Abstract
The present review highlights an association between autism, Alzheimer disease (AD), and fragile X syndrome (FXS). We propose a conceptual framework involving the amyloid-β peptide (Aβ), Aβ precursor protein (APP), and fragile X mental retardation protein (FMRP) based on experimental evidence. The anabolic (growth-promoting) effect of the secreted α form of the amyloid-β precursor protein (sAPPα) may contribute to the state of brain overgrowth implicated in autism and FXS. Our previous report demonstrated that higher plasma sAPPα levels associate with more severe symptoms of autism, including aggression. This molecular effect could contribute to intellectual disability due to repression of cell–cell adhesion, promotion of dense, long, thin dendritic spines, and the potential for disorganized brain structure as a result of disrupted neurogenesis and migration. At the molecular level, APP and FMRP are linked via the metabotropic glutamate receptor 5 (mGluR5). Specifically, mGluR5 activation releases FMRP repression of APP mRNA translation and stimulates sAPP secretion. The relatively lower sAPPα level in AD may contribute to AD symptoms that significantly contrast with those of FXS and autism. Low sAPPα and production of insoluble Aβ would favor a degenerative process, with the brain atrophy seen in AD. Treatment with mGluR antagonists may help repress APP mRNA translation and reduce secretion of sAPP in FXS and perhaps autism.
There is growing interest in associations between neurodevelopmental and neuropsychiatric disorders across the lifespan. Case in point is the association drawn between fragile X syndrome (FXS) and fragile X–associated tremor/ataxia syndrome (FXTAS) found in subsets of older adults harboring fragile X mental retardation 1 gene (FMR1) premutations.1 FXS is the most common inherited form of intellectual disability.2,3 FXTAS is a condition of progressive tremor and ataxia in individuals who show no premorbid cognitive deficits, developing over the age of 50. Dementia occurs in a subset of those with FXTAS. Macrocephaly is seen in children with FXS,4 as discussed below, and brain atrophy in the cerebrum, brainstem, and cerebellum is seen in FXTAS.2
Mutation of the 5′-untranslated region (UTR) of FMR1 (chromosome Xq27.3), consisting of expanded trinucleotide CGG repeats, causes both FXS and FXTAS. FXS is caused by the full mutation of FMR1 (>200 repeats). FMR1 premutation (between 55 and 200 repeats) leads to FXTAS in a subset of carriers, with greater preponderance in male carriers than in female carriers. The full mutation present in FXS promotes hypermethylation of the gene promoter and 5′ UTR leading to inhibition of gene transcription. The resulting lack of FMR1 protein (FMRP) leads to the disease phenotype. Conversely, patients with FXTAS carrying the FMR1 premutation have elevated FMR1 mRNA levels resulting from upregulation of transcription due to presumed feedback from translational deficits generated by the expanded CGG. Toxicity results from the high levels of expanded CGG repeat-containing mRNA.2 In summary, it is believed that FXS is caused by FMRP loss of function, and FXTAS is caused by a ribo-CGG mRNA gain of function toxicity.
The association of the above-mentioned clinically divergent disorders, occurring via dissimilar mechanisms involving the same gene, sets the stage for our discussion of the amyloid-β precursor protein (APP) in relation to Alzheimer disease (AD) and to the neurodevelopmental disorders of autism and FXS. APP is the parental molecule of neurotoxic amyloid-β peptide (Aβ), produced by amyloidogenic processing of APP and secreted in excess in AD.e1 When APP is processed alternatively via the nonamyloidogenic (α-secretase) pathway, the secreted alpha form of APP (sAPPα) is produced.
Based on data from our laboratory and a review of the current literature, we speculate that overproduction of sAPPα may contribute to autism and FXS phenotypes. We specifically hypothesize that the neurotrophic properties of sAPPαe1 may contribute to the state of brain overgrowth found in FXS and autism. We further review key features of FXS, autism, and AD, and discuss the recently formulated metabotropic glutamate receptor (mGluR) theory of FXS and autism,3 highlighting putative involvement of APP (unpublished data, 2011).5,6 We also discuss the involvement of APP in neurogenesis, cell proliferation, and migration as putative mechanisms underlying macrocephaly in FXS and autism. Finally, the roles of epigenetics and gene–environment interaction are discussed.
APP AND DERIVED METABOLITES
APP is a large (695–770 amino acid) glycoprotein produced in brain microglia, astrocytes, oligodendrocytes, and neurons.8 It has a large extracytoplasmic domain, a membrane-spanning domain containing the Aβ peptide, and a short intracytoplasmic domain.9 Mature APP is axonally transported and can be secreted from axon terminals in response to synaptic activation10 where it may play a role in neuronal maturation and synaptogenesis.11 APP undergoes proteolytic processing by secretase enzymes. Sequential cleavage by β-secretase (BACE1) and the γ-secretase complex releases sAPPβ and Aβ peptide, the major component of cerebral amyloid plaques found in AD and Down syndrome.e2 Promiscuous C-terminal cleavage of the Aβ domain in APP by the γ-secretase complex is responsible for the generation of 2 species: Aβ1-40 and Aβ1-42. Alternative cleavage by α-secretase and γ-secretase releases the nonamyloidogenic p3 peptide and sAPPα. This represents the predominant pathway for APP processing.e3 In mice, sAPPα has been shown to increase neurite outgrowth and memory and protect against multiple insults.11 Promotion of the nonamyloidogenic pathway has been considered a promising novel treatment in AD.12 Recently, there has been interest in the function of sAPPα in neurodevelopment and its relationship to autism13 and FXS.5
LINKING sAPP WITH AUTISM
We have reported high levels of total sAPP (including sAPPα) in plasma of a small sample of young children with severe autism and aggression.13 These children expressed sAPP at 2 or more times the levels of children without autism and up to 4 times more than children with mild autism. Overall, there was a trend toward higher levels of both sAPPα and total sAPP (sAPPβ is not a significant component of plasma total sAPP and was not measured) within children with autism, combined with a nonsignificant decrease in Aβ-40. This pointed toward the possibility of increased nonamyloidogenic (growth-promoting or anabolic) processing in autism, opposite what is seen in AD (degenerative or catabolic). While these findings are based on a small sample, they have been replicated and extended by an independent laboratory: elevated plasma sAPPα was found in 60% of known autistic children (n = 25) compared to healthy age-matched controls.6 Furthermore, a recent follow-up study by our laboratory in a separate, larger set of autistic and control patient plasma samples confirms our original finding of elevated sAPPα in the plasma of severely autistic patients without requiring coexistent aggression (unpublished data, 2011). Unlike our original study, we also observed significantly reduced levels of Aβ-40 and Aβ-42 in severe autism (unpublished data, 2011). Elevation in sAPPα was not found for children with mild autism in either study and may not be applicable to this population. This evidence and others16 have led us to the following model.
Higher levels of sAPPα produced via nonamyloidogenic processing may contribute to severe autistic and FXS phenotypes. Specifically, we postulate that high levels of sAPPα may contribute to macrocephaly, observed in both FXS and autism, through its associated neurotrophic activity. This activity of sAPPα may be partially mediated by interactions with adhesion modulators, such as β-catenin, thereby altering adhesion and migration of cortical neurons and promoting overgrowth. Seizures are seen in 10%–30% of individuals with autism and are observed frequently in those with FXS and AD.14,15 Based on recent work in mouse models,16 we also speculate that seizure etiology may involve overproduction of APP in these conditions.
FEATURES OF AUTISM, AD, AND FXS
Autism.
Autism is characterized by delayed speech development, impaired socialization, and rigid behavior including stereotypic movements.17 Neuropathologic findings in individuals with autism include age-related changes in cerebellar nuclei, inferior olives, and amygdala associated with cortical dysgenesis, and increased postmortem brain weight, especially in young autistic children.18 These features are accompanied by significant increases in cytokines.19,20 However, it is unknown if this inflammation is protective or destructive. Other studies have shown increased numbers of cortical pyramidal dendritic spines,e4 more numerous, narrower cortical minicolumns,e5 a reduction in size of the corpus callosum,e6 and abnormal connectivity between frontal and temporal lobes of the brain.e7 Altogether, these studies have been interpreted as an overabundance of white matter relative to gray matter, with overgrown short-range and reduced long-range brain connections.21
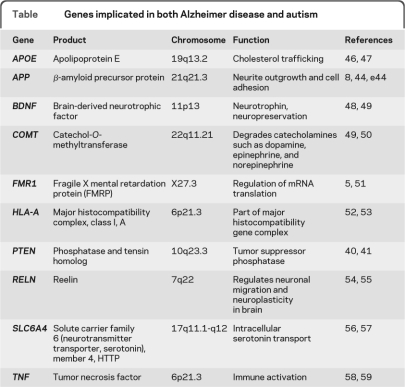
Macrocephaly has been one of the most widely replicated biological findings in autism, affecting up to 20% of all children with the condition22 and confirmed by MRI volumetric studies.23 Excessive brain growth occurs early, around the time symptoms appear, and then growth declines.22 Proposed mechanisms underlying brain enlargement include overproduction of synapses, failure of synaptic pruning, excessive neurogenesis and gliogenesis, or reduction in cell death.22 A prenatal cause of disease is supported by neuroanatomic and neuroimaging studies that show growth abnormalities suspected to occur during the first and second trimesters of pregnancy.24,25 Finally, neuronal cell adhesion derangement has recently been proposed as another mechanism of brain overgrowth.26 Adhesion genes NLGN1, ASTN2, and specific cadherins have recently been linked to autism. Cell adhesion suppresses brain growth, while abnormalities in adhesion promote growth or contribute to aberrant growth. APP may also play a role in the suppression of cell adhesion.27 Other genes implicated in growth currently being investigated in both AD and autism are listed in the table.
Table.
Genes implicated in both Alzheimer disease and autism
AD.
Dementia is progressive deterioration in multiple cognitive attributes severe enough to interfere with daily functioning.28 AD is the most prevalent of the dementias and is distinguished by progressive memory loss, deterioration of receptive speech in early stages, later deterioration of expressive speech, and social inappropriateness in elderly. Hallmark features of AD include deposition of extracellular Aβ peptide in neuritic plaques, presence of intracellular neurofibrillary tangles consisting of hyperphosphorylated tau protein, and resultant brain atrophy.9,10,29 The plaques and tangles become widely distributed throughout the cerebral cortex, with the medial temporal lobes (including the hippocampus and amygdala) and neocortical association areas severely affected. Soluble oligomers of Aβ are believed to induce early neuronal dysfunction and eventually contribute to the atrophy that is seen over time via MRI in these same brain regions.30 These changes are accompanied by increases in inflammation and in cytochemokine levels.28,30 Animal models and clinical studies strongly suggest that inflammation and oxidative stress significantly contribute to AD pathogenesis.10,29
FXS.
The key features of FXS are intellectual disability, social anxiety, gaze avoidance, sensory defensiveness, stereotypic movements, and delayed speech development.1 Males usually show more severe symptoms, while affected females usually show milder cognitive and social impairments. A consistent neuropathologic finding in FXS for both humans and Fmr1 knockout mice is a large number of abnormally long, thin, tortuous dendritic spines.31 The dysmorphic appearance of these dendrites is thought to represent abnormal synaptic plasticity, resulting in susceptibility to epilepsy, anxiety, and behavioral disorders.17 Studies of children with FXS have shown macrocephaly4 and derangement of white matter frontal-striatal pathways.32 A recent study4 showed head growth rate at 30 months was greater for FXS boys with autism (n = 22) than for FXS boys without autism (n = 22). Neuroimaging findings have consistently detected enlargement of the bilateral caudate nucleuse5,e8-e10 and larger caudate volumes associated with lower FMRP levels.e10,e11 Another consistent finding is decreased size in the cerebellar vermis, particularly the posterior segment,e10-e13 with larger posterior vermis size associated with higher FMRP levels.e10 Findings of enlarged caudatee14 and decreased cerebellar vermis (although not always replicatede15,e16) likewise have been reported for children with autism.e17,e18 While the amygdala is enlarged in younge17 and reduced in older children with autism,e19 it is reduced in both young and older children with FXS.e8,e20 There is speculation that regional enlargement seen in FXS may be due to lack of synapse maturation and pruning after birth and that smaller regional size differences could be due to prenatal insult to the brain related to FMRP deficiency.e21
RELATIONSHIPS AMONG NEURONAL PROTEINS IN AUTISM, AD, AND FXS
Role of APP in neurodevelopment and brain growth without guidance in autism.
Proliferation, migration, differentiation, myelination, and synaptogenesis are all steps involved in the generation of a mature neuron. Some of the known functions of APP in these processes include promotion of proliferation, cell–cell adhesion and migration,10 and synaptogenesis.33 APP is predominantly located at synapses,33 and sAPP is released from neurons in an activity-driven fashion.34 In fact, mGluR1/5 activation itself has been shown to increase secretion of sAPP in cell culture.9 The expression of APP appears to be developmentally controlled, with highest levels occurring early in synaptogenesis.33 APP levels are higher postnatally rather than prenatally but peak before 1 month of age in rodents.35 APP plays a functional role during growth cone development and has been implicated in neurite outgrowth.8,34 Further, APP works in opposition to NMDA and AMPA receptors with respect to glutamate's pruning effects on growth cone behaviors.34 Notably, sAPP blocks and reverses the ability of glutamate to inhibit dendrite outgrowth in embryonic rat hippocampal cell cultures.10
In animal models, full-length APP functions in normal migration of neuronal precursors into the cortical plate during brain development. Knockdown of APP inhibits neuronal migration from the cortical ventricular zone to the cortical plate in mice.36 Conversely, overexpression of APP accelerates migration of neuronal precursor cells into the cortex.36 In cell culture, APP has been linked to neuronal cell adhesion,10 with evidence suggesting that APP may play a role in its suppression. Therefore, the location of APP at the synapse and its developmental function in migration and suppression of cell adhesion support the hypothesis that dysregulated levels of sAPP contribute to brain growth without guidance as seen in autism.e21
FMRP.
FMRP is involved in both activity-dependent transport of target mRNAs and regulation of local protein synthesis at the synapse.e22 Local protein synthesis following synaptic activity is a phenomenon necessary for maintenance of some plastic changes at the synapse and also likely important for changes in spine morphology.e23 Therefore, FMRP-mediated regulation of local protein synthesis is presumably essential for normal memory and learning. FMRP can be synthesized locally in proximal dendrites,e24 or recruited to the synapse from more distant sites after mGluR activation.e25 FMRP is also present in the cytoplasm and nucleuse24,e26 and can function to escort associated mRNA from the soma into dendritic processes and spines.e22 mGluR1/5 receptors are positioned in the postsynaptic membrane, where they activate a Gq-coupled second messenger system which transduces glutamate release into downstream phosphorylation cascades. Other receptors systems also activate this second messenger system (e.g., cholinergic muscarinic M1 receptors).37 Activation can lead to either long-term potentiation (LTP) or long-term depression (LTD) depending on cell type and brain location. One component of the signaling pathway activated by receptor binding is induction of local protein synthesis necessary for some forms of LTP and LTD. In the resting state, FMRP binds to and inhibits dendritic translation of target mRNAs, including APP as discussed below.5 Activation of metabotropic glutamate receptor 5 (mGluR5) releases FMRP-mediated translation repression and results in protein synthesis-dependent LTD. In addition to APP, up to 4% of brain mRNAs associate with and may be regulated by FMRP, including mRNA encoding proteins involved in synaptic structural reorganization such as Arc, Rac1, and Map1b.e27 Synaptic synthesis of FMRP protein has itself been shown to be induced by mGluR activation.e28
The absence of FMRP, as observed in FXS patients and Fmr1 knockout mice, is accompanied by an increase in number of immature dendritic spines showing abnormal spine morphology.e23 Spine structure reflects the function and strength of the synapse and if disrupted leads to altered neuroplasticity with resultant behavioral and cognitive deficits.3 Fmr1-knockout mice show reduced LTP in cortex and amygdalae28-e30 and exaggerated mGluR-dependent LTD in hippocampus and cerebellum.e31-e34 The finding of exaggerated mGluR-LTD in the absence of FMRP suggests that LTD becomes uncoupled from mGluR5 activation and persists independent of FMRP-dependent new protein synthesis. Translation of normally FMRP-bound mRNA cargos in the dendrite becomes dysregulated and drives LTD independent of mGluR5 activation. Functional consequences of elevated mGluR signaling in absence of FMRP include prolongation of epileptic form bursts in hippocampal area CA3,6 elongation of dendritic spines on cultured hippocampal neurons,38 and LTD in hippocampal area CA1.e35 These findings may represent in vitro correlates of the following FXS clinical phenotypes: epilepsy, elongated and immature dendritic spines, and cognitive delay. Consequently, these findings led to the mGluR theory of FXS: disease phenotype is a result of excessive mGluR signaling arising from the absence of FMRP. The mGluR5 receptor has been proposed as a possible drug target for symptoms of FXS.3,39
mGluR induce activity-dependent protein synthesis by activating several pathways including the PI3K/mTOR pathway,e35,e36 which is a FMRP-dependent pathway. A regulator of the PI3K pathway is phosphatase and tensin homolog deleted on chromosome 10 (PTEN). There have been recent genetic associations found between cases of autism with pronounced macrocephaly and mutations in the PTEN gene.40–41 PTEN is a tumor suppressor that regulates cell cycle through its antagonistic actions on the PI3K/Akt/mTOR pathway. Specifically, PTEN is a phosphatase that counteracts PI3K by dephoshorylating PIP3. The mTOR signaling pathway is a central regulator of cell growth and proliferation that also regulates synaptic plasticity by modulating protein synthesis in an activity-dependent manner. Downstream mTOR effector S6 kinase appears to act as a direct FMRP kinase, influencing functional activity of FMRP via its phosphorylation state.e36 Interestingly, mTOR pathway signaling has been recently shown to be elevated in a Fmr1 knockout mouse model. PTEN activation was also enhanced in this model, perhaps as a compensatory mechanism.e37 Therefore, there appears to be signaling pathway crosstalk and modulation between 2 genes (PTEN and FMR1) implicated in autism spectrum disorders.
The relationship of APP to FMRP is mediated by mGluR.
Recent studies have characterized an important regulatory relationship among APP, FMRP, and mGluR5. Synaptoneurosomes from wild-type mice stimulated with a group I mGluR agonist demonstrated increased APP translation. This was not observed in synaptoneurosomes from Fmr1 knockout mice due to increased basal APP translation. In wild-type animals, RNA–protein complexes containing FMRP and APP mRNA were disrupted by mGluR agonist treatment.5 Soluble Aβ-40 and Aβ-42 were significantly higher in Fmr1 knockout mice compared to wild-type due to elevated APP expression.5 High levels of APP have also been found in another study of Fmr1 knockout mice.42
These results point to an activity-dependent regulatory relationship between FMRP and APP mediated by mGluR5 signaling and to loss of this FMRP-based regulation in FXS, thereby providing a link between neuronal proteins associated with AD and FXS (unpublished data, 2011).6,13,43 Specifically, FMRP inhibits APP translation under basal conditions, but when mGluR5 receptors are activated, this inhibitory effect is released. In FXS, where FMRP-dependent translational repression of APP is absent, high basal levels of APP and elimination of activity-dependent regulation of APP levels would be expected. Given trophic actions of sAPPα discussed above, we speculate that mGluR5 response may act as a master switch between the balance of catabolic and anabolic processes in nervous system development, which is maintained in part by regulating levels of APP and its metabolites (sAPPα) (figure 1). In this model, reducing or removing FMRP from the system favors anabolic activity (increased sAPPα), leading to the symptoms of FXS and autism (figure 2). Enhanced APP translation without stimulation of amyloidogenic processing of APP could provide more substrate for the α-secretase pathway and perhaps afford neuroprotection from AD. Notably, this would explain the lack of Aβ plaques observed in FXS and in autism.e38
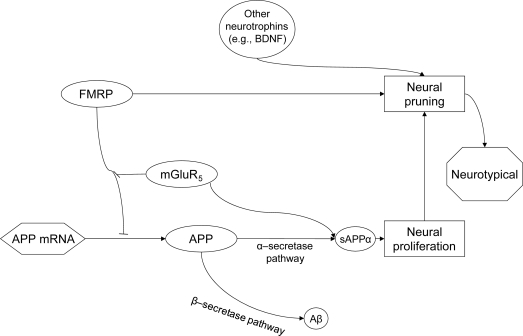
Figure 1. Amyloid-β (Aβ) precursor protein (APP), fragile X mental retardation protein (FMRP), metabotropic glutamate receptor 5 (mGluR) interaction cycle.
Interaction cycle of APP, FMRP, and mGluR in balance. APP mRNA translation is inhibited by FMRP binding to G quartets in the APP coding region.5 This binding is reversed by mGluR5 activation.5 Activity of mGluR5 also stimulates secretion of secreted α form of the amyloid-β precursor protein (sAPP) in neuron.9 In addition, FMRP stimulates neural pruning and synaptic plasticity through other intermediaries.e45 In a healthy system, FMRP activity and mGluR5 activity work in homeostasis and neural proliferation is balanced, leading to a neurotypical condition.
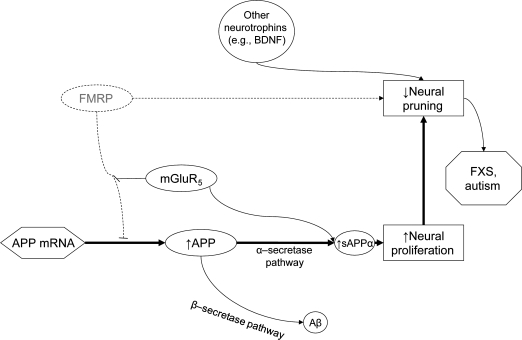
Figure 2. Fragile X mental retardation protein (FMRP) deficiency results in CNS overgrowth/deficiencies.
FMRP deficiency resulting in CNS overgrowth and developmental disorder. When FMRP is eliminated or diminished, translation of amyloid-β (Aβ) precursor protein (APP) mRNA is disinhibited resulting in elevated basal levels of APP protein and the elimination of activity-dependent dynamic APP production. Elevated neural proliferation is not balanced by increased neural pruning, in part due to FMRP deficiency. This could result in CNS neural overgrowth and leads to the symptoms of fragile X syndrome (FXS) or autism. mGluR5 = metabotropic glutamate receptor 5; sAPPα = secreted α form of the amyloid-β precursor protein. Bold arrows indicate predominant pathways.
ROLE OF GENE–ENVIRONMENT INTERACTION IN THE ETIOLOGY OF NEURODEVELOPMENTAL AND NEURODEGENERATIVE DISORDERS
We recognize that effects of any perturbation during development can be completely different from perturbations during adulthood. Indeed, various neurobiological disorders have diverse manifestations and symptomatology. Autism, FXS, and AD share uncertain etiologies, with opaque relationships between genes and the environment consistent with the recently proposed latent early-life associated regulation (LEARn) model, positing latent changes in expression of specific genes initially primed at the developmental stage of life.44 In this model, environmental agents epigenetically disturb gene regulation in a long-term manner, beginning at early developmental stages, but these perturbations might not have pathologic results until significantly later in life. APP has been recently shown to exhibit LEARn expression patterns following early lead (Pb) exposure.44 Other environmental perturbations may also be involved. Autism has been linked to greater paternal agee39 and prenatal stresse40 among other factors. Further, autism has been associated with DNA hypomethylation in parents.e41
SUMMARY, POSSIBLE DRUG TARGETS, AND FUTURE PERSPECTIVES
Recently, interest has increased regarding the function of APP in neurodevelopment and its relationship to autism6,13,43 and to FXS.5 The finding that FMRP regulates APP through an mGluR5-dependent process potentially links AD and FXS proteins at the synapse. Our findings of high levels of sAPPα in some children with autism (unpublished data, 2011)13,43 recently were corroborated in an independent laboratory.6 These results suggest that regulation of sAPP level could be an independent drug target for autism.13,43
We speculate that the anabolic effect of sAPPα contributes to the state of excess that underlies FXS and (severe) autism, especially in younger children. APP has been implicated in neurogenesis, which may set the stage for later prenatal to early postnatal overproliferation of neurons. As our previous report suggests, sAPPα excess may produce more severe symptoms of autism, including aggression.13 In AD, relatively lower sAPPα levels may contribute to symptoms that contrast to those of FXS and autism. Low sAPPα and production of insoluble Aβ-40/42 would favor a catabolic and degenerative process leading to brain atrophy.
Despite our contention that autism spectrum and AD may arise from converse disease mechanisms (especially with respect to APP metabolism), this does not preclude certain therapeutic modalities from proving beneficial in both disorders. As an example, memantine, an NMDA receptor antagonist, has been shown to improve symptomatology of both ADe42 and autism.e43 There have been reports of nonclassic effects mediated by memantine that include reductions in secretion of sAPP, sAPPα, and Aβ in cell culture.45 These wide-spectrum changes in APP processing modulate protein product levels in a direction that would be beneficial in both disorders according to our model and could partially explain the observed salutary effect of memantine in both disorders, aside from classic NMDAR antagonism.
Finally, treatment with mGluR antagonists, as proposed by Bear et al.,3 might reduce the phenotypic effects of sAPP protein produced in excess when FMRP is absent, as occurs in FXS (figure 3). By reducing excessive postsynaptic protein synthesis, including that of APP and subsequent sAPP secretion, we speculate that this strategy would also lead to improvement in autism symptoms.
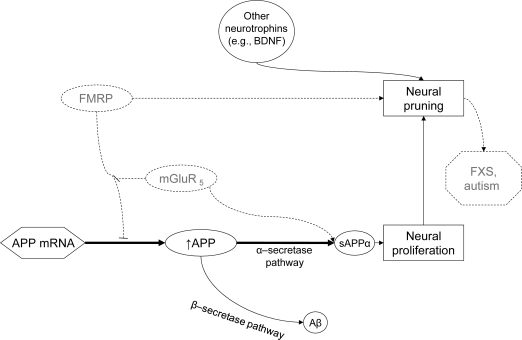
Figure 3. Overcoming fragile X mental retardation protein (FMRP) deficiency.
Use of metabotropic glutamate receptor 5 (mGluR5) inhibitors to ameliorate FMRP deficiency. mGluR5 activity in amyloid-β (Aβ) precursor protein (APP) mRNA translation is disinhibitory, so blocking mGluR5 is not likely to alter APP translation levels. Secretion of secreted α form of the amyloid-β precursor protein (sAPP) may, nevertheless, be reduced by reducing mGluR5 stimulation of sAPP secretion in neurons.9 Reduced sAPP secretion provides lower levels of available product of the α-secretase pathway, reducing symptoms of fragile X syndrome (FXS) or autism. Bold arrows indicate predominant pathways.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the Alzheimer's Association (Zenith award) and the NIH (AG18379 and AG18884) for grant support to D.K.L.
- Aβ
- amyloid-β
- AD
- Alzheimer disease
- APP
- Aβ precursor protein
- FMRP
- fragile X mental retardation protein
- FXS
- fragile X syndrome
- FXTAS
- fragile X–associated tremor/ataxia syndrome
- LEARn
- latent early-life associated regulation
- LTD
- long-term depression
- LTP
- long-term potentiation
- mGluR5
- metabotropic glutamate receptor 5
- sAPPα
- secreted α form of the amyloid-β precursor protein
- UTR
- untranslated region
Supplemental data at www.neurology.org
References e1–e45 are available on the Neurology® Web site at www.neurology.org.
DISCLOSURE
Dr. Sokol, B. Maloney, J.M. Long, and Dr. Ray report no disclosures. Dr. Lahiri is supported by the Zenith Award from Alzheimer's Association and the NIH.
REFERENCES
- 1. Hagerman RJ, Leehey M, Heinrichs W, et al. Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology 2001;57:127–130 [DOI] [PubMed] [Google Scholar]
- 2. Hagerman RJ. Lessons from fragile X regarding neurobiology, autism, and neurodegeneration. J Dev Behav Pediatr 2006;27:63–74 [DOI] [PubMed] [Google Scholar]
- 3. Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci 2004;27:370–377 [DOI] [PubMed] [Google Scholar]
- 4. Chiu S, Wegelin JA, Blank J, et al. Early acceleration of head circumference in children with fragile x syndrome and autism. J Dev Behav Pediatr 2007;28:31–35 [DOI] [PubMed] [Google Scholar]
- 5. Westmark CJ, Malter JS. FMRP mediates mGluR5-dependent translation of amyloid precursor protein. PLoS Biol 2007;5:e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bailey AR, Giunta BN, Obregon D, et al. Peripheral biomarkers in autism: secreted amyloid precursor protein-alpha as a probable key player in early diagnosis. Int J Clin Exp Med 2008;1:338–344 [PMC free article] [PubMed] [Google Scholar]
- 7. Deleted in proof.
- 8. Mullan M, Crawford F. Genetic and molecular advances in Alzheimer's disease. Trends Neurosci 1993;16:398–403 [DOI] [PubMed] [Google Scholar]
- 9. Jolly-Tornetta C, Gao ZY, Lee VM, Wolf BA. Regulation of amyloid precursor protein secretion by glutamate receptors in human Ntera 2 neurons. J Biol Chem 1998;273:14015–14021 [DOI] [PubMed] [Google Scholar]
- 10. Mattson MP. Secreted forms of beta-amyloid precursor protein modulate dendrite outgrowth and calcium responses to glutamate in cultured embryonic hippocampal neurons. J Neurobiol 1994;25:439–450 [DOI] [PubMed] [Google Scholar]
- 11. Stein TD, Johnson JA. Genetic programming by the proteolytic fragments of the amyloid precursor protein: somewhere between confusion and clarity. Rev Neurosci 2003;14:317–341 [DOI] [PubMed] [Google Scholar]
- 12. Bandyopadhyay S, Goldstein LE, Lahiri DK, Rogers JT. Role of the APP non-amyloidogenic signaling pathway and targeting alpha-secretase as an alternative drug target for treatment of Alzheimer's disease. Curr Med Chem 2007;14:2848–2864 [DOI] [PubMed] [Google Scholar]
- 13. Sokol DK, Chen D, Farlow MR, et al. High levels of Alzheimer beta-amyloid precursor protein (APP) in children with severely autistic behavior and aggression. J Child Neurol 2006;21:444–449 [DOI] [PubMed] [Google Scholar]
- 14. Hagerman RJ. The physical and behavioral phenotype. In: Hagerman RJ, Hagerman PJ. eds. Fragile X Syndrome: Diagnosis, Treatment, and Research, 3rd ed Baltimore: Johns Hopkins University Press; 2002:3–109 [Google Scholar]
- 15. Scarmeas N, Honig LS, Choi H, et al. Seizures in Alzheimer disease: who, when, and how common? Arch Neurol 2009;66:992–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Westmark CJ, Westmark PR, Beard AM, Hildebrandt SM, Malter JS. Seizure susceptibility and mortality in mice that over-express amyloid precursor protein. Int J Clin Exp Pathol 2008;1:157–168 [PMC free article] [PubMed] [Google Scholar]
- 17. Belmonte MK, Bourgeron T. Fragile X syndrome and autism at the intersection of genetic and neural networks. Nat Neurosci 2006;9:1221–1225 [DOI] [PubMed] [Google Scholar]
- 18. Pickett J, London E. The neuropathology of autism: a review. J Neuropathol Exp Neurol 2005;64:925–935 [DOI] [PubMed] [Google Scholar]
- 19. Molloy CA, Morrow AL, Meinzen-Derr J, et al. Elevated cytokine levels in children with autism spectrum disorder. J Neuroimmunol 2006;172:198–205 [DOI] [PubMed] [Google Scholar]
- 20. Chez MG, Dowling T, Patel PB, Khanna P, Kominsky M. Elevation of tumor necrosis factor-alpha in cerebrospinal fluid of autistic children. Pediatr Neurol 2007;36:361–365 [DOI] [PubMed] [Google Scholar]
- 21. Geschwind DH. Advances in autism. Annu Rev Med 2009;60:367–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McCaffery P, Deutsch CK. Macrocephaly and the control of brain growth in autistic disorders. Prog Neurobiol 2005;77:38–56 [DOI] [PubMed] [Google Scholar]
- 23. Sokol DK, Edwards-Brown M. Neuroimaging in autistic spectrum disorder (ASD). J Neuroimaging 2004;14:8–15 [PubMed] [Google Scholar]
- 24. Bauman ML, Kemper TL. Neuroanatomic observations of the brain in autism: a review and future directions. Int J Dev Neurosci 2005;23:183–187 [DOI] [PubMed] [Google Scholar]
- 25. Miller MT, Stromland K, Ventura L, Johansson M, Bandim JM, Gillberg C. Autism associated with conditions characterized by developmental errors in early embryogenesis: a mini review. Int J Dev Neurosci 2005;23:201–219 [DOI] [PubMed] [Google Scholar]
- 26. Glessner JT, Wang K, Cai G, et al. Autism genome-wide copy number variation reveals ubiquitin and neuronal genes. Nature 2009;459:569–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen Y, Bodles AM. Amyloid precursor protein modulates beta-catenin degradation. J Neuroinflammation 2007;4:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Qiu C, De Ronchi D, Fratiglioni L. The epidemiology of the dementias: an update. Curr Opin Psychiatry 2007;20:380–385 [DOI] [PubMed] [Google Scholar]
- 29. Lahiri DK, Farlow MR, Greig NH, Sambamurti K. Current drug targets for Alzheimer's disease treatment. Drug Dev Res 2002;56:267–281 [Google Scholar]
- 30. Ramani A, Jensen JH, Helpern JA. Quantitative MR imaging in Alzheimer disease. Radiology 2006;241:26–44 [DOI] [PubMed] [Google Scholar]
- 31. Irwin SA, Patel B, Idupulapati M, et al. Abnormal dendritic spine characteristics in the temporal and visual cortices of patients with fragile-X syndrome: a quantitative examination. Am J Med Genet 2001;98:161–167 [DOI] [PubMed] [Google Scholar]
- 32. Haas BW, Barnea-Goraly N, Lightbody AA, et al. Early white-matter abnormalities of the ventral frontostriatal pathway in fragile X syndrome. Dev Med Child Neurol 2009;51:593–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Priller C, Bauer T, Mitteregger G, Krebs B, Kretzschmar HA, Herms J. Synapse formation and function is modulated by the amyloid precursor protein. J Neurosci 2006;26:7212–7221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mattson MP, Furukawa K. Signaling events regulating the neurodevelopmental triad: glutamate and secreted forms of beta-amyloid precursor protein as examples. Perspect Dev Neurobiol 1998;5:337–352 [PubMed] [Google Scholar]
- 35. Lahiri DK, Nall C, Chen D, Zaphiriou M, Morgan C, Nurnberger JI., Sr. Developmental expression of the beta-amyloid precursor protein and heat-shock protein 70 in the cerebral hemisphere region of the rat brain. Ann NY Acad Sci 2002;965:324–333 [DOI] [PubMed] [Google Scholar]
- 36. Young-Pearse TL, Bai J, Chang R, Zheng JB, LoTurco JJ, Selkoe DJ. A critical function for beta-amyloid precursor protein in neuronal migration revealed by in utero RNA interference. J Neurosci 2007;27:14459–14469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Volk LJ, Pfeiffer BE, Gibson JR, Huber KM. Multiple Gq-coupled receptors converge on a common protein synthesis-dependent long-term depression that is affected in fragile X syndrome mental retardation. J Neurosci 2007;27:11624–11634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vanderklish PW, Edelman GM. Dendritic spines elongate after stimulation of group 1 metabotropic glutamate receptors in cultured hippocampal neurons. Proc Natl Acad Sci USA 2002;99:1639–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Robinson R. Glutamate blockade corrects fragile X syndrome in mice: human trials are planned. Neurol Today 2008;8:6 [Google Scholar]
- 40. Kerr F, Rickle A, Nayeem N, Brandner S, Cowburn RF, Lovestone S. PTEN, a negative regulator of PI3 kinase signalling, alters tau phosphorylation in cells by mechanisms independent of GSK-3. FEBS Lett 2006;580:3121–3128 [DOI] [PubMed] [Google Scholar]
- 41. Butler MG, Dasouki MJ, Zhou XP, et al. Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline PTEN tumour suppressor gene mutations. J Med Genet 2005;42:318–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. D'Agata V, Warren ST, Zhao W, Torre ER, Alkon DL, Cavallaro S. Gene expression profiles in a transgenic animal model of fragile X syndrome. Neurobiol Dis 2002;10:211–218 [DOI] [PubMed] [Google Scholar]
- 43. Sokol DK, Long J, Maloney B, Chen D, Lahiri DK. Potential Alzheimer's disease markers for autism? Beta amyloid precursor protein and acetylcholinesterase correlated with aggression in autism. Presented at the American Academy of Neurology, Seattle, 2009 [Google Scholar]
- 44. Lahiri DK, Maloney B, Zawia NH. The LEARn model: an epigenetic explanation for idiopathic neurobiological diseases. Mol Psychiatry 2009;14:992–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ray B, Banerjee PK, Greig NH, Lahiri DK. Memantine treatment decreases levels of secreted Alzheimer's amyloid precursor protein (APP) and amyloid beta (A beta) peptide in the human neuroblastoma cells. Neurosci Lett 2010;470:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Corder EH, Lannfelt L, Bogdanovic N, Fratiglioni L, Mori H. The role of APOE polymorphisms in late-onset dementias. Cell Mol Life Sci 1998;54:928–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Giunco CT, de Oliveira AB, Carvalho-Salles AB, et al. Association between APOE polymorphisms and predisposition for autism. Psychiatr Genet 2009;19:338. [DOI] [PubMed] [Google Scholar]
- 48. Feher A, Juhasz A, Rimanoczy A, Kalman J, Janka Z. Association between BDNF Val66Met polymorphism and Alzheimer disease, dementia with Lewy bodies, and Pick disease. Alzheimer Dis Assoc Disord 2009;23:224–228 [DOI] [PubMed] [Google Scholar]
- 49. Gadow KD, Roohi J, DeVincent CJ, Kirsch S, Hatchwell E. Association of COMT (Val158Met) and BDNF (Val66Met) gene polymorphisms with anxiety, ADHD and tics in children with autism spectrum disorder. J Autism Dev Disord 2009;39:1542–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sweet RA, Devlin B, Pollock BG, et al. Catechol-O-methyltransferase haplotypes are associated with psychosis in Alzheimer disease. Mol Psychiatry 2005;10:1026–1036 [DOI] [PubMed] [Google Scholar]
- 51. Hallmayer J, Pintado E, Lotspeich L, et al. Molecular analysis and test of linkage between the FMR-1 gene and infantile autism in multiplex families. Am J Hum Genet 1994;55:951–959 [PMC free article] [PubMed] [Google Scholar]
- 52. Zareparsi S, James DM, Kaye JA, Bird TD, Schellenberg GD, Payami H. HLA-A2 homozygosity but not heterozygosity is associated with Alzheimer disease. Neurology 2002;58:973–975 [DOI] [PubMed] [Google Scholar]
- 53. Torres AR, Sweeten TL, Cutler A, et al. The association and linkage of the HLA-A2 class I allele with autism. Hum Immunol 2006;67:346–351 [DOI] [PubMed] [Google Scholar]
- 54. Seripa D, Matera MG, Franceschi M, et al. The RELN locus in Alzheimer's disease. J Alzheimers Dis 2008;14:335–344 [DOI] [PubMed] [Google Scholar]
- 55. Persico AM, D'Agruma L, Maiorano N, et al. Reelin gene alleles and haplotypes as a factor predisposing to autistic disorder. Mol Psychiatry 2001;6:150–159 [DOI] [PubMed] [Google Scholar]
- 56. Li T, Holmes C, Sham PC, et al. Allelic functional variation of serotonin transporter expression is a susceptibility factor for late onset Alzheimer's disease. Neuroreport 1997;8:683–686 [DOI] [PubMed] [Google Scholar]
- 57. Klauck SM, Poustka F, Benner A, Lesch KP, Poustka A. Serotonin transporter (5-HTT) gene variants associated with autism? Hum Mol Genet 1997;6:2233–2238 [DOI] [PubMed] [Google Scholar]
- 58. Ramos EM, Lin MT, Larson EB, et al. Tumor necrosis factor alpha and interleukin 10 promoter region polymorphisms and risk of late-onset Alzheimer disease. Arch Neurol 2006;63:1165–1169 [DOI] [PubMed] [Google Scholar]
- 59. Li X, Chauhan A, Sheikh AM, et al. Elevated immune response in the brain of autistic patients. J Neuroimmunol 2009;207:111–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.