Abstract
Background:
Atypical language dominance is common in patients with temporal lobe epilepsy. We examined the association of left temporal hypometabolism with laterality of fMRI activation in a language task in a cross-sectional study.
Methods:
Thirty patients with temporal lobe epilepsy (mean age 32.4 ± 11.0 years [range 18–55]; epilepsy onset 15.3 ± 11.3 years [range 0.8–40]; 22 left focus, 8 right focus) had 18fluoro-deoxyglucose (FDG)-PET using noninvasive cardiac input function. After MRI-based partial volume correction, regional glucose metabolism (CMRglc) was measured and asymmetry index, AI = 2(l − R)/(L + R), calculated. fMRI language dominance was assessed with an auditory definition decision paradigm at 3 T. fMRI data were analyzed in SPM2 using regions of interest from Wake Forest PickAtlas (Wernicke area [WA], inferior frontal gyrus [IFG], middle frontal gyrus [MFG]) and bootstrap laterality index, LI = (l − R/L + R).
Results:
Nineteen patients had ipsilateral temporal hypometabolism; 3 of 4 patients with atypical language had abnormal FDG-PET. Increasing left midtemporal hypometabolism correlated with decreased MFG LI (r = −0.41, p < 0.05) and showed trends with WA LI (r = −0.37, p = 0.055) and IFG LI (r = −0.31, p = 0.099); these relationships became more significant after controlling for age at onset. Increasing hypometabolism was associated with fewer activated voxels in WA ipsilateral to the focus and more activated voxels contralaterally, but overall, activation amount in left WA was similar to subjects without left temporal hypometabolism (t = −1.39, p > 0.10).
Conclusions:
We did not find evidence of impaired blood oxygenation level–dependent response in hypometabolic cortex. Regional hypometabolism appears to be a marker for the temporal lobe dysfunction that leads to displacement of language function.
Atypical language dominance is common in patients with temporal lobe epilepsy (TLE), and language processing impairments are particularly common in left TLE.1 One-quarter of patients with left mesial TLE will have altered language representation even though the focus is remote from Wernicke area (WA). One-third of patients with left TLE who have normal MRI and no other known associated risk factors (i.e., left handedness, early onset) will also have atypical language dominance.1–3 The causes of language reorganization at a distance from the focus, and in the absence of a structural lesion, are unclear. Decreased glucose consumption (rCMRglc) identified by 18fluoro-deoxyglucose (FDG)-PET is common in patients with TLE.4 The hypometabolism typically involves lateral temporal neocortex and, less frequently, frontal regions, even in patients with a clear mesial focus.4–6 Functional changes in the temporal lobe could lead to impaired language processing. However, it is also possible that the ability of cortex to sustain the blood oxygenation level–dependent (BOLD) response needed for language mapping on fMRI is affected, while underlying cognitive processing is normal.
We aimed to examine the association of perturbations in glucose consumption with fMRI language lateralization as determined by a fMRI task targeted at both temporal and frontal language processing areas. We hypothesized that hypometabolic cortex would sustain BOLD response and that regional decreases in rCMRglc, an indicator of impaired neocortical function and capacity, would affect language dominance.
METHODS
Participants.
We performed a cross-sectional retrospective review of patients who were evaluated for epilepsy surgery who had both fMRI and FDG-PET between 2004 and 2009. We studied 30 patients with TLE confirmed by clinical characteristics, MRI, and ictal video-EEG monitoring. Twenty-four had intracranial monitoring or temporal resection. Twenty-two had a left seizure focus and 8 had a right focus. Fourteen women and 16 men participated; mean age at study was 32.4 ± 11.0 years (range 18–55 years); mean seizure onset age was 15.3 ± 11.3 years (range 0.8–40 years); and mean epilepsy duration was 17.1 ± 12.9 years (range 1–47 years). Patients underwent 3 T structural MRI (General Electric, Milwaukee, WI), fMRI with language activation paradigms, and FDG-PET. Nine patients had mesial temporal sclerosis (MTS) (7 left, 2 right), 2 a tumor or malformation of cortical development (MCD) (both left), and 19 had a normal MRI (13 left seizure focus, 6 right seizure focus). fMRI data were acquired from 49 healthy control subjects (24 women, 25 men; mean age 27.1 ± 7.9 years, range 20–53 years) who were free of neurologic and psychiatric disease, had normal neurologic examinations, and were deemed left hemisphere dominant for language (see fMRI below).
Standard protocol approvals, registrations, and patient consents.
The protocol was approved by the NIH Central Nervous System institutional review board; all patients provided written informed consent.
fMRI.
fMRI data were acquired at 3.0 T (General Electric Medical Systems, Milwaukee, WI) using EPI BOLD techniques. Acquisition parameters, paradigm presentation, and analysis methods have been described previously.1,7 We used an Auditory Description Decision Task with a 5-block design (30-second hemicycle). During the experimental condition, the participant heard a sentence that described, then named, an object (“a large pink bird is a flamingo”); 70% were correct definitions and 30% were foils. The control condition was reverse speech with an appended tone identification for 70% of reverse items. After normalization to the Montreal Neurological Institute anatomic atlas, language dominance was established by analyzing individual maps and employing the LI toolbox adapted for SPM2 to process by a bootstrap method.8 The fMRI tasks reliably elicit activation along the left superior temporal sulcus (Brodmann areas [BA] 21, 22, 39) implicated in “receptive” speech processing, the left inferior frontal gyrus implicated in “expressive” speech processing (BA 44, 45) and semantic decision (BA 47), and left middle frontal gyrus (BA 9, 46) implicated in verbal working memory.7,9 Regions of interest were based on the Wake Forest Pick Atlas, and included WA broadly defined along the superior temporal sulcus (BA 21, 22, 39; superior temporal gyrus [STG], middle temporal gyrus [MTG]), BA (BA 44, 45, 47; inferior frontal gyrus [IFG]), and middle frontal gyrus (MFG). A laterality index (LI) was calculated for each region where LI = (l − R)/(L + R). Left language (LL) dominance for each region was defined as LI ≥ 0.20; atypical language (AL) was defined as regional LI < 0.20. Patients were classified as having atypical language overall when either WA or IFG was right dominant (LI < −0.20), or when both regions were bilateral (LI < 0.20 ).10 Regional activation voxel counts were recorded at the individual mean LI threshold level used in the bootstrap method for all subjects.
FDG-PET.
FDG-PET acquisition and analysis have been described previously.11 Briefly, FDG-PET was performed following 6-hour fasting, in the awake quiet state, in a quiet, dim room with eyes closed and ears unconcluded. EEG was performed and patients observed to assure interictal studies. Five mCi FDG were injected, and the following 20-minute uptake period data were acquired on a GE Advance Tomograph (GE Healthcare; full width at half maximum, 6–7 mm). Absolute cerebral metabolic rates for glucose (CMRglc) were determined using a noninvasive input function based on cardiac radioactivity. Images were coregistered with a structural MRI and then corrected for partial volume effects. Regional CMRglc were then determined in anatomic regions drawn on the coregistered MRI following an anatomic atlas. Regions included, but were not limited to, the middle temporal gyrus and the mesial temporal region (hippocampal structures, including CA fields, dentate gyrus, presubiculum, and subiculum). An asymmetry index was calculated: AI = 2(l − R/L + R), with regional hypometabolism defined as >2 SD (the PET AI follows a different historical convention than fMRI).
Data analysis.
Statistical analysis was performed with SPSS: edition 12.0 for Windows. Descriptive statistics were used to characterize demographic, AI, and LI variables. To examine the relationships among these variables, the appropriate parametric and nonparametric analyses (depending on the nature and distribution) were conducted including t test and bivariate correlation. Regression analyses were conducted to determine if hypometabolism predicted language laterality after accounting for age at onset.
RESULTS
Language laterality and left temporal hypometabolism.
Hypometabolism was predominantly ipsilateral temporal; only a few patients had extratemporal or contralateral PET abnormalities (table e-1 on the Neurology® Web site at www.neurology.org). Nineteen patients (63%) had regional hypometabolism in midtemporal or mesial temporal areas ipsilateral to the seizure focus (table 1). Sixteen patients with ipsilateral temporal hypometabolism had a left focus and 3 patients had a right focus. Ten of 11 (91%) patients without ipsilateral temporal hypometabolism were left language dominant (LI > 0.20). Thirteen of 16 (81%) patients with ipsilateral left temporal hypometabolism were left language dominant (table 1). Four patients were classified with atypical language dominance, all of whom had a left seizure focus. Although 3 of 4 patients with atypical language had regional left temporal hypometabolism, most patients with regional hypometabolism remained left hemisphere dominant for language (figure 1).
Table 1.
Frequencies of typical and atypical language dominance in the presence and absence of ipsilateral temporal hypometabolism
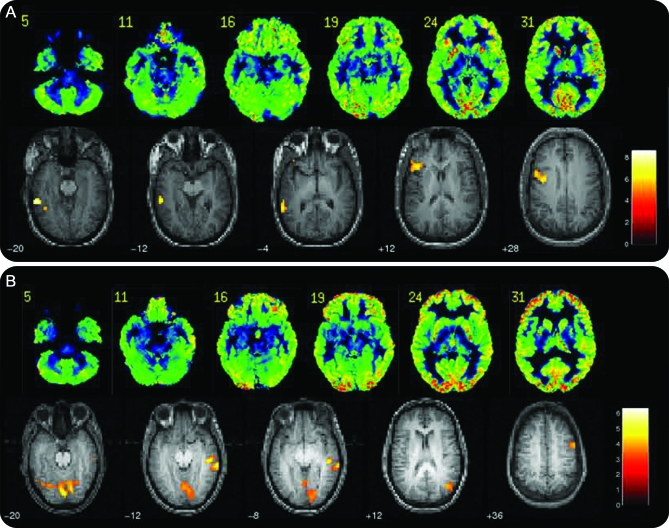
Figure 1. Language dominance.
(A) Subject with typical language dominance and left temporal hypometabolism. (B) Subject with atypical language dominance and left temporal hypometabolism. Images are shown in neurologic convention (left is left).
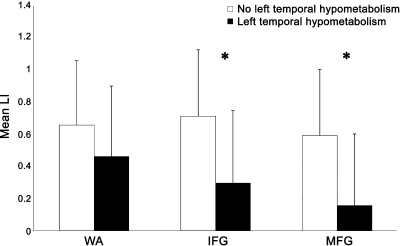
The few patients with atypical language prohibited statistical comparisons between groups based on language dominance; however, degree of language lateralization (LI) was examined in relation to hypometabolism (AI) in both midtemporal and mesial temporal regions. Greater left midtemporal hypometabolism was correlated with decreased MFG LI (r = −0.41, p < 0.05) and showed a trend with both WA LI (r = −0.37, p = 0.055) and IFG LI (r = −0.31, p = 0.099). Additionally, greater left mesial temporal (hippocampal) hypometabolism was correlated with decreased WA LI (r = −0.46, p < 0.05), but not MFG LI (r = −0.21, p > 0.10) or IFG LI (r = −0.15, p > 0.10). Midtemporal and mesial temporal hypometabolism were tightly linked, but not necessarily predictive of each other (r = 0.59, p < 0.001). Using a categorical classification of left temporal hypometabolism (middle or mesial)—defined as AI > 2 SD above normal—subjects with hypometabolism had lower MFG LI (t = 2.67, p < 0.05) and IFG LI (t = 2.53, p < 0.05), while WA LI was similar for subjects with and without hypometabolism (t = 1.21, p > 0.10) (table 2, figure 2).
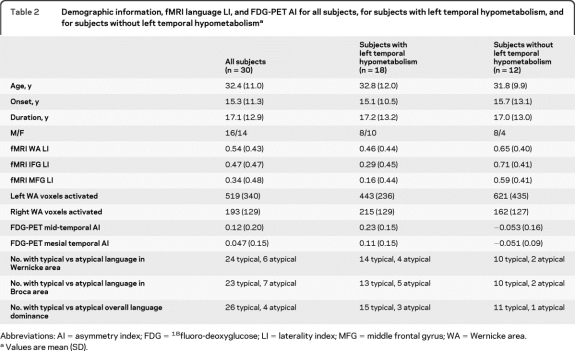
Table 2.
Demographic information, fMRI language LI, and FDG-PET AI for all subjects, for subjects with left temporal hypometabolism, and for subjects without left temporal hypometabolisma
Abbreviations: AI = asymmetry index; FDG = 18fluoro-deoxyglucose; LI = laterality index; MFG = middle frontal gyrus; WA = Wernicke area.
Values are mean (SD).
Figure 2. Mean fMRI laterality index (LI) in Wernicke area (WA), inferior frontal gyrus (IFG), and middle frontal gyrus (MFG) regions for patients with and without left temporal hypometabolism (*p < 0.05).
There were no differences in regional LI or degree of left temporal hypometabolism by subject age (p > 0.10). Degree of left temporal hypometabolism did not differ by gender (midtemporal AI: t = −0.61, p > 0.10; mesial temporal AI: t = −0.55, p > 0.10), nor did WA LI (t = 1.67, p > 0.10) or MFG LI (t = 1.36, p > 0.10), but IFG LI was lower for females than males (t = 2.44, p < 0.05).
fMRI BOLD signal and left temporal hypometabolism.
Increasing temporal hypometabolism was associated with fewer activated voxels ipsilateral to the hypometabolism and more activated voxels contralateral to the hypometabolism. Greater left midtemporal hypometabolism was correlated with fewer activated voxels in left WA (r = −0.45, p < 0.05). The amount of right WA voxel activation was not associated with degree of left midtemporal hypometabolism (r = 0.28, p > 0.10); however, this correlation was in the opposite direction from the ipsilateral correlation such that the tendency was for greater left hypometabolism to be associated with greater right activation. Left mesial temporal hypometabolism was correlated with decreased ipsilateral activation in left WA (r = −0.37, p = 0.05) and increased contralateral activation in right WA (r = 0.40, p < 0.05).
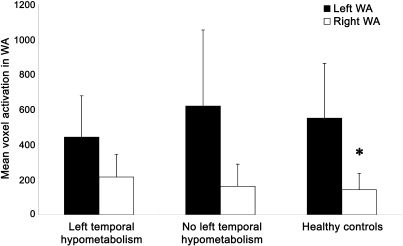
When divided into 2 groups by presence vs absence of left temporal hypometabolism above the clinical threshold of 2 standard deviations beyond normal, the amounts of left and right voxel activation in Wernicke area did not differ (left WA voxels: t = −1.39, p > 0.10; right WA voxels: t = 1.09, p > 0.10). Additionally, the relationship between fMRI activation and hypometabolism was not a function of overall depressed activation in patients with left temporal hypometabolism because the number of activated voxels in left Wernicke area did not differ from subjects without left temporal hypometabolism (t = −1.39, p > 0.10) or healthy controls (t = −1.27, p > 0.10) (figure 3). Patients with left temporal hypometabolism did have more activation in right WA than controls (t = 2.44, p < 0.05), while patients without hypometabolism did not differ from controls (t = 0.60, p > 0.10).
Figure 3. Mean left and right voxel activation in Wernicke area (WA).
Patients with left temporal hypometabolism, patients without left temporal hypometabolism, and healthy controls (*p < 0.05, amount of activation in right WA differs between controls and patients with left temporal hypometabolism).
Influence of age at onset on language laterality and hypometabolism.
Age at epilepsy onset was not correlated with degree of midtemporal or mesial temporal hypometabolism (p > 0.10), or with IFG LI or MFG LI (p > 0.10). However, earlier age at onset was correlated with lower WA LI (r = −0.39, p = 0.047) and moderated the relationship between left temporal hypometabolism and language laterality. After controlling for age at onset, greater left midtemporal hypometabolism was more strongly correlated with lower WA LI (r = −0.49, p = 0.01) and MFG LI (r = −0.43, p < 0.05), and became a stronger trend with IFG LI (r = −0.37, p = 0.06). Also, the strength of the relationship between increasing left mesial temporal hypometabolism and decreasing WA LI became more significant after controlling for age at onset (r = −0.54, p < 0.01), whereas MFG LI (r = −0.23, p > 0.10) and IFG LI (r = −0.18, p > 0.10) remained nonsignificant.
When divided into 2 groups by age at onset (early onset defined as ≤12 years, the median, for even statistical comparisons between groups), the relationship between WA LI and left temporal hypometabolism can be seen more clearly. Although the group numbers were small, patients with earlier onset (n = 13) had decreased WA LI in the presence of left temporal hypometabolism (mean WA LI = 0.51 ± 0.49) compared to no hypometabolism (mean WA LI = 0.80 ± 0.18). While these mean differences were not significant (t = 1.37, p = 0.197; Cohen d = 0.79, effect size r = 0.37), the medium effect size suggests that the effect of hypometabolism may become significant with more subjects. Patients with later onset epilepsy did not show these differences in WA LI based on the presence vs absence of left temporal hypometabolism (t = 0.04, p > 0.10, Cohen d = 0.02, effect size r = 0.01).
DISCUSSION
We found that greater regional temporal hypometabolism correlated with lessened strength of language dominance in the temporal region as assessed by fMRI. Furthermore, we found the relationship was related to younger age at epilepsy onset. These findings are not attributable to a disruption of the BOLD response used to determine language dominance. Our findings indicate that atypical language reflects left temporal functional pathology, which may be more pronounced with an earlier age at epilepsy onset.
These findings may also provide an explanation for the high incidence of atypical language dominance in patients with either normal MRI or MTS alone. Previous studies describe atypical language dominance in one-third of patients with TLE and normal MRI1 and in one-quarter of patients with MTS.1–3 The epileptogenic zone may be remote from the temporal neocortex that sustains receptive language processing. FDG-PET studies, however, show that functional metabolic abnormalities extend beyond the epileptogenic zone—often in mesial structures—and may be more pronounced in lateral temporal neocortex than in these mesial structures.4,5,12 Neocortical foci may be associated with hypometabolism that extends beyond the margin of the seizure focus.13 In our study, only one patient with atypical language and normal MRI had normal FDG-PET. There are both structural and PET data to suggest perturbation of a more widespread network involving basal ganglia and frontal cortex.12,14,15 The mechanism for regional hypometabolism, which is not fully understood, may be explained partially by neuronal loss in the hippocampal formation.16–19 However, functional disruption is an important factor. The widespread functional disruption identified by FDG-PET, frequently remote from the seizure focus, may provide an explanation for disruption of language processing in patients with neocortical TLE or a mesial seizure focus.
Postictal depression of neuronal function may impair the physiologic basis of BOLD and affect fMRI-based language maps.20 There are also case reports of language laterality reversing following successful temporal lobectomy.21 These data raise the possibility that metabolic perturbations by ictal and interictal activity may dampen the BOLD response on which fMRI measurements are based. There are other circumstances when the BOLD response is disrupted,22,23 including mass lesion effects and vascular steal. Furthermore, the relationship between blood flow and metabolism is altered in TLE neocortex.6,24 CBF is better preserved than rCMRglc, a divergence that may increase over time.25 Our data suggest that patients are able to mount a BOLD response in the interictal state, as hypometabolic areas generated a BOLD response comparable to normal volunteers and patients without hypometabolism. The reduced LI is not due solely to reduced left activation, and supports a shift of activity to the right. We do not have measures of CBF in our patients. The degree to which perturbations in perfusion, metabolism, and BOLD response exist may be determined by blood flow studies (arterial spin labeled fMRI or water PET) linked with FDG-PET.
A limitation of our study is that the lateral temporal ROI used for our fMRI and PET analyses overlap but are not entirely congruent across imaging modalities (Wernicke area [BA 21, 22, 39] compared to MTG). They are, however, those used for our standard clinical methods of assessing language dominance and regional hypometabolism, respectively. The relation between hippocampus (mesial temporal region) and neocortical lateral language processing would not be affected by our choice of regions. Although this issue may have a modest effect on lateral neocortical findings, the temporal hypometabolism extends beyond region boundaries.
Although only 8 patients had a right hemisphere seizure focus, they serve as a useful contrast to the patients with a left hemisphere focus, in showing the absence of an effect of right temporal hypometabolism on language. The degree of left temporal hypometabolism may be more sensitive than a binary categorization of its presence or absence in studying a relationship with fMRI laterality; therefore, including these subjects allows a greater range of glucose consumption with which to explore this relationship.
Ultimately, our findings support the notion that functional disruption in the ipsilateral temporal lobe may displace local cognitive processes.26–28 The earlier the onset of epilepsy or brain injury, the more likely are these functions to be affected.1,2,29 Hypometabolism in patients with early seizure onset had a more profound effect on language indices in temporal regions than in those with older onset. Older patients have lower LI values in general.30 There is some evidence that frequency of interictal discharges correlates with atypical language,31 but little data link interictal discharges to regional hypometabolism.32 We did not formally assess spike frequency in our studies. Furthermore, there is evidence that this disruption is not confined to temporal regions. Patients with temporal hypometabolism had lower LI in IFG and MFG. As the MFG is implicated in working memory, this finding may be a nonspecific effect of increased working memory demands as a result of generally compromised brain function, reflect compensation for greater demands on routine language function (in the way tasks perceived to be harder increase verbal working memory demands), or reflect compensation by working memory systems for disturbed hippocampal declarative memory function.33–35
The interplay between the seizure focus and widespread regional networks is complex and pervasive. There are functional consequences of focal pathology that may have widespread effects on brain function. In some instances they are associated with a shift of language dominance to the right hemisphere; in others, impaired function results in compensatory mechanisms for drawing upon a widely distributed network to perform essential cognitive functions.
Supplementary Material
Supplemental data at www.neurology.org
- AI
- asymmetry index
- AL
- atypical language
- BA
- Brodmann area
- BOLD
- blood oxygenation level–dependent
- CMRglc
- regional glucose metabolism
- FDG
- 18fluoro-deoxyglucose
- IFG
- inferior frontal gyrus
- LI
- laterality index
- LL
- left language
- MCD
- malformation of cortical development
- MFG
- middle frontal gyrus
- MTG
- middle temporal gyrus
- MTS
- mesial temporal sclerosis
- TLE
- temporal lobe epilepsy
- STG
- superior temporal gyrus
- WA
- Wernicke area
DISCLOSURE
Dr. Gaillard has served on a scientific advisory board for GE Healthcare and educational material advisory panels for Ovation Pharmaceuticals (now Lundbeck Inc.) and Questcor Pharmaceuticals, Inc.; served as an editor of Epilepsia; his department derives income from the clinical evaluation and management of children with epilepsy including performing EEG and vEEG; receives research support from Lundbeck Inc., King Pharmaceuticals, PRA International, Eisai Inc., Marinus Pharmaceuticals, Inc., the NIH (NINDS, NICHD, NIMH), and the CDC; and holds stock in Johnson & Johnson, Eli Lilly and Company, GlaxoSmithKline, and Pfizer Inc. Dr. Berl receives research support from the NIH. E.S. Duke reports no disclosures. Dr. Ritzl's spouse has served on the speakers' bureau for Wyeth. Dr. Miranda, Dr. Liew, A. Finegersh, A. Martinez, and I. Dustin report no disclosures. Dr. Sato receives research support and salary from NIH/NINDS DIR. Dr. Theodore serves as Co-editor-in-Chief of Epilepsy Research; receives research support and salary from the NIH/NINDS DIR; and holds stock in GE Healthcare.
REFERENCES
- 1. Gaillard WD, Berl MM, Moore EN, et al. Atypical language in lesional and nonlesional complex partial epilepsy. Neurology 2007;69:1761–1771 [DOI] [PubMed] [Google Scholar]
- 2. Woermann FG, Jokeit H, Luerding R, et al. Language lateralization by Wada test and fMRI in 100 patients with epilepsy. Neurology 2003;61:699–701 [DOI] [PubMed] [Google Scholar]
- 3. Thivard L, Hombrouck J, du Montcel ST, et al. Productive and perceptive language reorganization in temporal lobe epilepsy. Neuroimage 2005;24:841–851 [DOI] [PubMed] [Google Scholar]
- 4. Theodore WH, Gaillard WD, Sato S, Kufta C, Leiderman D. Positron emission tomographic measurement of cerebral blood flow and temporal lobectomy. Ann Neurol 1994;36:241–244 [DOI] [PubMed] [Google Scholar]
- 5. Hajek M, Antonini A, Leenders KL, Wieser WG. Mesiobasal versus lateral temporal lobe epilepsy: metabolic differences in the temporal lobe shown by interictal 18F-FDG positron emission tomography. Neurology 1993;43:79–86 [DOI] [PubMed] [Google Scholar]
- 6. Gaillard WD, Fazilat S, White S, et al. Interictal metabolism and blood flow are uncoupled in temporal lobe cortex of patients with complex partial epilepsy. Neurology 1995;45:1841–1847 [DOI] [PubMed] [Google Scholar]
- 7. Rosenberger LR, Zeck J, Berl MM, et al. Interhemispheric and intrahemispheric language reorganization in complex partial epilepsy. Neurology 2009;72:1830–1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wilke M, Schmithorst VJ. A combined bootstrap/histogram analysis approach for computing a lateralization index from neuroimaging data. Neuroimage 2006;33:522–530 [DOI] [PubMed] [Google Scholar]
- 9. Mbwana J, Berl MM, Ritzl EK, et al. Limitations to plasticity of language network reorganization in localization related epilepsy. Brain 2009;132:347–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gaillard WD, Balsamo L, Xu B, et al. Language dominance in partial epilepsy patients identified with an fMRI reading task. Neurology 2002;59:256–265 [DOI] [PubMed] [Google Scholar]
- 11. Hasler G, Bonwetsch R, Giovacchini G, et al. 5-HT1A receptor binding in temporal lobe epilepsy patients with and without major depression. Biol Psychiatry 2007;62:1258–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Henry TR, Frey KA, Sackellares JC, et al. In vivo cerebral metabolism and central benzodiazepine-receptor binding in temporal lobe epilepsy. Neurology 1993;43:1998–2006 [DOI] [PubMed] [Google Scholar]
- 13. Alkonyi B, Juhasz C, Muzik O, et al. Quantitative brain surface mapping of an electrophysiologic/metabolic mismatch in human neocortical epilepsy. Epilepsy Res 2009;87:77–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. DeCarli C, Hatta J, Fazilat S, Fazilat S, Gaillard WD, Theodore WH. Extratemporal atrophy in patients with complex partial seizures of left temporal origin. Ann Neurol 1998;43:41–45 [DOI] [PubMed] [Google Scholar]
- 15. Bernasconi N, Duchesne S, Janke A, Lerch J, Collins DL, Bernasconi A. Whole-brain voxel-based statistical analysis of gray matter and white matter in temporal lobe epilepsy. Neuroimage 2004;23:717–723 [DOI] [PubMed] [Google Scholar]
- 16. Semah F, Baulac M, Hasboun D, et al. Is interictal temporal hypometabolism related to mesial temporal sclerosis? A positron emission tomography/magnetic resonance imaging confrontation. Epilepsia 1995;36:447–456 [DOI] [PubMed] [Google Scholar]
- 17. Theodore WH, Gaillard WD, De Carli C, Bhatia S, Hatta J. Hippocampal volume and glucose metabolism in temporal lobe epileptic foci. Epilepsia 2001;42:130–132 [DOI] [PubMed] [Google Scholar]
- 18. Diehl B, LaPresto E, Najm I, et al. Neocortical temporal FDG-PET hypometabolism correlates with temporal lobe atrophy in hippocampal sclerosis associated with microscopic cortical dysplasia. Epilepsia 2003;44:559–564 [DOI] [PubMed] [Google Scholar]
- 19. Gaillard WD, Bhatia S, Bookheimer SY, Fazilat S, Sato S, Theodore WH. FDG-PET and volumetric MRI in the evaluation of patients with partial epilepsy. Neurology 1995;45:123–126 [DOI] [PubMed] [Google Scholar]
- 20. Jayakar P, Bernal B, Santiago Medina L, Altman N. False lateralization of language cortex on functional MRI after a cluster of focal seizures. Neurology 2002;58:490–492 [DOI] [PubMed] [Google Scholar]
- 21. Helmstaedter C, Fritz NE, Gonzalez Perez PA, Elger CE, Weber B. Shift-back of right into left hemisphere language dominance after control of epileptic seizures: evidence for epilepsy driven functional cerebral organization. Epilepsy Res 2006;70:257–262 [DOI] [PubMed] [Google Scholar]
- 22. Wellmer J, Weber B, Urbach H, Reul J, Fernandez G, Elger CE. Cerebral lesions can impair fMRI-based language lateralization. Epilepsia 2009;50:2213–2224 [DOI] [PubMed] [Google Scholar]
- 23. Gaillard WD. Functional MR imaging of language, memory, and sensorimotor cortex. Neuroimaging Clin N Am 2004;14:471–485 [DOI] [PubMed] [Google Scholar]
- 24. Lee DS, Lee JS, Kang KW, et al. Disparity of perfusion and glucose metabolism of epileptogenic zones in temporal lobe epilepsy demonstrated by SPM/SPAM analysis on 15O water PET, [18F]FDG-PET, and [99mTc]-HMPAO SPECT. Epilepsia 2001;42:1515–1522 [DOI] [PubMed] [Google Scholar]
- 25. Breier JI, Mullani NA, Thomas AB, et al. Effects of duration of epilepsy on the uncoupling of metabolism and blood flow in the complex partial seizures. Neurology 1997;48:1047–1053 [DOI] [PubMed] [Google Scholar]
- 26. Loring DW, Strauss E, Hermann BP, et al. Effects of anomalous language representation on neuropsychological performance in temporal lobe epilepsy. Neurology 1999;53:260–264 [DOI] [PubMed] [Google Scholar]
- 27. Oyegbile TO, Dow C, Jones J, et al. The nature and course of neuropsychological morbidity in chronic temporal lobe epilepsy. Neurology 2004;62:1736–1742 [DOI] [PubMed] [Google Scholar]
- 28. Kaaden S, Helmstaedter C. Age at onset of epilepsy as a determinant of intellectual impairment in temporal lobe epilepsy. Epilepsy Behav 2009;15:213–217 [DOI] [PubMed] [Google Scholar]
- 29. Springer JA, Binder JR, Hammeke TA, et al. Language dominance in neurologically normal and epilepsy subjects: a functional MRI study. Brain 1999;122:2033–2046 [DOI] [PubMed] [Google Scholar]
- 30. Szaflarski JP, Holland SK, Schmithorst VJ, Byars AW. fMRI study of language lateralization in children and adults. Hum Brain Mapp 2006;27:202–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Janszky J, Mertens M, Janszky I, Ebner A, Woermann FG. Left-sided interictal epileptic activity induces shift of language lateralization in temporal lobe epilepsy: an fMRI study. Epilepsia 2006;47:921–927 [DOI] [PubMed] [Google Scholar]
- 32. Koutroumanidis M, Binnie CD, Elwes RD, et al. Interictal regional slow activity in temporal lobe epilepsy correlates with lateral temporal hypometabolism as imaged with 18FDG PET: neurophysiological and metabolic implications. J Neurol Neurosurg Psychiatry 1998;65:170–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Berl MM, Balsamo LM, Xu B, et al. Seizure focus affects regional language networks assessed by fMRI. Neurology 2005;65:1604–1611 [DOI] [PubMed] [Google Scholar]
- 34. Dupont S, Van de Moortele PF, Samson S, et al. Episodic memory in left temporal lobe epilepsy: a functional MRI study. Brain 2000;123:1722–1732 [DOI] [PubMed] [Google Scholar]
- 35. Powell HW, Richardson MP, Symms MR, et al. Reorganization of verbal and nonverbal memory in temporal lobe epilepsy due to unilateral hippocampal sclerosis. Epilepsia 2007;48:1512–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.