Abstract
As a number of disease-modifying anti-rheumatic drugs often have side effects at high doses and/or during long-term administration, increased efficacy without increased toxicity is expected for combination therapy of rheumatoid arthritis (RA). The safety of long-term therapy of RA is very important as patients with RA are usually treated for two or more decades. This experimental overview is focused on some promising substances and their combinations with the standard antirheumatic drug – methotrexate (Mtx) for treatment of rheumatoid arthritis. The adjuvant arthritis model in Lewis rats was used for evaluation of antiinflammatory efficacy of the substances evaluated. Mtx was administered in the oral dose of 0.3 mg/kg b.w. twice a week. Natural and synthetic antioxidants were administered in the daily oral dose of 20 mg/kg b.w for coenzyme Q10 (CoQ10), 150 mg/kg b.w for carnosine (Carn), 15 mg/kg b.w. for stobadine dipalmitate (Stb) and its derivative SMe1.2HCl (SMe1), and 30 mg/kg b.w. for pinosylvin (Pin) or pterostilbene (Pte). Mtx in the oral dose of 0.4 mg/kg b.w. twice a week was combined with Pin in the oral daily dose of 50 mg/kg b.w. Clinical (hind paw volume – HPV), biochemical (activity of GGT in joint and level of TBARS in plasma), and immunological (IL-1 in plasma) parameters were assessed. Our results achieved with different antioxidants in monotherapies showed a reduction of oxidative stress in adjuvant arthritis independently of the chemical structure of the compounds. Pin was the most effective antioxidant tested in decreasing HPV. All combinations tested showed a higher efficacy in affecting biochemical or immunological parameters than Mtx administered in monotherapy. The findings showed the benefit of antioxidant compounds for their use in combination therapy with methotrexate.
Keywords: arthritis, methotrexate, combination therapy, oxidative stress, carnosine, coenzyme Q10, pyridoindoles, stilbenoids
Introduction
This experimental overview is focused on some promising substances and their combinations with the standard antirheumatic drug methotrexate (Mtx) for treatment of rheumatoid arthritis (RA). Preclinical research on animal models of RA is very important for alerting the healthcare and scientific community, as well as pharmaceutical companies, of the existence of new or “forgotten” molecules. Most antirheumatics have side-effects when used in higher doses and/or within long-term dosage. Combinatory therapy is expected to have a higher efficacy without increased toxicity. Mtx has become the main immunosuppressive substance used in the treatment of patients with RA (Weinblatt, 2003). The use of Mtx has to be limited due to its toxic manifestations, e.g. abdominal disorder, alopecia, oral ulcers, and cytopenia (Alarcon et al., 1989). Ineffectiveness of treatment can be also observed. In the survey of McKendry and Dale (1993), due to the risk of treatment termination was substantiated in 75% of patients with RA taking Mtx for 60 months. An adverse drug effect proved to be a more common reason for treatment termination (53%) compared to loss/lack of beneficial effect (22%), other reasons (16%), or lost to follow-up (9%). On the other hand, the therapeutic efficacy of Mtx can be increased by combination with other synthetic drugs or inhibitors of TNF-α (Smolen et al., 2010). Application of biological therapy (antibodies or soluble receptors of TNF-α, IL-1 and IL-6) represents a great progress in the therapy of RA, while biological treatment is also frequently combined with Mtx (Maini et al., 1998; Weinblatt et al., 1999). There are countless possibilities for combinations with Mtx. Many substances were neglected when they failed to show good efficacy in monotherapy compared to standard antirheumatics. They would not get a second chance if the expected reduction of clinical parameters (mainly edema of joints) did not materialize, despite the fact that they improved many biochemical disease markers. This overview is focused mostly on substances of natural origin possessing antiinflammatory and antioxidative properties along with minimal side effects when administered to animals. The safety of long-term therapy of RA is very important as patients with RA are usually treated for two or more decades.
In our experiments of the last five years, synthetic (pyridoindoles) and natural substances (polysaccharides: glucomannan, beta-glucan; endogenous molecules: coenzyme Q10 and carnosine; different substances and products of plant origin) with immunomodulatory and antioxidative effects were assessed in monotherapy by using experimental adjuvant arthritis (AA). These active substances decreased the progression of AA when administered to arthritic rats over the period of 28 days (Bauerova et al., 2005a; 2005b; 2008a; 2008b; 2009; Drabikova et al., 2009; Drafi et al., 2010; Jančinova et al., 2009; Kogan et al., 2005; Mačičkova et al., 2010; Poništ et al., 2010; Sotnikova et al., 2009; Štrosova et al., 2008, 2009). Recently we focused on combined therapy with Mtx and confirmed the potent effect of this drug in several experiments administered along with an appropriate antioxidative substance (Bauerova et al., 2010; Jančinova et al., 2010; Rovensky et al., 2009a).
The primary beneficial effect concerned the reduction of articular edema and normalization of biochemical parameters were also reported by other authors (Cuzzocrea et al., 2005; Kogure et al., 2010; Rovensky et al., 2002; 2008; 2009b).
In this overview, our results obtained in AA with endogenous (coenzyme Q10 and carnosine) and synthetic (stobadine and SMe1) antioxidants as well as two selected compounds from plants (pterostilbene and pinosylvin) will be completed with new unpublished results. The aim is to demonstrate the potential of antioxidant compounds for their use in combination therapy with Mtx.
Methods
Animals, experimental design and treatments
Male Lewis rats weighing 160–180 g were obtained from the Breeding Farm Dobra Voda, Slovakia. The rats had free access to standard pelleted diet and tap water. The experimental protocol was approved by the Ethics Committee of the Institute of Experimental Pharmacology and Toxicology and by the Slovak State Veterinary and Food Administration. Adjuvant arthritis (AA) was induced by a single intradermal injection of heat-inactivated Mycobacterium butyricum in incomplete Freund's adjuvant (Difco Laboratories, Detroit, MI, USA). The injection was performed near the tail base. The experiments included healthy animals (HC), arthritic animals not treated (AA), arthritic animals treated with methotrexate in the oral dose of 0.3 mg/kg b.w. twice a week (AA-Mtx), arthritic animals treated with coenzyme Q10 (CoQ10) in the daily oral dose of 20 mg/kg b.w (AA-CoQ10), arthritic animals treated with carnosine in the daily oral dose of 150 mg/kg b.w (AA-Carn), arthritic animals treated with the combination of CoQ10 and methotrexate (AA-CoQ10+Mtx), arthritic animals treated with stobadine dipalmitate (AA-Stb) or its derivative SMe1.2HCl (AA-SMe1) in the oral daily dose of 15 mg/kg b.w., arthritic animals treated with the combination of stobadine dipalmitate and methotrexate (AA-Stb+Mtx), arthritic animals treated with pinosylvin (AA-Pin) or pterostilbene (AA-Pte) in the oral daily dose of 30 mg/kg b.w., arthritic animals treated with the combination of pinosylvin and methotrexate (AA-Pin+Mtx). In the latter combination, arthritic animals were treated twice a week with methotrexate in the oral dose of 0.4 mg/kg b.w. and daily with pinosylvin in the oral dose of 50 mg/kg b.w.. Monotherapy was performed with the same doses.
Methotrexat® Lachema 50 sol. inj. was used. CoQ10 in the form of Li-Q-Sorb® was purchased from Tishcon Corp., USA. Carn was purchased from Hamary Chemicals Ltd., Japan. The pyridoindoles were synthetized by Ing. Vladimir Snirc, PhD. Pin and Pte were synthetized in the Institute of Organic Chemistry and Biochemistry, Prague, Czech Republic.
In each experimental group 8–10 animals were used. The duration of the experiments was 28 days. After the animals had been sacrificed under deep ketamin/xylasine anesthesia, blood for plasma preparation and tissue for hind paw joint homogenate preparation were taken on day 28. Plasma was stored at minus 70 °C until biochemical and immunological analysis.
Clinical parameter evaluated: hind paw volume
We monitored the basic clinical parameter: hind paw volume (HPV). The HPV increase was calculated as the percentage increase in HPV on the experimental day 28 relative to the HPV at the beginning of the experiment. Hind paw volume was recorded with the use of an electronic water plethysmometer (UGO BASILE, Comerio-Varese, Italy).
Rat IL-1α assay in plasma
For determination of IL-1α in plasma an ELISA kit from Bender MedSystems was used as described in the product manual Rat IL-1α ELISA BMS627 and BMS627TEN. The rat IL-1 ELISA is an enzyme-linked immunosorbent assay for quantitative detection of rat IL-1. Rat IL-1 present in the samples binds to anti-rat IL-1antibodies adsorbed to the microwells. The reaction of a secondary biotin-conjugated anti-rat IL-1 antibody is evaluated by Streptavidin-HRP. Tetramethyl-benzidine oxidation with HRP bound to the immune complex was measured at 490 nm against reference wavelength of 620 nm. The results were calculated from a standard calibration curve obtained for internal standards.
Tissue activity of cellular γ-glutamyltransferase from hind paw
The activity of cellular γ-glutamyltransferase (GGT) in hind paw joint tissue homogenate was measured by the method of Orlowski & Meister (1970) as modified by Ondrejickova et al. (1993). Samples were homogenized in a buffer at 1: 9 w/v (buffer composition: 2.6 mM NaH2HPO4; 50 mM Na2HPO4; 15 mM EDTA; 68 mM NaCl; pH 8.1) by Ultra Turax TP 18/10 (Janke & Kunkel, Germany) for 1 min at 0 °C. Substrates (8.7 mM γ-glutamyl-p-nitroanilide (γ-GPN); 44 mM methionine) were added in 65% isopropylalcohol to final concentrations of 2.5 mM and 12.6 mM, respectively. After incubation for 60 min at 37 °C, the reaction was stopped with 2.3 ml cold methanol and the tubes were centrifuged for 20 min at 5000 rpm. Absorbance of supernatant was measured in a Hewlett Packard Vectra 286/12 spectrophotometer in 0.5 cm cuvette at 406 nm. Reaction mixtures in the absence of either the substrate or acceptor were used as reference samples.
Thiobarbituric acid reactive substances in plasma
TBARS were measured in heparinized blood plasma (Brown & Kelly, 1996). The amount of 750 µl of 0.67% thiobarbituric acid, 750 µl of 20% trichloroacetic acid, 350 µl of phosphate buffer (pH 7.4) were added to 150 µl of plasma, then mixed and incubated in a water bath at 90 °C for 30 min. The reaction was stopped by dipping the test tubes into ice for 10 min. Samples were centrifuged at 3 000 rpm. The supernatant was removed and absorbance measured at 535 nm in a 0.5 cm cuvette.
Statistical analysis of data
The data for all parameters are expressed as arithmetic mean±S.E.M. For significance calculations unpaired Student‘s t-test was used with *p<0.05 (significant), *p<0.01 (very significant), ***p<0.001 (extremely significant). The arthritis group was compared with healthy control animals (*). The treated arthritis groups were compared with untreated arthritis (+). The combination treatment was compared to individual Mtx treatment (#).
Results
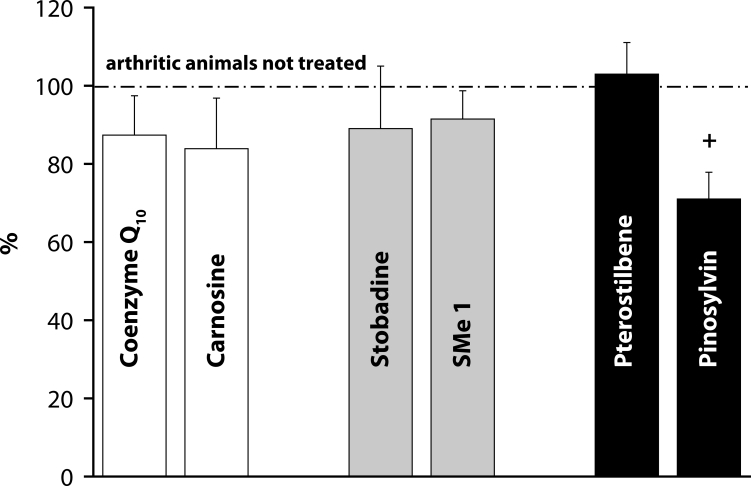
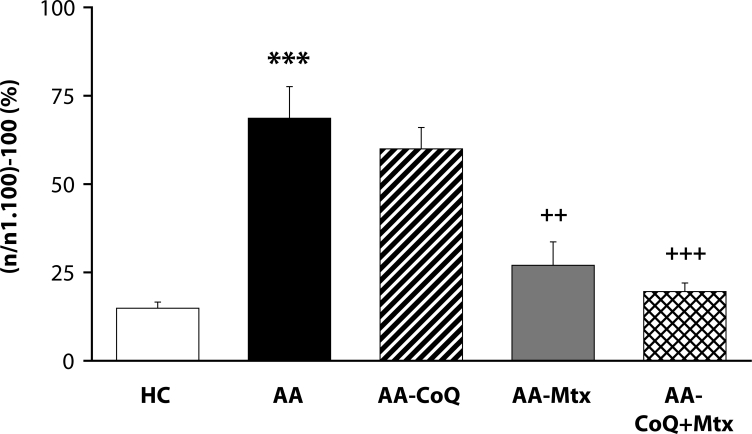
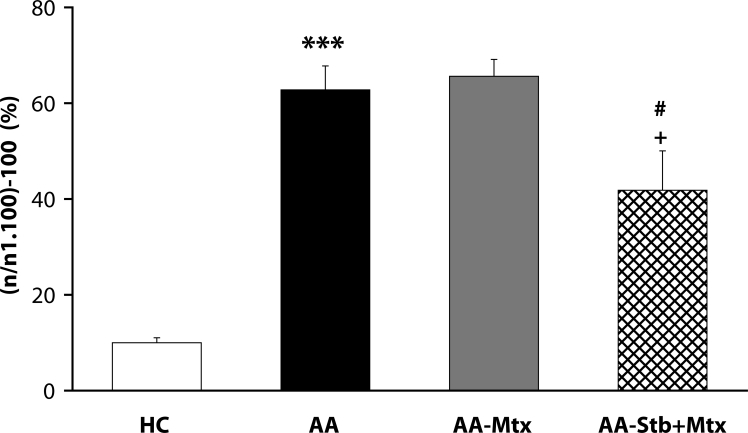
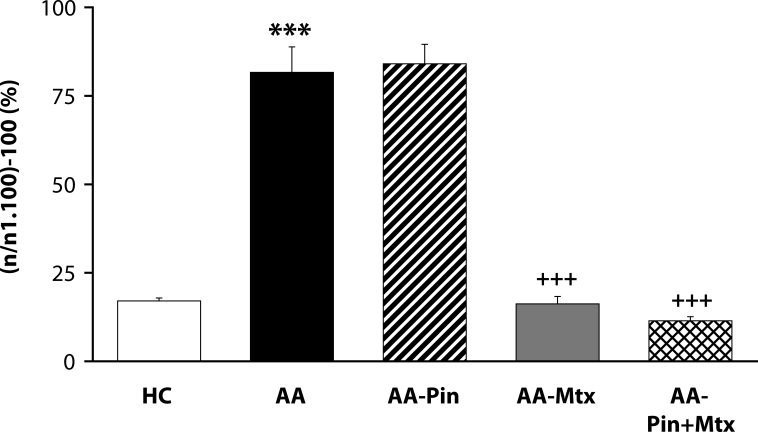
Of the antioxidants administered in monotherapy to arthritic rats only pinosylvin showed a significant reduction of HPV. There were no big differences in the effectivity of antioxidants in the groups compared (CoQ versus Carn, and Stb versus SMe1), except in the group of stilbenoids where Pin exhibited a notably higher efficacy than Pte (Figure 1). In all combination therapies, HPV was more effectively reduced than by Mtx alone. This effect was most noticeable for the combination of Mtx and Stb (Figures 2–4). All combination therapies achieved a significantly higher efficacy in affecting selected immunological (IL-1α – Figure 5) and biochemical (GGT – Figure 6 and TBARS – Figure 7) parameters compared to Mtx alone.
Figure 1.
Reduction of hind paw volume on day 28 – treatment with different antioxidants. Values are given as arithmetic mean of percentage ± S.E.M. of reduction of hind paw volume calculated to untreated arthritic rats (100% represented by dot-anddash line). Statistical significance was evaluated using unpaired Student′s t-test: +p<0.05 with respect to untreated arthritic animals.
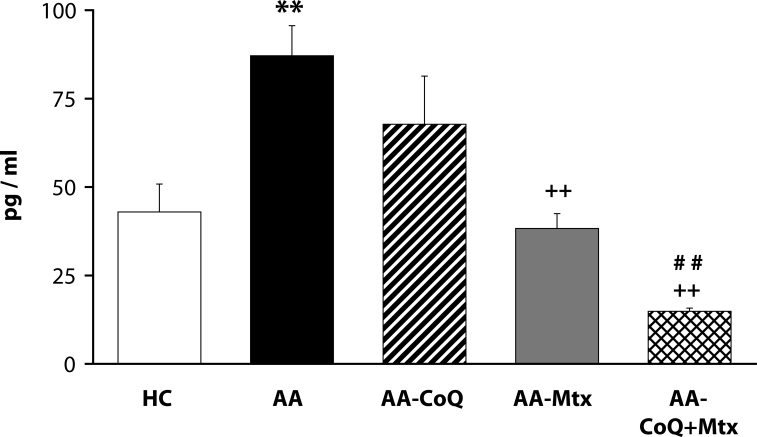
Figure 5.
Effect of combined therapy of methotrexate and coenzyme Q10 on plasmatic level of IL-1α assessed on day 28. Values are given as arithmetic mean ± S.E.M. Statistical significance was evaluated using unpaired Student′s t-test: **p<0.01 with respect to control healthy animals, ++p<0.01 with respect to untreated arthritic animals and ##p<0.01 for comparison of methotrexate monotherapy with combined therapy. The experiment included healthy control animals (HC), arthritic animals without any drug administration (AA), and arthritic animals with the administration of coenzyme Q10 (AA-CoQ), methotrexate (AA-Mtx) and combination of methotrexate and coenzyme Q10 (AA-CoQ+Mtx).
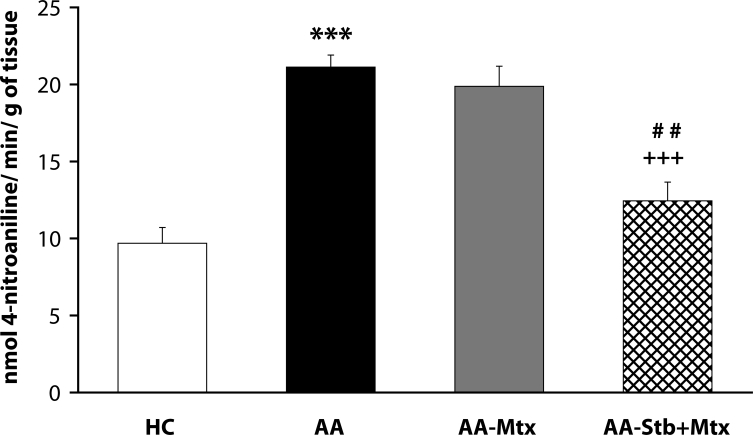
Figure 6.
Effect of combined therapy of methotrexate and stobadine on activity of GGT in joint homogenate assessed on day 28. Values are given as arithmetic mean ± S.E.M. Statistical significance was evaluated using unpaired Student′s t-test: ***p<0.001 with respect to control healthy animals, +++p<0.001 with respect to untreated arthritic animals and ##p<0.01 for comparison of methotrexate monotherapy with combined therapy. The experiment included healthy control animals (HC), arthritic animals without any drug administration (AA), and arthritic animals with the administration of methotrexate (AA-Mtx) and combination of methotrexate and stobadine (AA-Stb+Mtx).
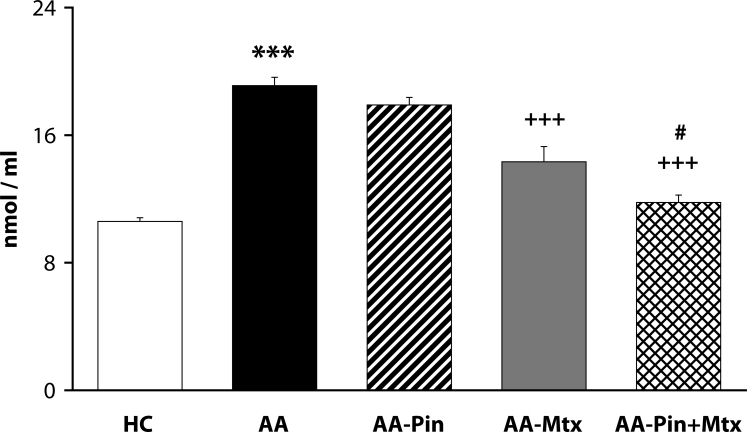
Figure 7.
Effect of combined therapy of methotrexate and pinosylvin on plasmatic level of TBARS assessed on day 28. Values are given as arithmetic mean ± S.E.M. Statistical significance was evaluated using unpaired Student′s t-test: ***p<0.001 with respect to control healthy animals, +++p<0.001 with respect to untreated arthritic animals and #p<0.05 for comparison of methotrexate monotherapy with combined therapy. The experiment included healthy control animals (HC), arthritic animals without any drug administration (AA), and arthritic animals with the administration of pinosylvin (AA-Pin), methotrexate (AA-Mtx) and combination of methotrexate and pinosylvin (AA-Pin+Mtx).
Figure 2.
Effect of combined therapy of methotrexate and coenzyme Q10 on hind paw volume assessed on day 28. Values are given as arithmetic mean ± S.E.M. Statistical significance was evaluated using unpaired Student′s t-test: ***p<0.001 with respect to control healthy animals, ++p<0.01 and +++p<0.001 with respect to untreated arthritic animals. The experiment included healthy control animals (HC), arthritic animals without any drug administration (AA), and arthritic animals with the administration of coenzyme Q10 (AA-CoQ), methotrexate (AA-Mtx) and combination of methotrexate and coenzyme Q10 (AA-CoQ+Mtx).
Figure 3.
Effect of combined therapy of methotrexate and stobadine on hind paw volume assessed on day 28. Values are given as arithmetic mean ± S.E.M. Statistical significance was evaluated using unpaired Student′s t-test: ***p<0.001 with respect to control healthy animals, +p<0.05 with respect to untreated arthritic animals and #p<0.05 for comparison of methotrexate monotherapy with combined therapy. The experiment included healthy control animals (HC), arthritic animals without any drug administration (AA), and arthritic animals with the administration of methotrexate (AA-Mtx) and combination of methotrexate and stobadine (AA-Stb+Mtx).
Figure 4.
Effect of combined therapy of methotrexate and pinosylvin on hind paw volume assessed on day 28. Values are given as arithmetic mean ± S.E.M. Statistical significance was evaluated using unpaired Student′s t-test: ***p<0.001 with respect to control healthy animals, +++p<0.001 with respect to untreated arthritic animals. The experiment included healthy control animals (HC), arthritic animals without any drug administration (AA), and arthritic animals with the administration of pinosylvin (AA-Pin), methotrexate (AA-Mtx) and combination of methotrexate and pinosylvin (AA-Pin+Mtx).
Discussion
Rheumatoid arthritis (RA) is a common severe joint disease affecting all age groups. It is thus of great importance to develop new strategies for its treatment. As a number of disease-modifying anti-rheumatic drugs (DMARDs) often have side effects at high doses and/or during long-term administration, increased efficacy without increased toxicity is expected for combination therapy of RA. Methotrexate (Mtx), a folic acid antagonist, has become the predominant immunosuppressive agent used in the treatment of patients with RA (Williams et al., 1985). Mtx acts mainly on actively proliferating cells during the S-phase of proliferation, suppresses macrophage function, modulates interleukin-1 (IL-1) and superoxide anion production, and inhibits neutrophil chemotaxis (Moreland et al., 1997). Furthermore, Mtx treatment was shown to decrease synovial collagenase gene expression in patients with RA (Genestier et al., 2000). The use of Mtx has been limited by some of its toxic manifestations, such as abdominal discomfort, alopecia, oral ulcerations, and cytopenia (Alarcon et al., 1989). In clinical studies, infliximab or etanercept have been used in combination with methotrexate to produce greater efficacy of the treatment of RA (Maini et al., 1998; Weinblatt et al., 1999). TNF-α blockers may be used alternatively with other candidates for RA combination therapy. As resulted from our previous experiments (Bauerova et al., 2005a; 2005b; 2008a; 2008b; 2009; Drábikova et al., 2009; Dráfi et al., 2010; Jančinova et al., 2009; Kogan et al., 2005; Mačičkova et al., 2010; Poništ et al., 2010; Sotnikova et al., 2009; Štrosova et al., 2008; 2009), all performed in the adjuvant arthritis model, substances with antioxidant properties have a high potency to be used in combination therapy with Mtx.
For our experiments, we chose substances with anti-oxidative properties and low toxicity. Administration of CoQ10 significantly improved changes of rat body mass, reduced HPV, and prevented CoQ imbalance in skeletal muscle mitochondria. The significantly improved myocardium mitochondrial function indicated that CoQ10 supplementation may protect cardiac function also in patients with RA (Bauerova et al., 2008b). Based on our results with mitochondrial energetics modification and the observed antiinflammatory and antioxidant effects (Gvozdjakova et al., 2004; Bauerova et al., 2005a; 2008b; Poništ et al., 2007), CoQ10 was selected as a candidate for combinatory therapy of RA. Another endogenous antioxidant used – carnosine, an essential endogenous molecule, has many physiological functions: radical scavenging, pH buffering, heavy metal chelating, anti-glycating, and neutralizing toxic aldehydes. Carn was found to have neuroprotective, hepatoprotective, cataract treating, and anti-aging abilities (Boldyrev, 2005), but its antiinflammatory potential in autoimmune systemic inflammatory diseases, as RA, has been scarcely investigated as yet. The first experiment with Carn in AA was performed in our laboratories. Carn beneficially affected HPV measured in time profile (14, 21 and 28 days), significantly on day 14 when the clinical manifestation of the disease started. Carn was able to delay the disease onset, however it was not so effective later on (days 21 and 28) when AA was fully developed. The markers of redox imbalance in plasma (TBARS and protein carbonyls) were significantly decreased, indicating the ability of Carn to restore redox balance in vivo (Dráfi et al., 2010). Stobadine dipalmitate (Stb) belongs to the group of naturally occurring carbolines (pyridoindoles – PI), tricyclic compounds of indole structure with a side ring comprising another nitrogen atom. Unlike α- and β-carbolines, it does not reveal any obvious toxic effects and possesses antioxidant activity. Stb, (–)-cis-2,8-dimethyl-2,3,4,4a,5,9b-hexahydro-1H-pyrido[4,3b]indole, was developed and synthesized in a search for new antidysrhythmic drugs at the Institute of Experimental Pharmacology, Slovak Academy of Sciences in Bratislava, Slovakia, in 1983 (Štolc et al., 1983). Later also the PI derivative SMe1.2HCl: 8-methoxy-2,3,4,4a,5,9b-hexahydro-1H-pyrido[4,3b]indole was synthesized at our institute. The first results with Stb in rat AA were obtained when its protective effect against indomethacin-induced gastroenteropathy was evaluated (Bauerova et al., 2004).
Pinosylvin [3′,5′-dihydroxystilbene] and pterostilbene [3,5-dimethoxy-4′-hydroxystilbene] are natural substances from the stilbenoid group, wide-spread in a variety of plants. They are chemically related to resveratrol. Both substances studied inhibited significantly the chemiluminescence (CL) of whole human blood and the CL of isolated human neutrophils (Perečko et al. 2008). The new information on the inhibitory effect of Pin and Pte on HPV, ROS and MPO activity suggests that the protective effect of Pin may be beneficial in controlling inflammation in experimental AA (Mačičkova et al., 2010).
The situation for adjuvant arthritis is complicated due to the dominant involvement of Th-1-driven autoimmune etiopathology. Oxidative stress (OS) in this animal model occurs as areaction to autoimmune processes. Under these conditions, control of OS is of secondary importance, although it could enhance immunomodulatory therapy of RA. We therefore administered CoQ10 (Bauerova et al., 2010) or Stb (our unpublished results) or Pin in combination with Mtx (Jančinova et al., 2010 and our unpublished results). The basic clinical parameter – HPV – was improved independently of the type of antioxidant used, although Pin seems to be more active than Stb or CoQ10 (Figure 1). Interestingly, on comparing all pairs of substances tested, namely CoQ10 with Carn, Stb with SMe1, and Pin with Pte (Figure 1), there were no significant differences between the two endogenous antioxidants or two pyridoindoles, but the two stilbenoids exhibited a great difference in their capacity to suppress the hind paw edema in AA. In all combination treatments, HPV was more effectively reduced than by individual Mtx treatment (Figures 2–4).
For further evaluation of the efficacy of CoQ10+Mtx administration, we analyzed IL-1α in plasma, which is one of the most important pro-inflammatory cytokines. IL-1 is closely related to inflammation and articular damage in several arthritis models and it is therefore generally accepted that IL-1 has a pivotal role in the pathophysiology of rheumatoid arthritis. In particular, IL-1 is a potent stimulator of synoviocytes, chondrocytes and osteoblasts. Moreover, IL-1 is a key mediator of synovial inflammation and pannus formation (Dinarello & Moldawer, 2002). Figure 5 shows the effects of the given treatments on AA-increased IL-1α levels. The improving effect on the increased cytokine plasmatic levels is rising in the order CoQ10, Mtx and CoQ10+Mtx. Furthermore, a statistically significant difference favored the combination of Mtx with CoQ10, compared to Mtx monotherapy.
GGT is an important component of inflammatory processes since its activity is closely connected with the overall antioxidant status of the organism. We found that the activity of GGT was approx. 3–6 times higher in AA animals than in healthy controls in the spleen and 1.4–2.3 higher in the joint (Bauerova et al., 2006; 2008a; 2009; Sotnikova et al., 2009). We assume that the increased activity of GGT in AA is a result of elevated systemic oxidative stress. Stb was significantly effective in suppressing the increased activity of GGT in the joint, as expected from its antioxidant potential (Poništ et al., 2010). The addition of Stb to Mtx caused significant improvement in lowering this biochemical parameter compared to Mtx monotherapy (Figure 6).
A frequently used marker of lipid peroxidation is MDA assessed as an adduct with TBA. Clinical studies have shown increased plasmatic levels of MDA in patients with RA (Baskol et al., 2005; 2006; Sarban et al., 2005). In animal models of AA, the level of MDA was elevated in the plasma of arthritic animals (Tastekin et al., 2007; He et al., 2006; Bauerova et al., 2008a; 2009; Štrosova et al., 2008; 2009; Sotnikova et al., 2009). The combination of Pin with Mtx was more effective in decreasing the plasmatic level of TBARS than was Mtx alone (Fig. 7). This finding is in good agreement with the results of Jančinova et al. (2010) showing the combination of the two substances as more active in suppressing spontaneous and stimulated chemiluminescence in blood of arthritic rats than achieved with Mtx administered alone.
Our results obtained with different antioxidants in monotherapies showed reduction of OS in adjuvant arthritis independently of the chemical structure of the compounds or their solubility in lipids (CoQ10) or water (Carn). Thus we may conclude that therapeutic benefits from antioxidant treatment are primarily bound to reduction of systemic OS. All combinations tested showed a higher efficacy in affecting biochemical or immunological parameters than Mtx administered in monotherapy. These results indicate that application of supporting immunosuppresive treatment represented by Mtx combined with an antioxidative substance appears to be a good decision. In our future experiments we will try to lower the dose of Mtx in combinations with the most effective antioxidants. We expect that a suboptimal dose of Mtx with an antioxidant could yield an as good efficacy as the optimal dose of Mtx alone in reducing of hind paw edema in AA. An effective combination of an antioxidant with a lowered dose of Mtx could also reduce the toxic side effect of Mtx treatment. Conclusion: We demonstrated the benefit of antioxidant compounds for their use in combination therapy with methotrexate.
Acknowledgements
The authors wish to thank Mrs. Denisa Komendova for technical assistance and Dr. Magda Kouřilová, PhD. for correction of the English language. This work was supported by grants VEGA 2/0045/11; VEGA 2/0090/08; APVV-51-017905; APVV 3015-07
REFERENCES
- Alarcón GS, Tracy IC, Blackburn WD., Jr. Methotrexate in rheumatoid arthritis. Toxic effects as the major factor in limiting long-term treatment. Arthritis Rheum. 1989;32(6):671–6. doi: 10.1002/anr.1780320603. [DOI] [PubMed] [Google Scholar]
- Baskol G, Demir H, Baskol M, Kilic E, Ates F, Kocer D, Muhtaroglu S. Assessment of paraoxonase 1 activity and malondialdehyde levels in patients with rheumatoid arthritis. Clin Biochem. 2005;38:951–965. doi: 10.1016/j.clinbiochem.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Baskol G, Demir H, Baskol M, Kilic E, Ates F, Karakukcu C, Ustdal M. Investigation of protein oxidation and lipid peroxidation in patients with rheumatoid arthritis. Cell Biochem Funct. 2006;24:307–311. doi: 10.1002/cbf.1257. [DOI] [PubMed] [Google Scholar]
- Bauerova K, Nosalova V, Mihalova D, Navarova J. Contribution to safe anti-inflammatory therapy with indomethacin. Cent Eur J Public Health. 2004;12:S8–S10. [PubMed] [Google Scholar]
- Bauerova K, Kucharska J, Mihalova D, Navarova J, Gvozdjakova A, Sumbalova Z. Effect of coenzyme Q(10) supplementation in the rat model of adjuvant arthritis. Biomed Pap. 2005a;149(2):501–503. doi: 10.5507/bp.2005.090. [DOI] [PubMed] [Google Scholar]
- Bauerova K, Valentova J, Poništ S, Navarova J, Komendova D, Mihalova D. Effect of copper complexes on the development of adjuvant arthritis: Therapeutic and toxicological aspects. Biologia. 2005b;60(17):65–68. [Google Scholar]
- Bauerova K, Poništ S, Ondrejíčkova O, Komendova D, Mihalova D. Association between tissue gamma glutamyl-transferase and clinical markers of adjuvant arthritis in Lewis rats. Neuroendocrinol Lett. 2006;27:172–175. [PubMed] [Google Scholar]
- Bauerova K, Poništ S, Navarova J, Dubničkova M, Paulovičova E, Pajtinka M, Kogan G, Mihalova D. Glucomannan in prevention of oxidative stress and inflammation occurring in adjuvant arthritis. Neuroendocrinology Lett. 2008a;29(5):691–696. [PubMed] [Google Scholar]
- Bauerova K, Kucharska J, Poništ S, Gvozdjakova A. In: Mitochondrial medicine: mitochodrial metabolism, diseases, diagnosis and therapy. Gvozdjakova , editor. Netherlands: Springer; 2008b. pp. 340–342. [Google Scholar]
- Bauerova K, Paulovičova E, Mihalova D, Švík K, Poništ S. Study of new ways of supplementary and combinatory therapy of rheumatoid arthritis with immunomodulators. Glucomannan and Imunoglukán® in adjuvant arthritis. Toxicology and Industrial Health. 2009;25(4–5):329–335. doi: 10.1177/0748233709102945. [DOI] [PubMed] [Google Scholar]
- Bauerova K, Paulovičova E, Mihalova D, Dráfi F, Štrosova M, Mascia C, Biasi F, Rovensky J, Kucharska J, Gvozdjakova A, Poništ S. Combined methotrexate and coenzyme Q10 therapy in adjuvant-induced arthritis evaluated using parameters of inflammation and oxidative stress. Acta Biochimica Polonica. 2010;57(3):347–354. [PubMed] [Google Scholar]
- Boldyrev A. Protection of proteins from oxidative stress A new illusion or a novel strategy? Ann N Y Acad Sci. 2005;1057:193–205. doi: 10.1196/annals.1356.013. [DOI] [PubMed] [Google Scholar]
- Brown RK, Kelly FJ. Peroxides and other products. In: Punchard NA, Kelly FJ, editors. Free Radicals, a practical approach. Oxford: Oxford University Press; 1996. pp. 119–131. [Google Scholar]
- Cuzzocrea S, Mazzon E, di Paola R, Genovese T, Muià C, Caputi AP, Salvemini D. Synergistic interaction between methotrexate and a superoxide dismutase mimetic: pharmacologic and potential clinical significance. Arthritis Rheum. 2005;52(12):3755–60. doi: 10.1002/art.21480. [DOI] [PubMed] [Google Scholar]
- Dinarello CA, Moldawer LL. Pro-inflammatory and anti-inflammatory cytokines in rheumatoid arthritis: a primer for clinicians. 3rd edn. Thousand Oaks, CA: Amgen; 2002. p. 351. [Google Scholar]
- Drábikova K, Perečko T, Nosál’ R, Bauerova K, Poništ S, Mihalova D, Kogan G, Jančinova V. Glucomannan reduces neutrophil free radical production in vitro and in rats with adjuvant arthritis. Pharmacological Research. 2009;59(6):399–403. doi: 10.1016/j.phrs.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Dráfi F, Bauerova K, Valachova K, Poništ S, Mihalova D, Juránek I, Boldyrev A, Hrabárova E, Šoltés L. Carnosine inhibits degradation of hyaluronan induced by free radical processes in vitro and improves the redox imbalance in adjuvant arthritis in vivo. Neuroendocrinol Lett. 2010;31(2):96–100. [PubMed] [Google Scholar]
- Genestier L, Paillot R, Quemeneur L, Izeradjene K, Revillard JP. Mechanisms of action of methotrexate. Immunopharmacology. 2000;47:247–257. doi: 10.1016/s0162-3109(00)00189-2. [DOI] [PubMed] [Google Scholar]
- Gvozdjakova A, Kucharska J, Tanaka S, Neradova B, Bauerova K. Coenzyme Q10 supplementation differently modulated heart and skeletal muscle mitochondrial function induced by adjuvant arthritis. Mitochondrion. 2004;4:20–21. [Google Scholar]
- He YH, Zhou J, Wang YS, Xiao C, Tong Y, Tang JC, Chan AS, Lu AP. Anti-inflammatory and anti-oxidative effects of cherries on Freund's adjuvant-induced arthritis in rats. Scand J Rheumatol. 2006;35:356–358. doi: 10.1080/03009740600704155. [DOI] [PubMed] [Google Scholar]
- Jančinova V, Perečko T, Nosál’ R, Koštálova D, Bauerova K, Drábikova K. Decreased activity of neutrophils in the presence of diferuloylmethane (curcumin) involves protein kinase C inhibition. Eur J Pharmacol. 2009;612(1–3):161–6. doi: 10.1016/j.ejphar.2009.03.080. [DOI] [PubMed] [Google Scholar]
- Jančinova V, Nosál’ R, Lojek A, Číz M, Ambrozova G, Mihalova D, Bauerova K, Harmatha J, Perečko T. Formation of reactive oxygen and nitrogen species in the presence of pinosylvin – an analogue of resveratrol. Neuroendocrinol Lett. 2010;31(2):79–83. [PubMed] [Google Scholar]
- Kogan G, Staško A, Bauerova K, Polovka M, Šoltés L, Brezova V, Navarova J, Mihalova D. Antioxidant properties of yeast (1→3)-β-D-glucan studied by electron paramagnetic resonance spectroscopy and its activity in adjuvant arthritis. Carbohydr Polym. 2005;61:18–28. [Google Scholar]
- Kogure T, Tatsumi T, Sato H, Oku Y, Kishi D, Ito T. Traditional herbal medicines (Kampo) for patients with rheumatoid arthritis receiving concomitant methotrexate: a preliminary study. Altern Ther Health Med. 2010;16:46–51. [PubMed] [Google Scholar]
- Mačičkova T, Drábikova K, Nosál’ R, Bauerova K, Mihalova D, Harmatha J, Pečivova J. In vivo effect of pinosylvin and pterostilbene in the animal model of adjuvant arthritis. Neuroendocrinol Lett. 2010;31(2):91–95. [PubMed] [Google Scholar]
- Maini RN, Breedveld FC, Kalden JR, Smolen JS, Davis D, Macfarlane JD, Antoni C, Leeb B, Elliott MJ, Woody JM, Schaible TF, Feldmann M. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor a monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum. 1998;41:1552–1563. doi: 10.1002/1529-0131(199809)41:9<1552::AID-ART5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- McKendry RJ, Dale P. Adverse effects of low dose methotrexate therapy in rheumatoid arthritis. J Rheumatol. 1993;20(11):1850–1856. [PubMed] [Google Scholar]
- Moreland LW, Heck LW, Jr, Koopman WJ. Biologic agents for treating rheumatoid arthritis: concepts and progress. Arthritis Rheum. 1997;40:397–409. doi: 10.1002/art.1780400302. [DOI] [PubMed] [Google Scholar]
- Ondrejičkova O, Ziegelhoeffer A, Gabauer I, Sotnikova R, Styk J, Gibala P, Sedlak J, Horákova L. Evaluation of ischemia-reperfusion injury by malondialdehyde, glutathione and gamma-glutamyl transpeptidase: lack of specific local effects in diverse parts of the dog heart following acute coronary occlusion. Cardioscience. 1993;4:225–230. [PubMed] [Google Scholar]
- Orlowski M, Meister A. The gamma-glutamyl cycle: a possible transport system for amino acids. Proc Natl Acad Sci. 1970;67:1248–1255. doi: 10.1073/pnas.67.3.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perečko T, Jančinova V, Drábikova K, Nosáĺ R, Harmatha J. Structure-efficiency relationship in derivatives of stilbene. Comparison of resveratrol, pinosilvin and pterostilbene. Neuroendocrinol Lett. 2008;29:802–805. [PubMed] [Google Scholar]
- Poništ S, Mihalova D, Jančinova V, Šnirc V, Ondrejičkova O, Mascia C, Poli G, Stančikova M, Nosál’ R, Bauerova K. Reduction of oxidative stress in adjuvant arthritis. Comparison of efficacy of two pyridoindoles: stobadine dipalmitate and SMe1.2HCl. Acta Biochim Pol. 2010;57(2):223–8. [PubMed] [Google Scholar]
- Poništ S, Kucharska J, Gvozdjakova A, Komendova D, Mihalova D, Bauerova K. Mitochondrial bioenergetics of skeletal muscle studied in adjuvant arthritis. Chem Listy. 2007;101:256–257. [Google Scholar]
- Smolen JS, Aletaha D, Bijlsma JW, Breedveld FC, Boumpas D, Burmester G, Combe B, Cutolo M, de Wit M, Dougados M, Emery P, Gibofsky A, Gomez-Reino JJ, Haraoui B, Kalden J, Keystone EC, Kvien TK, McInnes I, Martin-Mola E, Montecucco C, Schoels M, van der Heijde D. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010;69(4):631–7. doi: 10.1136/ard.2009.123919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotnikova R, Poništ S, Navarova J, Mihalova D, Tomekova V, Štrosova M, Bauerova K. Effects of sesame oil in the model of adjuvant arthritis. Neuroendocrinol Lett. 2009;30:22–24. [PubMed] [Google Scholar]
- Rovensky J, Švík K, Stančikova M, Istok R, Ebringer L, Ferenčik M. Treatment of experimental adjuvant arthritis with the combination of methotrexate and lyophilized Enterococcus faecium enriched with organic selenium. Folia Microbiol. 2002;47:573–578. doi: 10.1007/BF02818800. [DOI] [PubMed] [Google Scholar]
- Rovensky J, Švík K, Rovenska E, Štvrtinova V, Stančikova M. Effects of purified micronized flavonoid fraction (Detralex) on prophylactic treatment of adjuvant arthritis with methotrexate in rats. Isr Med Assoc J. 2008;10:377–380. [PubMed] [Google Scholar]
- Rovensky J, Stančikova M, Švík K, Bauerova K, Jurčovičova J. The effects of beta-glucan isolated from Pleurotus ostreatus on methotrexate treatment in rats with adjuvant arthritis. Rheumatol Int. 2009a doi: 10.1007/s00296-009-1258-z. [DOI] [PubMed] [Google Scholar]
- Rovensky J, Stančikova M, Rovenska E, Štvrtina S, Štvrtinova V, Švík K. Treatment of rat adjuvant arthritis with flavonoid (Detralex), methotrexate, and their combination. Ann N Y Acad Sci. 2009b;1173:798–804. doi: 10.1111/j.1749-6632.2009.04618.x. [DOI] [PubMed] [Google Scholar]
- Sarban A, Kocyigit A, Yazar M, Isikan UE. Plasma total antioxidant capacity, lipid peroxidation, and erythrocyte antioxidant enzyme activities in patients with rheumatoid arthritis and osteoarthritis. Clin Biochem. 2005;38:981–986. doi: 10.1016/j.clinbiochem.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Štolc S, Bauer V, Benes L, Tichy M. 1983. Medicine with antiarrhythmic and antihypoxic activity and its method of preparation. Patents: CS 229 067, SWED. 8204693–9, BELG. 894148, SWISS 651 754, BRD P-3231088, SPAIN 553 017, JAP. 151 4040. [Google Scholar]
- Štrosova M, Tomaškova I, Poništ S, Bauerova K, Karlovska J, Spickett CM, Horákova L. Oxidative impairment of plasma and skeletal muscle sarcoplasmic reticulum in rats with adjuvant arthritis – effects of pyridoindole antioxidants. Neuroendocrinol Lett. 2008;29:706–711. [PubMed] [Google Scholar]
- Štrosova M, Karlovska J, Spickett CM, Országhova Z, Poništ S, Bauerova K, Mihalova D, Horákova L. Modulation of SERCA in the chronic phase of adjuvant arthritis as a possible adaptation mechanism of redox imbalance. Free Radic Res. 2009;43:852–864. doi: 10.1080/10715760903089708. [DOI] [PubMed] [Google Scholar]
- Tastekin N, Aydogdu N, Dokmeci D, Usta U, Birtane M, Erbas H, Ture M. Protective effects of L-carnitine and alpha-lipoic acid in rats with adjuvant arthritis. Pharmacol Res. 2007;56:303–310. doi: 10.1016/j.phrs.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Weinblatt ME. Rheumatoid arthritis in 2003: where are we now with treatment? Ann Rheum Dis. 2003;62(2):94–6. doi: 10.1136/ard.62.suppl_2.ii94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinblatt ME, Kremer JM, Bankhurst AD, Bulpitt KJ, Fleischmann RM, Fox RI, Jackson CG, Lange M, Burge DJ. A trial of etanercept, a recombinant tumor necrosis factor receptor: Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med. 1999;340:253–259. doi: 10.1056/NEJM199901283400401. [DOI] [PubMed] [Google Scholar]
- Williams HJ, Willkens RF, Samuelson CO, Jr, Alarcon GS, Guttadauria M, Yarboro C, Polisson RP, Weiner SR, Luggen ME, Billingsley LM, Dahl SL, Egger MJ, Reading JC, Ward JR. Comparison of low-dose oral pulse methotrexate and placebo in the treatment of rheumatoid arthritis: a controlled clinical trial. Arthritis Rheum. 1985;28:721–730. doi: 10.1002/art.1780280702. [DOI] [PubMed] [Google Scholar]