Abstract
One of the factors proposed as mediators of vascular dysfunction observed in diabetes is the increased generation of reactive oxygen species (ROS). This provides support for the use of antioxidants as early and appropriate pharmacological intervention in the development of late diabetic complications. In streptozotocin (STZ)-induced diabetes in rats we observed endothelial dysfuction manifested by reduced endothelium-dependent response to acetylcholine of the superior mesenteric artery (SMA) and aorta, as well as by increased endothelaemia. Changes in endothelium-dependent relaxation of SMA were induced by injury of the nitric oxide radical (·NO)-signalling pathway since the endothelium-derived hyperpolarising factor (EDHF)-component of relaxation was not impaired by diabetes. The endothelial dysfunction was accompanied by decreased ·NO bioavailabity as a consequence of reduced activity of eNOS rather than its reduced expression. The results obtained using the chemiluminiscence method (CL) argue for increased oxidative stress and increased ROS production. The enzyme NAD(P)H-oxidase problably participates in ROS production in the later phases of diabetes. Oxidative stress was also connected with decreased levels of reduced glutathione (GSH) in the early phase of diabetes. After 10 weeks of diabetes, adaptational mechanisms probably took place because GSH levels were not changed compared to controls. Antioxidant properties of SMe1EC2 found in vitro were partly confirmed in vivo. Administration of SMe1EC2 protected endothelial function. It significantly decreased endothelaemia of diabetic rats and improved endothelium-dependent relaxation of arteries, slightly decreased ROS-production and increased bioavailability of ·NO in the aorta. Further studies with higher doses of SMe1EC2 may clarify the mechanism of its endothelium-protective effect in vivo.
Keywords: diabetes, ischaemia/reperfusion, pyridoindole antioxidans, SMe1EC2
Introduction
In the last decades, scientific research has revealed that deterioration of the endothelium with subsequent damage of smooth muscle reactivity results in generalised increase in vascular tone, platelet aggregation, thrombus formation, etc. The endothelium plays an important role in maintaining vascular homeostasis by synthesising and releasing several vasodilators, including prostacyclin, nitric oxide radical (·NO), and endothelium-derived hyperpolarising factor (EDHF) (Vanhoutte and Mombouli, 1996; Shimokawa, 1999). Endothelial dysfunction participates in the pathophysiology of various diseases like atherosclerosis, essential hypertension, diseases connected with ischaemia/reperfusion (I/R), rheumatoid arthritis, diabetic micro- and macrovasculopathies, etc. Among the mechanisms of the vascular dysfunction observed in these diseases, increased generation of reactive oxygen species (ROS) is emerging as a crucial factor. This provides support for the use of antioxidants in preventing or alleviating the above mentioned diseases.
In the last decades, more than 70 new derivatives (Štolc et al., 2010) of the pyridoindole antioxidant stobadine – (–)-cis-2,8-dimethyl-2,3,4,4a,5,9b-hexahydro-1H-pyrido[4,3-b]indole, (Štolc et al., 1983; Horáková and Štolc, 1998) were synthesised and tested in the Institute of Experimental Pharmacology and Toxicology, Slovak Academy of Sciences. The main reason for synthesising new derivatives was to enlarge the antioxidative and antiradical capacity of the parent substance and to eliminate the undesired α1-adrenolytic activity of stobadine (Štolc et al., 2006). SMe1EC2, one of these new compounds, exhibited an increase in its antioxidative activity achieved by substitution of the methyl group in position 8 of stobadine by a methoxy group. This is explained by the increased ability of the aromatic circle to delocalise electron density from the stable nitrogen radical on indole. Substitution of nitrogen on the piperidine circle by an ethoxycarbonyl group prevented protonisation of this atom and led to loss of undesirable α1-adrenolytic activity and to decreased toxicity of SMe1EC2 (Štolc et al., 2010; Štolc et al., 2006; Májeková et al., 2006, Ujházy et al., 2008). This was the reason why we selected this compound to study the possibility to improve endothelial dysfunction in several experimental models.
In our previous works, we documented endothelial dysfunction induced by different pathological situations in experimental models of ischaemia/reperfusion (I/R) (Sotníková et al., 1998; Nosálová et al., 2004; Szöcs, 2004; Sotníková et al., 2005; Nosálová et al., 2007), adjuvant arthrithis (Sotníková et al., 2009), in spontaneously hypertensive rats in conditions of social stress (Sotníková et al., 2006, 2007), in hypertriglyceridaemic rats (Sotníková et al., 2008), etc. The common feature proved to be impaired endothelium-dependent relaxation of the arteries manifested by decreased response to acetylcholine. In the model of I/R of the abdominal aorta, we demonstrated I/R-induced reversible ultrastructural changes both in endothelial and smooth muscle cells, predominantly in the mitochondria. Participation of ROS in vascular injury was confirmed directly on using oxidative fluorescent microtopography (Szöcs et al., 2003), by observation of increased lipid peroxidation in homogenates of I/R aortae and by the ability of the antioxidant stobadine to protect the abdominal aorta against I/R-induced changes (Sotníková et al., 1998). Further, decreased release of NOx and increased activity of lysosomal enzymes – N-acetyl-β-D-glucosaminidase (NAGA) and acid phosphatase (APH) – in aortic homogenates were observed (Sotníková et al., 1999). Lysosomes consist of a lipoprotein membrane surrounding potent acid hydrolases able to degrade all known constituents of cells. Leakage of lysosomal enzymes into cells and the surrounding extracellular space has been implicated in the pathogenesis of tissue injury (Waters et al., 1992). Pretreatment of rats with stobadine reduced the NAGA and APH release evoked by I/R (Sotníková et al., 1997, Navarová et al., 1998).
The use of the model of mesenteric I/R in rats provided evidence of ROS contribution to I/R-induced endothelial dysfunction. By the method of luminol enhanced chemiluminiscence (CL) we demonstrated increased generation of ROS in SMA tissue after 60-min ischaemia followed by 30-min reperfusion of the rat mesentery, which resulted in injury of both the ·NO and EDHF-endothelial relaxing systems of the SMA (Sotníková et al., 2005).
Endothelial dysfunction in experimental diabetes
Although the aetiology of vascular disorders in long lasting diabetes is not fully understood, it has been suggested that abnormal vascular function may underlie many of the associated vasculopathies. These are major causes of disability and death in patients with diabetes mellitus (Yan et al., 2003). Under physiological conditions, glucose is transported into the cells by means of glucose transporters. Transport of glucose to the endothelium and smooth muscle cells is not insulin-dependent, thus in the case of hyperglycaemia intracellular concentration of glucose in these cells increases and can induce their injury.
Reduction in endothelium-dependent relaxation is a common feature known to occur in both conduit and resistance arteries of experimental diabetic animals. Moreover, increased responsiveness of vessels to contractile agents in diabetes mellitus was also reported. Among the proposed mechanisms of vascular dysfunction in diabetes, the increased generation of ROS is emerging as a crucial factor. The above facts provide support for the use of antioxidants in prevention of diabetic complications (Karasu et al., 1997, Afanasjev, 2010).
In our previous experiments, we found impaired endothelial function induced by long-lasting streptozotocin (STZ)-induced diabetes in rats (Sotníková et al., 1999a). Dietary supplementation of the pyridoindole antioxidant stobadine reduced this impairment (Sotníková et al., 2001). The long-lasting hyperglycaemia had a detrimental yet reversible effect on the structure of the aorta, which was reduced by administration of antioxidants (Okruhlicová et al., 2001).
The experimental model of STZ-induced diabetes was used to study the ability of the pyridoindole antioxidant SMe1EC2 to prevent or delay the onset of diabetic vasculopathies. Diabetes was induced by STZ (30 mg/kg i.p.) administered for three consecutive days to male rats. Administration of repeated low doses of STZ is connected with an autoimmune process, insulitis and slow death of pancreatic beta-cells, a process mimicking human diabetes type 1 (Lukic et al., 1991). Changes in vascular function (the aorta and SMA) and of markers of endothelial injury were studied after 5 and 10 weeks from the last dose of STZ.
The first changes in vascular endothelial function could be observed 5 weeks after induction of diabetes and were demonstrated by increased endothelaemia (Table 1) and diminished endothelium-dependent relaxation of SMA to acetylcholine (Figure 1a). After prolonging the duration of diabetes to 10 weeks, the endothelial injury deepened and was observed also in the aorta (Zúrová et al. 2006).
Table 1.
Effect of SMe1EC2 on endothelaemia, on basal chemiluminiscence and on NOx and GSH levels in the aorta after 5 weeks of diabetes.
| Group | Endothelaemia | CL | NOx | GSH |
|---|---|---|---|---|
| C | 2.29 ± 0.29 | 0.573±0.13 | 108.82±11.21 | 4.34±0.70 |
| D | 3.71 ± 0.25* | 1.153±0.18 * | 61.90±14.42* | 1.70±0.66* |
| D – SMe1EC2 | 2.05 ± 0.51+ | 0.925±0.12 | 89.85±5.02 | 2.78±0.62 |
C – control group, D – diabetic group, D-SMe1EC2 – diabetic rats treateted with SMe1EC2. Endothelaemia is expressed in the cell number/10µl blood, chemiluminiscence (CL) as AUC (area under the curve) in mV/min/mg aortic tissue, NOx levels in nmol/mg protein, levels of reduced glutathione (GSH) in µg/mg protein. Results are means ± S.E.M., n=5–9,* p<0.05 versus control. + p<0.05 versus diabetes
Figure 1.
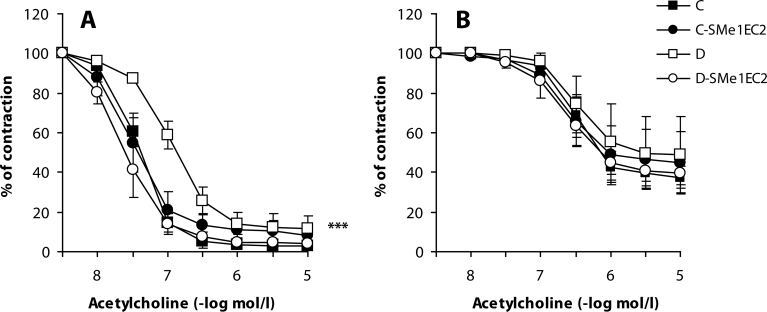
Effect of SMe1EC2 on endothelium-dependent relaxation of the superior mesenteric artery (SMA) ex vivo. Acetylcholine-induced responses of the phenylephrine (1µmol/l)-precontracted rings of SMA from control rats (C), control rats treated with 10mg/kg SMe1EC2 (C-SMe1EC2), diabetic rats (D), diabetic rats treated with SMe1EC2 (D-SMe1EC2). A – before blockade of eNOS with L-NAME and of prostaglandin synthesis with indomethacin; B – after blockade of eNOS with L-NAME and of prostaglandin synthesis with indomethacin. Data are means±S.E.M. of 8 experiments. ***p<0.001 versus C.
The reduced endothelium-dependent response can be mediated by several mechanisms: decreased bioavailability of ·NO, impaired diffusion of ·NO to smooth muscle cells, their decreased sensitivity or increased production of constrictory factors produced by the endothelium (Dai et al., 1993). In our experiments we demonstrated decreased ·NO concentration in the aorta after 5 weeks of diabetes (Table 1) accompanied by reduced response of SMA to acetylcholine. To find which endothelial factor was responsible for the reduction of acetylcholine-induced relaxation, we incubated arterial prepartions with the endothelial NO synthase (eNOS) inhibitor L-NG-nitroarginine methyl ester (L-NAME) and with the cyclooxygenase inhibitor indomethacin to obtain EDHF-dependent relaxation. In contrast to the results of Wigg et al. (2001), we did not find any changes in acetylcholine-induced relaxation after blockade of the ·NO-signalling pathway and prostaglandin synthesis (Figure 1b). This may be explained by the fact that those authors studied smaller branches of SMA where EDHF plays a more important role.
Diabetes-induced changes in ·NO-availability can be caused by changed production of ·NO. Adequate ·NO production depends on intact function of eNOS, with sufficiency of cofactors and substrates. Depressed production of ·NO can be the result of either decreased eNOS expression or its activity. The enzyme eNOS is localised in caveolae where it is coupled with caveolin-1, a22kDa protein, which is a physiological inhibitor of eNOS. After increasing intracellular calcium concentrations in endothelial cells, the complex caveolin-1 with eNOS breaks and eNOS is activated (Garcia-Cardena et al., 1997; Bucci et al., 2000). Using Western blot analysis, we did not find changes in eNOS expression, however expression of caveolin-1 was increased appr. to 140% of controls (p<0.05). We suggest that decreased ·NO bioavalability is not a consequence of reduced eNOS expression but of its activity caused by increased production of caveolin-1. These findings are in a good agreement with results of Bucci et al. (2004).
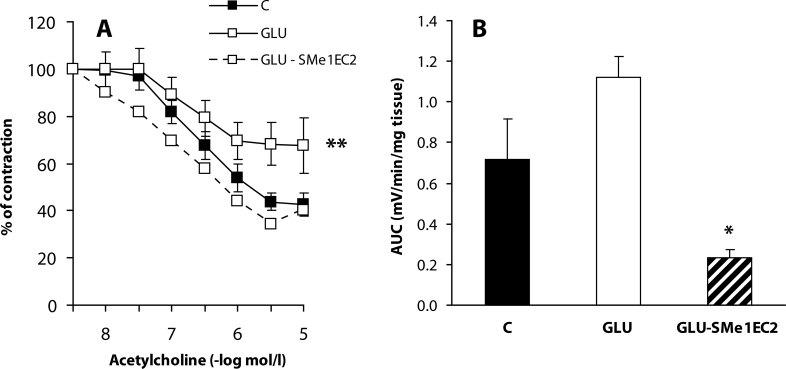
Besides reduced ·NO production, increased inactivation of ·NO by ROS may participate in decreased ·NO bioavailabity. In conditions of oxidative stress, ·NO quickly reacts with superoxide creating peroxinitrite, which is cytotoxic for endothelial cells and oxidises tetrahydrobiopterin, an essential cofactor of eNOS. Consecutively, eNOS is uncoupled and preferentially produces superoxide instead of ·NO (Maritim et al., 2003). Moreover, peroxinitrite is also metabolised to a highly reactive hydroxyl radical (Beckman et al., 1990), which contributes to diabetes-induced endothelial dysfunction (Dai et al., 1993). Long-lasting heperglycaemia is the most important factor responsible for endothelial dysfunction in diabetes. Cosentino et al. (2003) observed reduced levels of NOx caused by incubation of endothelial cells in solutions containing high concentrations of glucose. Similarly in our in vitro experiments, incubation of the aorta in the solution containing high concentration of glucose resulted in reduced endothelium-dependent relaxation (Figure 2a). We suggest that this is connected with oxidative stress since on using CL we found increased ROS production in these aortic preparations (Figure 2b).
Figure 2.
Effect of SMe1EC2 on endothelial dysfunction induced by a high concentration of glucose in vitro. C – control preparations incubated in Krebs solution, GLU – preparations incubated in Krebs solution containing 44 mmol/l glucose, GLU-SMe1EC2 – preparations incubated in Krebs solution containing 44 mmol/l glucose and 10 µmol/l SMe1EC2. A – responses of phenylephrine (1 µmol/l)-precontracted rings of the aorta to acetylcholine; B – chemiluminiscence expressed as AUC (area under the curve) in mV/min/mg of wet aortic tissue. Data are means±S.E.M. of 6 experiments. *p<0.05, **p<0.01 versus C.
One of the sources of ROS production is activation of proteinkinase C (PKC), which activates NAD(P)H-oxidase producing high amounts of superoxide in vessels (Cosentino et al., 2003). NAD(P)H-oxidase is a dominant source of superoxide in the vessels. It is a multicomponent enzyme found in phagocytes, endothelial and smooth muscle cells and in fibroblasts of vascular adventitia. It consists of the main catalytic subunit gp91phox (in vessels nox2) and of regulatory subunists p22phox, p47phox, p67phox, p40phox and GTPase Rac2. The subunits gp91phox and p22phox supply the transport of electrons from NAD(P)H to O2. Homologues of gp91phox were idenitified in vessels and they are termed nox1-5. Up to 45–56% they are identical with gp91phox (Babior, 1999). In our experiments, we found increased ROS production already after 5 weeks of diabetes (Table 1) but no changes in mRNA expression of subunits nox1 and nox4. One of the explanations could be that in the early phases of diabetes enzymatic NAD(P)H-oxidase activity is increased by accelerated association of individual components without increased expression of catalytic subunits. However Wendt et al. (2005) observed increased activity simultaneously with increased nox1 expression after 8 weeks of diabetes. Another explanation could be that in the early phases of diabetes an increased production of superoxide in vessels may be the cosequence of increased expression of nox2 (Hink et al., 2001). It thus seems that individual catalytic subunits of NAD(P)H-oxidase are exprimed at different time intervals, in different types of cells and they may also possess different functions. While nox2 subunit is responsible for ROS production in endothelial cells, nox1 subunit is a source of ROS in vascular smooth muscle cells. Angiotensin II increases nox1 expression, yet it has no effet on nox4 subunit (Mollnau et al., 2002). On the other hand, TNF-α increases nox4 subunit and does not influence nox1 expression (Moe et al., 2006). In our experiments, we observed increased nox4 expression when diabetes lasted a very long time, i.e. 5 months (p<0.05), while nox1 expression was not changed. Moreover, nox4 expression was many times higher than nox1 expression. We suggest that under conditions of our experimental model, nox4 plays a more important role.
Oxidative stress can result also from reduced activity of antioxidative systems. We observed decreased levels of reduced glutathione (GSH) after 5 weeks of diabetes (from control values of 5.34±0.70 to 2.70±0.76 µg/mg protein, p<0.05) which is in agreement with results of Tachi et al. (2001) and Yue et al. (2003). Changes in GSH levels probably result from increased ROS production induced by hyperglycaemia, which is in accordance with the observation of GSH depletion in vascular cells incubated with a high concentration of glucose (Urata et al., 1996). On the other hand, after 10 weeks of diabetes GSH concentration in the aorta was not changed (Zúrová-Nedelčevová et al., 2006). We propose that unchanged GSH levels in the aorta may reflect adaptation of the organism to chronic oxidative stress in the later phase of diabetes.
Effect of SMe1EC2
On using the described experimental model, an endothelium-protective effect of the antioxidant stobadine was observed. This finding confirmed the assumption of the important role of ROS in aetiopathology of diabetes-induced endothelial dysfunction (Sotníková et al., 2006a).
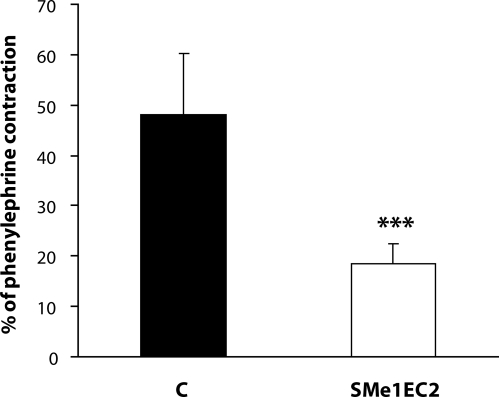
In the same model of STZ-diabetes, we further studied the effect of the stobadine derivative SMe1EC2 administered in the dose of 10 mg/kg every second day. A beneficial effect of SMe1EC2 on the vascular endothelium was found in our in vitro experiments, in which the aortal rings were incubated in a solution with a high concentration of glucose. The decreased endothelium-dependent relaxation was restored by this antioxidant (Figure 2). Searching for possible mechanisms of the SMe1EC2 protective effect, we found that it had no effect on basal tone of the arterial preparations or on contraction induced by depolarisation. It did not influence the concentration-response curves of phenylephrine or serotonin, indicating no effect on α1-adrenergic or 5-hydroxytryptamine receptors. Moreover, SMe1EC2 did not affect responses of the isolated aorta to the M3-agonist acetylcholine. Thus we propose that the endothelium-protective effect of SMe1EC2 is mediated by its antioxidant activity found also in the 1,1-diphenyl-2-picrylhydrazyl radical – (DPPH) test (Nosálová et al., 2010). With pD2 value of 5.67, SMe1EC2 exhibited a DPPH radical-scavenging activity higher than did stobadine. Indeed, under in vitro conditions, this compound suppressed production of ROS evoked by mesenteric I/R (Nosálová et al., 2010) and significantly reduced contraction of the aorta induced by ROS released from activated neutrophils (Bauer et al., 2008) (Figure 3).
Figure 3.
Effect of SMe1EC2 on the contraction induced by activated neutrophils in vitro. Responses of phenylephrine (1 µmol/l)-precontracted rings of the rat aorta to FMLP (N-formyl-methionyl-leucyl-phenylalanine, 10−7 mol/l)-activated neutrophils (106/ml). C – control rings, SMe1EC2 – rings pretreated with 10µmol/l SMe1EC2. Data are means±S.E.M. of 12 experiments. ***p<0.001 versus C.
We further found that in vivo administration of 10 mg/kg SMe1EC2 protected endothelial function. It significantly decreased endothelaemia of diabetic rats (Table 1) and improved endothelium-dependent relaxation of the aorta (Zúrová-Nedelčevová et al., 2006) and SMA without effect on the EDHF component (Figure 1). Therefore we assumed that this compound affected the ·NO-signalling pathway. Administration of SMe1EC2 slightly increased biological availability of ·NO in the aorta (Table 1), but it did not influence expression of eNOS or of caveolin-1. One of the explanations is that by its antioxidant properties SMe1EC2 inactivated ROS and thus increased ·NO bioavalability. This assumption was based also on the results of our in vitro experiments in which this compound intensively reduced CL increased by high glucose incubation and simultaneously protected endothelium-dependent relaxation of the aorta (Figure 2). In in vivo conditions, we observed a tendency of SMe1EC2 to decrease spontaneous ROS production and to increase levels of GSH in the aorta of diabetic rats (Table 1). These results may indicate that the dose of 10 mg/kg SMe1EC2 was not sufficient to observe all antioxidant properties in vivo. Further studies with higher doses of SMe1EC2 may clarify the mechanism of its endothelium-protective effect in STZ-diabetes in rats.
Acknowledgement
The authors would like to thank Dr. M. Kouřilová for correcting the English and for her critical reading of the manuscript. The authors thank Mrs. Dytrichová, Kollárová, Puškášová, Srnová, and Stojkovičová for their excellent technical assistance. This work was supported by grants VEGA No. 2/0050/09, 2/0086/08 and APVV-51-017905.
REFERENCES
- Afanasjev I. Signaling of reactive oxygen and nitrogen species in Diabetes mellitus. Oxid Med Cell Longev. 2010;3:361–373. doi: 10.4161/oxim.3.6.14415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior BM. NADPH-oxidase.An update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- Bauer V, Sotníková R, Gergel D. Comparison of the effects of activated neutrophils with the action of reactive oxygen species (ROS) on rat aortic smooth muscle (RASM) Prague Med Report. 2008;109:13–14. [Google Scholar]
- Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implication for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA. 1990;87:1620–1324. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci M, Gratton JP, Rudic RD, Acevedo L, Roviezzo F, Cirino G, Sessa WC. In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nat Med. 2000;6:1362–1367. doi: 10.1038/82176. [DOI] [PubMed] [Google Scholar]
- Bucci M, Roviezzo F, Brancaleone V, Lin MI, Di Lorenzo A, Cicala C, Pinto A, Sessa WC, Farneti S, Fiorucci S, Cirino G. Diabetic mouse angiopathy is linked to progressive sympathetic receptor deletion coupled to an enhanced caveolin-1 expression. Atheroscler Thromb Vasc Biol. 2004;24:721–726. doi: 10.1161/01.ATV.0000122362.44628.09. [DOI] [PubMed] [Google Scholar]
- Cosentino F, Eto M, De Paolis P, van der Loo B, Bachschmid M, Ullrich V, Kuoroedov A, Gatti CD, Joch H, Volpe M, Lüscher TF. High glucose causes upregulation of cyclooxygenase-2 and alters prostanoid profile in human endothelial cells. Circulation. 2003;107:1017–1023. doi: 10.1161/01.cir.0000051367.92927.07. [DOI] [PubMed] [Google Scholar]
- Dai FX, Diederich A, Skopec J, Diederich D. Diabetes-induced endothelial dysfunction in styreptozotocin-treated rats: role of prostaglandin endoperoxides and free radicals. J Am Soc Nephrol. 1993;4:1327–1336. doi: 10.1681/ASN.V461327. [DOI] [PubMed] [Google Scholar]
- Garcia-Cardena G, Martasek P, Masters BS, Skidd PM, Couet J, Li S, Lisanti MP, Sessa WC. Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the NOS caveolin binding domain in vivo. J Biol Chem. 1997;272:25437–25440. doi: 10.1074/jbc.272.41.25437. [DOI] [PubMed] [Google Scholar]
- Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, Skatchov M, Thaiss F, Stahl RAK, Warnholtz A, Meinertz T, Griedling K, Harrison DG, Forstermann U, Munzel T. Mechanism underlying endothelial dysfunction in diabetes mellitus. Circ Res. 2001;88:14–22. doi: 10.1161/01.res.88.2.e14. [DOI] [PubMed] [Google Scholar]
- Horáková L’, Štolc S. Antioxidant and pharmacodynamic effects of pyridoindole stobadine. Gen Pharmacol. 1998;30:627–638. doi: 10.1016/s0306-3623(97)00300-5. [DOI] [PubMed] [Google Scholar]
- Karasu C, Ozansoy G, Bozkurt O, Erdoǧan D, Omeroǧlu S. Antioxidant and triglyceride-lowering effects of vitamin E associated with the prevention of abnormalities in the reactivity and morphology of aorta from streptozotocin-diabetic rats. Antioxidants in Diabetes-Induced Complications (ADIC) Study Group. Metabolism. 1997;46:872–879. doi: 10.1016/s0026-0495(97)90072-x. [DOI] [PubMed] [Google Scholar]
- Lukic ML, Ejdus L, Pravica V, Shahin A, Atolic S, Mostarica M, Liew FW, Ramic Z, Badovinac V. Immunoregulation in Health and Disease. London/New York: Academic Press; 1998. Downregulation of Th-1 mediated autoimmune pathplogy; pp. 265–278. [Google Scholar]
- Májeková M, Šnirc V, Štolc S, Májek P, Bezáková Ž, Sotníková R. The alpha1-adrenolytic and structural evaluation of new pyridoindole derivatives. Central Eur J Med. 2006;1:370–378. [Google Scholar]
- Maritim AC, Sanders RA, Watkins JB III. Diabetes, oxidative stress, and antioxidants: A review. J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- Moe KT, Aulia S, Jiang F, Chua YL, Koh TH, Wong MC, Dusting GJ. Differential upregulation of Nox homologues of NADPH oxidase by tumor necrosis factor-α in human aortic smooth muscle and embryonic kidney cells. J Cell Mol Med. 2006;10:231–239. doi: 10.1111/j.1582-4934.2006.tb00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollnau H, Wendt M, Szocs K, Lassegue B, Schulz E, Oelze M, Li H, Bodenschatz M, August M, Kleschyov AL, Tsilimingas N, Walter U, Forstermann U, Meinertz T, Griendling K, Munzel T. Effects of angiotensin II infusion on the expression and function of NAD(P)H oxidase and components of nitric oxide/cGMP signaling. Circ Res. 2002;90:58–65. doi: 10.1161/01.res.0000012569.55432.02. [DOI] [PubMed] [Google Scholar]
- Navarová J, Sotníková R, Knezl V. Effect of stobadine on lysosomal enzymes in aorta and serum of rats following ischemia and reperfusion. Českoslov Fysiol. 1998;47:158. [in Slovak] [Google Scholar]
- Nosál'ová V, Sotníková R, Drábiková K, Fialová S, Koštálová D, Banášová S, Navarová J. Chemiluminescence response induced by mesenteric ischaemia/reperfusion: effect of antioxidative compounds ex vivo. Interdisc Toxicol. 2010;3:105–108. doi: 10.2478/v10102-010-0021-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosál'ová V, Navarová J, Mihalová D, Sotníková R. Mesenteric ischemia/reperfusion-induced intestinal and vascular damage: effect of stobadine. Meth Find Exp Clin Pharmacol. 2007;29:39–45. doi: 10.1358/mf.2007.29.1.1063495. [DOI] [PubMed] [Google Scholar]
- Nosál'ová V, Sotníková R, Mihalová D, Navarová J. Gut and vessel alterations induced by mesenteric ischemia/reperfusion. Central Eur J Pub Health. 2004;12:S70–S72. [PubMed] [Google Scholar]
- Okruhlicová L, Weismann P, Sotníková R, Beňuška J, Štefek M, Gajdošík A, Mráz P. Effect of stobadine and arginine on the structure of diabetic aorta. Sborník lékařský. 2001;102:315–316. [Google Scholar]
- Shimokawa H. Primary endothelial dysfunction: atherosclerosis. J Mol Cell Cardiol. 1999;31:23–37. doi: 10.1006/jmcc.1998.0841. [DOI] [PubMed] [Google Scholar]
- Sotníková R, Bernátová I, Nedelčevová J, Nosál'ová V, Navarová J. Proceedings of Genetic and Environmental Factors in Hypertension. Bratislava: Institute of Normal and Pathological Physiology SAS; 2007. Vascular effects of ProvinolsTM in spontaneously hypertensive rats exposed to crowding stress; pp. 35–40. [Google Scholar]
- Sotníková R, Drábiková K, Beňová M, Nosál'ová V, Nosál’ R, Rajská P. Increased chemiluminiscence accompanies ischaemia/reperfusion-induced endothelial dysfunction of rat superior mesenteric artery. Biologia. 2005;60:S145–S147. [Google Scholar]
- Sotníková J, Navarová R, Dytrichová V, Kollárová M. Drug Action on Reactive Oxygen Species with Special Attention to Stobadine. Abstracts. Bratislava: Institute of Experimental Pharmacology SAS; 1997. Biochemical changes in the rat abdominal aorta induced by ischemia – reperfusion; p. 55. [Google Scholar]
- Sotníková R, Navarová J, Kukan M, Prihelová E. Abstracts from Nitric oxide – From molecular level to clinical application. Bratislava; 1999. Jun 27–29, Endothelial damage of the rat abdominal aorta induced by ischemia/reperfusion: Effect of antioxidants. 1999. [Google Scholar]
- Sotníková R, Nosál'ová V, Štolc S, Gajdošík A, Gajdošíková A. Streptozotocin diabetes-induced changes in aorta, peripheral nerves and stomach of Wistar rats. Gen Physiol Biophys. 1999a;18:155–162. [PubMed] [Google Scholar]
- Sotníková R, Okruhlicová L’, Noskovič P. Endothelial protective effect of stobadine on ischaemia-reperfusion-induced injury. Gen Physiol Biophys. 1998;17:253–264. [PubMed] [Google Scholar]
- Sotníková R, Okruhlicová L’, Dlugošová K, Bernátová I, Bezek Š, Navarová J, Tribulová N. Effect of n-3 PUFA on endothelium-dependent relaxation of the superior mesenteric artery. In: Tribulová N, Okruhlicová L’, Slezák J, editors. Proceedings of experimental approaches in basic research and diagnostics of diseases: tailoring the treatment. Bratislava: Institute for Heart Research, Slovak Academy of Sciences and VEDA Publishing House; 2008. pp. 127–134. [Google Scholar]
- Sotníková R, Poništ S, Navarová J, Mihalová D, Tomeková V, Štrosová M, Bauerová K. Effects of sesame oil in the model of adjuvant arthritis. Neuro Endocrinol Lett. 2009;30:S22–S24. [PubMed] [Google Scholar]
- Sotníková R, Skalská S, Okruhlicová L’, Navarová J, Kysel'ová Z, Zúrová J, Štefek M, Hozová R, Nosál'ová V. Changes in the function and ultrastructure of vessels in the rat model of multiple low dose streptozotocin-induced diabetes. Gen Physiol Biophys. 2006a;25:289–302. [PubMed] [Google Scholar]
- Sotníková R, Štefek M, Okruhlicová L’, Navarová J, Bauer V, Gajdošík A, Gajdošíková A. Dietary supplementation of the pyridoindole antioxidant stobadine reduces vascular impairment in streptozotocin-diabetic rats. Meth Find Exp Clin Pharmacol. 2001;23:121–129. doi: 10.1358/mf.2001.23.3.627943. [DOI] [PubMed] [Google Scholar]
- Sotníková R, Zúrová J, Bernátová I. Differences in the response of the mesenteric artery of normotensive and spontaneously hypertensive rast exposed to long-term social stress. In: Bernátová I, Kristek F, Pecháňová O, Török J, editors. Mechanisms and derangements of blood pressure rehulation. Bratislava: Comenius University; 2006. pp. 61–69. [in Slovak] [Google Scholar]
- Szöcs K, Žilka N, Sotníková R. Proceedings of the second Drobnica memorial. 2003. Nov 12–14, Detection of superoxide and analysis of functional changes in the superior mesenterica artery induced by ischemia and reperfusion. 2003, Senec. pp.36–37 [in Slovak] [Google Scholar]
- Szöcs K. Endothelial dysfunction and reactive oxygen species. Production in ischemia/reperfusion and nitrate tolerance. Gen Physiol Biophys. 2004;23:265–295. [PubMed] [Google Scholar]
- Štolc S, Bauer V, Beneš L, Tichý M. 1983. Czechoslovak Patent. AO-229067. [Google Scholar]
- Štolc S, Považanec F, Bauer V, Májeková M, Wilcox AL, Šnirc V, Račková L, Sotníková R, Štefek M, Gáspárová-Kvaltínová Z, Gajdošíková A, Mihalová D. Slovak patent registration. 2010. Pyridoindole derivatives with antioxidative properties, their preparation and use in therapeutic practice; p. 287650. [Google Scholar]
- Štolc S, Šnirc V, Májeková M, Gáspárová Z, Gajdošíková A, Štvrtina S. Development of the new group of indole-derived neuroprotective drugs affecting oxidative stress. Cell Mol Neurobiol. 2006;26:1495–1504. doi: 10.1007/s10571-006-9037-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachi Y, Okuda Y, Bannai C, Bannai S, Shinohara M, Shimpuku H, Yamashita K, Ohura K. Hyperglycemia in diabetic rats reduces the glutathione content in the aortic tissue. Life Sci. 2001;69:1039–1047. doi: 10.1016/s0024-3205(01)01183-3. [DOI] [PubMed] [Google Scholar]
- Ujházy E, Dubovický M, Ponechalová V, Navarová J, Brucknerová I, Šnirc V, Mach M. Prenatal developmental toxicity study of the pyridoindole antioxidant SMe1EC2 in rats. Neuro Endocrinol Lett. 2008;29:639–643. [PubMed] [Google Scholar]
- Urata Y, Yamamoto H, Goto S, Tsushima H, Akazawa S, Yamashita S, Nagataki S, Kondo T. Long exposure to high glucose concentration impairs the responsive expression of gamma-glutamylcysteine synthetase by interleukin-1 beta and tumor necrosis factor-alpha in mouse endothelial cells. J Biol Chem. 1996;271:15146–15152. doi: 10.1074/jbc.271.25.15146. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM, Mombouli JV. Vascular endothelium: vasoactive mediators. Prog Cardiovasc Dis. 1996;39:229–238. doi: 10.1016/s0033-0620(96)80003-x. [DOI] [PubMed] [Google Scholar]
- Waters PJ, Flyn MD, Corrall RJM, Pennock CA. Increases in plasma lysosomal enzymes in type 1 (insulin-dependent) diabetes mellitus: relationship to diabetic complications and glycaemic control. Diabetologia. 1992;35:991–995. doi: 10.1007/BF00401431. [DOI] [PubMed] [Google Scholar]
- Wendt MC, Daider A, Kleschyov AL, Mülch A, Sydow K, Schulz E, Chen K, Keaney JF, Lassegue B, Walter U, Griendling KK, Münzel T. Differential effect of diabetes on the expression of the gp91phox homologues nox1 and nox4. Free Radic Biol Med. 2005;39:381–391. doi: 10.1016/j.freeradbiomed.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Wigg SJ, Tare M, Tonta MA, O‘Brien RC, Meredith IT, Parkington HC. Comparison pf effects of diabetes mellitus on an EDHF-dependent and an EDHF-independent artery. Am J Physiol Heart Circ Physiol. 2001;281:H232–H240. doi: 10.1152/ajpheart.2001.281.1.H232. [DOI] [PubMed] [Google Scholar]
- Yan SF, Ramasamy R, Naka Y, Schmidt AM. Glycation, inflammation, and RAGE. A scaffold for the microvasular complications of diabetes and beynod. Circ Res. 2003;93:1159–1169. doi: 10.1161/01.RES.0000103862.26506.3D. [DOI] [PubMed] [Google Scholar]
- Yue KKM, Lee KW, Chan KKC, Leung KSY, Leung AWN, Cheng CHK. Danshen prevents the occurrence of oxidative stress in the eye and aorta of diabetic rats without affecting the hyperglycemic state. J Ethnopharmacol. 2006;106:136–141. doi: 10.1016/j.jep.2005.12.026. [DOI] [PubMed] [Google Scholar]
- Zúrová-Nedelčevová J, Navarová J, Drábiková K, Jančinová V, Petríková M, Bernátová I, Kristová V, Šnirc V, Nosálová V, Sotníková R. Participation of reactive oxygen species in diabetes-induced endothelial dysfunction. Neuro Endocrinol Lett. 2006;27:68–171. [PubMed] [Google Scholar]