Abstract
Background:
Welding exposes workers to manganese (Mn) fumes, but it is unclear if this exposure damages dopaminergic neurons in the basal ganglia and predisposes individuals to develop parkinsonism. PET imaging with 6-[18F]fluoro-l-dopa (FDOPA) is a noninvasive measure of nigrostriatal dopaminergic neuron integrity. The purpose of this study is to determine whether welding exposure is associated with damage to nigrostriatal neurons in asymptomatic workers.
Methods:
We imaged 20 asymptomatic welders exposed to Mn fumes, 20 subjects with idiopathic Parkinson disease (IPD), and 20 normal controls using FDOPA PET. All subjects were examined by a movement disorders specialist. Basal ganglia volumes of interest were identified for each subject. The specific uptake of FDOPA, Ki, was generated for each region using graphical analysis method.
Results:
Repeated measures general linear model (GLM) analysis demonstrated a strong interaction between diagnostic group and region (F4,112 = 15.36, p < 0.001). Caudate Kis were lower in asymptomatic welders (0.0098 + 0.0013 minutes−1) compared to control subjects (0.0111 + 0.0012 minutes−1, p = 0.002). The regional pattern of uptake in welders was most affected in the caudate > anterior putamen > posterior putamen. This uptake pattern was anatomically reversed from the pattern found in subjects with IPD.
Conclusions:
Active, asymptomatic welders with Mn exposure demonstrate reduced FDOPA PET uptake indicating dysfunction in the nigrostriatal dopamine system. The caudate Ki reduction in welders may represent an early (asymptomatic) marker of Mn neurotoxicity and appears to be distinct from the pattern of dysfunction found in symptomatic IPD.
There are approximately 466,000 full-time welding and soldering workers in the United States1 and over 1 million workers who perform welding as part of their job functions.2 Concerns about the exposure to welding fumes and their constituents, particularly manganese (Mn), and subsequent neurologic complications including parkinsonism and idiopathic Parkinson disease (IPD) have increased over recent years.3 Many welders are regularly exposed to Mn levels beyond the American Conference of Governmental Industrial Hygienists threshold limit value of 0.2 mg/m3.4,5 While an exposure-response relationship between Mn or welding fume and parkinsonism has not yet been determined, demonstration of neurotoxicity in exposed workers would have substantial public health impact for the US workforce and economy.
6-[18F]Fluoro-l-dopa (FDOPA) PET imaging noninvasively measures in vivo dopaminergic presynaptic nerve terminal dysfunction.6 Previous FDOPA PET studies in patients with Mn toxicity have been limited and the results conflicting. FDOPA PET in 4 smelter workers with high levels of Mn exposure and clinical parkinsonism demonstrated normal FDOPA uptake.7 Subsequent studies have found presynaptic dopaminergic dysfunction in Mn-exposed subjects with symptomatic parkinsonism in a pattern similar to subjects with IPD8–10; however, coincident IPD in these subjects cannot be excluded. To avoid this confound, this study investigates dopaminergic function using FDOPA PET in a group of relatively young, healthy, asymptomatic Mn-exposed welders in whom coincident IPD is unlikely. Demonstration of dopaminergic dysfunction in these workers would have substantial public health impact for worker safety and could provide a critical link in understanding the relationship among environmental Mn exposure, parkinsonism, and IPD.
METHODS
Standard protocol approvals, registrations, and patient consents.
This study was approved by the Washington University School of Medicine Human Research Protection Office and all subjects signed a written consent form. Welder data are presented in aggregate to protect individual subject confidentiality.
Subjects.
Welders were recruited from 2 Midwestern shipyards and one fabrication company from April 16, 2007, through November 6, 2009, where they were primarily engaged in ship building/repair and heavy industrial equipment fabrication. The welding processes most commonly used by these welders were flux core arc welding, shielded metal arc welding (“stick” welding), and gas metal arc welding, mainly on carbon steel. Subjects with IPD and healthy controls were recruited through the Movement Disorders Center at Washington University for a 1:1:1 comparison with the welder group. All subjects were evaluated by a movement disorders specialist with an examination that included a Unified Parkinson's Disease Rating Scale motor subsection 3 (UPDRS3).11 Welders were excluded from participation if they had less than 100 hours of welding exposure or if they had comorbid neurologic disease that affected the UPDRS3 rating. Welders and control subjects were also required to be asymptomatic, defined as never previously seeking treatment or evaluation for parkinsonism or tremor. Control subjects were excluded for a total UPDRS3 score >3 or rest, postural, or action tremor >1. All subjects were screened for prior drug (prescription and recreational) exposures and were excluded for neuroleptic or amphetamine use. Subjects with IPD had probable PD according to the criteria proposed by Gelb et al.12 Welding subjects completed a validated exposure questionnaire and provided blood samples for Mn levels.13
MRI and FDOPA PET studies.
A high-resolution 3-dimensional magnetization-prepared rapid gradient echo (MPRAGE) image (1 × 1 × 1.25 mm voxels) was acquired on each subject using a Siemens Magnetom Symphony, Sonata, or Trio scanner (Erlangen, Germany). A reviewer blinded to the clinical status of the subject outlined volumes of interest (VOIs) including the caudate, globus pallidus (GP), anterior and posterior putamen, and occipital regions on individual MPRAGE images. Striatal VOIs outlined the entire structure while the control regions comprised a pair of semicylinders on either side of midline in occipital cortex. Mn exposure is associated with increased T1 signal within the GP, and the intensity of the signal is traditionally measured in terms of a pallidal index (PI), defined as the ratio of T1 signal in the GP to a white matter reference region. PIs were calculated from the MPRAGE images as previously described14:
 |
The MPRAGE image then was coregistered to a composite FDOPA PET image for each subject using Automated Image Registration (AIR).15
PET images were acquired using a Siemens/CTI 953B, ECAT EXACT HR, or EXACT HR+ scanner (Knoxville, TN). Dopaminergic medications were held for 12 hours prior to the study. All subjects were given carbidopa (Lodosyn®, Merck & Co., Inc., Whitehouse Station, NJ) 200 mg orally 1 hour prior to FDOPA administration. Attenuation was measured using 68Ga-68Ge rotating rod sources. Dynamic emission scans were acquired in 3-dimensional mode following injection of FDOPA (3–5 mCi). Emission scans were corrected using individual attenuation and model-based scatter correction and reconstructed using filtered back projection with a ramp filter cutoff at the Nyquist frequency. Individual frames were aligned to each other to correct for movement between frames using AIR.16,17
The PET scanners, EXACT HR and HR+, have similar 3-dimensional spatial resolutions of approximately 4.5 mm full width at half-maximum in all 3 dimensions for central regions of the field of view while the older 953B has a 3-dimensional spatial resolution of approximately 6 mm. To correct for different partial volume effects due to spill-out of higher gray matter (GM) activity and to spill-in from lower activity of adjacent white matter (WM) and CSF, we corrected regional PET counts as follows. The MPRAGE was segmented into GM, WM, and CSF using Statistical Parametric Mapping (SPM5, 2005). These individual tissue images were smoothed to the resolution of the PET using a 3-dimensional Gaussian filter prior to coregistration and reslicing to the PET images. We assumed that CSF has no FDOPA activity and that WM has uniform FDOPA uptake. While we corrected control regions to reflect GM activity, we did not correct striatal VOIs for any internal WM. Therefore, the computed values of Ki represent FDOPA uptake for the entire structure relative to nonspecific GM uptake in occipital cortex. The partial-volume–corrected GM FDOPA activity of each VOI was computed as follows:
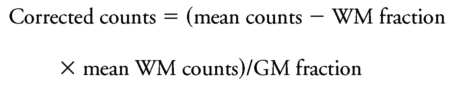 |
where counts are decay-corrected PET activity, and the WM fraction, GM fraction, and mean WM counts were determined as described above.
The transformation matrix generated by coregistering MPRAGE to PET was used to resample magnetic resonance (MR)–defined VOIs. VOIs for PET comprised voxels in which at least 50% of each voxel was within the corresponding MR-defined VOI. Decay-corrected regional PET counts were extracted from dynamic PET images using these VOIs. Net FDOPA uptake (Ki) was computed using Patlak graphical analysis of the time-activity data from 24 to 94 minutes postinjection and reflects levodopa transport, decarboxylase activity, and dopamine storage capacity.18 For our power calculation, we assumed that exposed welders would have a posterior putamen Ki of 0.011 ± 0.001, representing a 10% reduction from normal subjects. A sample size of 20 per group, assuming loss of 2 subjects per group, provided over 90% power to detect a 10% difference between welders and normal subjects.
Statistical analysis.
Age, UPRDS3 scores, and PIs were compared among the 3 diagnostic groups using analysis of variance (ANOVA). If ANOVA demonstrated an overall significance at p < 0.05, a Scheffe test was used to examine differences between diagnostic groups. A Fisher exact test was used to analyze the relationship between gender and diagnostic categories. Effects of gender and age on regional Ki were separately examined within the control group. Variables with significant effects (p ≤ 0.05) on regional control Kis were considered for inclusion as covariates into the main GLM analysis. Within the welder cohort, relationships among Mn blood levels, welding exposure hours, PIs, and Kis were examined by Pearson correlation. Repeated-measures GLM analysis was used to examine the effects of diagnostic group and region on regional Ki values. In addition, the interaction between diagnostic group (between-subjects variable) and region (within-subject variable) from the repeated-measures GLM analysis was used to examine differences in the patterns of FDOPA Kis across regions in the 3 diagnostic groups. Post hoc analyses of covariance (ANCOVAs) were performed to compare differences between diagnostic groups for each region. The statistical software SPSS for Windows v17.0 (Chicago, IL) was used for data analysis.
RESULTS
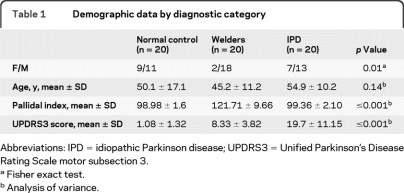
There was no difference in age among the 3 groups (ANOVA, F = 2.06, p = 0.14). Gender was unevenly distributed between diagnostic categories (Fisher exact test, p = 0.01) with women underrepresented in the welder group. Demographic characteristics for the 3 diagnostic categories are in table 1. Welders had mean lifetime exposures of 30,968 ± 26,842 welding hours and average Mn levels 2 times the upper limits of normal (20.7 ± 13.6 μg/L, normal values <10.8 μg/L). The PI was higher in welders compared to controls and subjects with IPD (ANOVA, F = 101. 36, p < 0.001) (table 1). PIs increased with exposure hours (Pearson correlation, r = 0.41, p = 0.07) but were less strongly linked with Mn levels (Pearson correlation, r = 0.17, p = 0.52) within the welder group. UPDRS3 scores differed by diagnostic group (ANOVA, F = 37.69, p < 0.001). Asymptomatic welders demonstrated mildly elevated average UPDRS3 scores (8.33 ± 3.82) despite no clinical symptoms and were different from the control group (post hoc Tukey HSD, p = 0.004) (table 1). UPDRS3 scores did not correlate with PIs, exposure hours, or Mn levels.
Table 1.
Demographic data by diagnostic category
Abbreviations: IPD = idiopathic Parkinson disease; UPDRS3 = Unified Parkinson's Disease Rating Scale motor subsection 3.
Fisher exact test.
Analysis of variance.
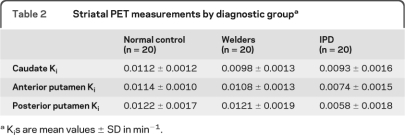
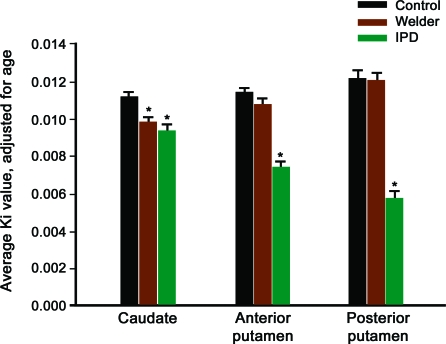
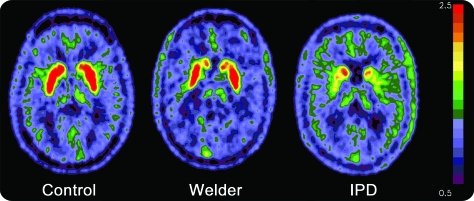
The average estimated FDOPA-PET Ki by region (left and right side combined) are in table 2. Neither gender nor age had a significant effect on Ki values within the control group. However, given previous concerns that age affects FDOPA uptake, age was included in the GLM analysis as a covariate.19 Repeated-measures GLM analysis demonstrated a main effect of diagnostic group, F2,56 = 58.26, p < 0.001, but not of region, F2,55 = 1.88, p = 0.16. There was also a strong interaction between region and group within the model, F4,112 = 15.36, p < 0.001. To further understand the interaction, we performed follow-up ANCOVA analyses, again controlling for age, with pairwise comparisons between diagnostic groups. There were differences in Kis between groups for all regions (caudate, F = 6.73, p = 0.001; anterior putamen, F = 36.40, p < 0.001; posterior putamen, F = 54.67, p < 0.001). FDOPA uptake in welders was lower than controls in the caudate (p = 0.002) region but not the anterior (p = 0.13) or posterior putamen (p = 0.99). Diagnostic group comparisons for all regions and the patterns of uptake by group are illustrated in figure 1. The regional pattern of uptake in welders was most affected in caudate > anterior putamen > posterior putamen. This pattern was reversed from the IPD subject pattern, which is also represented in figure 1. Representative FDOPA PET images from each of the diagnostic groups are in figure 2. There was no consistent relationship between PI, Mn levels, UPDRS3 scores, and Ki by region.
Table 2.
Striatal PET measurements by diagnostic groupa
Kis are mean values ± SD in min−1.
Figure 1. Average FDOPA PET Ki by region by group adjusted for age.
Average FDOPA PET Ki by region for controls, welders, and subjects with idiopathic Parkinson disease (IPD) adjusted for age. *Different from controls, p < 0.01.
Figure 2. FDOPA PET images of decay-corrected counts from 24 to 94 minutes.
FDOPA PET composite images of decay-corrected counts from 24 to 94 minutes from a representative control, welder, and subject with idiopathic Parkinson disease (IPD) normalized to the reference region. FDOPA uptake is reduced in the caudate region of the welder in comparison to the control subject while the posterior putamen is the most affected region in the subject with IPD.
DISCUSSION
Our study demonstrates a mean reduction of 11.71% in caudate Ki of asymptomatic Mn-exposed welders, suggesting presynaptic nigrostriatal dysfunction. These results differ from previous case reports7,9,10; however, the total number of Mn-exposed FDOPA PET cases in the literature is 7 symptomatic patients. Therefore these studies were not powered to detect a difference on the order of 10%. Our current study has the advantage of including 20 asymptomatic Mn-exposed subjects, providing much greater power to detect a small change in striatal FDOPA uptake, a critical point in trying to detect evidence of subclinical neurotoxicity. Most importantly, studying younger, asymptomatic Mn-exposed workers markedly reduces the likelihood of coincident IPD that could bias the findings. This study provides compelling evidence for abnormal striatal uptake of FDOPA in asymptomatic, Mn-exposed welders.
Although our study demonstrates dopaminergic dysfunction, these in vivo measures cannot distinguish a neurotoxic effect of welding fume on nigrostriatal neurons from a regulatory effect on processes that alter FDOPA uptake. Recent studies in Mn-exposed nonhuman primates may provide some insight into the underlying neuropathology associated with the reduced FDOPA uptake in these welders. Mn exposure in primates caused a marked reduction (51%) in amphetamine-induced displacement of [11C]raclopride, suggesting an abnormality in presynpatic dopaminergic neurons.20,21 These primate findings support our conclusion that chronic Mn exposure produces clinical parkinsonism through presynaptic dopamine terminal dysfunction. Determining if a similar mechanism is responsible for our human subjects' reduced striatal FDOPA uptake will require further research.
The second important finding of this study is the distinct pattern of FDOPA Ki reductions in welders across regions. In subjects with IPD, FDOPA uptake in the basal ganglia is decreased compared to normal control subjects, with the greatest reduction in the posterior putamen.22 In general, welders demonstrated an anterior-posterior Ki gradient with FDOPA uptake most affected in the caudate > anterior putamen > posterior putamen, which was anatomically reversed from the pattern demonstrated in subjects with IPD. There are several interpretations of differences in these regional Ki patterns. The anterior predominant Ki gradient found in these welders may represent a distinct pattern of dopaminergic dysfunction specific to Mn exposure and could provide some insight into the atypical clinical characteristics of Mn toxicity. Clinical-pathologic case series in humans link lesions in the putamen to traditional motor symptoms of parkinsonism, while caudate lesions are more associated with psychiatric and cognitive changes.23 Previous FDOPA studies in IPD have found lower caudate Kis are associated with poor performance on cognitive testing including reduced attentional functioning and working memory.24 Classic descriptions of severe manganese toxicity include cognitive impairment, depression, and hallucinations, which are unusual in early IPD.25 Similarly, welders with Mn exposure have difficulties with attention, concentration, and cognitive function.26 Although this study focused on motor abnormalities in Mn-exposed welders, future investigation into the neuropsychological symptoms associated with these FDOPA PET findings may provide a pathophysiologic basis for Mn-associated cognitive and behavior abnormalities.
Alternatively, the anterior striatal predominant pattern observed in our study may reflect a presymptomatic pattern that shifts to affect posterior striatum preferentially in more advanced, symptomatic stages. Unfortunately, there are little FDOPA data on the progression of the regional pattern differences in presymptomatic subjects at risk for parkinsonism. Older studies report reduced FDOPA uptake in asymptomatic family members in a pattern similar to sporadic IPD (putamen > caudate).27,28 However, a recent study found that FDOPA uptake in the anterior putamen of 4 asymptomatic heterozygous parkin gene carriers (Ki = 0.0108 ± 0.0018) was lower than 15 control subjects (Ki = 0.0132 ± 0.0012), whereas there were no significant differences between caudate or posterior putamen.29 These preliminary findings raise the question of whether the pattern of striatal dysfunction changes as degeneration progresses. Follow-up studies in these asymptomatic subjects would address this issue.
There are several potential limitations of this study. Welding fumes contain a number of elements and gases30 and this study cannot exclude a contribution from other substances in welding fumes. However, Mn is most strongly associated with parkinsonism and these welders had both elevated blood Mn levels and the increased pallidal signal characteristic of Mn exposure. Moreover, this study demonstrates an association between common types of welding exposures and dopaminergic dysfunction but did not investigate causation. Although our study suggests that welding-exposed workers have a characteristic pattern of dopaminergic dysfunction, demonstration of a dose response between welding fume exposure and striatal uptake of FDOPA would provide even stronger evidence for cause and effect. Welders demonstrated a small but significant elevation in UPRDS3 scores as compared to controls; however, there was no correlation between the UPDRS3 score and regional Kis. Detecting a significant correlation may require a broader cross-section of welders with a wider range of UPDRS3 scores. The clinical implications of these modest elevations in UPDRS3 will require additional study. Finally, striatal FDOPA uptake reflects dopa decarboxylase activity which upregulates in dopamine-deficient states, thus underestimating the degree of nigrostriatal neuronal loss. Ideally, imaging with a less regulated presynaptic marker of dopaminergic dysfunction such as (+)-11C-dihydrotetrabenazine (11C-DTBZ) would provide additional proof of our findings.
Editorial, page 1286
- AIR
- Automated Image Registration
- ANCOVA
- analysis of covariance
- ANOVA
- analysis of variance
- FDOPA
- 6-[18F]fluoro-l-dopa
- GLM
- general linear model
- GM
- gray matter
- GP
- globus pallidus
- IPD
- idiopathic Parkinson disease
- MPRAGE
- magnetization-prepared rapid gradient echo
- MR
- magnetic resonance
- PI
- pallidal index
- UPDRS3
- Unified Parkinson's Disease Rating Scale motor subsection 3
- VOI
- volume of interest
- WM
- white matter.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. Susan R. Criswell.
DISCLOSURE
Dr. Criswell receives/has received research support from Merck Serono, Chiltern International, Teva Pharmaceutical Industries Ltd., Medivation, Inc., the NIH, and the American Parkinson Disease Association. Dr. Perlmutter serves on scientific advisory boards for the American Parkinson Disease Association, the Dystonia Medical Research Foundation, MO Chapter of the Dystonia Medical Research Fund, and Greater St. Louis Chapter of the APDA; serves on the editorial board of Neurology®; has received funding for travel and speaker honoraria from Ceregene; has received honoraria from Parkinson Disease Study Group for grant reviews; has received fellowship support from Medtronic, Inc.; and receives research support from the NIH, the Huntington Disease Society of American Center of Excellence, Michael J. Fox Foundation, CHDI (formerly HiQ Foundation), McDonnell Center for Higher Brain Function, Greater St. Louis Chapter of the American Parkinson Disease Association, American Parkinson Disease Association, Bander Foundation for Medical Ethics and Advanced PD Research Center at Washington University, and the Barnes Jewish Hospital Foundation. Dr. Videen receives support from the NIH/NINDS and the Michael J. Fox Foundation. Dr. Moerlein serves on a scientific advisory board for the National Institute of Biomedical Imaging and Bioengineering (NIH); serves as Consultant Editor for Evaluations of Drug Interactions; and has received research from Amgen. H.P. Flores reports no disclosures. A.M. Birke holds stock in Merck Serono and Bristol-Myers Squibb. Dr. Racette has received research support from Teva Pharmaceutical Industries Ltd., Eisai Inc., Solvay Pharmaceuticals, Inc., SCHWARZ PHARMA, Solstice Neurosciences, Inc., Allergan, Inc., Neurogen Corporation, the NIH (NIEHS), BJHF/ICTS, and the Michael J. Fox Foundation; and has served as a consultant in medico-legal cases.
REFERENCES
- 1. Welding, soldering, and brazing workers: United States Department of Labor, Bureau of Labor Statistics 2009. Available at: http://www.bls.gov/oco/ocos226.htm Accessed July 15, 2010
- 2. Antonini JM. Health effects of welding. Crit Rev Toxicol 2003;33:61–103 [DOI] [PubMed] [Google Scholar]
- 3. Meeker JD, Susi P, Flynn MR. Manganese and welding fume exposure and control in construction. J Occup Environ Hyg 2007;4:943–951 [DOI] [PubMed] [Google Scholar]
- 4. Korczynski RE. Occupational health concerns in the welding industry. Appl Occup Environ Hyg 2000;15:936–945 [DOI] [PubMed] [Google Scholar]
- 5. Susi P, Goldberg M, Barnes P, Stafford E. The use of a task-based exposure assessment model (T-BEAM) for assessment of metal fume exposures during welding and thermal cutting. Appl Occup Environ Hyg 2000;15:26–38 [DOI] [PubMed] [Google Scholar]
- 6. Yee RE, Irwin I, Milonas C, et al. Novel observations with FDOPA-PET imaging after early nigrostriatal damage. Mov Disord 2001;16:838–848 [DOI] [PubMed] [Google Scholar]
- 7. Wolters EC, Huang CC, Clark C, et al. Positron emission tomography in manganese intoxication. Ann Neurol 1989;26:647–651 [DOI] [PubMed] [Google Scholar]
- 8. Racette BA, Antenor JA, McGee-Minnich L, et al. [18F]FDOPA PET and clinical features in parkinsonism due to manganism. Mov Disord 2005;20:492–496 [DOI] [PubMed] [Google Scholar]
- 9. Racette BA, McGee-Minnich L, Moerlein SM, Mink JW, Videen TO, Perlmutter JS. Welding-related parkinsonism: clinical features, treatment, and pathophysiology. Neurology 2001;56:8–13 [DOI] [PubMed] [Google Scholar]
- 10. Kim Y, Kim JW, Ito K, et al. Idiopathic parkinsonism with superimposed manganese exposure: utility of positron emission tomography. Neurotoxicology 1999;20:249–252 [PubMed] [Google Scholar]
- 11. Fahn S, Elton RL, members of the UPDRS Development Committee Unified Parkinson's Disease Rating Scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB. eds. Recent Developments in Parkinson's Disease. New York: Macmillan; 1987:153–163 [Google Scholar]
- 12. Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol 1999;56:33–39 [DOI] [PubMed] [Google Scholar]
- 13. Hobson AJ, Sterling DA, Emo B, et al. Validity and reliability of an occupational exposure questionnaire for parkinsonism in welders. J Occup Environ Hyg 2009;6:324–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Spahr L, Butterworth RF, Fontaine S, et al. Increased blood manganese in cirrhotic patients: relationship to pallidal magnetic resonance signal hyperintensity and neurological symptoms. Hepatology 1996;24:1116–1120 [DOI] [PubMed] [Google Scholar]
- 15. Woods RP, Mazziotta JC, Cherry SR. MRI-PET registration with automated algorithm. J Comput Assist Tomogr 1993;14:536–546 [DOI] [PubMed] [Google Scholar]
- 16. Woods RP, Cherry SR, Mazziotta JC. Rapid automated algorithm for aligning and reslicing PET images. J Comput Assist Tomogr 1992;16:620–633 [DOI] [PubMed] [Google Scholar]
- 17. Andersson JL. How to obtain high-accuracy image registration: application to movement correction of dynamic positron emission tomography data. Eur J Nucl Med 1998;25:575–586 [DOI] [PubMed] [Google Scholar]
- 18. Heiss WD, Hilker R. The sensitivity of 18-fluorodopa positron emission tomography and magnetic resonance imaging in Parkinson's disease. Eur J Neurol 2004;11:5–12 [DOI] [PubMed] [Google Scholar]
- 19. Kumakura Y, Vernaleken I, Buchholz HG, et al. Age-dependent decline of steady state dopamine storage capacity of human brain: an FDOPA PET study. Neurobiol Aging 2010;31:447–463 [DOI] [PubMed] [Google Scholar]
- 20. Guilarte TR, Burton NC, McGlothan JL, et al. Impairment of nigrostriatal dopamine neurotransmission by manganese is mediated by pre-synaptic mechanism(s): implications to manganese-induced parkinsonism. J Neurochem 2008;107:1236–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guilarte TR, Chen MK, McGlothan JL, et al. Nigrostriatal dopamine system dysfunction and subtle motor deficits in manganese-exposed non-human primates. Exp Neurol 2006;202:381–390 [DOI] [PubMed] [Google Scholar]
- 22. Brooks DJ. The early diagnosis of Parkinson's disease. Ann Neurol 1998;44:S10–S18 [DOI] [PubMed] [Google Scholar]
- 23. Bhatia KP, Marsden CD. The behavioural and motor consequences of focal lesions of the basal ganglia in man. Brain 1994;117:859–876 [DOI] [PubMed] [Google Scholar]
- 24. Rinne JO, Portin R, Ruottinen H, et al. Cognitive impairment and the brain dopaminergic system in Parkinson disease: [18F]fluorodopa positron emission tomographic study. Arch Neurol 2000;57:470–475 [DOI] [PubMed] [Google Scholar]
- 25. Rodier J. Manganese poisoning in Moroccan miners. Brit J Industr Med 1955;12:21–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bowler RM, Nakagawa S, Drezgic M, et al. Sequelae of fume exposure in confined space welding: a neurological and neuropsychological case series. Neurotoxicology 2007;28:298–311 [DOI] [PubMed] [Google Scholar]
- 27. Piccini P, Morrish PK, Turjanski N, et al. Dopaminergic function in familial Parkinson's disease: a clinical and 18F-dopa positron emission tomography study. Ann Neurol 1997;41:222–229 [DOI] [PubMed] [Google Scholar]
- 28. Sawle GV, Wroe SJ, Lees AJ, Brooks DJ, Frackowiak RS. The identification of presymptomatic parkinsonism: clinical and [18F]dopa positron emission tomography studies in an Irish kindred. Ann Neurol 1992;32:609–617 [DOI] [PubMed] [Google Scholar]
- 29. Hilker R, Klein C, Hedrich K, et al. The striatal dopaminergic deficit is dependent on the number of mutant alleles in a family with mutations in the parkin gene: evidence for enzymatic parkin function in humans. Neurosci Lett 2002;323:50–54 [DOI] [PubMed] [Google Scholar]
- 30. NIOSH Criteria for a Recommended Standard: Occupational Exposure to Welding, Brazing, and Thermal Cutting: Publication No. 88_110. Cincinnati, OH: US Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health, DHHS (NIOSH); 1988 [Google Scholar]