Abstract
Ataxin-1 (ATXN1), a causative factor for spinocerebellar ataxia type 1 (SCA1), and the related Brother of ATXN1 (BOAT1) are human proteins involved in transcriptional repression. So far, little is known about which transcriptional pathways mediate the effects of ATXN1 and BOAT1. From our analyses of the properties of BOAT1 in Drosophila and of both proteins in mammalian cells, we report here that BOAT1 and ATXN1 are components of the Notch signalling pathway. In Drosophila, BOAT1 compromises the activities of Notch. In mammalian cells, both ATXN1 and BOAT1 bind to the promoter region of Hey1 and inhibit the transcriptional output of Notch through direct interactions with CBF1, a transcription factor that is crucial for the Notch pathway. Our results suggest that, in addition to their involvement in SCA1, ATXN1 and BOAT1 might participate in several Notch-controlled developmental and pathological processes.
Keywords: ATXN1, BOAT1, Notch, CBF1, Hey1
Introduction
The human protein ataxin-1 (ATXN1) originally drew attention because glutamine tract expansion in ATXN1 causes spinocerebellar ataxia type 1 (SCA1; Orr et al, 1993; Banfi et al, 1994), a late-onset and dominantly inherited neurological disorder. ATXN1 is also required for cognitive function, motor coordination and processing of β-amyloid precursor protein (Matilla et al, 1998; Crespo-Barreto et al, 2010; Zhang et al, 2010). Recent studies have revealed that, along with the toxicity caused by a long glutamine repeat, inherent properties of ATXN1 also contribute to the pathogenesis of SCA1 (Fernandez-Funez et al, 2000; Mizutani et al, 2005; Zoghbi & Orr, 2009; Crespo-Barreto et al, 2010). These diverse observations about ATXN1 highlight the need to understand its native functions more comprehensively.
Several lines of evidence from our own and other recent research have shown that ATXN1 is involved in transcriptional repression. ATXN1 associates with members of the transcriptional corepressor SMRT (silencing mediator of retinoid and thyroid receptors) family in both mammalian cells and Drosophila (Tsai et al, 2004). It also selectively forms protein complexes with histone deacetylase (HDAC) 3 and 4 (Tsai et al, 2004; Bolger et al, 2007). Additionally, ATXN1 interacts with various transcription factors, including Capicua, and cofactors such as LANP that are implicated in transcriptional repression (Lam et al, 2006; Riley & Orr, 2006; Cvetanovic et al, 2007). Our characterization of a protein related to ATXN1, Brother of ATXN1 (BOAT1; Mizutani et al, 2005), supports the idea that these proteins are involved in transcriptional repression. We have shown that BOAT1 directly interacts with the SMRT-family proteins, forms a protein complex with HDAC3, and has repressive activity in cell-based assays. What is not known, however, is which pathways are regulated by ATXN1 or BOAT1 in connection with SMRT and HDAC3.
In this study, through experiments with Drosophila and mammalian cells, we report that BOAT1 and ATXN1 are integral components of the Notch pathway—an evolutionarily conserved signalling pathway controlling a range of developmental processes, including nervous system development (Lai, 2004; Bray, 2006; Kopan & Ilagan, 2009). Our data show that BOAT1 and ATXN1 take part in the Notch pathway through interactions with CBF1, a SMRT-associating transcription factor that is central to the Notch pathway. The involvement of ATXN1 and BOAT1 in the Notch pathway suggests that these proteins might participate in several Notch-controlled processes during animal development and disease progression.
Results And Discussion
BOAT1 inhibits Notch activity in Drosophila
The neurotoxicity associated with glutamine-repeat expanded forms of ATXN1 has been studied in Drosophila, mouse and cultured mammalian cells (Zoghbi & Orr, 2009). However, less is known about the biological properties of ATXN1. Ectopic expression of either ATXN1 or BOAT1 in fly tissues causes specific phenotypes (Fernandez-Funez et al, 2000; Mizutani et al, 2005), the morphological features of which might indicate the pathways that these proteins are involved in. First, we analysed the phenotypes caused by the less-toxic BOAT1 in adult flies, because expression of ATXN1 causes lethality or severe cell loss in larval tissues.
Directed expression of BOAT1 in the posterior compartment of various fly tissues, as in hedgehog (hh)>Boat1, is not lethal but does cause defects, most prominently in the wings. As Drosophila wing development is well-characterized, we focused on this tissue to investigate the properties of BOAT1. The defects in the hh>Boat1 wings include an expanded longitudinal vein 5 (LV5) and loss of the posterior crossvein (Fig 1A). Broadened LV5 is a typical phenotype of Notch-mutant wings (Fig 1A; De Celis, 2003). This correlation led us to speculate that Notch activity might be compromised by BOAT1 in the wing. To test this hypothesis, we crossed hh>Boat1 flies with two Notch mutant lines (1 and N-8) and examined the phenotypes of their progenies. Few Notch−/+; hh>Boat1/+ females (less than 10% of the total number of scored females) survived to adult stage. In addition, the surviving escapers showed more severe wing defects, including a further expanded LV5 (Fig 1A), indicating that Boat1 interacts phenotypically with Notch in the Drosophila wing.
Figure 1.
BOAT1 inhibits Notch activity in the Drosophila wings. (A) The BOAT1-mediated wing-vein phenotype is enhanced by Notch mutation. Wings were prepared from adult female flies with the indicated genotypes; hh-Gal4 line directs protein expression in the posterior compartment; GFP was used as a negative control; N1 is a loss-of-function allele and Df(1)N-8 is a deficiency line; the positions of the LV5 and the PCV are marked with arrowheads and asterisks, respectively. (B) BOAT1 inhibits E(spl)mβ expression. Wing discs isolated from late third instar larvae with the indicated genotypes were immunostained with antibodies against β-galactosidase and BOAT1, and then DAPI stained; E(spl)mβ-lacZ is a reporter line; ap-Gal4 line directs protein expression in the dorsal compartment. The anterior (A), posterior (P), dorsal (D) and ventral (V) compartments are marked. (C) The BOAT1-mediated LV5 phenotype is suppressed by Su(H) mutation. Su(H)1 is a loss-of-function allele; the positions of LV5 and PCV are marked with arrowheads and asterisks, respectively. ap, apterous; BOAT1, Brother of ataxin-1; DAPI, 4,6-diamidino-2-phenylindole; GFP, green fluorescent protein; hh, hedgehog; LV5, longitudinal wing vein 5; PCV, posterior crossvein; Su(H), Suppressor of Hairless; wt, wild type.
BOAT1 reduces E(spl)mβ expression
The width of the wing vein is restricted by Notch through a mechanism known as lateral inhibition, in which Notch activates an array of target genes, including members of the enhancer of split complex such as E(spl)mβ (De Celis, 2003). In the wing discs (the precursor of adult wings), activated Notch signalling induces E(spl)mβ expression specifically in cells bordering the proveins (Fig 1B). When Notch is mutated, this expression pattern of E(spl)mβ is reduced or lost. To determine whether the BOAT1-mediated wing vein phenotype involves E(spl)mβ, we examined the expression of E(spl)mβ in wing regions expressing BOAT1. Indeed, in hh>Boat1/+ wing discs, the expression of E(spl)mβ-lacZ, a reporter for endogenous E(spl)mβ, is downregulated in the posterior compartment where BOAT1 is expressed (Fig 1B). Directed expression of BOAT1 in the dorsal compartment, as in apterous (ap)>Boat1/+ wing discs, also reduces E(spl)mβ expression (Fig 1B). Thus, forced expression of BOAT1 in the Drosophila wing disrupts the Notch pathway.
Su(H) suppresses the BOAT1-mediated wing phenotype
The expression of E(spl)mβ is directly regulated by Suppressor of Hairless (Su(H)), a transcription factor that is important for the Notch pathway (Furukawa et al, 1992; Schweisguth & Posakony, 1992). In the presence or absence of activated Notch, Su(H) can function as either an activator or a repressor, respectively. Having observed negative regulation of E(spl)mβ by BOAT1, we decided to investigate the relationship between BOAT1 and Su(H) by crossing hh>Boat1 flies with two Su(H) mutant alleles (1 and Δ47). We found that the BOAT1-mediated broadened LV5 phenotype is significantly suppressed in hh>Boat1/+; Su(H)−/+ flies (Fig 1C, data not shown for Δ47). By comparison, the BOAT1-mediated posterior crossvein phenotype is less sensitive to Su(H) mutation.
The broadened LV5 phenotype caused by BOAT1 is modulated by Su(H) and Notch mutations in an inverse manner. As Su(H) functions as a transcriptional repressor in the absence of activated Notch signalling, the results from our fly experiments led us to postulate that BOAT1 disrupts the Notch pathway by enhancing the transcriptional repressive activity of Su(H). This proposed mode of action of BOAT1 (as a transcriptional corepressor of Su(H)) provides an explanation for the enhancement of BOAT1-mediated wing phenotype by Notch mutation and its suppression by Su(H) mutation. In the former case, the transcriptional repressive effect of the Su(H)–BOAT1 complex is increased, whereas in the latter case, its repressive effect is alleviated.
BOAT1 and ATXN1 bind to CBF1
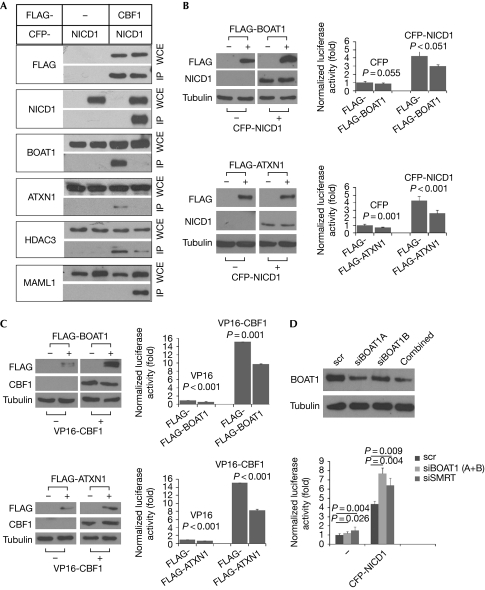
BOAT1 is a human protein. We therefore investigated whether the highly conserved Notch pathway is affected by BOAT1 in mammalian cells also. On the basis of our observations of BOAT1 and Su(H) in Drosophila, we speculated that a similar relationship might exist between BOAT1 and CBF1 (also called RBP Jκ), the vertebrate homologue of Su(H) (Matsunami et al, 1989). We first investigated whether BOAT1 and, by extension, ATXN1 are associating factors of CBF1 in mammalian cells. We carried out coimmunoprecipitation experiments, using FLAG antibody, on cell extracts prepared from human embryonic kidney (HEK)293 cells that express FLAG, FLAG-CBF1, FLAG-Su(H) and FLAG-HES1, respectively. The precipitated products were analysed for their constituents with western blots, using antibodies against BOAT1, ATXN1, SMRT (a positive control) and Sin3A (a negative control). In this experiment, we used blank FLAG and FLAG-tagged HES1, a human E(spl) homologue, as negative controls. As shown in Fig 2A, both BOAT1 and ATXN1, similarly to the control SMRT, are present in the precipitated CBF1, as well as in the precipitated Su(H) complex. As only a small amount of BOAT1 and ATXN1 is present in the control precipitated HES1 complex, BOAT1 and ATXN1 preferentially bind to members of the CBF1 family.
Figure 2.
BOAT1 and ATXN1 are direct CBF1-interacting factors. (A) BOAT1 and ATXN1 associate with CBF1 in mammalian cells. HEK293 cells expressing the indicated FLAG-tagged proteins were subject to coimmunoprecipitation experiments using FLAG antibody. The precipitants were analysed with western blot using the indicated antibodies; HES1 is a human homologue of E(spl); SMRT and Sin3A were used as controls. (B) Yeast two-hybrid assays show that BOAT1 and ATXN1 interact with CBF1. AH109 yeast cells were transformed with the indicated pGBT9- and pGAD424-based plasmids; SD-Leu/-Trp are growth plates and SD-Leu/-Trp/-His/-Ade are selection plates. ATXN1(1–703) has basal transcriptional activity owing to its lack of the (704–816) region of ATXN1; SMRT construct was used as a positive control. ATXN1, ataxin-1; BOAT1, Brother of ataxin-1; IP, immunoprecipitation; HEK, human embryonic kidney; Su(H), Suppressor of Hairless; WCE, whole-cell extract.
Next, we carried out yeast two-hybrid and glutathione S-transferase (GST) pull-down assays to determine whether the interaction between CBF1 and BOAT1 or ATXN1 observed in mammalian cells is direct or indirect, as their interactions in cells could be bridged through mutual interactions with SMRT (Tsai et al, 2004; Mizutani et al, 2005). For our yeast two-hybrid assays, AH109 cells were transformed with plasmids expressing full-length CBF1 (fused with a Gal4-activation domain) and each of the tested BOAT1 or ATXN1 variants (fused with a Gal4-DNA-binding domain), shown in Fig 2B. In this assay, positive protein–protein interactions enable transformed yeast to grow on the selection plate. On the basis of the results shown in Fig 2B, both BOAT1 and ATXN1, similarly to the positive control SMRT, are interacting factors of CBF1. These results are supported by data from the GST-pull-down assays (supplementary Fig S1 online).
Additionally, it appears that BOAT1 and ATXN1 compete with each other to bind to CBF1. These results, along with our discussion of a unique feature of ATXN1 in yeast, are included in the supplementary information online.
NICD disrupts CBF1–BOAT1/ATXN1 interactions
Similarly to Su(H), CBF1 has also been characterized as a bimodal transcription factor (Kadesch, 2004; Kopan & Ilagan, 2009). The switch of CBF1 from a repressor to an activator depends on its association with the Notch intracellular domain (NICD). In the absence of NICD, CBF1 represses transcription owing to its association with corepressors such as the SMRT-family proteins and HDAC3 (Kao et al, 1998; Perissi et al, 2008). On association with NICD, however, CBF1 becomes an activator due to its release of corepressors and concomitant recruitment of coactivators.
As BOAT1 and ATXN1 are direct interacting factors of CBF1, we tested whether their interactions with CBF1 are influenced by NICD. We performed coimmunoprecipitation experiments on HEK293 cells expressing FLAG and FLAG-CBF1 in the absence or presence of an active form of Notch1 (NICD1; Schroeter et al, 1998). The precipitated products from these experiments were analysed with western blots, using antibodies against BOAT1, ATXN1, HDAC3 and Mastermind-like 1 (MAML1), respectively. HDAC3 and MAML1 were used as controls in this study because HDAC3 is a corepressor of CBF1 (Perissi et al, 2008) and MAML1 is a coactivator of CBF1 (Wu et al, 2000). As shown in Fig 3A, the amount of endogenous BOAT1 and ATXN1 detected in the precipitated FLAG–CBF1 complex is significantly decreased in the presence of NICD1. This indicates that NICD1 binding disrupts the interaction between CBF1 and BOAT1 or ATXN1.
Figure 3.
BOAT1 and ATXN1 inhibit Notch activity in mammalian cells. (A) Notch disrupts the interaction between CBF1 and BOAT1 or ATXN1 in mammalian cells. Western blot analysis, using the indicated antibodies, was performed on precipitated products from HEK293 cells expressing the indicated proteins; HDAC3 and MAML1 were used as controls. (B,C) BOAT1 and ATXN1 inhibit the transcriptional activity of Notch and CBF1. Reporter assays were performed on cell extracts prepared from HEK293 cells transfected with 4XCBS-luc, along with the indicated expression plasmids. 4XCBS-luc is a Notch-responsive reporter; CFP-NICD1 is a fusion protein formed by NICD1 and CFP; VP16-CBF1 is a fusion protein formed by CBF1 and VP16; the reporter activity was normalized with β-galactosidase activity; western blots show the levels of expressed proteins used in reporter assays; tubulin was used as a loading control. Error bars indicate s.d. (n=3). (D) Notch activity increases as a result of reduced BOAT1 expression. Reporter assays were performed on HEK293 cells transfected with a 4XCBS-luc reporter along with the indicated siRNA. Western blot shows the reduced expression of BOAT1 by the tested Boat1 siRNA; for our reporter assays, we used a mixture of two Boat1 siRNAs. Error bars indicate s.d. (n=3). ATXN1, ataxin-1; BOAT1, Brother of ataxin-1; HDAC3, histone deacetylase 3; HEK, human embryonic kidney; MAML1, Mastermind-like 1; NICD, Notch intracellular domain; scr, scrambled RNA; siRNA, short-interfering RNA.
BOAT1 and ATXN1 inhibit CBF1 activity
Both BOAT1 and ATXN1 bind to SMRT and form protein complexes with HDAC3 (Tsai et al, 2004; Mizutani et al, 2005). These properties of BOAT1 and ATXN1, combined with our observations of BOAT1 in flies, led us to predict that these proteins inhibit the transcriptional output of Notch/CBF1. To test this prediction, we carried out reporter assays for HEK293 cells that were transfected with 4XCBS-luc and plasmids expressing NICD1, BOAT1 or ATXN1. 4XCBS-luc is a reporter construct in which the luciferase (luc) gene is regulated by multimerized CBF1-binding sites (Ong et al, 2008). As expected, this 4XCBS-luc reporter can be induced by NICD1, as well as by VP16-CBF1, a constitutively active form of CBF1. In the presence of BOAT1 or ATXN1, however, the reporter activity of 4XCBS-luc induced by NICD1 or VP16-CBF1 is reduced (Fig 3B,C). Similar results were obtained when we used another Notch-responsive reporter, Hey1-luc (Maier & Gessler, 2000; data not shown). These results indicate that both BOAT1 and ATXN1 negatively influence the transcriptional output of Notch/CBF1.
We also carried out reporter assays for 4XCBS-luc in cells expressing a reduced level of BOAT1 or ATXN1. For this purpose, we deployed short-interfering RNAs (siRNAs) to knock down the expression of BOAT1 or ATXN1. Although we were able to reduce the expression of endogenous BOAT1 in HEK293 cells with two independent siRNAs (Fig 3D), none of our tested Atxn1 siRNAs yielded satisfactory results. We therefore carried out our reporter assays in cells transfected with Boat1 siRNA, Smrt siRNA (a positive control) and scrambled siRNA (a negative control), respectively. As shown in Fig 3D, a decrease in BOAT1 levels led to an increase in reporter activity for 4XCBS-luc, both in the absence and presence of NICD1. This behaviour of BOAT1 is similar to that of the control SMRT, suggesting that BOAT1, like SMRT, acts as a transcriptional corepressor of CBF1.
BOAT1 and ATXN1 are recruited to the Hey1 promoter
Notch signalling influences the proliferation and differentiation of many tissues and cell types. In the case of C2C12 myoblasts, for example, activated Notch signalling prevents this cell line from differentiating into myotubes (Kopan et al, 1994; Kuroda et al, 1999). The ability of Notch to inhibit myogenesis is achieved in part by activating several direct target genes, including Hey1 (Hairy/enhancer-of-split related YRPW motif protein 1) (Buas et al, 2009). The regulatory region of Hey1 contains two consensus CBF1-binding sites (Maier & Gessler, 2000). We therefore asked whether BOAT1 and ATXN1, through interactions with CBF1, are naturally recruited to the Hey1 promoter in C2C12 cells.
First, we determined whether BOAT1 and ATXN1 are expressed in C2C12 cells. Western blot analysis was performed on cell extracts prepared from C2C12 cells that were grown in growth medium (marked as day 0, with high Notch activity) and in differentiating medium (with reduced Notch activity) for 1, 3 and 5 days, respectively. As shown in Fig 4A, both BOAT1 and ATXN1 are expressed in C2C12 cells, and their expression is not significantly altered by the length of differentiating medium treatment. By contrast, continued incubation of C2C12 cells in differentiating medium led to a gradual increase of Myosin heavy chain (an indicator of myogenesis) (Fig 4A), accompanied by a sharp decline in Hey1 expression (an indicator of reduced Notch activity; Fig 4B). These results are in accordance with previous reports (Kuroda et al, 1999; Iso et al, 2001).
Figure 4.
ATXN1 and BOAT1 are recruited to the Hey1 promoter. (A) C2C12 cells express both BOAT1 and ATXN1. Cell extracts prepared from C2C12 cells grown in growth medium (marked as day 0) and in DM at three time points were analysed by western blots using the indicated antibodies. Tubulin is a loading control; MHC is a marker for myogenesis. (B) Hey1 expression is reduced in C2C12 cells that were cultured in DM. Reverse transcription–PCR was performed on total RNA isolated from the four indicated C2C12 cell populations; the level of Hey1 transcript, a direct target gene of CBF1/Notch, is normalized with the level of GAPDH transcript. Error bars indicate s.d. (n=3). (C) BOAT1 and ATXN1 bind to the Hey1 promoter in differentiated C2C12 cells. Schematic diagram shows the Hey1 promoter region that harbours two CBF1-binding sites. Primers used for PCR are marked with arrows. Chromatin prepared from the indicated C2C12 cell populations was immunoprecipitated with the marked antibodies or the control IgG; SMRT antibody was used as a positive control and acetylated-histone H3 antibody was used as a marker for open chromatin and gene activation; the intensity of PCR products correlates with the amount of proteins recruited to (or the modifications at) the Hey1 promoter. (D) A proposed model for the roles of BOAT1 and ATXN1 in the Notch signalling pathway. Both BOAT1 and ATXN1 function as transcriptional corepressors of CBF1. When Notch signalling is not activated, BOAT1 and ATXN1, along with their associated SMRT and HDAC3, are recruited to the Hey1 promoter through their interactions with CBF1. When Notch is activated, upon the formation of the CBF1–NICD complex, BOAT1 and ATXN1 are dissociated from CBF1 and coactivators, such as MAML, are recruited by CBF1. ATXN1, ataxin-1; BOAT1, Brother of ataxin-1; DM, differentiating medium; HDAC3, histone deacetylase 3; Hey1, Hairy/enhancer-of-split related YRPW motif protein 1; IgG, immunoglobulin G; MAML, Mastermind-like protein; MHC, myosin heavy chain; NICD, Notch intracellular domain.
Having established that both BOAT1 and ATXN1 are expressed in C2C12 cells, we carried out a chromatin immunoprecipitation assay using antibodies against BOAT1 or ATXN1, followed by PCR, to determine whether these proteins bind to the Hey1 promoter. In these assays, we used CBF1 and SMRT antibodies as positive controls, immunoglobulin G (IgG) as a negative control and acetyl-histone H3 antibody as a marker to assess the state of the chromatin structures flanking the Hey1 promoter region. The results shown in Fig 4C suggest that the Hey1 promoter is occupied by not only the control CBF1 and SMRT, but also BOAT1 and ATXN1. These results confirm our long-held view that BOAT1 and ATXN1 are chromatin-binding factors (Tsai et al, 2004; Mizutani et al, 2005).
Interestingly, whereas binding of CBF1 to the Hey1 promoter is persistent, the binding of SMRT, BOAT1 and ATXN1 to the Hey1 promoter becomes prominent only when C2C12 cells are cultured in differentiating medium. The occupation of the Hey1 promoter by BOAT1, ATXN1 and SMRT coincides with a decreased level of acetyl-histone H3 (Fig 4C) and also with a reduced expression of Hey1 (Fig 4B). This suggests that BOAT1 or ATXN1 might work together with SMRT to assist CBF1 in repressing Hey1 expression.
The chromatin-binding profiles for BOAT1 and ATXN1 at the Hey1 promoter, although similar, are not identical. As shown in Fig 4C, binding of ATXN1 to the Hey1 promoter is transient, and is diminished with longer incubation of C2C12 cells in differentiating medium. This chromatin-binding profile is unique to ATXN1, because BOAT1 and SMRT remain bound to the Hey1 promoter under the same conditions. As the expression level of ATXN1 in C2C12 cells is not significantly altered by longer exposure to differentiating medium (Fig 4A), the dissociation of ATXN1 from the Hey1 promoter probably involves a different mechanism. Whether post-translational modifications of ATXN1 are responsible for its release from the Hey1 promoter after long-term treatment with differentiating medium remains to be determined.
CONCLUSIONS
ATXN1 has been known as a causative factor for SCA1 for more than 18 years (Orr et al, 1993; Banfi et al, 1994), but our understanding of the biological properties of ATXN1 and the related protein BOAT1 remains limited. By analysing the properties of BOAT1 in Drosophila and of BOAT1 and ATXN1 in mammalian cells, we show here that both BOAT1 and ATXN1 are components of the Notch signalling pathway. On the basis of these results, we propose that both BOAT1 and ATXN1, similarly to SMRT and HDAC3, act as transcriptional corepressors of CBF1. The interaction between BOAT1 or ATXN1 and CBF1 takes place in the absence of NICD and is disrupted when CBF1 binds to NICD (see the model shown in Fig 4D). Our identification of BOAT1 and ATXN1 as integral components of the Notch pathway has broad implications. On the one hand, it raises the possibility that aberrant Notch signalling contributes to the pathogenesis of SCA1. On the other hand, it implies that ATXN1/BOAT1-family proteins might participate in diverse Notch-controlled pathways in species throughout the metazoans.
Methods
Drosophila stock and experiment. UAS-Boat1 line was described previously (Mizutani et al, 2005). w1118, hh-Gal4, ap-Gal4, Notch and Su(H) fly lines were obtained from the Bloomington Stock Center or from K. Irvine's lab at Rutgers University.
Cell culture and transient transfection. HEK293 cells or C2C12 (mouse myoblast) cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin (Mediatech, USA). To induce differentiation of C2C12 cells, cells were grown to near 85% confluence and then shifted to differentiation medium (DMEM containing 2% horse serum (Invitrogen)). Transfection was performed using Lipofectamine 2000 (Invitrogen) following the manufacturer’ instructions.
Yeast two-hybrid assays. AH109 yeast cells were transformed with pGBT9- and pGAD424-based constructs according to the manufacturer's instructions (Clontech). After transformation, yeast cells were grown on SD-Leu/-Trp plates to select for transformants. To assay protein–protein interaction, the transformed yeast cells were first grown in SD-Leu/-Trp liquid media until saturation. Tenfold serial dilutions of each yeast culture in the volume 5 μl were then spotted onto SD-Leu/-Trp/-His/-Ade selection plates or onto SD-Leu/-Trp control growth plates. Growth differences were recorded following incubation of the plates for 2–4 days at 30°C.
Additional materials and methods are included in the supplementary information.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank K. Irvine, J. Posakony, H. Bellen and the Bloomington Stock Center for fly lines; R. Kopan, S. Stifani, M. Gessler, S.D. Hayward and R. Evans for constructs; and R. Head, H.Y. Kao and K. Irvine for commenting on this manuscript. This research is supported by a grant from the National Institutes of Health (5R01NS050365).
Footnotes
The authors declare that they have no conflict of interest.
References
- Banfi S, Servadio A, Chung MY, Kwiatkowski TJ Jr, McCall AE, Duvick LA, Shen Y, Roth EJ, Orr HT, Zoghbi HY (1994) Identification and characterization of the gene causing type 1 spinocerebellar ataxia. Nat Genet 7: 513–520 [DOI] [PubMed] [Google Scholar]
- Bolger TA, Zhao X, Cohen TJ, Tsai CC, Yao TP (2007) The neurodegenerative disease protein ataxin-1 antagonizes the neuronal survival function of myocyte enhancer factor-2. J Biol Chem 282: 29186–29192 [DOI] [PubMed] [Google Scholar]
- Bray SJ (2006) Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol 7: 678–689 [DOI] [PubMed] [Google Scholar]
- Buas MF, Kabak S, Kadesch T (2009) Inhibition of myogenesis by Notch: evidence for multiple pathways. J Cell Physiol 218: 84–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo-Barreto J, Fryer JD, Shaw CA, Orr HT, Zoghbi HY (2010) Partial loss of ataxin-1 function contributes to transcriptional dysregulation in spinocerebellar ataxia type 1 pathogenesis. PLoS Genet 6: e1001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvetanovic M, Rooney RJ, Garcia JJ, Toporovskaya N, Zoghbi HY, Opal P (2007) The role of LANP and ataxin 1 in E4F-mediated transcriptional repression. EMBO Rep 8: 671–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Celis JF (2003) Pattern formation in the Drosophila wing: the development of the veins. Bioessays 25: 443–451 [DOI] [PubMed] [Google Scholar]
- Fernandez-Funez P et al. (2000) Identification of genes that modify ataxin-1-induced neurodegeneration. Nature 408: 101–106 [DOI] [PubMed] [Google Scholar]
- Furukawa T, Maruyama S, Kawaichi M, Honjo T (1992) The Drosophila homolog of the immunoglobulin recombination signal-binding protein regulates peripheral nervous system development. Cell 69: 1191–1197 [DOI] [PubMed] [Google Scholar]
- Iso T, Sartorelli V, Chung G, Shichinohe T, Kedes L, Hamamori Y (2001) HERP, a new primary target of Notch regulated by ligand binding. Mol Cell Biol 21: 6071–6079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadesch T (2004) Notch signaling: the demise of elegant simplicity. Curr Opin Genet Dev 14: 506–512 [DOI] [PubMed] [Google Scholar]
- Kao HY, Ordentlich P, Koyano-Nakagawa N, Tang Z, Downes M, Kintner CR, Evans RM, Kadesch T (1998) A histone deacetylase corepressor complex regulates the Notch signal transduction pathway. Genes Dev 12: 2269–2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopan R, Ilagan MX (2009) The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 137: 216–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopan R, Nye JS, Weintraub H (1994) The intracellular domain of mouse Notch: a constitutively activated repressor of myogenesis directed at the basic helix-loop-helix region of MyoD. Development 120: 2385–2396 [DOI] [PubMed] [Google Scholar]
- Kuroda K, Tani S, Tamura K, Minoguchi S, Kurooka H, Honjo T (1999) Delta-induced Notch signaling mediated by RBP-J inhibits MyoD expression and myogenesis. J Biol Chem 274: 7238–7244 [DOI] [PubMed] [Google Scholar]
- Lai EC (2004) Notch signaling: control of cell communication and cell fate. Development 131: 965–973 [DOI] [PubMed] [Google Scholar]
- Lam YC et al. (2006) ATAXIN-1 interacts with the repressor Capicua in its native complex to cause SCA1 neuropathology. Cell 127: 1335–1347 [DOI] [PubMed] [Google Scholar]
- Maier MM, Gessler M (2000) Comparative analysis of the human and mouse Hey1 promoter: Hey genes are new Notch target genes. Biochem Biophys Res Commun 275: 652–660 [DOI] [PubMed] [Google Scholar]
- Matilla A, Roberson ED, Banfi S, Morales J, Armstrong DL, Burright EN, Orr HT, Sweatt JD, Zoghbi HY, Matzuk MM (1998) Mice lacking ataxin-1 display learning deficits and decreased hippocampal paired-pulse facilitation. J Neurosci 18: 5508–5516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunami N, Hamaguchi Y, Yamamoto Y, Kuze K, Kangawa K, Matsuo H, Kawaichi M, Honjo T (1989) A protein binding to the J kappa recombination sequence of immunoglobulin genes contains a sequence related to the integrase motif. Nature 342: 934–937 [DOI] [PubMed] [Google Scholar]
- Mizutani A, Wang L, Rajan H, Vig PJ, Alaynick WA, Thaler JP, Tsai CC (2005) Boat, an AXH domain protein, suppresses the cytotoxicity of mutant ataxin-1. EMBO J 24: 3339–3351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong CT, Sedy JR, Murphy KM, Kopan R (2008) Notch and presenilin regulate cellular expansion and cytokine secretion but cannot instruct Th1/Th2 fate acquisition. PLoS ONE 3: e2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr HT, Chung MY, Banfi S, Kwiatkowski TJ Jr, Servadio A, Beaudet AL, McCall AE, Duvick LA, Ranum LP, Zoghbi HY (1993) Expansion of an unstable trinucleotide CAG repeat in spinocerebellar ataxia type 1. Nat Genet 4: 221–226 [DOI] [PubMed] [Google Scholar]
- Perissi V, Scafoglio C, Zhang J, Ohgi KA, Rose DW, Glass CK, Rosenfeld MG (2008) TBL1 and TBLR1 phosphorylation on regulated gene promoters overcomes dual CtBP and NCoR/SMRT transcriptional repression checkpoints. Mol Cell 29: 755–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley BE, Orr HT (2006) Polyglutamine neurodegenerative diseases and regulation of transcription: assembling the puzzle. Genes Dev 20: 2183–2192 [DOI] [PubMed] [Google Scholar]
- Schroeter EH, Kisslinger JA, Kopan R (1998) Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature 393: 382–386 [DOI] [PubMed] [Google Scholar]
- Schweisguth F, Posakony JW (1992) Suppressor of Hairless, the Drosophila homolog of the mouse recombination signal-binding protein gene, controls sensory organ cell fates. Cell 69: 1199–1212 [DOI] [PubMed] [Google Scholar]
- Tsai CC, Kao HY, Mitzutani A, Banayo E, Rajan H, McKeown M, Evans RM (2004) Ataxin 1, a SCA1 neurodegenerative disorder protein, is functionally linked to the silencing mediator of retinoid and thyroid hormone receptors. Proc Natl Acad Sci USA 101: 4047–4052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Aster JC, Blacklow SC, Lake R, Artavanis-Tsakonas S, Griffin JD (2000) MAML1, a human homologue of Drosophila mastermind, is a transcriptional co-activator for NOTCH receptors. Nat Genet 26: 484–489 [DOI] [PubMed] [Google Scholar]
- Zhang C, Browne A, Child D, Divito JR, Stevenson JA, Tanzi RE (2010) Loss of function of ATXN1 increases amyloid β-protein levels by potentiating β-secretase processing of β-amyloid precursor protein. J Biol Chem 285: 8515–8526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoghbi HY, Orr HT (2009) Pathogenic mechanisms of a polyglutamine-mediated neurodegenerative disease, spinocerebellar ataxia type 1. J Biol Chem 284: 7425–7429 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.