Abstract
Objective:
Temporal lobe resection is an established treatment for medication-resistant temporal lobe epilepsy, which in recent years has increasingly been performed in children. However, little is known about the long-term outcome in these children. The aim of this study was to characterize intellectual and psychosocial functioning of children after temporal lobe resection as they progress into late adolescence and adulthood.
Methods:
We report the long-term follow-up of 42 children who underwent temporal lobe surgery after an average postoperative period of 9 years. Longitudinal change in IQ was documented, psychosocial outcome including quality of life was assessed, and preoperative and postoperative T1-weighted MRI brain scans were evaluated quantitatively. A well-matched nonsurgical comparison group of 11 children with similar clinical characteristics was also assessed.
Results:
At follow-up, 86% of the surgical group were seizure-free, and 57% were no longer taking antiepileptic medication. A significant increase in IQ was found in the surgical group after an extended follow-up period of >5 years. This IQ change was not found in the nonsurgical comparison group. IQ increases were associated with cessation of antiepileptic medication and changes in MRI-derived gray matter volume. The surgical group also reported better psychosocial outcome including quality of life, which was more strongly associated with seizure freedom rather than surgery per se.
Conclusions:
Surgery for temporal lobe epilepsy performed in childhood results in excellent long-term seizure control and favorable cognitive outcome along with positive effects on brain development.
Classification of evidence:
This study provides Class III evidence that temporal lobectomy in children with temporal lobe epilepsy is associated with improved long-term intellectual outcomes compared with those undergoing standard medical treatment.
Medication-resistant temporal lobe epilepsy (TLE) is often caused by brain abnormalities such as tumors or hippocampal sclerosis. Surgical removal of such lesions results in excellent seizure control.1 When surgical treatment for TLE in children is considered, factors such as the impact of seizures and medication on brain development and function have to be taken into account.2 Because children with poor seizure control show a decrease in intellectual functioning over time,3 it is thought that early surgical intervention could reduce the severity of cognitive impairment.4
Short-term intellectual outcome in children after temporal lobe surgery is relatively well documented. Although many follow-up studies span up to 2 years after surgery, showing little or no group changes in IQ,5–8 long-term follow-up studies commonly extrapolate outcome from only a few individuals because of considerable within-study variation in follow-up duration,9–11 ranging from 6 months to 10 years. Previous studies have suggested that seizure frequency,12 memory function,13 and intelligence11 may show late changes after temporal lobe surgery. Long-term follow-up studies are therefore required to determine the more stable levels of cognitive functioning, allowing for postsurgical recovery and functional reorganization.
Because previous studies have shown a relationship between IQ in childhood-onset TLE and brain tissue volumes,14 we set out to explore this relationship. Finally, longer-term outcome studies can also serve to answer questions regarding the psychosocial outcome of children with temporal lobe surgery as they progress into adulthood.
METHODS
Participants.
All patients who underwent temporal lobe resection for epilepsy associated with hippocampal sclerosis or dysembryoplastic neuroepithelial tumors between 1992 and 2002 at Great Ormond Street Hospital NHS Trust (GOSH) were contacted for participation in this study. Inclusion criteria were preoperative medication-resistant epilepsy, single pathologic diagnosis, a minimum follow-up period of 5 years, and minimum age of 16 years at follow-up. All surgical patients underwent temporal lobe resection performed by the same neurosurgeon (W.H.). Of 60 patients identified as potential candidates for this study, 42 (70%) participated in the research. The mean interval between surgery and follow-up was 9 years (range, 5–15 years). Of the 18 patients who did not participate, 6 could not be located, and 12 declined. Demographic details of study participants and those lost to follow-up are given in table 1. The 18 surgery patients lost to follow-up did not differ significantly from those recruited in terms of preoperative seizure burden and level of cognitive functioning. Of the 12 who declined, 4 patients had a degree of disability that impeded them from taking part.
Table 1.
Demographic data for surgical and nonsurgical groups recruited into the study and for surgical patients who were lost to follow-upa
Abbreviations: DNT = dysembryoplastic neuroepithelial tumor; HS = hippocampal sclerosis.
No significant group differences were found.
In addition, all patients who underwent preoperative investigations for epilepsy surgery at GOSH between 1992 and 2002 but did not undergo surgery at our center were identified as potential control subjects for this study and were contacted for participation. Inclusion criteria were age 16 years or older at follow-up, medication-resistant epilepsy at the time of initial investigation for epilepsy surgery, a minimum follow-up period of 5 years after initial investigation, IQ data available from time of initial assessment, and focal temporal lobe abnormalities seen on MRI with corroborating findings from EEG investigations, functional imaging studies, or both. Eleven control participants were recruited into the study. In 5 participants, evidence for unilateral hippocampal volume loss was visible on MRI. This control group was comparable to the surgical group in terms of age at onset of epilepsy and duration of follow-up as well as preoperative IQ (table 1). In the majority of participants, surgery was not offered because of discordance of EEG and SPECT or PET changes in comparison with MRI findings.
Standard protocol approvals, registrations, and patient consents.
Ethics permission for this study was granted by the GOSH Ethics Committee, and fully informed written consent was obtained from each participant.
Procedures.
Preoperative and postoperative clinical assessments.
Clinical workup.
All patients underwent a presurgical evaluation, which included neuropsychologic assessment, ictal and interictal EEG, and MRI. For the surgical group, all available presurgical MRI scans (available for n = 31, 74%) were obtained from records. These MRI scans were acquired on a 1.5-T Siemens Vision System (Siemens, Erlangen, Germany) and included a volume T1-weighted scan using a 3-dimensional magnetization-prepared rapid gradient echo sequence (repetition time = 10 msec; echo time = 4 msec; flip angle = 12 degrees; voxel size = 1.0 × 1.0 × 1.25 mm).
Neurodevelopment/cognition.
Data from all previous IQ assessments performed at GOSH were collected. Throughout the follow-up period, a number of different Wechsler intelligence scales were used (Wechsler Intelligence Scale for Children [WISC]–Revised, WISC-III, Wechsler Adult Intelligence Scale [WAIS]–Revised, and WAIS-III). The intercorrelations between the different test versions observed in this sample were high: WISC to WISC (n = 37): R = 0.89; WISC to WAIS (n = 32): R = 0.91; and WAIS to WAIS (n = 17): R = 0.89 (all p < 0.001). Preoperative IQ data were available in all but 4 patients (one patient was too young and 3 were not testable). Thirty-seven patients underwent intermediate testing (often at 1, 3, and 5 years after surgery). Baseline IQ refers to the IQ scores obtained most immediately preceding surgery. In 2 patients in whom initial surgery did not yield seizure control and more extensive surgery was undertaken at a later date, baseline IQ was obtained immediately preceding the first surgery. For the nonsurgical control group, baseline IQ denotes the first assessment performed at our center.
Long-term follow-up assessment.
Neurodevelopment/cognition.
Intellectual functioning was measured using the WAIS-III UK,15 yielding a full-scale IQ (FSIQ), verbal IQ (VIQ), and performance IQ (PIQ).
MRI.
Investigations were performed on a 1.5-T Siemens Avanto system. Three-dimensional volume T1-weighted scans were acquired using a 3-dimensional fast low-angle shot sequence (repetition time = 11 msec; echo time = 5 msec; flip angle = 15 degrees; voxel size = 1.0 × 1.0 × 1.0 mm).
Quality of life and socioeconomic outcome.
The Quality of Life in Epilepsy Questionnaire (QOLIE-36 UK)16 assesses illness-specific aspects, including seizure and medication concerns, as well as more general traits (e.g., energy levels and emotional well-being). Seizure-independent quality of life was also calculated, using only items of the QOLIE-36 UK that did not inquire about seizure status and medication. Socioeconomic outcome was measured using the Socioeconomic Development Questionnaire.17
MRI analysis.
For the surgical group, presurgical and postsurgical gray matter and white matter volumes were obtained using the VBM toolbox (developed by C. Gaser, University Jena, Germany, dbm.neuro.uni-jena.de/vbm/) for SPM5 (Wellcome Department of Imaging Neuroscience, www.fil.ion.ucl.ac.uk). This program uses the unified segmentation procedure implemented in SPM5,18 modified to include a Hidden Markov Field model as an additional spatial constraint (dbm.neuro.uni-jena.de/vbm/markov-random-fields/).19 The segmentation algorithm is based on a Gaussian mixture model, but in contrast with the default SPM5 algorithm, the final tissue probabilities are estimated without tissue priors.20,21 All segments were visually inspected for accuracy. For measurement of resection volumes, lesion maps were created using MRIcro software (C. Rorden, www.cabiatl.com/mricro/). The resection was manually traced on the volume T1-weighted images, using as reference the intact hemisphere contralateral to the resected temporal lobe.
Statistical analysis.
Group differences in demographic, cognitive, and psychosocial data were tested using independent sample t tests, analysis of variance, and χ2 or Fisher exact tests where appropriate. Changes across time in IQ scores were tested using repeated-measures analysis of variance. Changes in IQ and brain volume were calculated by subtracting baseline from follow-up values. All positive changes thereby index increases, whereas negative changes index decrements. Partial correlations and multiple linear regression analyses were used to identify factors associated with IQ change. Factors entered into the regression model for IQ change were preoperative FSIQ, number of prior IQ assessments, current use of antiepileptic medication, age at onset, duration of epilepsy, surgery, time since last seizure, and current seizure status. Patients with missing values were excluded from analyses. Diagnostic analyses included examination of influential points, normality of residuals, and multicollinearity.
RESULTS
Group representativeness and matching.
The surgical group recruited and the group of patients lost to follow-up did not differ in terms of age at surgery, age at seizure onset, or preoperative FSIQ (all p > 0.185) (table 1). Furthermore, surgical and nonsurgical groups did not differ in terms of age at seizure onset, age at follow-up, or preoperative FSIQ (all p > 0.147).
Seizure outcome and medication.
At baseline, 45% of the surgical group and 27% of the nonsurgical control group experienced daily seizures, with the remainder of participants experiencing seizures less than once a day (χ2 = 2.3, p = 0.314, not significant). Both groups showed improved seizure control at follow-up. However, as expected, fewer control participants (36%) compared with surgical patients (86%) were seizure-free at follow-up (Fisher exact test, p = 0.002). When seizure types were considered, the control group reported a greater proportion of complex partial seizures than the surgery group (Fisher exact test, p = 0.023), but no difference in simple partial or generalized seizures was found. Furthermore, the proportions of surgical patients and control participants who were taking antiepileptic drugs (AEDs) at follow-up were 43% and 73%, respectively (Fisher exact test, p = 0.076). The nonsurgical group used on average a greater number of AEDs at baseline and follow-up than the surgery group (table e-1 on the Neurology® Web site at www.neurology.org).
Changes in intellectual functioning.
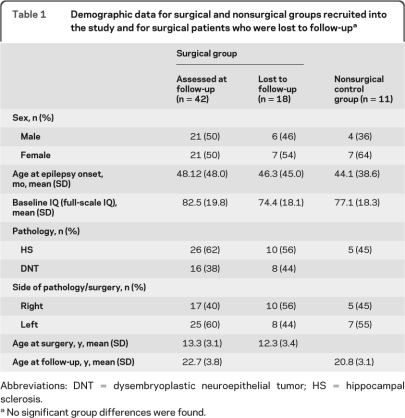
Preoperatively, the distribution in IQ scores was skewed toward the low end of the spectrum (figure 1A). The distribution at long-term follow-up has a resemblance to a normal distribution. Indeed, the mean FSIQ improved in surgical participants but remained unchanged in control participants (group by time interaction: F1,47 = 4.8, p = 0.033) (figure 1B).
Figure 1. Full-scale IQ (FSIQ) changes from baseline to long-term follow-up.
(A) FSIQ distribution of the surgical group at baseline (white bars) and at long-term follow-up (black bars). (B, inset) Mean FSIQ of surgical and nonsurgical control groups over the period of follow-up. (C, inset) IQ change scores for surgery patients grouped according to preoperative IQ range. The Kruskal-Wallis test indicated differences between IQ categories (p = 0.032). Case A had an IQ reduction by 22 points before a second operation was required because of continuing severe seizures (see text).
At an individual level, a gain in FSIQ of at least 10 points was observed in 17 surgery patients (41%) and in one control participant (9%). The individual change scores for the surgery group are shown in figure 1C, grouped according to preoperative IQ range. Only one patient, who had undergone a second surgical procedure due to poor seizure control after the first, showed an equivalently high loss (case A in figure 1C). However, he had had an IQ reduction by 22 points before this second procedure, which eventually rendered him seizure-free. The overall loss of 13 points at follow-up therefore includes an improvement of 9 points since the initial surgical procedure.
Hemisphere-dependent changes.
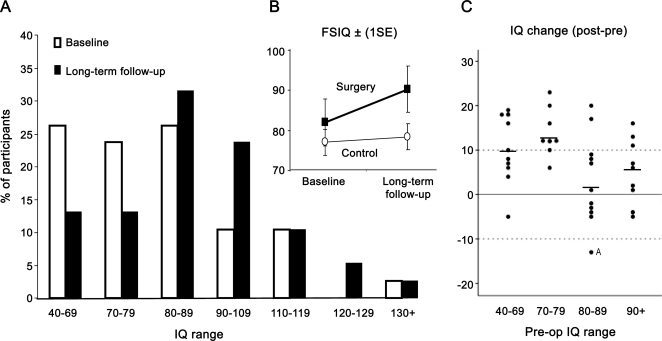
Changes in VIQ and PIQ were dependent on the side of surgery (interaction of task by side, F2,46 = 5.1, p = 0.01) (figure 2). Post hoc t tests showed that PIQ improved in both left- and right-sided surgery groups, but VIQ improved only in the left-sided surgery group.
Figure 2. Hemisphere-dependent changes in verbal IQ (VIQ) and performance IQ (PIQ).
Preoperative to postoperative changes in VIQ and PIQ scores in nonsurgical control and left- and right-sided surgery groups. *Significant IQ changes (post hoc one-sample t tests, p < 0.01).
Longitudinal analysis of the surgery group.
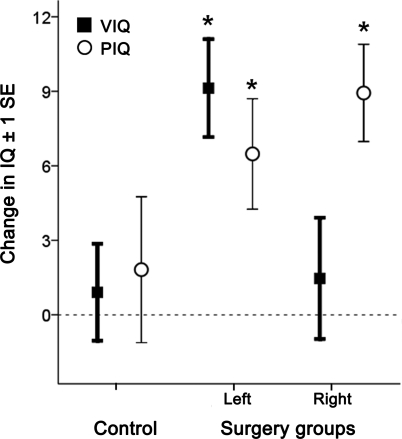
The IQ profile over time varied greatly among the individual patients. In those who had more than one postoperative IQ assessment, only 23% of the variance in long-term FSIQ change was predicted by FSIQ change at the initial postoperative assessment (mean = 1.5 years) after 2 patients with repeated surgery were excluded (R = 0.48, p = 0.011). No significant change in FSIQ was present during the follow-up periods up to 6 years (one-sample t tests, all p > 0.637) (figure 3). Group changes in IQ were only apparent at 6–8 years (t = 2.2, p = 0.05) and longer than 8 years (t = 4.6, p < 0.001). To investigate whether changes in Wechsler IQ test versions could account for these findings, we restricted the longitudinal analysis to changes measured using the same test version. These findings, albeit in a smaller subgroup of surgical patients, mirror the longitudinal profile observed across the whole sample.
Figure 3. Longitudinal profile of IQ changes.
Preoperative to postoperative full-scale IQ changes across time after surgery, shown for subsequent 2-year periods (positive values denote IQ gains). Note that only a proportion of all patients were assessed during each time period (indicated as a percentage of the total group). *Significant changes (one-sample t tests, p ≤ 0.05).
Brain structural correlates of FSIQ change.
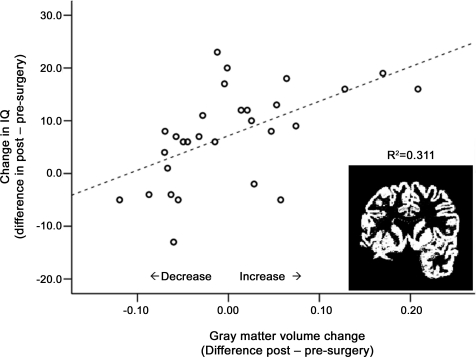
There were no volume differences according to pathology or side of surgery in any of the preoperative and postoperative tissue and brain volumes or for the volume of resection (table e-2). Only interindividual variability in presurgical to postsurgical tissue volume changes will be considered here, because different MRI scanners were used at baseline and follow-up. Partial correlations, controlling for time between scans, sex, and volume of resection, revealed associations for change in gray matter volume (i.e., the difference in preoperative to postoperative volume) and change in FSIQ (R = 0.57, p = 0.002) (figure 4) but no relationship with change in white matter volume (R = 0.24, p = 0.244).
Figure 4. Brain gray matter volume and IQ change.
Changes in gray matter volume (residuals after controlling for time between scans, sex, and lesion volume, in liters) in relation to full-scale IQ change in the surgery group (n = 31). (Inset) Extracted gray matter segment of a patient with a left temporal lobe resection.
Furthermore, at each time point, partial correlations with FSIQ (controlled for age at scan and sex) were significant for total gray matter volume (preoperative: R = 0.57, p = 0.002; follow-up: R = 0.43, p = 0.019) and white matter volume (preoperative: R = 0.55, p = 0.003; follow-up: R = 0.57, p = 0.001).
Predictors of FSIQ change.
Stepwise multiple regression analysis was used to determine predictors of FSIQ change in the whole cohort (excluding surgical and MRI variables). Negative predictors (F2,46 = 8.0, p = 0.001, R2 = 0.26) were current antiepileptic medication (β = −0.47) and preoperative FSIQ (β = −0.32), the latter reflecting the relationship shown in figure 1C. Clinical variables including age at onset of epilepsy, duration of epilepsy, number of prior IQ assessments, surgery, time since last seizure, and current seizure status were not significant. Repeating this analysis in seizure-free patients as well as in the surgical cohort (with inclusion of age at surgery and pathology type) identified the same predictors. An exploratory regression analysis of the different AED types in the total sample at follow-up (table e-1) revealed that use of topiramate was a single negative factor associated with IQ change (β = −0.43, p = 0.002). An exploration of seizure types at follow-up (simple partial, complex partial, and generalized) did not reveal any significant contribution to IQ change.
A further analysis was performed to assess the contribution of gray and white matter volume changes to FSIQ change in the surgery group. Only change in gray matter volume (β = 0.55) was a predictor of FSIQ change (F1,27 = 11.5, p = 0.002, R2 = 0.30). Volume of resection, change in white matter volume, and the above clinical variables were not predictive.
Psychosocial and quality-of-life outcome.
Total quality-of-life scores were higher in the surgery group (table e-3). A stepwise regression analysis, which included FSIQ, AED use, surgery, and seizure status as factors, confirmed that total quality of life was mainly determined by seizure freedom (β = 0.44, p = 0.001). No other outcome measure differed between groups. The same measures were explored in relation to seizure freedom across both groups. A higher proportion of participants with continuing seizures held a statement of disability (75% compared with 23% in seizure-free participants; χ2 = 10.5, p = 0.001), whereas no difference was seen at baseline (31% and 42%, respectively). Seizure freedom was also associated with a higher seizure-independent QOLIE-36 UK score (p = 0.045).
DISCUSSION
We find improved intellectual functioning in long-term follow-up of individuals who underwent temporal lobe resection in childhood; this outcome was not observed in a well-matched nonsurgical control group. At a group level, this improvement was observed only after a postoperative period of 6 years or more. The increase in FSIQ was associated with cessation of antiepileptic medication and correlated with changes in total brain gray matter volume. Furthermore, there was a high rate of seizure control after surgery, which was related to improved quality of life.
Children at the low end of the IQ spectrum improved more after surgery than those with average and high average IQs. Whereas the possible statistical confound of regression toward the mean22 cannot be excluded in our study, this effect was not observed with FSIQ changes at the first postoperative assessment. Greater postsurgical cognitive improvements in children with lower preoperative IQs have also been noted in previous studies.6,10,11,23 IQ changes in this cohort were only seen 6 or more years after surgery, similar to findings in other cohorts.11,24 In comparison, the fact that studies with shorter duration of follow-up typically do not find improvements in intellectual functioning5,25–28 suggests that a prolonged period is required for cognitive recovery and subsequent development.
We found a robust relationship between change in gray matter volume and FSIQ. The lack of a contribution of resection volume to this relationship may seem paradoxical at first glance; however, the average temporal lobe resection represents only about 1.5% of total brain volume. The relationship between IQ and gray matter changes in our cohort is consistent with other longitudinal data29,30 (see appendix e-1 for further discussion). This observation is also strengthened by the cross-sectional finding that brain tissue volumes correlated with IQ at both preoperative and postoperative assessments, in agreement with a previous report.14 The neuroanatomic basis of the observed gray matter changes is not known but may consist of a mixture of changes in cortical thickness, surface area, and volume changes in subcortical regions that have been documented in patients with chronic TLE.31
Cessation of antiepileptic medication was the strongest predictor of IQ increase, even after exclusion of patients with continuing seizures, which lends support for the concerns about the potential impact of long-term AED use (in particular of topiramate) on cognitive development.32 Given recent evidence for the detrimental effects of such drugs on neurodevelopmental processes, such as apoptosis and neurogenesis,33 our findings call for controlled investigations into the timing of drug withdrawal after epilepsy surgery and seizure cessation.
Seizure freedom was the most significant predictor of enhanced psychosocial outcome in this study, associated with higher quality-of-life scores and a lower percentage of patients with a statement of disability. These findings are in agreement with other studies in adults5,34,35 and children.2,36,37
It is worth highlighting some limitations to this study, including the relatively small sample size and the retrospective nature of the information on AED use and seizure burden. Surgical and nonsurgical groups are likely to differ in a number of aspects, including pathologic diagnosis. The control group may have presented with a more complex seizure disorder or less focal seizure onsets than the surgical group, as evidenced by the number of discordant or bilateral EEG findings. The study was developmental in nature, and the same psychometric tests were not used at baseline and follow-up, which may have resulted in systematic changes in IQ.6 However, our nonsurgical comparison group allowed us to estimate the effect of these potential confounds. Although Wechsler intelligence scales are susceptible to practice effects,38 FSIQ change was not correlated to the number of postoperative IQ assessments conducted with each individual. Furthermore, VIQ improvements showed side specificity, seen only in patients undergoing left temporal lobectomy, suggesting that practice effects and switching between test versions are unlikely to fully account for the IQ changes seen. These changes may reflect a reversal of preoperative VIQ decline.3,39 A similar pattern has also been reported in adults after left temporal lobe surgery.40
Overall, intellectual gains observed in surgically treated patients with TLE in our study are striking when viewed in the context of the risk of cognitive deterioration documented over the medium term in conventionally treated patients with early-onset TLE.3,39
Supplementary Material
ACKNOWLEDGMENT
The authors thank all members of the Epilepsy Surgery Team at GOSH for providing the baseline clinical data. They also thank Tina Banks and Wendy Norman for MRI scanning and Johanna Gaiottino and Nisa Bitsch for help with data collection.
Supplemental data at www.neurology.org
- AED
- antiepileptic drug
- FSIQ
- full-scale IQ
- GOSH
- Great Ormond Street Hospital NHS Trust
- PIQ
- performance IQ
- QOLIE-36 UK
- Quality of Life in Epilepsy Questionnaire
- TLE
- temporal lobe epilepsy
- VIQ
- verbal IQ
- WAIS
- Wechsler Adult Intelligence Scale
- WISC
- Wechsler Intelligence Scale for Children
AUTHOR CONTRIBUTIONS
Statistical analyses were conducted by Dr. Torsten Baldeweg and Caroline Skirrow.
DISCLOSURE
C. Skirrow has received research support from the Medical Research Council (UK) and the National Institute for Health Research (UK). Prof. Cross serves on a scientific advisory board for GlaxoSmithKline; has received funding for travel from Eisai Inc. and UCB; serves on the editorial boards of Developmental Medicine, Child Neurology, Epileptic Disorders, and European Journal of Paediatric Neurology; has received educational grants from Janssen, UCB, Eisai Inc., and Nutricia; and has received research support from Matthews Friends, Epilepsy Research (UK), and Action Medical Research (UK). Dr. Cormack reports no disclosures. Mr. Harkness has served as a consultant for Forth Medical Ltd. Prof. Vargha-Khadem has received research support from the Medical Research Council (UK), Epilepsy Research (UK), Action Medical Research (UK), Wellcome Trust (UK), and the NIH. Dr. Baldeweg has received research support from Action Medical Research (UK), Royal Society (UK), Medical Research Council (UK), Epilepsy Research (UK), the Volkswagenstiftung (Germany), and Great Ormond Street Hospital Children's Charity.
REFERENCES
- 1. Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med 2001;345:311–318 [DOI] [PubMed] [Google Scholar]
- 2. Spencer S, Huh L. Outcomes of epilepsy surgery in adults and children. Lancet Neurol 2008;7:525–537 [DOI] [PubMed] [Google Scholar]
- 3. Bjornaes H, Stabell K, Henriksen O, Loyning Y. The effects of refractory epilepsy on intellectual functioning in children and adults: a longitudinal study. Seizure 2001;10:250–259 [DOI] [PubMed] [Google Scholar]
- 4. Cross JH. Epilepsy surgery in childhood. Epilepsia 2002;43(suppl 3):65–70 [DOI] [PubMed] [Google Scholar]
- 5. Williams J, Griebel ML, Sharp GB, Boop FA. Cognition and behavior after temporal lobectomy in pediatric patients with intractable epilepsy. Pediatr Neurol 1998;19:189–194 [DOI] [PubMed] [Google Scholar]
- 6. Gleissner U, Clusmann H, Sassen R, Elger CE, Helmstaedter C. Postsurgical outcome in pediatric patients with epilepsy: a comparison of patients with intellectual disabilities, subaverage intelligence, and average-range intelligence. Epilepsia 2006;47:406–414 [DOI] [PubMed] [Google Scholar]
- 7. Korkman M, Granstrom ML, Kantola-Sorsa E, et al. Two-year follow-up of intelligence after pediatric epilepsy surgery. Pediatr Neurol 2005;33:173–178 [DOI] [PubMed] [Google Scholar]
- 8. Westerveld M, Sass KJ, Chelune GJ, et al. Temporal lobectomy in children: cognitive outcome. J Neurosurg 2000;92:24–30 [DOI] [PubMed] [Google Scholar]
- 9. Lewis DV, Thompson RJ, Jr, Santos CC, et al. Outcome of temporal lobectomy in adolescents. J Epilepsy 2007;9:198–205 [Google Scholar]
- 10. Miranda C, Smith ML. Predictors of intelligence after temporal lobectomy in children with epilepsy. Epilepsy Behav 2001;2:13–19 [DOI] [PubMed] [Google Scholar]
- 11. Freitag H, Tuxhorn I. Cognitive function in preschool children after epilepsy surgery: rationale for early intervention. Epilepsia 2005;46:561–567 [DOI] [PubMed] [Google Scholar]
- 12. Jarrar RG, Buchhalter JR, Meyer FB, Sharbrough FW, Laws E. Long-term follow-up of temporal lobectomy in children. Neurology 2002;59:1635–1637 [DOI] [PubMed] [Google Scholar]
- 13. Alpherts WC, Vermeulen J, van Rijen PC, da Silva FH, van Veelen CW. Verbal memory decline after temporal epilepsy surgery? A 6-year multiple assessments follow-up study. Neurology 2006;67:626–631 [DOI] [PubMed] [Google Scholar]
- 14. Hermann B, Seidenberg M, Bell B, et al. The neurodevelopmental impact of childhood-onset temporal lobe epilepsy on brain structure and function. Epilepsia 2002;43:1062–1071 [DOI] [PubMed] [Google Scholar]
- 15. Wechsler D. Wechsler Adult Intelligence Scale, 3rd ed. London: 1999 [Google Scholar]
- 16. Cramer JA, Perrine K, Devinsky O, Bryant-Comstock L, Meador K, Hermann B. Development and cross-cultural translations of a 31-item quality of life in epilepsy inventory. Epilepsia 1998;39:81–88 [DOI] [PubMed] [Google Scholar]
- 17. Lendt M, Helmstaedter C, Elger CE. Pre- and postoperative socioeconomic development of 151 patients with focal epilepsies. Epilepsia 1997;38:1330–1337 [DOI] [PubMed] [Google Scholar]
- 18. Ashburner J, Friston KJ. Unified segmentation. Neuroimage 2005;26:839–851 [DOI] [PubMed] [Google Scholar]
- 19. Cuadra MB, Cammoun L, Butz T, Cuisenaire O, Thiran JP. Comparison and validation of tissue modelization and statistical classification methods in T1-weighted MR brain images. IEEE Trans Med Imaging 2005;24:1548–1565 [DOI] [PubMed] [Google Scholar]
- 20. Gaser C, Altaye M, Wilke M, Holland SK. Unified segmentation without tissue priors. Neuroimage 2007;36(suppl 1):S68 [Google Scholar]
- 21. Altaye M, Holland SK, Wilke M, Gaser C. Infant brain probability templates for MRI segmentation and normalization. Neuroimage 2008;43:721–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hermann BP, Wyler AR, VanderZwagg R, et al. Predictors of neuropsychological change following anterior temporal lobectomy: role of regression toward the mean. J Epilepsy 1991;4:139–148 [Google Scholar]
- 23. Bittar RG, Rosenfeld JV, Klug GL, Hopkins IJ, Simon HA. Resective surgery in infants and young children with intractable epilepsy. J Clin Neurosci 2002;9:142–146 [DOI] [PubMed] [Google Scholar]
- 24. Lindsay J, Glaser G, Richards P, Ounsted C. Developmental aspects of focal epilepsies of childhood treated by neurosurgery. Dev Med Child Neurol 1984;26:574–587 [DOI] [PubMed] [Google Scholar]
- 25. Smith ML, Elliott IM, Lach L. Cognitive, psychosocial, and family function one year after pediatric epilepsy surgery. Epilepsia 2004;45:650–660 [DOI] [PubMed] [Google Scholar]
- 26. Bjornaes H, Stabell KE, Henriksen O, Roste G, Diep LM. Surgical versus medical treatment for severe epilepsy: consequences for intellectual functioning in children and adults: a follow-up study. Seizure 2002;11:473–482 [DOI] [PubMed] [Google Scholar]
- 27. Jambaque I, Dellatolas G, Fohlen M, et al. Memory functions following surgery for temporal lobe epilepsy in children. Neuropsychologia 2007;45:2850–2862 [DOI] [PubMed] [Google Scholar]
- 28. Gilliam F, Wyllie E, Kashden J, et al. Epilepsy surgery outcome: comprehensive assessment in children. Neurology 1997;48:1368–1374 [DOI] [PubMed] [Google Scholar]
- 29. Gothelf D, Penniman L, Gu E, Eliez S, Reiss AL. Developmental trajectories of brain structure in adolescents with 22q11.2 deletion syndrome: a longitudinal study. Schizophr Res 2007;96:72–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shaw P, Greenstein D, Lerch J, et al. Intellectual ability and cortical development in children and adolescents. Nature 2006;440:676–679 [DOI] [PubMed] [Google Scholar]
- 31. Dabbs K, Jones J, Seidenberg M, Hermann B. Neuroanatomical correlates of cognitive phenotypes in temporal lobe epilepsy. Epilepsy Behav 2009;15:445–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Marsh ED, Brooks-Kayal AR, Porter BE. Seizures and antiepileptic drugs: does exposure alter normal brain development? Epilepsia 2006;47:1999–2010 [DOI] [PubMed] [Google Scholar]
- 33. Stefovska VG, Uckermann O, Czuczwar M, et al. Sedative and anticonvulsant drugs suppress postnatal neurogenesis. Ann Neurol 2008;64:434–445 [DOI] [PubMed] [Google Scholar]
- 34. Keene DL, Higgins MJ, Ventureyra EC. Outcome and life prospects after surgical management of medically intractable epilepsy in patients under 18 years of age. Childs Nerv Syst 1997;13:530–535 [DOI] [PubMed] [Google Scholar]
- 35. Markand ON, Salanova V, Whelihan E, Emsley CL. Health-related quality of life outcome in medically refractory epilepsy treated with anterior temporal lobectomy. Epilepsia 2000;41:749–759 [DOI] [PubMed] [Google Scholar]
- 36. van Empelen R, Jennekens-Schinkel A, van Rijen PC, Helders PJ, van Nieuwenhuizen O. Health-related quality of life and self-perceived competence of children assessed before and up to two years after epilepsy surgery. Epilepsia 2005;46:258–271 [DOI] [PubMed] [Google Scholar]
- 37. Sabaz M, Lawson JA, Cairns DR, et al. The impact of epilepsy surgery on quality of life in children. Neurology 2006;66:557–561 [DOI] [PubMed] [Google Scholar]
- 38. Chelune GJ, Naugle RI, Luders H, Sedlak J, Awas IA. Individual change after epilepsy surgery: practice effects and base-rate information. Neuropsychology 1993;7:41–52 [Google Scholar]
- 39. Seidenberg M, Pulsipher DT, Hermann B. Cognitive progression in epilepsy. Neuropsychol Rev 2007;17:445–454 [DOI] [PubMed] [Google Scholar]
- 40. Alpherts WC, Vermeulen J, Hendriks MP, et al. Long-term effects of temporal lobectomy on intelligence. Neurology 2004;62:607–611 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.