of arabinose (the inducer). When OD600 reached 0.2 U/ml, the cells were shifted into fresh MOPS medium containing glucose as the carbon source. Relative RNA concentrations were calculated as the ratio of absorbance of DnaseI-treated and re-precipitated cell lysates at 260 nm to the optical density of the culture at 460 nm. At the time of nutrient up-shift to glucose, both the induced and uninduced cultures had similar RNA concentrations (Fig 4, 0 time point), indicating—in accordance with the finding of Jinks-Robertson et al (1983)—that induction of plasmid-borne rRNA does not lead to increased ribosome production. Both cultures show an initial increase in RNA concentrations on nutritional up-shift, corresponding to the stable phase of tagged ribosomes. Cultures constantly growing in the presence of glucose did not show such an increase in RNA concentrations (data not shown), indicating that the RNA concentration increases shown in Fig 4 are caused by increasing growth rates on nutritional up-shift (Bremer & Dennis, 2008). The phase of increasing RNA concentration is followed by a relatively rapid reduction in RNA concentration, which precedes the entry of cultures into the stationary phase and corresponds in time to the degradation of tagged ribosomes. Finally, RNA concentrations are mostly stable in the stationary growth phase. These results demonstrate that degradation of tagged ribosomes corresponds well to the reduction in the concentration of total cellular RNA.
In theory, RNA concentrations could be decreased by diluting the existing ribosomes, instead of ribosome degradation. According to this hypothesis, when cell growth slows on exit from exponential phase, de novo ribosome-synthesis rates should fall faster than growth rates. However, a recent work by Scott et al (2010), showing that active ribosome concentration is rate-limiting for cell growth, argues against this possibility. In addition, we performed an experiment in which we labelled cells with 5-min [3H]-uridine pulses across the growth curve and measured label incorporation into the trichloroacetic acid-precipitable macromolecular RNA fraction. The results indicate significant de novo RNA synthesis while ribosomes are degraded (data not shown). Taken together, these results suggest that the cause of the observed reduction in total RNA concentration is ribosome degradation, and that degradation is not confined to tagged ribosomes.
In conclusion, we have shown that whereas E. coli ribosomes are stable during exponential growth and in the stationary phase, degradation occurs between these stages. To our knowledge, this is the first direct demonstration of ribosome degradation in bacterial cells that have not been subjected to stress for the study. The intermediate growth state preceding the stationary phase is characterized by slowing of growth and complex changes in cellular physiology that collectively prepare the cell for safe entry into the stationary phase. The best-studied mechanism for stationary-phase preparation is the stringent response, which is triggered by ribosomes starved of amino acids (Potrykus & Cashel, 2008). The stringent response is triggered by the alarmone ppGpp(p) synthesis by RelA and SpoT proteins and is inhibited by ppGpp(p) hydrolysis by SpoT. The stringent response leads to widespread changes in gene expression, including repression of new ribosome synthesis and diversion of resources from growth and division to preparation for the stationary phase. To test the functional connection between ribosome degradation and the stringent-response pathway, we measured ribosomal stability in batch cultures in RelA and RelA/SpoT genetic backgrounds (supplementary Fig S6 online). In both experiments ribosomes were degraded similarly to in wild-type cells, suggesting that ribosome degradation is not connected to a ppGpp(p)-dependent mechanism.
Methods
The ribosome-degradation experiment. MG1655 cells transformed with pBAD-23t-ara, pBAD-16t-ara, pBB-23t-rha or pBAD-hybrid plasmid (see supplementary information online) were grown at 37°C in MOPS medium supplemented with 0.5% glycerol and 0.2% arabinose or 0.2% rhamnose, respectively. At the indicated optical density, 10 μl of 5,6-[3H]-uridine (1 μCi/μl, specific activity 35.6 Ci/mmol) was inoculated to 2-ml culture, which was then grown for a further 30 min at 37°C. Cells were subsequently pelleted and resuspended in 2 ml fresh MOPS medium supplemented with 0.5% glucose, and growth was resumed at 37°C. At the indicated time points, 200 μl aliquots were collected and stored at −85°C. In control experiments, 10 μl [3H]-uridine was added 10 min after shifting the cells to glucose-containing media. Before lysis, each aliquot was mixed with 20 ml of culture grown under inducing conditions in non-radioactive medium until OD600 reached 0.5 U/ml. RNA was extracted by incubation of cells resuspended in 600 μl TEN buffer containing 1% SDS, 0.6% Na-deoxycholate and 1% Brij in equal volume of phenol pH 5.5 at 65°C for 15 min, followed by phenol:chloroform extraction and ethanol precipitation. Tagged 23S rRNAs were purified as described in Leonov et al (2003; see supplementary methods online for details). Radioactivity in each sample was scintillation-counted and normalized to the A260 value of the sample.
For the turbidostat experiments, the induction of tagged RNAs and pulse labelling with 5,6-[3H]-uridine was performed in 4 ml batch cultures at an optical density of 0.2 U/ml as described above. After repression in 4 ml fresh MOPS glucose, the cultures were transferred into the pre-warmed turbidostat and grown for 5 h and optical density of the culture was not allowed to exceed 0.45 U/ml. Volumes of medium that were collected for lysis after every 30 min were increased in proportion to the increases in culture volumes as fresh medium was added to keep the culture turbidity constant. Radioactive cells were mixed with normalization cultures and treated as in the batch culture experiments.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank Arvi Jõers and Gemma Atkinson for critically reading the manuscript, Jaanus Remme for useful discussions and Dmitri Lubenets for technical help. We are grateful to Rachel Green for her kind gift of plasmids pEYspur MS2 and pMBP-MS2-His, and to Niilo Kaldalu for the gift of the RelA/SpoT deletion strain. This work was supported by the Estonian Science Foundation grant 7505 (Ü.M.), the European Union Social Fund (A.P.) and the European Regional Development Fund through the Centre of Excellence in Chemical Biology.
Footnotes
The authors declare that they have no conflict of interest.
References
- Bessarab DA, Kaberdin VR, Wei CL, Liou GG, Lin-Chao S (1998) RNA components of Escherichia coli degradosome: evidence for rRNA decay. Proc Natl Acad Sci USA 95: 3157–3161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer H, Dennis PP (2008) Modulation of chemical composition and other parameters of the cell at different exponential growth rates. In EcoSal—Escherichia coli and Salmonella: Cellular and Molecular Biology, Böck A, Curtiss R, III, Kaper JB, Karp PD, Neidhardt FC, Nyström T, Slauch JM, Squires CL, Ussery D (eds), Ch. 5.2.3. Washington, DC: ASM [DOI] [PubMed] [Google Scholar]
- Cole SE, LaRiviere FJ, Merrikh CN, Moore MJ (2009) A convergence of rRNA and mRNA quality control pathways revealed by mechanistic analysis of nonfunctional rRNA decay. Mol Cell 34: 440–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BD, Luger SM, Tai PC (1986) Role of ribosome degradation in the death of starved Escherichia coli cells. J Bacteriol 166: 439–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher MP (2003) Degradation of stable RNA in bacteria. J Biol Chem 278: 45041–45044 [DOI] [PubMed] [Google Scholar]
- Deutscher MP (2009) Maturation and degradation of ribosomal RNA in bacteria. Prog Mol Biol Transl Sci 85: 369–391 [DOI] [PubMed] [Google Scholar]
- Dong H, Nilsson L, Kurland CG (1995) Gratuitous overexpression of genes in Escherichia coli leads to growth inhibition and ribosome destruction. J Bacteriol 177: 1497–1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sharoud WM (2004) Ribosome inactivation for preservation: concepts and reservations. Sci Prog 87: 137–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii K, Kitabatake M, Sakata T, Miyata A, Ohno M (2009) A role for ubiquitin in the clearance of nonfunctional rRNAs. Genes Dev 23: 963–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gausing K (1977) Regulation of ribosome production in Escherichia coli: synthesis and stability of ribosomal RNA and of ribosomal protein messenger RNA at different growth rates. J Mol Biol 115: 335–354 [DOI] [PubMed] [Google Scholar]
- Hsu D, Shih LM, Zee YC (1994) Degradation of rRNA in Salmonella strains: a novel mechanism to regulate the concentrations of rRNA and ribosomes. J Bacteriol 176: 4761–4765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson RW, DeMoss JA (1965) Effects of toluene on Escherichia coli. J Bacteriol 90: 1420–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson A, Gillespie D (1968) Metabolic events occurring during recovery from prolonged glucose starvation in Escherichia coli. J Bacteriol 95: 1030–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinks-Robertson S, Gourse RL, Nomura M (1983) Expression of rRNA and tRNA genes in Escherichia coli: evidence for feedback regulation by products of rRNA operons. Cell 33: 865–876 [DOI] [PubMed] [Google Scholar]
- Kaplan R, Apirion D (1975) Decay of ribosomal ribonucleic acid in Escherichia coli cells starved for various nutrients. J Biol Chem 250: 3174–3178 [PubMed] [Google Scholar]
- LaRiviere FJ, Cole SE, Ferullo DJ, Moore MJ (2006) A late-acting quality control process for mature eukaryotic rRNAs. Mol Cell 24: 619–626 [DOI] [PubMed] [Google Scholar]
- Leonov AA, Sergiev PV, Bogdanov AA, Brimacombe R, Dontsova OA (2003) Affinity purification of ribosomes with a lethal G2655C mutation in 23 S rRNA that affects the translocation. J Biol Chem 278: 25664–25670 [DOI] [PubMed] [Google Scholar]
- Potrykus K, Cashel M (2008) (p)ppGpp: still magical? Annu Rev Microbiol 62: 35–51 [DOI] [PubMed] [Google Scholar]
- Pulk A, Liiv A, Peil L, Maivali U, Nierhaus K, Remme J (2010) Ribosome reactivation by replacement of damaged proteins. Mol Microbiol 75: 801–814 [DOI] [PubMed] [Google Scholar]
- Roostalu J, Joers A, Luidalepp H, Kaldalu N, Tenson T (2008) Cell division in Escherichia coli cultures monitored at single cell resolution. BMC Microbiol 8: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M, Gunderson CW, Mateescu EM, Zhang Z, Hwa T (2010) Interdependence of cell growth and gene expression: origins and consequences. Science 330: 1099–1102 [DOI] [PubMed] [Google Scholar]
- Shen V, Bremer H (1977) Chloramphenicol-induced changes in the synthesis of ribosomal, transfer, and messenger ribonucleic acids in Escherichia coli B/r. J Bacteriol 130: 1098–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers JA, Champney WS (2005) Accumulation and turnover of 23S ribosomal RNA in azithromycin-inhibited ribonuclease mutant strains of Escherichia coli. Arch Microbiol 184: 66–77 [DOI] [PubMed] [Google Scholar]
- Suzuki H, Kilgore WW (1967) Decomposition of ribosomal particles in Escherichia coli treated with mitomycin C. J Bacteriol 94: 666–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngman EM, Green R (2005) Affinity purification of in vivo-assembled ribosomes for in vitro biochemical analysis. Methods 36: 305–312 [DOI] [PubMed] [Google Scholar]
- Yuan D, Shen V (1975) Stability of ribosomal and transfer ribonucleic acid in Escherichia coli B/r after treatment with ethylenedinitrilotetraacetic acid and rifampicin. J Bacteriol 122: 425–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zundel MA, Basturea GN, Deutscher MP (2009) Initiation of ribosome degradation during starvation in Escherichia coli. RNA 15: 977–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.