Abstract
The haploid pathogenic yeast Candida glabrata is the second most common Candida species isolated from cases of bloodstream infection. The clinical relevance of C. glabrata is enhanced by its reduced susceptibility to fluconazole. Despite this, little is known of the epidemiology or population structure of this species. We developed a multilocus sequence typing (MLST) scheme for C. glabrata and used it to fingerprint a geographically diverse collection of 107 clinical isolates and 2 reference strains. Appropriate loci were identified by amplifying and sequencing fragments of the coding regions of 11 C. glabrata genes in 10 unrelated isolates. The 6 most variable loci (FKS, LEU2, NMT1, TRP1, UGP1, and URA3) were sequenced in the collection of 109 isolates. From the 3,345 bp sequenced in each isolate, 81 nucleotide sites were found to be variable. These defined 30 STs among the 109 strains. The technique was validated by comparison with random amplified polymorphic DNA and the complex DNA fingerprinting probes Cg6 and Cg12. MLST identified 5 major clades among the isolates studied. Three of the clades exhibited significant geographical bias. Our data demonstrate for the first time, with such a large geographically diverse strain collection, that distinct genetic clades of C. glabrata prevail in different geographical regions.
Candida spp. now represent the fourth most commonly isolated organisms from nosocomial bloodstream infections (8). In the past, the majority of infections were caused by Candida albicans. However, the proportion of infections caused by non-C. albicans species is increasing (1, 14, 29, 37). Though some geographical variations exist, Candida glabrata is now considered the most commonly isolated of the non-C. albicans species (6, 30), and the incidence of C. glabrata bloodstream infections is rising (31, 32). C. glabrata is also emerging as the second most common cause of vaginitis (39). This increase in the incidence of C. glabrata infections is noteworthy because of its decreased susceptibility to fluconazole (30, 31) and the high crude mortality rate of bloodstream infection (13, 43)
In spite of its increased prominence, little is known of the population structure, epidemiology, and basic biology of C. glabrata. Recently, however, several studies have been reported that shed light on the developmental capabilities of this organism. These have included the description of phenotypic switching (19, 20), the demonstration of pseudohypha and tube formation (4, 19), and the identification and characterization of a mating system that is similar to that of Saccharomyces cerevisiae (41, 44). Studies of the population structure of the organism have also begun to emerge (5). To facilitate the latter, we have developed and begun to characterize a fingerprinting method based upon multilocus sequencing.
A number of different genetic fingerprinting methods have previously been applied to C. glabrata (40), including random amplification of polymorphic DNA (RAPD) (22, 24), pulsed-field gel electrophoresis (PFGE) (17, 42), multilocus enzyme electrophoresis (MLEE) (5), and fingerprinting with complex DNA probes (24). To date, there has only been one example of direct sequence comparison for typing C. glabrata (38). This study involved an analysis of variation in the mitochondrial COX2 gene. To obtain a higher resolution DNA fingerprinting system based on direct sequence comparison, we developed a multilocus sequence typing (MLST) system, in which data from alleles at multiple loci are combined to assess genetic relatedness. Though this system is based on the same principles as MLEE, MLST has the advantage in that genetic variation based upon differences in DNA sequences is greater than that assessed by differences in protein mobility. Hence, fewer loci are needed to achieve the same level of discrimination as MLEE. However, the major advantage of MLST over many other typing techniques is the unambiguous nature of the data generated. This allows laboratories to easily compare data and allows for the construction of large international internet-accessible databases such as those for the bacterial pathogens Neisseria meningitidis and Streptococcus pneumoniae (available at www.mlst.net). MLST schemes now exist for a number of important bacterial pathogens including N. meningitidis (26), S. pneumoniae (10), Staphylococcus aureus (11), Streptococcus pyogenes (9), and Campylobacter jejuni (7). The technique has also been successfully used to assess genetic relatedness among strains of C. albicans (3), Aspergillus flavus (12), and Coccidioides immitis (18).
We describe here the development of an MLST scheme for the haploid pathogenic yeast C. glabrata by using sequences from 6 genes to type a diverse collection of 109 isolates. We compared this MLST method with current typing techniques to validate the system (40). We have also used the MLST method to examine the genetic relatedness of isolates from different geographical locales and isolates with different levels of fluconazole resistance.
MATERIALS AND METHODS
Isolates.
A total of 107 clinical isolates and two reference strains were used (Table 1). The clinical isolates were geographically diverse, comprising 63 from Europe (29 from the United Kingdom, 16 from Spain, 8 from Belgium, 7 from Germany, 2 from Switzerland, and 1 from The Netherlands), 28 from the United States, 14 from Japan, and 2 from Chile. Isolates from a wide variety of clinical sites, including the bloodstream and the genitourinary and gastrointestinal tracts, were represented in the collection. Twenty-six isolates had previously been typed by RAPD and Southern blotting with the complex C. glabrata-specific DNA probes Cg6 and Cg12 (24). Twenty-one isolates had previously been analyzed for MICs of fluconazole by published methods (21). Eight isolates were fluconazole resistant (MIC ≥ 64 μg/ml) by the criteria of Rex et al. (36).
TABLE 1.
Details of isolates used in this studya
| Identification no. | Location or strain | Clinical source | Identification no. | Location strain | Clinical course | |
|---|---|---|---|---|---|---|
| UK-01 | Newcastle, UK | Blood | ||||
| UK-02 | Chester, UK | Vagina | ||||
| UK-03 | Salford, UK | Vagina | ||||
| UK-04 | Hartlepool, UK | Blood | ||||
| UK-05 | Cumberland, UK | Urine | ||||
| UK-06 | Salford, UK | Throat | ||||
| UK-07 | Queen's Park, UK | Blood | ||||
| UK-08 | Salford, UK | Urine | ||||
| UK-09 | Salford, UK | Throat | ||||
| UK-10 | Liverpool, UK | Blood | ||||
| UK-11 | Burnley, UK | Blood | ||||
| UK-12 | Salford, UK | Blood | ||||
| UK-13 | Worksop, UK | Sputum | ||||
| UK-14 | Salford, UK | Bronchial wash | ||||
| UK-15 | Salford, UK | Blood | ||||
| UK-16 | Stockport, UK | NK | ||||
| UK-17 | Salford, UK | Throat | ||||
| UK-18 | Salford, UK | Femoral line | ||||
| UK-19 | Newcastle, UK | Abscess | ||||
| UK-20 | Ormskirk, UK | Blood | ||||
| UK-21 | Salford, UK | Abdominal abscess | ||||
| UK-22 | Rochdale, UK | Vagina | ||||
| UK-23 | Bolton, UK | Vagina | ||||
| UK-24 | Salford, UK | Tracheal aspirate | ||||
| UK-25 | Antrim, UK | Blood | ||||
| UK-26 | Tameside, UK | Blood | ||||
| UK-27 | Withington, UK | Line tip | ||||
| UK-28 | Salford, UK | Femoral line | ||||
| UK-29 | Hartlepool, UK | NK | ||||
| CE-01 | Switzerland | NK | ||||
| CE-02 | Switzerland | NK | ||||
| CE-03 | Belgium | Fecal sample | ||||
| CE-04 | Belgium | Vagina | ||||
| CE-05 | Germany | Oral cavity | ||||
| CE-06 | Germany | Oral cavity | ||||
| CE-07 | Germany | Oral cavity | ||||
| CE-08 | Germany | Oral cavity | ||||
| CE-09 | Germany | Oral cavity | ||||
| CE-10 | Germany | Oral cavity | ||||
| CE-11 | Germany | Oral cavity | ||||
| CE-12 | Belgium | Anus | ||||
| CE-13 | Belgium | Vagina | ||||
| CE-14 | Belgium | Vagina | ||||
| CE-15 | Belgium | Vagina | ||||
| CE-16 | Belgium | Vagina | ||||
| CE-17 | Belgium | NK | ||||
| CE-18 | The Netherlands | Blood | ||||
| SP-01 | Galicia, Spain | Blood | ||||
| SP-02 | Castilla-LaMancha, Spain | Blood | ||||
| SP-03 | Almeria, Spain | NK | ||||
| SP-04 | Vizcaya, Spain | NK | ||||
| SP-05 | Canary Islands | NK | ||||
| SP-06 | Asturias, Spain | NK | ||||
| SP-07 | Albacete, Spain | NK | ||||
| SP-08 | Barcelona, Spain | NK | ||||
| SP-09 | Alicante, Spain | NK | ||||
| SP-10 | Madrid, Spain | NK | ||||
| SP-11 | Alicante, Spain | NK | ||||
| SP-12 | Tarragona, Spain | NK | ||||
| SP-13 | Pontevedra, Spain | NK | ||||
| SP-14 | Albacete, Spain | NK | ||||
| SP-15 | Madrid, Spain | NK | ||||
| SP-16 | Zamora, Spain | NK | ||||
| SA-01 | Chile | NK | ||||
| SA-02 | Chile | Catheter | ||||
| US-01 | United States | Blood | ||||
| US-02 | United States | Blood | ||||
| US-03 | Richmond, Va. | Oral cavity | ||||
| US-04 | Iowa City, Iowa | Oral cavity | ||||
| US-05 | Iowa City, Iowa | Oral cavity | ||||
| US-06 | Iowa City, Iowa | Oral cavity | ||||
| US-07 | Iowa City, Iowa | Oral cavity | ||||
| US-08 | Iowa City, Iowa | Oral cavity | ||||
| US-09 | Iowa City, Iowa | Oral cavity | ||||
| US-10 | Detroit, Mich. | Vagina | ||||
| US-11 | Detroit, Mich. | Vagina | ||||
| US-12 | Detroit, Mich. | Vagina | ||||
| US-13 | Iowa City, Iowa | NK | ||||
| US-14 | Iowa City, Iowa | Oral cavity | ||||
| US-15 | Detroit, Mich. | Vagina | ||||
| US-16 | Detroit, Mich. | Vagina | ||||
| US-17 | Detroit, Mich. | Vagina | ||||
| US-18 | Iowa City, Iowa | Oral cavity | ||||
| US-19 | Detroit, Mich. | Vagina | ||||
| US-20 | Detroit, Mich. | Vagina | ||||
| US-21 | Richmond, Va. | Oral cavity | ||||
| US-22 | Richmond, Va. | Oral cavity | ||||
| US-23 | Richmond, Va. | Oral cavity | ||||
| US-24 | Richmond, Va. | Oral cavity | ||||
| US-25 | Richmond, Va. | Oral cavity | ||||
| US-26 | Richmond, Va. | Oral cavity | ||||
| US-27 | Richmond, Va. | Oral cavity | ||||
| US-28 | Richmond, Va. | Oral cavity | ||||
| J-01 | Japan | Oral cavity | ||||
| J-02 | Japan | Oral cavity | ||||
| J-03 | Japan | Blood | ||||
| J-04 | Japan | Blood | ||||
| J-05 | Japan | Sputum | ||||
| J-06 | Japan | Sputum | ||||
| J-07 | Japan | Nasal discharge | ||||
| J-08 | Japan | Nasal discharge | ||||
| J-09 | Japan | Fecal sample | ||||
| J-10 | Japan | Fecal sample | ||||
| J-11 | Japan | Secretion | ||||
| J-12 | Japan | Secretion | ||||
| J-13 | Japan | Pus | ||||
| J-14 | Japan | Pus | ||||
| ATCC | ACTC 90030 | NK | ||||
| NCPF | NCPF3309 | Fecal sample |
Isolates are listed with the identification number used in this study, where the prefix denotes the region from which the isolate was obtained. UK, United Kingdom; CE, other Continental Europe; SP, Spain; SA, South America; US, United States; J, Japan; NK, not known; ATCC, American Type Culture Collection; NCPF, National Collection of Pathogenic Fungi.
DNA extraction and PCR amplification.
DNA was extracted by using the Qiagen DNA tissue kit (Qiagen, Crawley, United Kingdom) according to the manufacturer's instructions. Oligonucleotide primers (Table 2) were designed by using GenBank sequences of C. glabrata genes as templates. PCRs were performed in 25-μl volumes containing 2.5 ng of C. glabrata DNA, 1 U of Taq DNA polymerase (Q-Biogene, Harefield, United Kingdom), 2.5 μl of 10× PCR buffer plus 1.5 mM MgCl2 (provided with Taq DNA polymerase), 0.2 mM concentrations of combined deoxynucleoside triphosphates (Amersham Pharmacia Biotech, Little Chalfont, United Kingdom), and 0.2 μM concentrations of each primer (Table 2). The reaction conditions were as follows: 7 min at 94°C, 30 cycles of 1 min at 94°C, 1 min at the relevant annealing temperature (Table 2), and 1 min at 74°C, followed by 10 min at 74°C. The reactions were performed on a Perkin Elmer GeneAmp PCR system 2400 thermal cycler (Perkin Elmer, Norwalk, Conn.). Purification of the PCR product was done with the Genelute PCR clean-up kit (Sigma-Aldrich Life Sciences, Poole, United Kingdom) according to the manufacturer's instructions.
TABLE 2.
Oligonucleotide primers for amplification and sequencing of loci used in MLST scheme
| Locus | Gene product | GenBank accession no. | Primer | Primer sequence (5′-3′) | Annealing temp (°C) |
|---|---|---|---|---|---|
| FKS | 1,3-Beta-glucan synthase | AF229171 | FKSF1 | GTCAAATGCCACAACAACAACCT | 55.0 |
| FKSR1 | AGCACTTCAGCAGCGTCTTCAG | ||||
| LEU2 | 3-Isopropylmalate dehydrogenase | U90626 | LEU2F1 | TTTCTTGTATCCTCCCATTGTTCA | 54.0 |
| LEU2R1 | ATAGGTAAAGGTGGGTTGTGTTGC | ||||
| NMT1 | Myristoyl-CoA, protein N-myristoyltransferasea | AF073886 | NMT1F1 | GCCGGTGTGGTGTTGCCTGCTC | 59.0 |
| NMT1R1 | CGTTACTGCGGTGCTCGGTGTCG | ||||
| TRP1 | Phosphoribosyl-anthranilate isomerase | U31471 | TRP1F1 | AATTGTTCCAGCGTTTTTGT | 50.0 |
| TRP1R1 | GACCAGTCCAGCTCTTTCAC | ||||
| UGP1 | UTP-glucose-1-phosphate uridylyltransferase | AB037186 | UGP1F1 | TTTCAACACCGACAAGGACACAGA | 57.0 |
| UGP1R1 | TCGGACTTCACTAGCAGCAAATCA | ||||
| URA3 | Orotidine-5′-phosphate decarboxylase | L13661 | URA3F1 | AGCGAATTGTTGAAGTTGGTTGA | 53.0 |
| URA3R1 | AATTCGGTTGTAAGATGATGTTGC |
CoA, coenzyme A.
Sequencing.
All loci were sequenced in both the forward and reverse directions with the same primers as those used for the PCRs. Sequencing reactions were performed in a 20-μl volume with 3 pmol of oligonucleotide primer, 25 ng of template, 4 μl of BigDye terminator cycle sequencing ready reaction mix (version 1; ABI, Warrington, United Kingdom), and 2 μl of 5× sequencing buffer (80 mM Tris-Cl [lpH 9.0], 2 mM MgCl2 [final concentrations]). Excess chromophore was removed by ethanol precipitation. The reaction products were analyzed with an ABI Prism 377 DNA sequencer.
Selection of suitable loci.
Comparisons were made of the sequence variation of DNA fragments of the coding regions of ADE2, ERG11, FKS, HEM2, LEU2, NEP1, NMT1, PSA1, TRP1, UGP1, and URA3 in 10 unrelated isolates. Nucleotide sequences were determined by alignment of the forward and reverse sequences with the Genebuilder program of the Bionumerics package (Applied Maths, Sint-Martens-Latem, Belgium). All novel polymorphisms were confirmed visually by examination of the sequencing traces. Single base pair differences were considered significant. Therefore, each allele was defined by a unique sequence. Unique alleles were assigned arbitrary numbers as they were identified. Sequence types (STs) were defined by combining the allelic data obtained from a number of loci. The combination of the alleles gave an allelic profile, which was used to assign a ST. Therefore, each ST was described by a unique combination of alleles. STs were numbered in order of their identification, with no reference to relatedness. Those loci showing the most variation, and combinations of which generated the most STs, were chosen for use in the MLST scheme. The six genes selected for the MLST scheme to analyze the collection of 109 isolates were FKS, LEU2, NMT1, TRP1, UGP1, and URA3. To determine whether the addition of further loci could increase the discrimination of the scheme, the loci ERG11, NEP1, and PSA1 (which had shown a degree of variability in initial screening) and a further 6 loci (MSH4, CCA1, RAP1, HIS3, SNF1, and RRN1) were sequenced from 24 selected isolates representing 4 STs. For all of the loci studied, only coding regions were considered for analysis.
Data analysis.
The dendrogram of the whole collection was constructed from the matrix of pairwise similarity from the 3,345-bp concatenated DNA sequence (a composite of the sequences from all 6 loci) by using the unweighted pair group method with arithmetic averages (UPGMA) computed by the Bionumerics package. Assessment of the significance of the nodes was done by bootstrapping with 1,000 randomizations. Only the polymorphic sites were used for the bootstrap analysis. STs were also grouped based upon allelic profiles by using the BURST program (available at www.mlst.net), defining a group as those STs sharing alleles at 4 or more of the 6 analyzed loci. Assessment of the likelihood of selective pressure at each of the loci was calculated by the ratio of nonsynonymous to synonymous nucleotide substitutions (dN/dS) by using the method of Nei and Gojobori (28) implemented in the START program (available at http://outbreak.ceid.ox.ac.uk/software.shtml). Dendrograms for the isolates typed by RAPD and Southern blotting with the probes Cg6 and Cg12 were produced by using previously published methods (24). To enable comparison, dendrograms were generated from MLST data with DENDRON software (40), with the nucleotides at polymorphic sites used to represent bands.
RESULTS
Sequence variability.
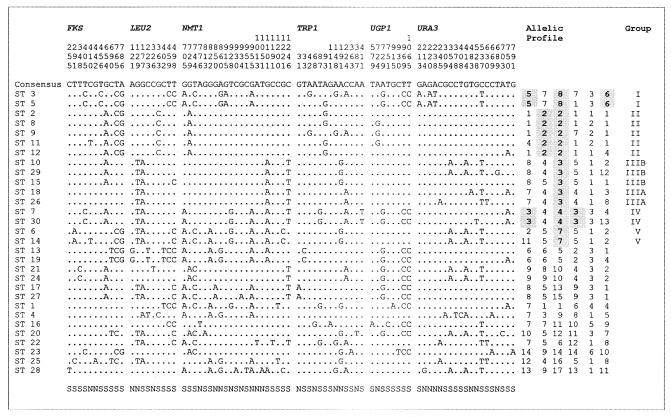
For MLST analysis, sequences from 6 loci were analyzed in 109 isolates of C. glabrata. A total of 3,345 bp were sequenced in the 6 loci in each isolate. The sizes of the 6 fragments ranged between 419 bp (TRP1) and 616 bp (UGP1), as shown in Table 3. No insertions, deletions, or heterozygosities were detected in any of the sequenced DNA fragments. Eighty-one (2.5%) polymorphic sites were identified among the 6 loci (Fig. 1). The number of variable nucleotide sites per locus ranged between 6 (1.3%, UGP1) and 21 (3.5%, NMT1). Data for all 6 loci are shown in Table 3 and Fig. 1. The polymorphisms defined between 8 (UGP1) and 17 (NMT1) alleles per locus. The ratio of synonymous to nonsynonymous nucleotide substitutions (dN/dS) as calculated by the method of Nei and Gojobori (28) was below 1 for all 6 loci (Table 3), suggesting that none were under positive selective pressure. The three loci with the highest dN/dS ratios (NMT1, TRP1, and URA3) were also the three that exhibited the greatest percentage of variable sites.
TABLE 3.
Variability of loci used in MLST scheme in the 109 isolates tested
| Locus | No. of bp sequenced | No. of variable sites (%) | No. of alleles defined | dN/dSa |
|---|---|---|---|---|
| FKS | 589 | 11 (1.9) | 14 | 0.037 |
| LEU2 | 512 | 9 (1.8) | 9 | 0.096 |
| NMT1 | 607 | 21 (3.5) | 17 | 0.145 |
| TRP1 | 419 | 13 (3.1) | 14 | 0.214 |
| UGP1 | 616 | 8 (1.3) | 6 | 0.036 |
| URA3 | 602 | 19 (3.2) | 13 | 0.118 |
dN/dS refers to the ratio of nonsynonymous to synonymous nucleotide substitutions as calculated by the method of Nei and Gojobori (28).
FIG. 1.
Polymorphic sites of the 6 loci used in the MLST scheme. The polymorphisms comprising each of the 30 STs found in the 109 isolates tested are shown. The STs are arranged in the 5 groups defined in the study, followed by the pairs of STs differing at only one allele, followed finally by the STs not fitting these criteria. The allelic profiles and groups of the STs are also shown. The allelic profiles are given in the order FKS, LEU2, NMT1, TRP1, UGP1, and URA3. Synaptomorphic alleles are indicated in boldface type. The letters under the figure indicate whether the polymorphism is synonymous (S) or nonsynonymous (N). The position of the polymorphism in the coding sequence is given by reading the numbers vertically.
Sequence typing and grouping of isolates.
STs were assigned by combining the data from each of the 6 loci sequenced per isolate. Each ST represents a unique combination of alleles. STs were named as they were described, with no reference to relatedness. A total of 30 STs could be defined by using the 6 loci (Fig. 1). It was necessary to include all 6 loci to differentiate all 30 STs. The number of isolates with each ST varied widely, from 26 with ST3, to 17 STs represented by a single isolate (Fig. 2). Eight pairs of STs were separated by differences at only one locus (Fig. 1). Of these, 5 were different by only one base (Fig. 1). Pairwise similarities were calculated between each pair of concatenated DNA sequences. The similarities were used to generate a UPGMA dendrogram. In the dendrogram generated for the whole collection of isolates (Fig. 2), 12 nodes presented greater than 60% confidence in bootstrap analysis. These nodes were all found above a DNA sequence similarity threshold of 99.6%. Thus, we used this arbitrary threshold to distinguish 5 major groups (I to V) in the collection of isolates analyzed. Four of the five groups had bootstrap values of 98% or higher. However, the value of 64% for group III was relatively low. To better assess the integrity of group III, the maximum-parsimony method was applied. It confirmed the integrity of this group (data not shown). Partitioning of STs based on the alleles themselves (with the BURST program) defined the same groups, with the exception of group III, which was subdivided into 2 smaller groups by this method (designated IIIA and IIIB), again suggesting this group to be less homogeneous than the others. In addition, a number of synaptomorphic alleles were associated with each of the five groups (Fig. 1). Alleles were deemed synaptomorphic when they were shared by all members of a given group and were not present in any other isolates in the collection analyzed (35). The alleles FKS-5, NMT1-8, and URA3-6 were group I specific and were identified in all members of group I. Group II members were the only isolates with LEU2-2 or NMT1-2. NMT1-3 was found only in group III isolates. Group IV isolates shared three synaptomorphic alleles, FKS-3, NMT1-4, and TRP1-3. NMT1-7 was synaptomorphic for group V isolates. It should be noted that each of the five groups had an NMT1 synaptomorphic allele and that in this collection the NMT1 data alone were sufficient to distinguish the groups. Together, these data suggest that the five groups described represent genuine clades, even if group III shows higher heterogeneity.
FIG. 2.
Dendrogram showing the relationships of all 109 isolates typed by MLST. The dendrogram was generated by using the UPGMA method from the concatenated sequence obtained from all 6 loci used in the MLST scheme. Bootstrap values of >60% are shown. The STs to which the isolates belong are shown. Groups defined by a sequence similarity of >99.6% (shown by a dashed line) are also shown. Isolates from Europe are shown in blue, those from Japan are shown in red, and those from the United States are shown in green. All other isolates are shown in black. The 8 isolates known to be fluconazole resistant (MIC ≥ 64 μg/ml) are underlined.
Analysis of additional loci.
To test whether the discriminatory power of the MLST scheme could be improved by the addition of other loci, fragments of the genes CCA1, ERG11, HIS3, MSH4, NEP1, PSA1, RAP1, RRN1, and SNF1 were sequenced in 24 selected isolates. The loci ERG11, NEP1, and PSA1 had previously been discarded following the initial screen for loci appropriate for the MLST scheme. They nevertheless exhibited a degree of variability warranting further evaluation. The isolates selected for further sequencing were taken from 4 frequently occurring STs (5 from ST2, 11 from ST3, 4 from ST7, and 4 from ST18). The sequence variability of the 9 loci and a comparison with the variability of the 6 loci used in the MLST scheme are shown in Table 4. In the 24 isolates sequenced, the percentage of variable sites in the additional loci ranged between 0.1 and 2.3%, which was similar to the variability of the original 6 MLST loci in these isolates of 0.5 to 1.5%. Overall, taking all of the 9 additional loci into account, 0.9% of the 5,894 nucleotide sites sequenced were variable, in comparison to 1% of the 3,345 sites sequenced for the 6 MLST loci. This resulted in 53 polymorphic sites analyzed with the additional loci versus 34 polymorphic sites analyzed for the original 6 MLST loci. In the 24 isolates, there were between 2 and 4 alleles per locus with both sets of loci. The distribution of the alleles was such that even with a greater number of polymorphic sites analyzed for the additional loci, none of the loci were able to further subdivide any of the STs identified by using the original 6 MLST loci. This suggests that the genetic variability obtained through the analysis of the original 6 loci used in the MLST scheme was close to the limit of discrimination that could be achieved.
TABLE 4.
Genetic variability observed in the 6 MLST loci and the 9 additional loci sequenced in 24 isolates
| Locus | MLST or additional locusa | No. of bp sequenced | No. of variable sites | % Variable sites | No. of alleles |
|---|---|---|---|---|---|
| FKS | M | 589 | 5 | 0.8 | 4 |
| LEU2 | M | 512 | 5 | 1.0 | 3 |
| NMT1 | M | 607 | 9 | 1.5 | 4 |
| TRP1 | M | 419 | 6 | 1.4 | 4 |
| UGP1 | M | 616 | 3 | 0.5 | 2 |
| URA3 | M | 602 | 6 | 1.0 | 4 |
| Total for MLST loci | 3,345 | 34 | 1.0 | 21 | |
| CCA1 | A | 815 | 8 | 1.0 | 4 |
| ERG11 | A | 614 | 3 | 0.5 | 3 |
| HIS3 | A | 572 | 13 | 2.3 | 4 |
| MSH4 | A | 682 | 8 | 1.2 | 3 |
| NEP1 | A | 522 | 3 | 0.6 | 3 |
| PSA1 | A | 597 | 3 | 0.5 | 4 |
| RAP1 | A | 598 | 1 | 0.2 | 2 |
| RRN1 | A | 857 | 4 | 0.5 | 3 |
| SNF | A | 637 | 10 | 1.6 | 4 |
| Total for additional loci | 5,894 | 53 | 0.9 | 30 |
The 6 loci comprising the MLST scheme used to type the full collection of 109 isolates are indicated by the letter M; the 9 additional loci sequenced in 24 isolates only are indicated by the letter A.
Comparison of the clustering ability of MLST and other DNA fingerprinting methods.
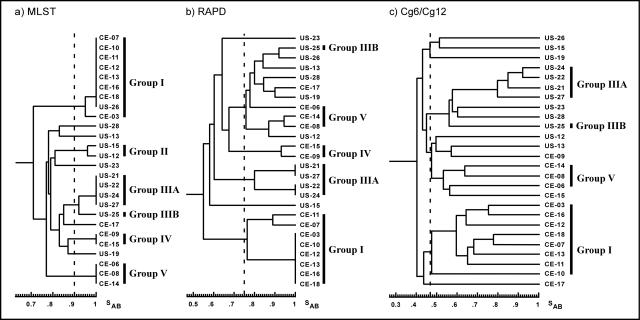
To verify the ability of a typing technique to correctly cluster related isolates, it is necessary to compare the clusters obtained by the scheme in question with those generated by other unrelated typing techniques (40). Of the 109 isolates typed in this study, 26 formed a representative subset of the 39 used by Lockhart et al. (24) to develop and verify DNA fingerprinting probes for C. glabrata. Dendrograms constructed for these 26 isolates generated from MLST and the results of previous typing with RAPD (24) and DNA fingerprinting with the combined results of the complex probes Cg6 and Cg12 (24) are presented for comparison in Fig. 3a, b, and c, respectively. An SAB threshold of 0.9 defined the groups described from the MLST data. Arbitrary thresholds of 0.75 and 0.47 were used to cluster the isolates typed by RAPD and by combined Cg6 and Cg12 fingerprinting, respectively. Eight of the nine group I isolates clustered in the RAPD and Cg6/Cg12 dendrograms, with the one exception being isolate US-26 (Fig. 3). The two isolates in group II (US-12 and US-15) did not cluster in the RAPD or Cg6/Cg12 dendrogram (Fig. 3). All four isolates in group IIIA of the MLST dendrogram also clustered in the RAPD and Cg6/Cg12 dendrograms (Fig. 3). The two isolates in group IV of the MLST dendrogram clustered in the RAPD dendrogram but not in the Cg6/Cg12 dendrogram (Fig. 3). Finally, all three isolates in group V of the MLST dendrogram appear in the same clusters in the RAPD and Cg6/Cg12 dendrograms. Of the isolates that did not cluster in the RAPD and Cg6/Cg12 dendrograms, three isolates clustered differently with each of the three typing techniques (US-12, US-15, and US-26). Strains CE-09 and CE-15, both from group IV, clustered with RAPD but not with Cg6/Cg12, whereas US-25 clustered with other isolates of group III when typed with Cg6/Cg12 but not with RAPD. The similar grouping of the majority of the isolates by RAPD and Cg6/Cg12 fingerprinting, which can be assumed to involve different DNA markers, supported the ability of the MLST scheme developed to cluster genetically related isolates.
FIG. 3.
Dendrograms based on the computed SABs between the 26 isolates typed by MLST (a), RAPD (b), and combined data obtained by Southern blotting with the DNA probes Cg6 and Cg12 (c). The arbitrary SAB thresholds used to define clusters are shown by dashed lines.
Geographical distribution.
Table 5 shows the geographical distribution of the 103 clinical isolates from individual patients from Europe, Japan, and the United States with respect to the 5 groups. Fisher's exact tests were used to statistically assess differences in distribution of the groups among the three populations. Despite the relatively small numbers of isolates in these geographical groupings, significant (P < 0.05) differences were observed in the distribution of three of the groups. Group I was significantly overrepresented in the European population with respect to the Japanese and United States populations. This situation was reversed in group III, which was significantly underrepresented among European isolates with respect to those from the United States, although with respect to Japan, the distribution just failed to reach significance (P = 0.0509). Japanese isolates, when compared to either the United States or European populations, had a significant bias toward group IV. Seven (54%) of the Japanese isolates were group IV in comparison to none from the United States and only 4 (6%) from Europe. Group II consisted of 19 isolates, none of which were Japanese. While this distribution just failed to reach significance, with P values of 0.057 and 0.077 for the distribution of Japanese isolates versus European and United States isolates, respectively, it suggested a trend. Together, these results suggest geographical specificity in some of the clades.
TABLE 5.
Geographical distribution of groupsa
| Location (n) | % of strains in
group:
|
|||||
|---|---|---|---|---|---|---|
| I | II | III | IV | V | Others | |
| Europe (63) | 37 | 21 | 10 | 6 | 10 | 17 |
| United States (27) | 11 | 22 | 30 | 0 | 11 | 26 |
| Japan (13) | 0 | 0 | 31 | 54 | 8 | 8 |
| Total (103) | 25 | 18 | 17 | 11 | 10 | 18 |
The table excludes reference strains (2), multiple isolates from the same patient (1 from Japan and 1 from the United States), and Chilean isolates (2). Percentages given are with respect to geographical regions. Significant (P < 0.05, Fisher's exact test) over- or underrepresentation of a group within a geographical region is marked in boldface type. Note that, as percentages are rounded to the nearest whole number, they may not total 100%.
Association of genotype with clinical site and fluconazole resistance.
Of the 109 isolates typed, 21 had been analyzed for fluconazole resistance. Eight isolates had a MIC of fluconazole of ≥64 μg/ml and, hence, were deemed fluconazole resistant (36). These resistant isolates fell into 6 different STs (Fig. 2). Two STs contained two resistant isolates each (ST 3 and ST6). When analyzed as groups, resistant isolates were found in four of the five groups. Two isolates belonged to STs not associated with a group. The most common ST, ST3, contained five isolates for which the MICs ranged from 6.25 to 128 μg/ml. Though the numbers were too small to perform any meaningful statistical analysis, it appeared that fluconazole resistance was not related to ST or group. The data on the clinical site of origin of the isolates were incomplete. Thus, no statistical analysis was attempted, though there seemed to be no obvious association between clinical site and genotype.
DISCUSSION
MLST is considered a highly effective method for DNA fingerprinting microorganisms, since it fulfills the requirement set forth by some population geneticists that a method must be based on the identification of discrete alleles for each analyzed locus. Here, we have developed for the first time an MLST scheme for the yeast pathogen C. glabrata. We chose 6 loci that defined 30 STs among the 109 isolates analyzed. In an attempt to improve resolution, we tested nine additional loci. The addition of these loci did not increase discrimination, suggesting that we were approaching the limit of resolution for this method. The MLST system developed for C. albicans (3) revealed similar ranges for the percentage of variable sites (1.5 to 4.0% for C. albicans compared to 1.5 to 3.5% for C. glabrata). The MLST system developed for C. albicans (3), however, appears to be more discriminating than the system developed for C. glabrata, even though more polymorphic sites were analyzed in the C. glabrata system (81 in C. glabrata versus 68 in C. albicans). This is probably due to heterozygosity at the tested loci of C. albicans, which, in contrast to haploid C. glabrata, is diploid.
To verify the efficacy of the MLST system we developed for C. glabrata, we compared its capacity to cluster isolates in a test collection of 26 isolates with that of two other independent DNA fingerprinting methods. We found that the majority (80%) of isolates that formed clusters defined by an arbitrary threshold in the MLST dendrogram also formed similar clusters in the RAPD and Cg6/Cg12 dendrograms. One group (group II) in the MLST dendrogram did not remain intact in either the RAPD or Cg6/Cg12 dendrogram. These results demonstrated that the MLST method was effective in distinguishing deep-rooted clusters and, in fact, may be more effective than the other two methods in examining population structure. However, the MLST method did not, for the most part, discriminate between isolates in a group. The RAPD method exhibited a higher degree of discrimination, but neither the MLST method nor the RAPD method discriminated between the great majority of group I isolates. In contrast, the Cg6/Cg12 method discriminated between all isolates in all groups, including group I isolates. Therefore, for analyzing microevolution and studies of nosocomial transmission, Southern blot hybridization with Cg6/Cg12 (24) is the superior method.
An analysis of the 103 test isolates according to geographical origin revealed that a degree of geographical specificity existed for the different clades, as has recently been demonstrated for C. albicans (2, 34). We found that the most representative C. glabrata groups in Europe, the United States, and Japan were groups I and II, groups II and III, and groups III and IV, respectively. The population structure of C. glabrata is believed to be predominantly clonal (5). The results we have obtained on geographical specificity tend to support the conclusion that mixing between clades is depressed. This is also suggested by the robustness of the MLST clades and the presence of synapomorphic alleles associated with each of the groups. It is also believed that the population structure of C. albicans is predominantly clonal (33), but the discovery of mating type loci (15) and the demonstration of fusion and mating (16, 25, 23, 27) has refocused attention to the possibility of low levels of recombination, which was suggested in most studies of population structure. Similarly, the mating type loci of C. glabrata were recently identified and characterized (41, 44), suggesting that recombination also takes place in this species. Detailed studies of the population structure of C. glabrata are, therefore, warranted.
Finally, we found no association between fluconazole resistance and either ST or group. de Meeûs et al. (5) also found no correlation between fluconazole resistance and genotypes derived by MLEE. However, our results do not exclude the possibility that the capacity to become resistant through drug exposure is a function of ST or group.
In summary, we have developed an MLST system for DNA fingerprinting of the yeast pathogen C. glabrata. This system is highly effective in cluster analysis directed at population structure but is not suited for studies of nosocomial infection or microevolution. Our results further suggest specificity of particular clades to particular geographical locales. We have also shown that NMT1 data alone may be sufficient to ascertain groups. Therefore, a reduced number of loci may provide a straightforward method for further study of the geographical distribution of C. glabrata.
Acknowledgments
This work was funded by Wellcome Trust Medical Microbiology Research Fellowship 064466 to A.R.D. and National Institutes of Health grant DE014219 to D.R.S.
We thank C. B. Moore, S. R. Lockhart, J. Bille, J. L. Rodriguez-Tudela, and S. Kohno for the strains used in this study; M. J. Anderson for help with the manuscript; and M. Bond and P. Fullwood from the University of Manchester Sequencing Facility for help with sequencing.
REFERENCES
- 1.Abi-Said, D., E. Anaissie, O. Uzun, I. Raad, H. Pinzcowski, and S. Vartivarian. 1997. The epidemiology of hematogenous candidiasis caused by different Candida species. Clin. Infect. Dis. 24:1122-1128. [DOI] [PubMed] [Google Scholar]
- 2.Blignaut, E., C. Pujol, S. Lockhart, S. Joly, and D. R. Soll. 2002. Ca3 fingerprinting of Candida albicans isolates from human immunodeficiency virus-positive and healthy individuals reveals a new clade in South Africa. J. Clin. Microbiol. 40:826-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bougnoux, M. E., S. Morand, and C. d'Enfert. 2002. Usefulness of multilocus sequence typing for characterization of clinical isolates of Candida albicans. J. Clin. Microbiol. 40:1290-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Csank, C., and K. Haynes. 2000. Candida glabrata displays pseudohyphal growth. FEMS Microbiol. Lett. 189:115-120. [DOI] [PubMed] [Google Scholar]
- 5.de Meeûs, T., F. Renaud, E. Mouveroux, J. Reynes, G. Galeazzi, M. Mallie, and J. M. Bastide. 2002. Genetic structure of Candida glabrata populations in AIDS and non-AIDS patients. J. Clin. Microbiol. 40:2199-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diekema, D. J., S. A. Messer, A. B. Brueggemann, S. L. Coffman, G. V. Doern, L. A. Herwaldt, and M. A. Pfaller. 2002. Epidemiology of candidemia: 3-year results from the emerging infections and the epidemiology of Iowa organisms study. J. Clin. Microbiol. 40:1298-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dingle, K. E., F. M. Colles, D. R. A. Wareing, R. Ure, A. J. Fox, F. E. Bolton, H. J. Bootsma, R. J. L. Willems, R. Urwin, and M. C. J. Maiden. 2001. Multilocus sequence typing system for Campylobacter jejuni. J. Clin. Microbiol. 39:14-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edmond, M. B., S. E. Wallace, D. K. McClish, M. A. Pfaller, R. N. Jones, and R. P. Wenzel. 1999. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin. Infect. Dis. 29:239-244. [DOI] [PubMed] [Google Scholar]
- 9.Enright, M. C., B. G. Spratt, A. Kalia, J. H. Cross, and D. E. Bessen. 2001. Multilocus sequence typing of Streptococcus pyogenes and the relationships between emm type and clone. Infect. Immun. 69:2416-2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Enright, M. C., and B. G. Spratt. 1998. A multilocus sequence typing scheme for Streptococcus pneumoniae: identification of clones associated with serious invasive disease. Microbiology 144:3049-3060. [DOI] [PubMed] [Google Scholar]
- 11.Enright, M. C., N. P. J. Day, C. E. Davies, and S. J. Peacock. 2000. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 38:1008-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geiser, D. M., J. I. Pitt, and J. W. Taylor. 1998. Cryptic speciation and recombination in the aflatoxin-producing fungus Aspergillus flavus. Proc. Natl. Acad. Sci. USA 95:388-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gumbo, T., C. M. Isada, G. Hall, M. T. Karafa, and S. M. Gordon. 1999. Candida glabrata fungemia. Clinical features of 139 patients. Medicine (Baltimore) 78:220-227. [DOI] [PubMed] [Google Scholar]
- 14.Hazen, K. C. 1995. New and emerging yeast pathogens. Clin. Microbiol. Rev. 8:462-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hull, C. M., and A. D. Johnson. 1999. Identification of a mating type-like locus in the asexual pathogenic yeast Candida albicans. Science 285:1271-1275. [DOI] [PubMed] [Google Scholar]
- 16.Hull, C. M., R. M. Raisner, and A. D. Johnson. 2000. Evidence for mating of the “asexual” yeast Candida albicans in a mammalian host. Science 289:307-310. [DOI] [PubMed] [Google Scholar]
- 17.Kaufmann, C. S., and W. G. Merz. 1989. Electrophoretic karyotypes of Torulopsis glabrata. J. Clin. Microbiol. 27:2165-2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koufopanou, V., A. Burt, and J. W. Taylor. 1997. Concordance of gene genealogies reveals reproductive isolation in the pathogenic fungus Coccidioides immitis. Proc. Natl. Acad. Sci. USA 94:5478-5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lachke, S. A., S. Joly, K. Daniels, and D. R. Soll. 2002. Phenotypic switching and filamentation in Candida glabrata. Microbiology 148:2661-2674. [DOI] [PubMed] [Google Scholar]
- 20.Lachke, S. A., T. Srikantha, L. K. Tsai, K. Daniels, and D. R. Soll. 2000. Phenotypic switching in Candida glabrata involves phase-specific regulation of the metallothionein gene MT-II and the newly discovered hemolysin gene HLP. Infect. Immun. 68:884-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Law, D., C. B. Moore, H. M. Wardle, L. A. Ganguli, M. G. Keaney, and D. W. Denning. 1994. High prevalence of antifungal resistance in Candida spp. from patients with AIDS. J. Antimicrob. Chemother. 34:659-668. [DOI] [PubMed] [Google Scholar]
- 22.Lehmann, P. F., D. Lin, and B. A. Lasker. 1992. Genotypic identification and characterization of species and strains within the genus Candida by using random amplified polymorphic DNA. J. Clin. Microbiol. 30:3249-3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lockhart, S. R., K. J. Daniels, R. Zhao, D. Wessels, and D. R. Soll. 2003. Cell biology of mating in Candida albicans. Eukaryotic Cell 2:49-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lockhart, S. R., S. Joly, C. Pujol, J. D. Sobel, M. A. Pfaller, and D. R. Soll. 1997. Development and verification of fingerprinting probes for Candida glabrata. Microbiology 143:3733-3746. [DOI] [PubMed] [Google Scholar]
- 25.Magee, B. B., and P. T. Magee. 2000. Induction of mating in Candida albicans by construction of MTLa and MTLalpha strains. Science 289:310-313. [DOI] [PubMed] [Google Scholar]
- 26.Maiden, M. C. J., J. A. Bygraves, E. Feil, G. Morelli, J. E. Russell, R. Urwin, Q. Zhang, J. Zhou, K. Zurth, D. A. Caugant, I. M. Feavers, M. Achtman, and B. G. Spratt. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. USA 95:3140-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller, M. G., and A. D. Johnson. 2002. White-opaque switching in Candida albicans is controlled by mating-type locus homeodomain proteins and allows efficient mating. Cell 110:293-302. [DOI] [PubMed] [Google Scholar]
- 28.Nei, M., and T. Gojobori. 1986. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol. Biol. Evol. 3:418-426. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen, M. H., J. E. Peacock, Jr., A. J. Morris, D. C. Tanner, M. L. Nguyen, D. R. Snydman, M. M. Wagener, M. G. Rinaldi, and V. L. Yu. 1996. The changing face of candidemia: emergence of non-Candida albicans species and antifungal resistance. Am. J. Med. 100:617-623. [DOI] [PubMed] [Google Scholar]
- 30.Pfaller, M. A., D. J. Diekema, R. N. Jones, H. S. Sader, A. C. Fluit, R. J. Hollis, S. A. Messer, and the SENTRY Participant Group. 2001. International surveillance of bloodstream infections due to Candida species: frequency of occurrence and in vitro susceptibilities to fluconazole, ravuconazole, and voriconazole of isolates collected from 1997 through 1999 in the SENTRY antimicrobial surveillance program. J. Clin. Microbiol. 39:3254-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfaller, M. A., R. N. Jones, G. V. Doern, H. S. Sader, S. A. Messer, A. Houston, S. Coffman, R. J. Hollis, and the SENTRY Participant Group. 2000. Bloodstream infections due to Candida species: SENTRY antimicrobial surveillance program in North America and Latin America, 1997-1998. Antimicrob. Agents Chemother. 44:747-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pfaller, M. A., S. A. Messer, R. J. Hollis, R. N. Jones, G. V. Doern, M. E. Brandt, and R. A. Hajjeh. 1999. Trends in species distribution and susceptibility to fluconazole among blood stream isolates of Candida species in the United States. Diagn. Microbiol. Infect. Dis. 33:217-222. [DOI] [PubMed] [Google Scholar]
- 33.Pujol, C., J. Reynes, F. Renaud, M. Raymond, M. Tibayrenc, F. J. Ayala, F. Janbon, M. Mallie, and J. M. Bastide. 1993. The yeast Candida albicans has a clonal mode of reproduction in a population of infected human immunodeficiency virus-positive patients. Proc. Natl. Acad. Sci. USA 90:9456-9459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pujol, C., M. Pfaller, and D. R. Soll. 2002. Ca3 fingerprinting of Candida albicans bloodstream isolates from the United States, Canada, South America, and Europe reveals a European clade. J. Clin. Microbiol. 40:2729-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pujol, C., S. Joly, S. R. Lockhart, S. Noel, M. Tibayrenc, and D. R. Soll. 1997. Parity among the randomly amplified polymorphic DNA method, multilocus enzyme electrophoresis, and Southern blot hybridization with the moderately repetitive DNA probe Ca3 for fingerprinting Candida albicans. J. Clin. Microbiol. 35:2348-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rex, J. H., M. A. Pfaller, J. N. Galgiani, M. S. Bartlett, A. Espinel-Ingroff, M. A. Ghannoum, M. Lancaster, F. C. Odds, M. G. Rinaldi, T. J. Walsh, and A. L. Barry. 1997. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and Candida infections. Subcommittee on Antifungal Susceptibility Testing of the National Committee for Clinical Laboratory Standards. Clin. Infect. Dis. 24:235-247. [DOI] [PubMed] [Google Scholar]
- 37.Samra, Z., J. Bishara, S. Ashkenazi, S. Pitlik, M. Weinberger, M. Lapidoth, M. Yardeni, and I. Levy. 2002. Changing distribution of Candida species isolated from sterile and nonsterile sites in Israel. Eur. J. Clin. Microbiol. Infect. Dis. 21:542-545. [DOI] [PubMed] [Google Scholar]
- 38.Sanson, G. F., and M. R. Briones. 2000. Typing of Candida glabrata in clinical isolates by comparative sequence analysis of the cytochrome c oxidase subunit 2 gene distinguishes two clusters of strains associated with geographical sequence polymorphisms. J. Clin. Microbiol. 38:227-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sobel, J. D., and W. Chaim. 1997. Treatment of Torulopsis glabrata vaginitis: retrospective review of boric acid therapy. Clin. Infect. Dis. 24:649-652. [DOI] [PubMed] [Google Scholar]
- 40.Soll, D. R. 2000. The ins and outs of DNA fingerprinting the infectious fungi. Clin. Microbiol. Rev. 13:332-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Srikantha, T. A., S. A. Lachke, and D. R. Soll. 2003. Three mating type-like loci of Candida glabrata. Eukaryotic Cell. 2:328-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vazquez, J. A., A. Beckley, S. Donabedian, J. D. Sobel, and M. J. Zervos. 1993. Comparison of restriction enzyme analysis versus pulsed-field gradient gel electrophoresis as a typing system for Torulopsis glabrata and Candida species other than C. albicans. J. Clin. Microbiol. 31:202120-202130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Viscoli, C., C. Girmenia, A. Marinus, L. Collette, P. Martino, B. Vandercam, C. Doyen, B. Lebeau, D. Spence, V. Krcmery, B. De Pauw, and F. Meunier. 1999. Candidemia in cancer patients: a prospective, multicenter surveillance study by the Invasive Fungal Infection Group (IFIG) of the European Organization for Research and Treatment of Cancer (EORTC). Clin. Infect. Dis. 28:1071-1079. [DOI] [PubMed] [Google Scholar]
- 44.Wong, S., M. A. Fares, W. Zimmermann, G. Butler, and K. H. Wolfe. 23 January 2003, posting date. Evidence from comparative genomics for a complete sexual cycle in the ‘asexual’ pathogenic yeast Candida glabrata. Genome Biol. 4:R10. [Online.] http://www.genomebiology.com. [DOI] [PMC free article] [PubMed] [Google Scholar]