Abstract
At least 15% of human malignant diseases are attributable to the consequences of persistent viral or bacterial infection. Chronic infection with oncogenic human papillomavirus (HPV) types is a necessary, but insufficient, cause in the development of more cancers than any other virus. Currently available prophylactic vaccines have no therapeutic effect for established infection or for disease. Early disease is characterised by tissue sequestration. However, because a proportion of intraepithelial HPV-associated disease undergoes immune-mediated regression, the development of immunotherapeutic strategies is an opportunity to determine proof-of-principle for therapeutic vaccines. In this Review, we discuss recent progress in this field and priorities for future clinical investigations.
Introduction
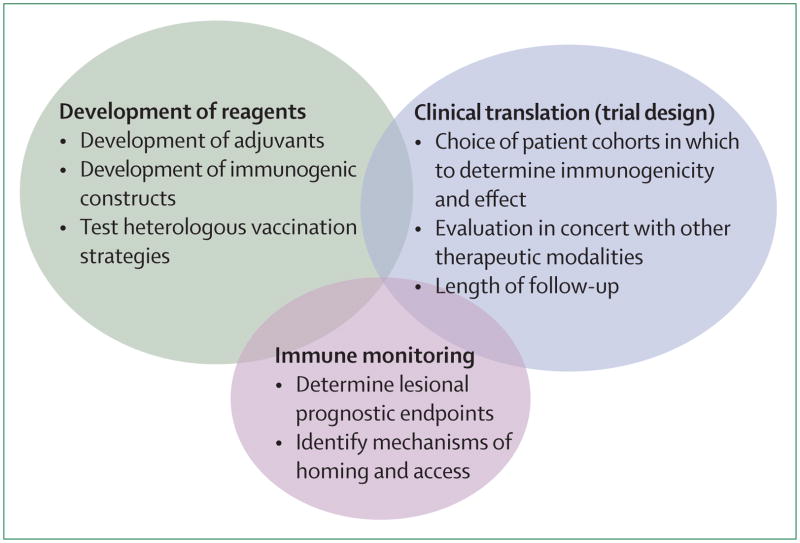
Persistent infection with human papillomavirus (HPV), most commonly type 16, is the proximate cause of 10% of malignant diseases in women, and 5% of the total global cancer burden,1 including cancers of the cervix, vagina, vulva, anus, and oropharynx.2 Of these cancers, cervical cancer is the most common. Current screening strategies for preinvasive disease of the cervix (ie, high-grade dysplasia or cervical intraepithelial neoplasia [CIN] 2/3), including cytology, HPV testing, and direct visualisation and immediate triage (see-and-treat), all need infrastructure and funding that are well beyond the resources available in much of the world. Consequently, on a global scale, cervical cancer remains the second leading cause of cancer death in women.3 Although recently available prophylactic vaccines represent a public-health milestone, they provide no therapeutic effect for prevalent infection, or for already established HPV-associated disease. Furthermore, because implementation of these prophylactic vaccines presents many of the same challenges posed by screening and treatment, the global burden of HPV disease is unlikely to decrease in the near future. The figure summarises the challenges that need to be addressed in the development of immune-based therapies for HPV disease.
HPV-associated neoplasia represents an excellent opportunity to test antigen-specific immunotherapies, because expression of two viral antigens, E6 and E7, are needed to initiate and maintain high-grade squamous intraepithelial lesions, the immediate precursors to invasive cervical cancer, and overtly invasive disease.4 Integration of viral DNA into the host genome is strongly associated with persistent HPV infection and disease progression, although both episomal and integrated viral DNA can be present in the same lesion.5–7 In most immunocompetent individuals, HPV infection will eventually clear without immune intervention. Immuno-suppression by drugs or HIV infection significantly reduces clearance of infection8 and clearance correlates with the development of specific CD4-T-cell immunity to the papillomavirus E2 and E6 proteins.9 Moreover, a subset of persistent HPV disease is susceptible to immune manipulation; topical application of an inducer of innate immune responses, imiquimod, a toll-like receptor (TLR) 7 agonist, is approved by the US Food and Drug Administration (FDA) as first-line therapy for external genital warts, most of which are caused by HPV-6 and 11. HPV-associated disease is an ideal test of antigen-specific immunotherapy, because disease is common and expression of viral oncoproteins is obligate for persisting tumour growth, providing tumour-specific non-self antigenic targets. Furthermore, pre invasive intraepithelial precursor lesions are identifiable and clinically indolent, and a proportion of dysplastic lesions undergo spontaneous immune-mediated regression. Thus, the development of immunotherapeutic strategies for patients with HPV-associated intraepithelial neoplasia represents an ideal opportunity to determine proof-of-principle of immune-based therapeutic interventions for epithelial cancer. Because the lower genital tract is relatively accessible, the effect of immune interventions on both the systemic circulation and the target tissue can be studied. Furthermore, the opportunity to study the lesion microenvironment over time after therapeutic inter ventions can provide extra insights into how immunotherapy might work for persisting viral infection.
Naturally occurring immune responses to HPV antigens
The lifetime risk of genital infection on at least one occasion with an oncogenic strain of human papilloma-virus is thought to be greater than 80%.10 In immunocompetent hosts, more than 90% of genital HPV infections become undetectable without intervention.11 Because HPV infections are asymptomatic, and the timeframe for clearance is in the order of months to years, the herd burden of HPV is essentially endemic. Dysplasia develops from a chronic, mucosally-sequestered infection with HPV, and is associated with ineffective immune responses to viral non-structural proteins. Naturally occurring systemic humoral and adaptive responses to HPV antigens, even in cohorts with documented type-specific mucosal infections that have become undetectable, are hard to detect in peripheral blood.12–14 Type-specific serum antibodies to capsid proteins are detectable in less than half of women in whom cervical HPV infections of known serotype have cleared. Nonetheless, data from cohorts undergoing prophylactic vaccination show an anamnestic response to a single dose of virus-like particle (VLP)-vaccine in previously infected individuals.15 The antibody to the E7 protein can be measured in people with invasive cancer, but not in those with early stage disease.16
Women with intraepithelial HPV lesions rarely have systemic T-cell responses to HPV E6 or E7 that can be detected directly ex vivo, which probably reflects the low antigen load and tissue-compartmentalisation of early disease. By contrast to immune responses to other viral infections, the frequency of systemic memory CD8+ T cells in individuals with a known previous cervical HPV infection that has subsequently become undetectable is vanishingly low. For example, by use of direct ex-vivo assays, the frequency of systemic virus-specific CD8+ T cells after primary infection with cytomegalovirus or hepatitis C virus can be up to 5%.17,18 By contrast, in patients with CIN, the frequency of HPV-specific T cells is two to three orders of magnitude lower, in the range of 0·1–0·01%.19 Detection of systemic, HPV-specific T-cell responses in patients with intraepithelial neoplasia requires in-vitro sensitisation.20–24 Various methods have been developed to identify responses to E6 and E7, including: assessment of proliferation of blood lymphocytes after incubation with HPV E6 or E7 peptides and interleukin (IL)-2 for 21 days21 or with E7 20-mers for 7 days;25 use of recombinant adenoviruses encoding HPV oncoproteins for secondary in-vitro restimulation for 21 days to identify cytotoxic-T-lymphocyte responses;26 interferon-γ enzyme-linked immunosorbent spot (ELISPOT) assays after 4 days of in-vitro sensitisation with long E6 and E7 overlapping peptides;24 and major histocompatibility complex class I tetramer analysis done directly ex vivo by use of a fluorogenic human leucoctye antigen (HLA)-A*0201-HPV16-E711–20 construct.19 After in-vitro stimulation with HPV antigens, peptide-specific T-cell frequencies increase in people with concurrent disease at the time of blood sampling, compared with individuals with no evidence of disease.19,26 However, although amplification can identify qualitative responses to HPV antigens, this method is likely to have limited use in accurately distinguishing quantitative differences between individuals, either in the course of a natural infection, or in those with intraepithelial disease. Responses identified only after in-vitro restimulation would represent expansion of previously induced memory immune responses, rather than an ongoing response at the time of sampling. From a practical standpoint, two conclusions can be drawn concerning T-cell responses in women with intraepithelial neoplasia; first, natural infection with HPV fails to elicit a potent systemic immune response; and second, the size of natural HPV-specific T-cell responses measured in the peripheral blood of individual patients does not reliably predict lesion regression.
Natural history of HSIL
High-grade dysplasia is associated with integration of the viral genome into the host genome.27 Nonetheless, a proportion of established cervical high-grade lesions do undergo regression over a relatively short timeframe. Both retrospective and prospective studies suggest that across all HPV types, the rate of regression of cervical high-grade squamous intraepithelial lesions (HSIL) in 4–6 months is around 35%.28,29 Cervical HSIL associated with HPV-16 is less likely to regress than CIN2/3 associated with HPV types other than 16.29,30 However, because it is not possible to distinguish lesions that are likely to regress from those that are not, the standard of care for a biopsy-proven high-grade dysplasia is surgical excision. Over the same timeframe, 4–6 months, high-grade cervical dysplasia is unlikely to progress, even in women who are immuno-compromised.31 Thus, the clinical indolence of intra-epithelial lesions in combination with the fact that they can be directly visualised represents an opportunity to monitor lesions long after the active treatment window of any given immunotherapeutic intervention.
Vulvar and vaginal dysplasias are clinically more recalcitrant than cervical HSIL. Although progression to invasive carcinoma is low, in the range of 9% over a timeframe of years, spontaneous regression is rare, in the region of 1%.32 Finally, despite the fact that high-grade anal dysplasia is less common than intraepithelial HPV lesions elsewhere in the genital tract, the incidence of this disease is increasing, both in men and women.33
Identification of viral epitopes recognised in natural infection, preinvasive disease, and invasive cancer can inform the monitoring of immune therapies in these patient cohorts. However, useful antigenic targets for induced immune responses are unlikely to be identified by study of HPV-antigen-specific cells in the systemic circulation. Development of improved methods to identify HPV-specific T-cell responses could enable monitoring of the functional polarisation of immune T cells in HPV-associated clinical lesions, and of the correlation of tissue and systemic immune responses to HPV proteins.
Summary of therapeutic vaccine approaches
Despite the fact that the epithelial compartment of cervical cancers and also preinvasive anogenital lesions can express up to nine papillomavirus-encoded proteins (L1, L2, and E1–E7), the E6 and E7 proteins are of specific interest for vaccine development, not only because of their functional role in the neoplastic process, but also because natural immune responses to these antigens, although limited, have been identified in relation to disease. Up to now, delivery systems tested clinically have included fusion proteins used alone and with adjuvant, encapsulated polynucleotides, protein with adjuvant, recombinant viruses, DNA constructs, dendritic cells, and chimeric VLP constructs (table). These vaccines have been tested in a spectrum of patient cohorts, from patients with end-stage cervical cancer to those with intraepithelial neoplasia of the cervix, vulva, or perianal area who are otherwise healthy. Overall, these investigations have established the safety, feasibility, tolerability, and limited immunogenicity of these vaccine constructs. However, despite optimistic preclinical data, evidence of therapeutic benefit from induced T-cell responses in humans has been limited. Early clinical trials were done in patients with late-stage disease, who were immunocompromised both by disease and by previous treatment for disease. Moreover, late-stage disease is probably a poor target for antigen-specific therapies as a stand-alone modality, because these tumours commonly have mutations in addition to deletions of genes involved in antigen processing and presentation.52,53
Table.
Clinical trials of human papillomavirus (HPV)-specific immunotherapy
| Delivery system | Antigen | Disease group | Immunogenicity | Clinical outcome | |
|---|---|---|---|---|---|
| Double-blind placebo-controlled trial34 | Fusion protein (TA-CIN) | HPV-16 L2–E6–E7 fusion protein (no adjuvant) | Healthy volunteers (N=40) | Antibody, T cell, interferon γ, and ELISPOT all detected | No HPV infections |
| Open-label uncontrolled trial (warts not HPV-16+)35 | HSP fusion protein (HSP-E7) | HPV-16 E7 peptide | Genital warts (N=22) | ND | Regression of warts: 3/14 CRs and 10/14 PRs |
| Open-label uncontrolled trials (anal dysplasia and HPV-16+ cervical dysplasia)36–38 | Encapsulated polynucleotide (ZYC101) | HPV-16 E7 | Anal and cervical dysplasia; HPV-16+; HLA-A2 (anal: N=12, cervical: N=15) | Most individuals ELISPOT positive; induction of E2-specific immunity | Regression of AIN: 3/12 PRs; regression of CIN: 5/15 CRs |
| Multicentre, double-blind, randomised, placebo-controlled trial (CIN2/3, any HPV type)38,39 | Encapsulated polynucleotide (ZYC101) | HPV-16 E7 peptide | CIN2/3, any HPV type (assessable N=127) | Most individuals ELISPOT positive; induction of E2-specific immunity | Lesion regression higher in patients <25 years of age; not restricted to HPV-16 or 18+ lesions |
| Randomised placebo-controlled trial39,40 | Protein/Iscomatrix adjuvant (E6, E7–IMX) | HPV-16 E6–E7 fusion protein | CIN (N=31) | Antibody, DTH, CTLs | HPV-type-specific reduction in HPV infection: 7/14 CRs and 7/14 PRs/no clinical regression |
| Open-label phase I/II uncontrolled trial41 | Vaccinia virus (TA-HPV) | E6–E7 fusion protein | Late-stage cervical cancer (N=8) | CTLs (1/8), antibody (3/8) | Outcome not documented |
| Open-label uncontrolled trial42 | Vaccinia virus (TA-HPV) | E6–E7 fusion protein | Vulval HPV/VIN (N=18) | Antibody, CMI (13/18) | 50% reduction in disease in 8/18; loss of viral load in 12/18 |
| Open-label uncontrolled trial43 | Vaccinia virus (TA-HPV) | E6–E7 fusion protein | VIN (N=12) | T-helper cell ELISPOT increase (6/10); vaccinia response in all patients | >50% reduction in disease in 5/12 |
| Open-label uncontrolled trial44 | Peptide/oil plus water adjuvant | E7 peptides | Refractory cervical cancer; HPV-16+; HLA-A201 (N=19) | No CTL response | 2/19 stable disease |
| Open-label uncontrolled trial45 | Protein/algammulin adjuvant | E7–GST fusion protein | Cervical cancer (N=24) | Antibody, DTH | No alteration in natural history of disease |
| Open-label uncontrolled, trial HPV-16+46 | Peptide plus IFA | E7 A0201 peptide | VIN/CIN; HPV-16+; HLA-A2 (N=18) | CTLs 10/16, no DTH | 3/18 CRs and 6/18 PRs |
| Open-label uncontrolled, trial47 | VLPs | L1 | Genital warts (N=33) | Antibody, DTH | Regression of warts: 25/33 CRs |
| Open-label uncontrolled, trial48 | Dendritic cells | HPV-16 E7 and HPV-18 E7 | Cervical cancer, stage IV (N=15) | Antibody, proliferation, ELISPOT (3/11) | No objective clinical response |
| Randomised placebo-controlled trial49 | Chimeric virus-like particles (CVLP) | HPV-16 1 E7 protein | CIN2/3; HPV-16 only (N=39) | Antibody, CTL | 39% histological improvement in vaccinated patients vs 25% in placebo group; 59% of responders became HPV-16 DNA-negative |
| Open-label uncontrolled, trial50 | Peptide montanide ISA-51 adjuvant | HPV-16 E6 combined or separated from HPV-16 E7 overlapping long peptides | End-stage cervical cancer (N=35) | ELISPOT | Immunity against E6 in patients vaccinated with E6 and E7 at the same site; greater response to E7 in patients vaccinated with E6 in one limb and with E7 in a different limb |
| Open-label uncontrolled, trial51 | DNA vaccine | Sig-E7(detox)-heat-shock protein-70 fusion protein | CIN2/3; HPV-16+; (N=15) | ELISPOT | Complete histological regression in 33% (3/9) in the highest dose cohort; new responses to E7 at 6 months |
IFN=interferon. ELISPOT=enzyme-linked immunosorbent spot. ND=not done. CR=complete response. PR=partial response. HSP=heat shock protein. HLA-A2=human leucocyte antigen serotype A2. AIN=anal intraepithelial neoplasia. CIN=cervical intraepithelial neoplasia. DTH=delayed hypersensitivity reaction. CTL=cytotoxic T lymphocyte. VIN=vulvar intraepithelial neoplasia. CMI=cell-mediated immunity. GST=glutathione-S-transferase. IFA=incomplete Freund’s adjuvant. VLP=virus-like particle.
More recently, the design of trials testing HPV immunotherapies has undergone a paradigm shift towards testing immunisation strategies in patients with preinvasive disease. An emerging body of evidence from clinical studies testing a non-HPV-specific immune modulator, imiquimod, on intraepithelial HPV disease suggests several potentially crucial insights for the design of subsequent clinical trials. 54,55
The use of topical imiquimod on high-grade vulvar intraepithelial neoplasia (VIN) has been reported with a complete response in nine (34·6%) of 26 patients over a 12-month period.54 The expected rate of spontaneous regression in this timeframe is less than 1%. The use of sequential imiquimod and photodynamic therapy has also been reported in this patient cohort, with six (30%) of 20 patients showing a complete response in a 12-month period.55 Notably, in both of these studies, patients with detectable systemic HPV-specific T-cell responses before study intervention were more likely to have a clinical response to manipulation of the lesion microenvironment than patients who did not have a detectable HPV-specific response at study entry. This finding suggests that combinatorial regimens should incorporate both induction of virus-specific T-cell responses and subsequent manipulation of the target lesion microenvironment. Both studies also reported an increase in the ratio of CD8:Foxp3 cells in lesional lymphocytes, in patients who responded to imiquimod. It is also worth noting for future clinical trial design that clinical responses were noted a long time after the completion of the intervention phase of the trials. These data suggest that clinical trials testing immunotherapies in this patient cohort should include monitoring of the lesion compartment, in addition to the more conventional systemic measures of immunological parameters. Although eliciting an HPV-specific T-cell response is a rational proxy measure of vaccine efficacy, the development of immunotherapeutic strategies for HPV disease should also include immunological monitoring of the lesion site.
Animal models of therapy for HPV disease
Preclinical data to support clinical trials of HPV-specific immunotherapy have emerged from three different models: naturally occurring animal papillomavirus infections in cows, dogs, and rabbits; murine transplantable tumours expressing HPV antigens; and mice transgenic for HPV genes. Natural animal papillomavirus infections have limited use, because most disease regresses spontaneously. Bovine papillomavirus (BPV)-associated oesophageal tumours develop in cattle fed bracken fern, but do not express papillomavirus-encoded antigens,56 whereas BPV-associated sarcoids in horses, which express some papillomavirus antigens,57 are not widely available for preclinical research. Studies of canine oral papillomavirus infections, which are generally benign and self-limiting, have confirmed that immune responses to two viral early proteins (E2 and E6) correlate with lesion regression.58 Rabbit oral papillomavirus infections and cottontail rabbit papillomavirus infections are self-limiting in most strains; chronic infection leads to localised skin cancers, for which regression can be induced by immunotherapy.59 However, the mechanism of regression remains unclear, due to the low availability of reagents and of genetically homogenous rabbits. Several transplantable murine tumours expressing the early open reading frames of HPV have been established, and are generally susceptible to a wide range of antigen-specific immunotherapy.60,61 However, immunotherapeutics effective in these animal models, have, when tested in humans, shown limited or no clinical efficacy. These data suggest that transplantable tumour models are not representative of persistent papillomavirus infection in humans, perhaps because the 2% of humans that develop persistent infection do so because of specific genetic predisposition. In transgenic mice, HPV early proteins expressed in the epithelium from a keratin promoter can be induced with oestrogen to develop cervical carcinoma.62 These mice develop partial tolerance to the papillomavirus-encoded transgenic proteins, and are unable to generate papillomavirus antigen-specific cytotoxic T cells after immunisation.63 However, such mice might model the requirements for induction of papillomavirus protein-specific immunity in humans already tolerant of papillomavirus proteins from extended exposure during infection. Skin from papillomavirus E6 and E7 transgenic mice is not spontaneously rejected when grafted to immunocompetent recipients,64,65 by contrast to the skin from animals expressing other transgenes from the same promoter,65 and such grafts provide a further model for assessing papillomavirus protein-specific immunotherapy. E7-expressing grafts are not rejected after immunisation with E7, although such immunisation induces a specific immune response sufficient to reject an E7-expressing transplantable tumour.66 This finding suggests that papillomavirus E6 and E7 proteins are either poorly immunogenic or interfere with their own immunogenicity. Local immuno-regulation in skin, attributable in part to properties of the E7 protein, and in part to the immuno-regulatory properties of hyperproliferative epithelium, also contributes to the failure of immunotherapy in resulting in E7 graft rejection. In this model, possible mechanisms allowing persistent HPV infection can be identified (figure), which might be relevant to persisting HPV infection in some apparently immunocompetent patients. These mechanisms include inhibition of interferon-γ mediated upregulation of antigen presentation, a requirement for local inflammation, inducible by topical imiquimod therapy, for optimum effector T-cell function, and cellular inhibitors of antigen-specific CD8 T-cell induction and effector function, including regulatory CD4 T cells, and natural killer T cells.
Figure.
Identifying barriers to therapeutic vaccination for human papillomavirus disease
Conclusions
Clinical trials that have been done up to now have been moderately successful in eliciting cell-mediated immune responses to HPV E6 and E7 in patients with a spectrum of HPV-associated disease. However, clinical responses have not been consistent. Advances in immunotherapies for HPV-associated disease will be predicated on the development of instruments for measuring tissue-localised mucosal immune parameters. Mechanisms of immune-cell traffcking to the genital mucosa, and, more generally to epithelial surfaces in the absence of local inflammation, are incompletely understood, and this knowledge would provide valuable direction for optimising vaccination strategies. Clinical assessment of nasal, oral, rectal, intramuscular, and intravaginal immunisation suggest that, although intravaginal priming and boosting is the most effective schema for eliciting detectable antigen-specific genital immune responses, nasal immunisation also generates genital immune responses.67 Nasal immunisation has certain practical advantages over genital immunisation, including more straightforward logistics, and a greater likelihood of cultural acceptability.
The ability to identify mechanisms of immune dysfunction in the lesion milieu could allow temporary systemic immune modulation in conjunction with strategies to elicit E6-specific or E7-specific T-cell responses. The development of methods to quantitate immunological parameters in the mucosal micro environment could also allow the identification of predictors of lesions that are likely to respond either to vaccination or to manipulation of the local immune environment.
Acknowledgments
CLT has received grants from the National Cancer Institute, Bethesda, MD, USA, from the Maryland Cigarette Restitution Fund, and from the Dana Foundation. IHF has received grants from the National Health and Medical Research Council of Australia, the Cancer Research Institute New York, Wellcome foundation, the Australian Cancer Research Foundation, and the Cancer Council Australia.
Footnotes
Conflicts of interest
IHF receives royalties from sales of HPV prophylactic vaccines, and is a consultant for Merck, GlaxoSmithKine, and Commonweath Serum Laboratories on papillomavirus vaccine development. CLT declared no conflicts of interests.
Contributor Information
Cornelia L Trimble, Johns Hopkins University Hospital, Baltimore, MD, USA.
Prof Ian H Frazer, Diamantina Institute for Cancer, Immunology, and Metabolic Medicine, Woolloongabba, Australia.
References
- 1.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030–44. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 2.Frazer IH, Lowy DR, Schiller JT. Prevention of cancer through immunization: prospects and challenges for the 21st century. Eur J Immunol. 2007;37 (suppl 1):S148–55. doi: 10.1002/eji.200737820. [DOI] [PubMed] [Google Scholar]
- 3.HPV and cervical cancer in the 2007 report. Vaccine. 2007;25 (suppl 3):C1–230. doi: 10.1016/S0264-410X(07)01183-8. [DOI] [PubMed] [Google Scholar]
- 4.Hudson J, Bedell M, McCance D, Laiminis L. Immortalization and altered differentiation of human keratinocytes in vitro by the E6 and E7 open reading frames of human papillomavirus type 18. J Virol. 1990;64:519–26. doi: 10.1128/jvi.64.2.519-526.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li W, Wang W, Si M, et al. The physical state of HPV16 infection and its clinical significance in cancer precursor lesion and cervical carcinoma. J Cancer Res Clin Oncol. 2008;134:1355–61. doi: 10.1007/s00432-008-0413-3. [DOI] [PubMed] [Google Scholar]
- 6.Hudelist G, Manavi M, Pischinger KI, et al. Physical state and expression of HPV DNA in benign and dysplastic cervical tissue: different levels of viral integration are correlated with lesion grade. Gynecol Oncol. 2004;92:873–80. doi: 10.1016/j.ygyno.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 7.Cricca M, Morselli-Labate AM, Venturoli S, et al. Viral DNA load, physical status and E2/E6 ratio as markers to grade HPV16 positive women for high-grade cervical lesions. Gynecol Oncol. 2007;106:549–57. doi: 10.1016/j.ygyno.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Koshiol JE, Schroeder JC, Jamieson DJ, et al. Time to clearance of human papillomavirus infection by type and human immunodeficiency virus serostatus. Int J Cancer. 2006;119:1623–29. doi: 10.1002/ijc.22015. [DOI] [PubMed] [Google Scholar]
- 9.Welters MJ, van der Logt P, van den Eeden SJ, et al. Detection of human papillomavirus type 18 E6 and E7-specific CD4+ T-helper 1 immunity in relation to health versus disease. Int J Cancer. 2006;118:950–56. doi: 10.1002/ijc.21459. [DOI] [PubMed] [Google Scholar]
- 10.Koutsky LA, Galloway DA, Holmes KK. Epidemiology of genital human papillomavirus infection. Epidemiol Rev. 1988;10:122–63. doi: 10.1093/oxfordjournals.epirev.a036020. [DOI] [PubMed] [Google Scholar]
- 11.Bosch FX, Burchell AN, Schiffman M, et al. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine. 2008;26 (suppl 10):K1–16. doi: 10.1016/j.vaccine.2008.05.064. [DOI] [PubMed] [Google Scholar]
- 12.Frazer IH. Interaction of human papillomaviruses with the host immune system: a well evolved relationship. Virology. 2009;384:410–14. doi: 10.1016/j.virol.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Konya J, Dillner J. Immunity to oncogenic human papillomaviruses. Adv Cancer Res. 2001;82:205–38. doi: 10.1016/s0065-230x(01)82007-8. [DOI] [PubMed] [Google Scholar]
- 14.Carter JJ, Koutsky LA, Hughes JP, et al. Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J Infect Dis. 2000;181:1911–19. doi: 10.1086/315498. [DOI] [PubMed] [Google Scholar]
- 15.Villa LL, Ault KA, Giuliano AR, et al. Immunologic responses following administration of a vaccine targeting human papillomavirus Types 6, 11, 16, and 18. Vaccine. 2006;24:5571–83. doi: 10.1016/j.vaccine.2006.04.068. [DOI] [PubMed] [Google Scholar]
- 16.Jochmus-Kudielka I, Schneider A, Braun R, et al. Antibodies against the human papillomavirus type 16 early proteins in human sera: correlation of anti-E7 reactivity with cervical cancer. J Natl Cancer Inst. 1989;81:1698–704. doi: 10.1093/jnci/81.22.1698. [DOI] [PubMed] [Google Scholar]
- 17.Lechner F, Gruener NH, Urbani S, et al. CD8+ T lymphocyte responses are induced during acute hepatitis C virus infection but are not sustained. Eur J Immunol. 2000;30:2479–87. doi: 10.1002/1521-4141(200009)30:9<2479::AID-IMMU2479>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 18.Sester M, Sester U, Gartner BC, Girndt M, Meyerhans A, Kohler H. Dominance of virus-specific CD8 T cells in human primary cytomegalovirus infection. J Am Soc Nephrol. 2002;13:2577–84. doi: 10.1097/01.asn.0000030141.41726.52. [DOI] [PubMed] [Google Scholar]
- 19.Youde SJ, Dunbar PR, Evans EM, et al. Use of fluorogenic histocompatibility leukocyte antigen-A*0201/HPV 16 E7 peptide complexes to isolate rare human cytotoxic T-lymphocyte-recognizing endogenous human papillomavirus antigens. Cancer Res. 2000;60:365–71. [PubMed] [Google Scholar]
- 20.Ressing ME, van Driel WJ, Celis E, et al. Occasional memory cytotoxic T-cell responses of patients with human papillomavirus type 16-positive cervical lesions against a human leukocyte antigen-A *0201-restricted E7-encoded epitope. Cancer Res. 1996;56:582–88. [PubMed] [Google Scholar]
- 21.Kadish AS, Ho GY, Burk RD, et al. Lymphoproliferative responses to human papillomavirus (HPV) type 16 proteins E6 and E7: outcome of HPV infection and associated neoplasia. J Natl Cancer Inst. 1997;89:1285–93. doi: 10.1093/jnci/89.17.1285. [DOI] [PubMed] [Google Scholar]
- 22.Kadish AS, Timmins P, Wang Y, et al. Regression of cervical intraepithelial neoplasia and loss of human papillomavirus (HPV) infection is associated with cell-mediated immune responses to an HPV type 16 E7 peptide. Cancer Epidemiol Biomarkers Prev. 2002;11:483–88. [PubMed] [Google Scholar]
- 23.Nimako M, Fiander AN, Wilkinson GW, Borysiewicz LK, Man S. Human papillomavirus-specific cytotoxic T lymphocytes in patients with cervical intraepithelial neoplasia grade III. Cancer Res. 1997;57:4855–61. [PubMed] [Google Scholar]
- 24.van der Burg SH, Ressing ME, Kwappenberg KM, et al. Natural T-helper immunity against human papillomavirus type 16 (HPV16) E7-derived peptide epitopes in patients with HPV16-positive cervical lesions: identification of 3 human leukocyte antigen class II-restricted epitopes. Int J Cancer. 2001;91:612–18. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1119>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 25.de Gruijl TD, Bontkes HJ, Walboomers JM, et al. Differential T helper cell responses to human papillomavirus type 16 E7 related to viral clearance or persistence in patients with cervical neoplasia: a longitudinal study. Cancer Res. 1998;58:1700–06. [PubMed] [Google Scholar]
- 26.Bontkes HJ, de Gruijl TD, van den Muysenberg AJ, et al. Human papillomavirus type 16 E6/E7-specific cytotoxic T lymphocytes in women with cervical neoplasia. Int J Cancer. 2000;88:92–98. [PubMed] [Google Scholar]
- 27.Pett M, Coleman N. Integration of high-risk human papillomavirus: a key event in cervical carcinogenesis? J Pathol. 2007;212:356–67. doi: 10.1002/path.2192. [DOI] [PubMed] [Google Scholar]
- 28.Melnikow J, Nuovo J, Willan AR, Chan BK, Howell LP. Natural history of cervical squamous intraepithelial lesions: a meta-analysis. Obstet Gynecol. 1998;92 (4 Pt 2):727–35. doi: 10.1016/s0029-7844(98)00245-2. [DOI] [PubMed] [Google Scholar]
- 29.Trimble CL, Piantadosi S, Gravitt P, et al. Spontaneous regression of high-grade cervical dysplasia: effects of human papillomavirus type and HLA phenotype. Clin Cancer Res. 2005;11:4717–23. doi: 10.1158/1078-0432.CCR-04-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schlecht NF, Platt RW, Duarte-Franco E, et al. Human papillomavirus infection and time to progression and regression of cervical intraepithelial neoplasia. J Natl Cancer Inst. 2003;95:1336–43. doi: 10.1093/jnci/djg037. [DOI] [PubMed] [Google Scholar]
- 31.Heard I, Tassie JM, Kazatchkine MD, Orth G. Highly active antiretroviral therapy enhances regression of cervical intraepithelial neoplasia in HIV-seropositive women. Aids. 2002;16:1799–802. doi: 10.1097/00002030-200209060-00013. [DOI] [PubMed] [Google Scholar]
- 32.van Seters M, van Beurden M, de Craen AJ. Is the assumed natural history of vulvar intraepithelial neoplasia III based on enough evidence? A systematic review of 3322 published patients. Gynecol Oncol. 2005;97:645–51. doi: 10.1016/j.ygyno.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 33.Chiao EY. Duration of anal human papillomavirus infection among immunocompetent women: clues to anal cancer epidemiology and possible prevention strategies. Clin Infect Dis. 2009;48:547–49. doi: 10.1086/596759. [DOI] [PubMed] [Google Scholar]
- 34.de Jong A, O’Neill T, Khan AY, et al. Enhancement of human papillomavirus (HPV) type 16 E6 and E7-specific T-cell immunity in healthy volunteers through vaccination with TA-CIN, an HPV16 L2E7E6 fusion protein vaccine. Vaccine. 2002;20:3456–64. doi: 10.1016/s0264-410x(02)00350-x. [DOI] [PubMed] [Google Scholar]
- 35.Goldstone SE, Palefsky JM, Winnett MT, Neefe JR. Activity of HspE7, a novel immunotherapy, in patients with anogenital warts. Dis Colon Rectum. 2002;45:502–07. doi: 10.1007/s10350-004-6229-6. [DOI] [PubMed] [Google Scholar]
- 36.Klencke B, Matijevic M, Urban RG, et al. Encapsulated plasmid DNA treatment for human papillomavirus 16-associated anal dysplasia: a phase I study of ZYC101. Clin Cancer Res. 2002;8:1028–37. [PubMed] [Google Scholar]
- 37.Sheets EE, Urban RG, Crum CP, et al. Immunotherapy of human cervical high-grade cervical intraepithelial neoplasia with microparticle-delivered human papillomavirus 16 E7 plasmid DNA. Am J Obstet Gynecol. 2003;188:916–26. doi: 10.1067/mob.2003.256. [DOI] [PubMed] [Google Scholar]
- 38.Garcia F, Petry KU, Muderspach L, et al. ZYC101a for treatment of high-grade cervical intraepithelial neoplasia: a randomized controlled trial. Obstet Gynecol. 2004;103:317–26. doi: 10.1097/01.AOG.0000110246.93627.17. [DOI] [PubMed] [Google Scholar]
- 39.Frazer IH. Cancer Vaccine Collaborative. Vol. 2003. New York, New York, USA: 2002 6, Feb 6, Immunotherapy for HPV associated pre-cancer; p. 25. [Google Scholar]
- 40.Frazer IH, Quinn M, Nicklin JL, et al. Phase 1 study of HPV16-specific immunotherapy with E6E7 fusion protein and ISCOMATRIX adjuvant in women with cervical intraepithelial neoplasia. Vaccine. 2004;23:172–81. doi: 10.1016/j.vaccine.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 41.Borysiewicz LK, Fiander A, Nimako M, et al. A recombinant vaccinia virus encoding human papillomavirus types 16 and 18, E6 and E7 proteins as immunotherapy for cervical cancer. Lancet. 1996;347:1523–27. doi: 10.1016/s0140-6736(96)90674-1. [DOI] [PubMed] [Google Scholar]
- 42.Davidson EJ, Boswell CM, Sehr P, et al. Immunological and clinical responses in women with vulval intraepithelial neoplasia vaccinated with a vaccinia virus encoding human papillomavirus 16/18 oncoproteins. Cancer Res. 2003;63:6032–41. [PubMed] [Google Scholar]
- 43.Baldwin PJ, van der Burg SH, Boswell CM, et al. Vaccinia-expressed human papillomavirus 16 and 18 e6 and e7 as a therapeutic vaccination for vulval and vaginal intraepithelial neoplasia. Clin Cancer Res. 2003;9:5205–13. [PubMed] [Google Scholar]
- 44.van Driel WJ, Ressing ME, Kenter GG, et al. Vaccination with HPV16 peptides of patients with advanced cervical carcinoma: clinical evaluation of a phase I–II trial. Eur J Cancer. 1999;35:946–52. doi: 10.1016/s0959-8049(99)00048-9. [DOI] [PubMed] [Google Scholar]
- 45.Frazer I. Immunology of HPV infection. In: Tindle R, editor. Vaccines for human papillomavirus infection and anogenital disease. Austin, TX: RG Landes Company; 1999. pp. 13–31. [Google Scholar]
- 46.Muderspach L, Wilczynski S, Roman L, et al. A phase I trial of a human papillomavirus (HPV) peptide vaccine for women with high-grade cervical and vulvar intraepithelial neoplasia who are HPV 16 positive. Clin Cancer Res. 2000;6:3406–16. [PubMed] [Google Scholar]
- 47.Zhang LF, Zhou J, Chen S, et al. HPV6b virus like particles are potent immunogens without adjuvant in man. Vaccine. 2000;18:1051–58. doi: 10.1016/s0264-410x(99)00351-5. [DOI] [PubMed] [Google Scholar]
- 48.Ferrara A, Nonn M, Sehr P, et al. Dendritic cell-based tumor vaccine for cervical cancer II: results of a clinical pilot study in 15 individual patients. J Cancer Res Clin Oncol. 2003;129:521–30. doi: 10.1007/s00432-003-0463-5. [DOI] [PubMed] [Google Scholar]
- 49.Kaufmann AM, Nieland JD, Jochmus I, et al. Vaccination trial with HPV16 L1E7 chimeric virus-like particles in women suffering from high grade cervical intraepithelial neoplasia (CIN 2/3) Int J Cancer. 2007;121:2794–800. doi: 10.1002/ijc.23022. [DOI] [PubMed] [Google Scholar]
- 50.Kenter GG, Welters MJ, Valentijn AR, et al. Phase I immunotherapeutic trial with long peptides spanning the E6 and E7 sequences of high-risk human papillomavirus 16 in end-stage cervical cancer patients shows low toxicity and robust immunogenicity. Clin Cancer Res. 2008;14:169–77. doi: 10.1158/1078-0432.CCR-07-1881. [DOI] [PubMed] [Google Scholar]
- 51.Trimble CL, Peng S, Kos F, et al. A phase I trial of a human papillomavirus DNA vaccine for HPV16+ cervical intraepithelial neoplasia 2/3. Clin Cancer Res. 2009;15:361–67. doi: 10.1158/1078-0432.CCR-08-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brady CS, Bartholomew JS, Burt DJ, et al. Multiple mechanisms underlie HLA dysregulation in cervical cancer. Tissue Antigens. 2000;55:401–11. doi: 10.1034/j.1399-0039.2000.550502.x. [DOI] [PubMed] [Google Scholar]
- 53.Evans M, Borysiewicz LK, Evans AS, et al. Antigen processing defects in cervical carcinomas limit the presentation of a CTL epitope from human papillomavirus 16 E6. J Immunol. 2001;167:5420–28. doi: 10.4049/jimmunol.167.9.5420. [DOI] [PubMed] [Google Scholar]
- 54.van Seters M, van Beurden M, ten Kate FJ, et al. Treatment of vulvar intraepithelial neoplasia with topical imiquimod. N Engl J Med. 2008;358:1465–73. doi: 10.1056/NEJMoa072685. [DOI] [PubMed] [Google Scholar]
- 55.Winters U, Daayana S, Lear JT, et al. Clinical and immunologic results of a phase II trial of sequential imiquimod and photodynamic therapy for vulval intraepithelial neoplasia. Clin Cancer Res. 2008;14:5292–99. doi: 10.1158/1078-0432.CCR-07-4760. [DOI] [PubMed] [Google Scholar]
- 56.Campo MS, Moar MH, Sartirana ML, Kennedy IM, Jarrett WF. The presence of bovine papillomavirus type 4 DNA is not required for the progression to, or the maintenance of, the malignant state in cancers of the alimentary canal in cattle. Embo J. 1985;4:1819–25. doi: 10.1002/j.1460-2075.1985.tb03856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nasir L, Reid SW. Bovine papillomaviral gene expression in equine sarcoid tumours. Virus Res. 1999;61:171–75. doi: 10.1016/s0168-1702(99)00022-2. [DOI] [PubMed] [Google Scholar]
- 58.Jain S, Moore RA, Anderson DM, Gough GW, Stanley MA. Cell-mediated immune responses to COPV early proteins. Virology. 2006;356:23–34. doi: 10.1016/j.virol.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 59.Brandsma JL, Shlyankevich M, Zelterman D, Su Y. Therapeutic vaccination of rabbits with a ubiquitin-fused papillomavirus E1, E2, E6 and E7 DNA vaccine. Vaccine. 2007;25:6158–63. doi: 10.1016/j.vaccine.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frazer IH. Prevention of cervical cancer through papillomavirus vaccination. Nat Rev Immunol. 2004;4:46–54. doi: 10.1038/nri1260. [DOI] [PubMed] [Google Scholar]
- 61.Kanodia S, Da Silva DM, Kast WM. Recent advances in strategies for immunotherapy of human papillomavirus-induced lesions. Int J Cancer. 2008;122:247–59. doi: 10.1002/ijc.23252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Riley RR, Duensing S, Brake T, Munger K, Lambert PF, Arbeit JM. Dissection of human papillomavirus E6 and E7 function in transgenic mouse models of cervical carcinogenesis. Cancer Res. 2003;63:4862–71. [PubMed] [Google Scholar]
- 63.Frazer IH, Fernando GJ, Fowler N, et al. Split tolerance to a viral antigen expressed in thymic epithelium and keratinocytes. Eur J Immunol. 1998;28:2791–800. doi: 10.1002/(SICI)1521-4141(199809)28:09<2791::AID-IMMU2791>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 64.Matsumoto K, Leggatt GR, Zhong J, et al. Impaired antigen presentation and effectiveness of combined active/passive immunotherapy for epithelial tumors. J Natl Cancer Inst. 2004;96:1611–19. doi: 10.1093/jnci/djh301. [DOI] [PubMed] [Google Scholar]
- 65.Zhong J, Hadis U, De Kluyver R, Leggatt GR, Fernando GJ, Frazer IH. TLR7 stimulation augments T effector-mediated rejection of skin expressing neo-self antigen in keratinocytes. Eur J Immunol. 2008;38:73–81. doi: 10.1002/eji.200737599. [DOI] [PubMed] [Google Scholar]
- 66.Dunn LA, Evander M, Tindle RW, et al. Presentation of the HPV16E7 protein by skin grafts is insufficient to allow graft rejection in an E7-primed animal. Virology. 1997;235:94–103. doi: 10.1006/viro.1997.8650. [DOI] [PubMed] [Google Scholar]
- 67.Kozlowski PA, Williams SB, Lynch RM, et al. Differential induction of mucosal and systemic antibody responses in women after nasal, rectal, or vaginal immunization: influence of the menstrual cycle. J Immunol. 2002;169:566–74. doi: 10.4049/jimmunol.169.1.566. [DOI] [PubMed] [Google Scholar]