Abstract
In RNA silencing, microRNA (miRNA)-mediated translational repression occurs through mechanisms that do not invoke messenger-RNA (mRNA) target cleavage by Argonaute proteins. The nature of these mechanisms is unclear, but several recent studies have proposed that a direct interaction between the mRNA–cap and the middle (MID) domain of Argonautes is involved. Here, we present crystallographic and NMR data demonstrating that cap analogues do not bind significantly to the isolated MID domain of human Argonaute 2 (hAGO2) and are found in the miRNA 5′-nucleotide binding site in an implausible binding mode. Additionally, in vitro pull-down experiments with full-length hAGO2 indicate that the interaction with cap analogues is nonspecific.
Keywords: Argonaute, miRNA, MID domain, cap, translational repression
Introduction
MicroRNAs (miRNAs) are a class of genomically expressed small RNAs that post-transcriptionally regulate gene expression by recognizing partially complementary sequences, found mostly in the 3′-untranslated region of target messenger RNAs (mRNA; Bartel, 2009). miRNAs associate with Argonaute proteins (AGOs) to form the so-called miRNA-induced silencing complex (miRISC). The miRNA imparts sequence specificity to the miRISC and guides the complex to specific target mRNAs for post-transcriptional gene silencing. AGOs consist of amino-terminal, PAZ, MID (middle) and PIWI domains, in which the PAZ and MID domains interact with the 3′- and 5′-ends of miRNAs, respectively (Hutvagner & Simard, 2008). In small RNAs that are complementary to the target, such as many short-interfering RNAs (siRNAs), the catalytic PIWI domain cleaves the target mRNA strand. However, most miRNAs show only partial complementarity towards the target, which disrupts cleavage activity, and in these situations mRNA repression occurs by other mechanisms.
Considerable efforts have been made to investigate the mechanisms by which miRISC represses protein synthesis of target mRNAs. It is well established that miRNAs cause mRNA degradation through deadenylation mediated by interactions with GW182 (Fabian et al, 2010). In fact, Guo et al (2010) recently used ribosome profiling and simultaneous measurements of mRNA levels to show that mRNA degradation accounts for most (⩾84%) of the miRNA-mediated decrease in protein levels. However, it seems that miRNAs can also hinder gene expression by inhibiting mRNA translation (Chekulaeva & Filipowicz, 2009; Fabian et al, 2010), although the mechanism of translational repression remains controversial, with different, often conflicting results found in the literature (Chekulaeva & Filipowicz, 2009; Fabian et al, 2010).
One model posits that the 5′-cap structure of the target mRNA interacts directly with AGO in miRISC, thereby competing with the cap-binding translation factor eIF4E and leading to translational repression at the initiation stage (Kiriakidou et al, 2007; Boland et al, 2010; Djuranovic et al, 2010). Kiriakidou et al (2007) suggested that two conserved residues (F470 and F505) in the human AGO2 (hAGO2) MID domain bind to the cap by stacking interactions analogous to those found in eIF4E and other cap-binding proteins. Mutating these residues prevented the cap interaction and inhibited translational repression. Subsequent studies have challenged these results by showing that F470 and F505 are buried in the hydrophobic core (Kinch & Grishin, 2009) and that mutating them also prevented AGO from interacting with both GW182 and miRNAs (Eulalio et al, 2008).
More recently, however, another study that supported the AGO–cap interaction hypothesis was published. Djuranovic et al (2010) found that binding of Drosophila AGO1 (dmAGO1) to m7 guanosine triphosphate (GTP)-Sepharose resin was enhanced in the presence of miRNAs and that the interaction was localized to the MID domain. This led to a model of an allosterically regulated cap-binding site in AGOs, in which an initial interaction of miRNA with the 5′-phosphate binding site in the MID domain triggers a conformational change in AGO that makes available a second binding site specific for the cap (Djuranovic et al, 2010). In support of this model, the crystal structure of the MID domain from Neurospora Argonaute QDE-2 identified a sulphate ion occupying a binding site in close proximity to the 5′-nucleotide binding site (Boland et al, 2010).
Recently, we reported crystal structures of the hAGO2 MID domain in complex with nucleoside monophosphates (NMPs) that revealed the structural basis for the observed bias of uracil or adenine nucleotides at the 5′-end of eukaryotic miRNAs (Frank et al, 2010). Notably, the hAGO2 MID domain presented no evidence for a second binding site and no conformational changes were observed on nucleotide binding. However, it is possible that conformational changes were masked by the constraints of the crystal lattice or that the isolated MID domain was insufficient to engage the cap. This prompted us to analyse in more detail the interaction between the cap and the hAGO2 MID domain, by using biophysical methods and biochemical experiments with full-length hAGO2. We found that cap analogues do not significantly interact with either the isolated MID domain or full-length hAGO2, in the conditions used here.
Results and Discussion
Biophysical analysis of hAGO2 MID domain–cap interaction
We had previously reported the affinities of NMPs to the MID domain and found that uridine monophosphate (UMP) and adenosine monophosphate (AMP) interact with an affinity of 125 and 250 μM, respectively, whereas cytidine monophosphate (CMP) and guanosine monophosphate (GMP) bind more weakly, with approximately 3 mM affinity (Frank et al, 2010). Thus, we began by determining the dissociation constants of cap analogues with MID domain using 1H–15N heteronuclear single-quantum coherence (HSQC) NMR titration experiments. Both dinucleotide cap analogues used in this study, m7GpppG and m7GpppA, bound to the MID domain with similar affinities of 1.57 and 1.37 mM, respectively (Fig 1A,B,D; supplementary Fig S1 online). Both affinities were lower than for the corresponding single nucleotides (m7GTP, GTP and ATP). This observation is in contrast to what is expected for a binding site specific for interaction with a capped mRNA. Furthermore, such weak affinities suggest that the interaction is not physiologically relevant, as comparable interactions of the cap with eIF4E and other bona fide cap-binding proteins are in the nM range (Cai et al, 1999; Worch et al, 2005). However, the affinities determined here for dinucleotide cap analogues are between those of weakly binding mononucleotides (GMP/CMP) and higher-affinity mononucleotides (UMP/AMP) at the miRNA 5′-end.
Figure 1.
Binding affinity analysis of cap analogues with hAGO2 MID domain. (A) 1H–15N HSQC NMR titration experiments with hAGO2 MID domain and m7GpppG. Significantly shifting peaks selected for analysis are marked with arrows. (B) Chemical shift differences were calculated and were plotted as a function of the molar ratio (nucleotide/protein). The data for multiple peaks were fit using the maximum shift and dissociation constant as adjustable parameters. (C) Chemical structure of the mRNA cap and miRNA 5′-nucleotide. (D) Dissociation constants (KD) extracted from NMR titration experiments. Standard deviations from the fitting procedures of three different peaks are shown. GMP, guanosine monophosphate; GTP, guanosine triphosphate; hAGO2, human Argonaute 2; HSQC, heteronuclear single-quantum coherence; MID, middle; miRNA, micro RNA; mRNA, messenger RNA.
To determine the source of the stronger affinity for cap analogues over that of GMP, we solved the crystal structure of the complex between the hAGO2 MID domain and m7GpppG (see supplementary information online). The cap structure consists of a 7-methylguanosine linked to the first nucleotide of the mRNA by a 5′–5′ triphosphate bridge (Fig 1C). The crystal structure showed that m7GpppG binds to the highly conserved, positively charged 5′-nucleotide binding site that is responsible for interaction of AGOs with small RNAs (Fig 2A,B). Clear electron density was observed for the α-phosphate of the bound nucleotide, and only fragmented density was visible for one of the sugar moieties and base. The α-phosphate group interacts with the conserved phosphate-binding residues, as shown previously for NMPs (Frank et al, 2010). The β-phosphate was also partly visible and makes electrostatic interactions with Y529, K533 and K570. These additional phosphate contacts are probably responsible for the stronger affinities of cap analogues over those of GMP as the binding affinities of both ATP and GTP were also substantially increased relative to AMP and GMP, respectively (Fig 1D). The crystal structure of the MID domain in complex with ATP confirmed the additional interactions with the β-phosphate (supplementary Fig S2 online).
Figure 2.
Crystal structure of the hAGO2 MID domain in complex with cap analogue. (A) Charged surface representation. The nucleotide specificity loop is highlighted in yellow. (B) Difference electron density contoured at 2σ before inclusion of m7GpppG. Black sticks show the unmodelled portion of the cap. α-, β- and γ-phosphates are labelled in red. (C) Schematic view of potential cap binding compared with miRNAs. The surface of the hAGO2 MID domain is shown in grey. A small RNA 5′-nucleotide bound in the nucleotide-binding pocket is shown in cyan. The position of the second nucleotide of a bound small RNA is shown as white sticks, while the hypothetical position of a bound cap-like dinucleotide is shown as grey sticks. hAGO2, human Argonaute 2; MID, middle; miRNA, microRNA.
No electron density was observed for the second base of the cap. Due to the almost symmetrical nature of the m7GpppG molecule (Fig 1C) and lack of clear electron density for the base, its binding orientation could not be determined. Notably, no electron density that might indicate the presence of a second binding site was evident at other regions on the MID domain and no conformational changes relative to the nucleotide-free state were observed, consistent with our previously published structures of MID domain:NMP complexes (Frank et al, 2010).
The reduced affinity of m7GpppA and m7GpppG compared with the mononucleotides ATP, GTP and m7GTP (Fig 1D) is probably due to unfavourable interactions with the second base of the dinucleotide cap analogues (in miRNAs, the second nucleotide is linked by a 5′–3′ linkage rather than 5′–5′, see Fig 2C) on the surface surrounding the 5-nucleotide binding pocket. We expect these restrictions on cap binding to be accentuated in the context of full-length AGOs (Wang et al, 2008) due to the presence of additional domains adjacent to the 5′-nucleotide binding site. These domains are positioned in a manner that prevents an additional 5′–5′-linked nucleotide from occupying this site (supplementary Fig S3 online). This reasoning extends to the additional phosphates present in nucleotide triphosphates relative to NMPs, thus confining the miRNA 5′-binding site to interact only with its physiological ligands, namely monophosphorylated small RNAs. Indeed, chemical modification of the monophosphorylated 5′-end of miRNAs significantly reduces AGO cleavage activity (Nykänen et al, 2001; Martinez et al, 2002).
Next, we measured the affinity of m7GTP, to assess the effect of the methyl modification of the cap. Titration of m7GTP into the MID domain caused the same characteristic NMR peak shifts as observed with GTP (supplementary Fig S4 online), but slightly weaker affinity was observed for the interaction (Fig 1D). This confirms that m7GTP also binds to the 5′-nucleotide binding pocket in solution and suggests that a specific interaction between the cap and this site does not occur. In studies of bona fide cap-interacting proteins such as eIF4E or the cap-binding complex, the presence of the 7-methyl modification results in substantially increased affinity of cap analogues over guanosine (Cai et al, 1999; Worch et al, 2005).
To investigate the possible presence of a second miRNA-dependent allosteric binding site for the cap (supplementary Fig S5 online), we carried out 1H–15N HSQC NMR experiments in which the MID domain was initially saturated with 5 mM UMP (miRNA mimic), followed by the addition of 3 mM m7GpppG. The presence of a separate binding site specific for the cap would predict additional chemical shift changes after UMP saturation. This should be the case even in the presence of the high concentrations of UMP used here (which could in theory occupy the potential second binding site as well), as the addition of the cap should displace the UMP at the putative second site, which is ostensibly specific for the cap. We found that addition of m7GpppG to UMP-saturated MID domain did not cause any additional chemical shift changes in the NMR spectra (Fig 3). Furthermore, Hill coefficients determined from binding curves are close to 1, indicating no cooperativity of binding (supplementary Table S2 online).
Figure 3.
1H–15N HSQC NMR experiments assessing the presence of a second allosteric binding site. (A) MID domain alone (grey) and with 5 mM UMP (red). Significant peaks shifts due to UMP addition are marked with arrows. (B) MID with 5 mM UMP (red) overlaid with 3 mM m7GpppG (blue). HSQC, heteronuclear single-quantum coherence; MID, middle.
In vitro pull-down experiments
The Djuranovic et al (2010) study showed that there is an increase in the binding of full-length dmAGO1 to m7GTP-Sepharose in the presence of miRNA, leading to the postulation of a second allosteric binding site for the cap. Conversely, this effect was not seen for either GTP/GMP-agarose beads suggesting that this is a cap-specific interaction. It should be noted that m7GTP-Sepharose beads (from GE Healthcare) differ in their chemical conjugation from GMP/GTP-agarose (from Sigma) in that m7GTP is linked to the matrix by the γ-phosphate, whereas both GMP and GTP are attached by either their 2′-OH or the 3′-OH of the ribose sugar. The effect of these different linkages on binding experiments is unclear, given the nature of the binding mode of ligands with the miRNA 5′-nucleotide binding sites, as described above. Additionally, differences between bead-linked nucleotides and free nucleotides used in other experiments could also contribute to the inconsistencies in the MID domain–cap interactions reported here.
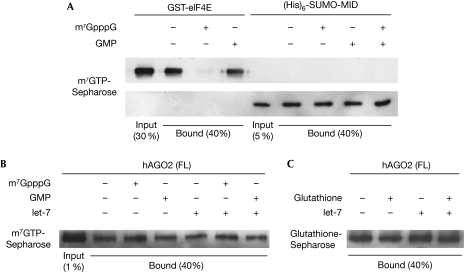
Our biophysical analyses were restricted to the isolated hAGO2 MID domain because of the difficulties associated with studying full-length eukaryotic AGOs using structural techniques. In order to address this, and the inconsistencies with the Djuranovic et al (2010) study, we performed pull-down experiments of full-length hAGO2 as well as the isolated MID domain with m7GTP-conjugated beads, using concentrations and conditions similar to those in the Djuranovic et al (2010) study, where possible. We performed pull-down experiments using 5 μg of hAGO2 MID domain as a hexahistidine/SUMO-fusion protein (approximately 0.75 μM in a 200 μl reaction) and 30 μl of m7GTP-Sepharose beads (approximately 75 μM ligand concentration according to the manufacturer). Binding of MID domain to cap resin was nonspecific, as addition of free cap analogue did not abolish the interaction, which is observed in the case of the known cap-binding protein eIF4E (Fig 4A). Moreover, no increased binding was observed in the presence of GMP, and the presence of both GMP and m7GpppG did not alter the amount of recovered protein.
Figure 4.
Full-length hAGO2 binds to m7GTP-Sepharose nonspecifically in in vitro pull-down experiments and at low levels, indicating background binding. (A) Pull-down experiments using recombinant GST-eIF4E and hexahistidine-SUMO-tagged hAGO2 MID domain with m7GTP-Sepharose. Western blots using eIF4E or His-tag antibodies are shown, respectively. (B) Recovery of hAGO2 during in vitro pull-down experiments with m7GTP-Sepharose is less than 1%. Binding is not affected by the presence of 10-fold excess of let-7 miRNA or by 500 μM m7GpppG as a competitor. (C) Binding to glutathione-Sepharose as a negative control gives similar results to experiments with m7GTP-Sepharose, confirming that binding represents background levels. Full-length hAGO2 was detected on western blots using hAGO2 antibody. GMP, guanosine monophosphate; GST, glutathione-S-transferase; GTP, guanosine triphosphate; hAGO2, human Argonaute 2; MID, middle.
We proceeded to express and purify full-length hAGO2 (S34 to the carboxy-terminus, lacking the N-terminal proline-rich tract) from baculovirus-infected insect cells (supplementary Fig S6 online) for use in pull-down experiments. This protein was properly folded and active, as assessed by RNA cleavage experiments (supplementary Fig S6 online). We incubated approximately 1 μg (approximately 50 nM in a 200 μl reaction) of purified hAGO2 with 30 μl of m7GTP-Sepharose beads in the presence or absence of m7GpppG and/or let-7 miRNA. We found that regardless of the combination of competitors included in the binding reactions, the same amount of hAGO2 was recovered (Fig 4B). Furthermore, recovery of hAGO2 from the resin was consistently less than 1% of the input, suggesting that this reflects background binding only. To confirm this result, we repeated the same set of experiments in the context of glutathione-conjugated Sepharose beads (i.e., nonspecific binding control). We obtained a similar recovery of hAGO2 (Fig 4C), suggesting that the interaction of hAGO2 with m7GTP-Sepharose beads is not specific. Notably, there was no increase of hAGO2 binding to m7GTP-Sepharose beads in the presence of miRNA.
Conclusions
Here, we present crystallographic and NMR data using the hAGO2 MID domain together with pull-down experiments using full-length hAGO2. The observation that cap analogue binds to the known miRNA 5′-nucleotide binding site in the MID domain together with the low affinity of the interaction suggests that cap interaction with the hAGO2 MID domain is a consequence of its capacity to accommodate nucleotides. Additionally, comparison of the affinities of cap-like nucleotides (m7GpppG, m7GpppA and m7GTP) with non-cap-like nucleotides (ATP, GTP, AMP and GMP) shows that there is no specificity for a cap structure at this site. In vitro pull-down experiments suggest that the interaction of the mRNA cap structure with hAGO2 is not specific and reflects background binding to the Sepharose resin, rather than a specific interaction with m7GTP.
Our results are in disagreement with m7GTP-Sepharose pull-down experiments reported by Djuranovic et al (2010). Their study presented pull-down experiments with full-length and MID domain constructs from dmAGO1, which supported the notion of an allosterically regulated cap-binding site located in the MID domain, separate from the miRNA 5′-nucleotide binding site. One possibility for the observed discrepancies is that dmAGO1 and hAGO2 are inherently different in their mechanistic behaviours. However, analogous pull-down studies, previously performed by Eulalio et al (2008) and Iwasaki et al (2009) on dmAGO1, revealed that the interaction of dmAGO1 with cap-resin is non-specific, consistent with our findings here. Taken together, our results show that the isolated MID domain is insufficient to engage the cap and, moreover, that full-length AGO, under the conditions used here, cannot specifically bind to the cap. However, we do not discount the possibility that in vivo, there might be other cellular factors that could allow accommodation of capped mRNA by the miRISC.
Methods
Chemical syntheses and protein preparation. Syntheses and purification of the cap analogues were performed as described previously (Darzynkiewicz et al, 1985).
The hAGO2 MID domain (residues 439–578 for crystallographic experiments and residues 432–578 for NMR studies) was cloned into the BamHI and NotI sites of a pSMT3 vector (Höck et al, 2007), which contains an N-terminal Ulp1 cleavable His6-Sumo tag. The protein was bacterially expressed using standard protocols and purified by Ni-affinity chromatography, cleavage of the tag, anion exchange chromatography and Superdex-75 size-exclusion chromatography in 25 mM Tris pH 8.0, 150 mM NaCl and 3 mM DTT (for crystallization), or in 25 mM MES pH 6.5, 200 mM NaCl and 3 mM DTT (for NMR). For pull-down experiments the hexahistidine/SUMO fusion protein was not cleaved with Ulp-1 and was purified by Ni-affinity and anion exchange chromatography.
Full-length hAGO2 (S34 to the C-terminus) was cloned into the BamHI and NotI sites of the pFastBac HTb vector for expression in baculovirus-infected insect cells. Infected cells were harvested 72 h post-infection and protein was extracted by Ni-affinity chromatography, cleavage of the tag using tobacco etch virus protease and Superdex-200 size-exclusion chromatography in 25 mM Tris pH 8.0, 300 mM NaCl, 3 mM DTT and 10% glycerol.
Crystallization, data collection and structure determination. Crystals (size: approximately 0.2 mm × 0.2 mm × 0.6 mm) of hAGO2 MID domain (439–578) were grown by hanging-drop vapour diffusion at 4°C. Protein at 10–15 mg ml−1 was mixed 1:1 with a well solution containing 0.1 M imidazole pH 8.0, 0.2 M NaCl, 0.46 M NaH2PO4 and 1.84 M K2HPO4. Protein-ligand crystals were obtained by soaking native crystals in a drop containing 0.2 M (NH4)2SO4, 0.1 M Na-Cacodylate pH 6.5, 15% PEG8000, 20% glycerol and 20 mM ligand. Diffraction data for nucleotide-containing crystals were collected on a Rigaku rotating copper-anode generator and processed using HKL2000 (Otwinowski, 1997). The nucleotide complexes were solved by difference Fourier analysis using the native structure and refined using CNS (Brunger et al, 1998) and Phenix (Adams et al, 2010). For the m7GpppG complex, we arbitrarily chose to model the m7Gpp portion of the cap analogue, as the quality of the density for the base does not allow us to discern the orientation of the dinucleotide. The α-phosphate and G nucleotide portion were placed so as to minimize close contacts, and their occupancies were set to 0 during refinement.
NMR titration experiments. All NMR experiments were performed at 293 K using a Bruker 600 MHz spectrometer. NMR titrations were carried out by acquiring 1H–15N HSQC spectra on samples of 0.10–0.25 mM 15N-labelled hAGO2 MID domain (432–578), with the addition of increasing amounts of unlabelled ligand. Chemical shift differences were calculated as Δδ=[(ΔδH)2+(0.2*ΔδN)2]1/2, where ΔδH and ΔδN are the observed chemical shift changes for 1H and 15N, respectively. For determination of dissociation constants, Δδ was plotted as a function of the molar ratio (nucleotide/protein) and the data for multiple peaks were fit, using the maximum shift and dissociation constant as adjustable parameters.
For saturation with UMP and subsequent addition of m7GpppG, the MID domain was first saturated with 5 mM UMP by addition from a stock solution of 0.5 M UMP in water and a spectrum was recorded. After addition of 3 mM m7GpppG (from a 0.3-M stock solution of m7GpppG in water) to the UMP-saturated sample, another spectrum was recorded.
In vitro pull-down experiments. In all, 0.5 μg of GST-eIF4E, 5 μg of hexahistidine/SUMO-hAGO2 MID domain fusion protein and 1 μg of recombinant hAGO2 (S34 to the C-terminus) were diluted in PBS with 5 mM MgCl2. Where indicated, we added a 10-fold molar excess of let-7 miRNA or nucleotides at a final concentration of 500 μM. Samples were then incubated for 15 min at 4°C before addition of 30 μl of m7GTP-Sepharose beads (GE Healthcare) or glutathione-Sepharose, followed by rotation for 2 h at 4°C. Samples were washed four times with 600 μl PBS containing 2 mM MgCl2 and heparin (0.5 mg/ml) and bound proteins were eluted with SDS-loading buffer and analysed by western blot. Pull-down experiments were performed at least twice and representative results are shown.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank Katalin Illes for technical support in the production of full-length human AGO2, K. Gehring for comments on the manuscript and R. Szittner for technical support. NMR data were collected at the Québec/Eastern Canada High Field NMR facility. The hAGO2 antibody was a kind gift from Zissimos Mourelatos. B.N. is supported by a Canada Research Chair, a Career Development Award from the Human Frontiers Science Program (0018/2006-C/1) and an operating grant from the Canadian Institutes of Health Research (CIHR; grant MOP-82929). N.S. is funded by a CIHR grant. F.F. is supported by a Boehringer Ingelheim Fonds PhD Fellowship. E.D. is a Howard Hughes Medical Institute International Scholar (Grant No. 55005604). E.D., J.S. and J.J. are supported by the Polish Ministry of Science and Higher Education (Grant No. N N301 096339).
Footnotes
References
- Adams PD et al. (2010) PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr 66: 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136: 215–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland A, Tritschler F, Heimstädt S, Izaurralde E, Weichenrieder O (2010) Crystal structure and ligand binding of the MID domain of a eukaryotic Argonaute protein. EMBO Rep 11: 522–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunger AT et al. (1998) Crystallography & NMR System (CNS), a new software suite for macromolecular structure determination. Acta Crystallogr D Biol Crystallogr 54: 905–921 [DOI] [PubMed] [Google Scholar]
- Cai A, Jankowska-Anyszka M, Centers A, Chlebicka L, Stepinski J, Stolarski R, Darzynkiewicz E, Rhoads RE (1999) Quantitative assessment of mRNA cap analogues as inhibitors of in vitro translation. Biochemistry 38: 8538–8547 [DOI] [PubMed] [Google Scholar]
- Chekulaeva M, Filipowicz W (2009) Mechanisms of miRNA-mediated post-transcriptional regulation in animal cells. Curr Opin Cell Biol 21: 452–460 [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz E, Ekiel I, Tahara SM, Seliger LS, Shatkin AJ (1985) Chemical synthesis and characterization of 7-methylguanosine cap analogues. Biochemistry 24: 1701–1707 [Google Scholar]
- Djuranovic S, Zinchenko MK, Hur JK, Nahvi A, Brunelle JL, Rogers EJ, Green R (2010) Allosteric regulation of Argonaute proteins by miRNAs. Nat Struct Mol Biol 17: 144–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulalio A, Huntzinger E, Izaurralde E (2008) GW182 interaction with Argonaute is essential for miRNA-mediated translational repression and mRNA decay. Nat Struct Mol Biol 15: 346–353 [DOI] [PubMed] [Google Scholar]
- Fabian MR, Sonenberg N, Filipowicz W (2010) Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem 79: 351–379 [DOI] [PubMed] [Google Scholar]
- Frank F, Sonenberg N, Nagar B (2010) Structural basis for 5′-nucleotide base-specific recognition of guide RNA by human AGO2. Nature 465: 818–822 [DOI] [PubMed] [Google Scholar]
- Guo H, Ingolia NT, Weissman JS, Bartel DP (2010) Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 466: 835–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höck J, Weinmann L, Ender C, Rüdel S, Kremmer E, Raabe M, Urlaub H, Meister G (2007) Proteomic and functional analysis of Argonaute-containing mRNA-protein complexes in human cells. EMBO Rep 8: 1052–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutvagner G, Simard MJ (2008) Argonaute proteins: key players in RNA silencing. Nat Rev Mol Cell Biol 9: 22–32 [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Kawamata T, Tomari Y (2009) Drosophila argonaute1 and argonaute2 employ distinct mechanisms for translational repression. Mol Cell 34: 58–67 [DOI] [PubMed] [Google Scholar]
- Kinch LN, Grishin NV (2009) The human Ago2 MC region does not contain an eIF4E-like mRNA cap binding motif. Biol Direct 4: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiriakidou M, Tan GS, Lamprinaki S, De Planell-Saguer M, Nelson PT, Mourelatos Z (2007) An mRNA m7G cap binding-like motif within human Ago2 represses translation. Cell 129: 1141–1151 [DOI] [PubMed] [Google Scholar]
- Martinez J, Patkaniowska A, Urlaub H, Lührmann R, Tuschl T (2002) Single-stranded antisense siRNAs guide target RNA cleavage in RNAi. Cell 110: 563–574 [DOI] [PubMed] [Google Scholar]
- Nykänen A, Haley B, Zamore PD (2001) ATP requirements and small interfering RNA structure in the RNA interference pathway. Cell 107: 309–321 [DOI] [PubMed] [Google Scholar]
- Otwinowski Z (1997) Processing of X-ray diffraction data collected in oscillation mode. Meth Enzymol 276: 307–326 [DOI] [PubMed] [Google Scholar]
- Wang Y, Sheng G, Juranek S, Tuschl T, Patel DJ (2008) Structure of the guide-strand-containing argonaute silencing complex. Nature 456: 209–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worch R, Niedzwiecka A, Stepinski J, Mazza C, Jankowska-Anyszka M, Darzynkiewicz E, Cusack S, Stolarski R (2005) Specificity of recognition of mRNA 5′ cap by human nuclear cap-binding complex. RNA 11: 1355–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.