Abstract
CD3ζ is a subunit of the CD3 molecule that, until recently, appeared restricted to T-cells and natural killer cells. However, experimental studies have demonstrated a role of CD3ζ in dendritic outgrowth in the visual system as well as in synaptic plasticity. Given the increasing evidence for uncharacteristic recapitulation of neurodevelopmental processes in neurodegenerative diseases, in this study, we evaluated brains from subjects with Parkinson's disease and Lewy body dementia for evidence of aberrant CD3 expression. Our data shows marked CD3ζ in association with the α-synuclein containing pathological lesions, i.e., Lewy bodies and Lewy neurites, in the brains of subjects with Parkinson's disease and Lewy body dementia. This finding raises the novel concept of CD3 dysregulation in these disorders as a pathogenic factor and also furthers the increasing evidence that the recall of aberrant neurodevelopmental processes underlies the pathogenesis of neurodegenerative diseases.
Keywords: alpha-synuclein, CD3, Lewy bodies, Lewy body dementia, Lewy neurites, neurodevelopment, Parkinson's disease
Introduction
Evidence of similarities in molecular signaling between neuroectodermal cells and the immune system is accumulating, which in turn suggests novel neuronal functions for molecules previously restricted to the immune system (Boulanger and Shatz 2004; Baudouin et al. 2008). It is already established that dendritic development is regulated by a number of factors, such as neuronal activity (Lohmann et al. 2002), diffusible molecules (Polleux et al. 2000), and signaling molecules (Fink et al. 2003), processes that are also involved in the differentiation and function of immune cells. The diffusible signaling molecules, semaphorins and slits, for example, involved in dendritic outgrowth, are required for the maturation, migration, and activation of T lymphocytes and follicular dendritic cells (Polleux et al. 2000). Such commonalities emphasize the similarities of such ostensibly divergent cellular phenotypes as neurons and lymphocytes.
Conversely, CD3ζ, a subunit to the CD3 antigen and a transmembrane signaling protein that, until recently, had only been characterized in T lymphocytes and natural killer cells (Lanier 2001; Pitcher and van Oers 2003), has been implicated in the development of retinogeniculate projections in the visual system and synaptic plasticity (Huh et al. 2000; Barco et al. 2005). These findings raise the possibility of a fundamental neurodevelopmental role of CD3ζ, and therefore, the possibility that abnormalities in CD3 transcription, translation, or expression with age or disease may be involved in neurological diseases.
To address the role of CD3 in human neurologic disease, we examined brains of subjects with Parkinson's disease and Lewy body dementia for CD3 expression.
Materials and Methods
Tissue from six cases with Lewy body dementia (age range 59-82), six with Parkinson's disease (age range 53-81), and four control cases (age range 67-86) were fixed in either 10% formalin or methacarn (methanol/chloroform/acetic acid, 60:30:10 by volume). Following fixation tissue was embedded in paraffin and 6 μm thick sections were mounted on silane-coated slides (Sigma, St. Louis, MO). Sections were then hydrated through descending ethanol. Endogenous peroxidase activity was eliminated by incubation in 3% H2O2 in Tris-buffered saline (TBS; 50mM Tris-HCl, 150 mM NaCl, pH 7.6) for 30 min. To reduce non-specific binding, sections were incubated for 30 min with 10% normal goat serum (NGS) in TBS. After rinsing briefly with 1% NGS, sections were incubated overnight with primary antibody, CD3ζ (rabbit, Dako North America, Carpinteria, CA) (Zhan et al. 2005) or α-synuclein (mouse; Abcam, Cambridge, MA) (Zhu et al. 2006). As previously described (Smith et al. 1994a; Smith et al. 1994b), cells were stained with the peroxidase antiperoxidase method (Sternberger 1986) using 3,3′-diaminobenzidine (DAB) as a chromogen (Dako Corporation, Carpinteria, CA, USA).
Results
Brains from six Lewy body dementia subjects showed α-synuclein pathology in the form of Lewy bodies and Lewy neurites, in the brainstem and neocortex, indicative of Lewy body dementia, and six brains from subjects with the clinical diagnosis of Parkinsonism and Lewy bodies within nigral dopaminergic neurons at autopsy (i.e., Parkinson's disease) were also examined. Pathology was highlighted using α-synuclein immunohistochemistry as described previously (Zhu et al. 2006).
CD3 immunohistochemistry was remarkable for widespread immunoreactivity limited only to brains of subjects with Parkinson's disease and Lewy body dementia. The immunoreactivity showed precise overlap with α-synuclein pathology, reacting with brainstem and neocortical Lewy bodies and Lewy neurites, including the CA-2,3 linear dystrophic neurites characteristic of Lewy body dementia (Figure 1). No staining of histologically unaffected neurons was apparent nor was there labeling of neurons in control brain sections.
Figure 1.
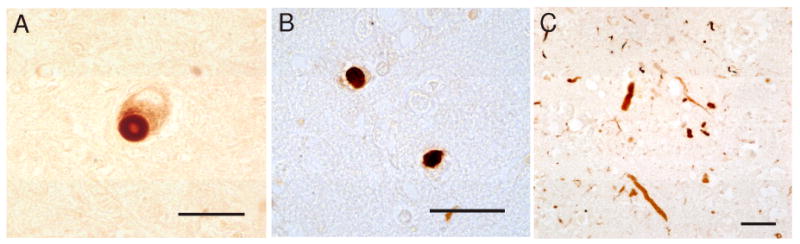
CD3 immunohistochemistry of the pars compacta region of the substantia nigra in a subject with Parkinson disease (A), temporal neocortex in a subject with Lewy body dementia, (B), and hippocampal pyramidal layer (CA2-3) in a subject with Lewy body dementia (C) are shown. Strong immunoreactivity of Lewy bodies within pars compacta neurons was apparent (A), as well as neocortical Lewy bodies (B), and CA2-3 dystrophic neurites (C). (Scale bars = 20 μm).
Discussion
In this study, we noted a precise overlap between immunoreactivity for CD3 and that for α-synuclein in cases of Lewy body dementia and Parkinson's disease. This is the first demonstration of CD3 immunoreactivity in neurodegenerative diseases and raises interesting possibilities in terms of disease pathogenesis.
CD3 is a polypeptide complex comprised of four distinct chains (CD3γ, CD3δ, CD3ε, and CD3ζ) associated with the T-cell receptor (TCR), and has been demonstrated in T cells and natural killer cells (Kuhns et al. 2006). The CD3 subunits are structurally related members of the immunoglobulin superfamily containing a single extracellular immunoglobulin domain, encoded by closely linked genes on chromosome 11. The CD3 components have long cytoplasmic tails which associate with cytoplasmic signal transduction molecules. The transmembrane region of the CD3 chains contains a negatively charged aspartic acid which allows these chains to associate with the positively charged TCR chains (TCRα and TCRβ). The intracellular tails of CD3 complexes possess a single conserved motif known as an immunoreceptor tyrosine-based activation motif (ITAM), essential to the signaling capability of the TCR. CD3 may be a factor influencing TCR induced growth arrest, cell survival, and proliferation. After the TCR recognizes an antigen-major histocompatibility complex (MHC) its associated CD3 complex functions as a signal transducer, activating the signaling cascade of the T cell (Abbas et al. 2007).
While previously restricted to the hematopoietic system, the CD3 molecule, and in particular the CD3ζ subunit, has been implicated as a product of neuronal metabolism in the setting of neurodevelopment. CD3ζ deficient mice show arrest of intrathymic T cell differentiation as expected (Malissen et al. 1993), but, in addition, show marked abnormalities in retinal ganglion cell projection to the lateral geniculate nucleus (Huh et al. 2000). Additionally, defects in synaptic plasticity with alterations in long term potentiation have been demonstrated in CD3ζ deficient mice (Barco et al. 2005). Taken together, CD3ζ appears to play a key role in neurodevelopment and synaptic plasticity.
Our finding that CD3 expression is altered, favoring accumulation within poorly soluble intraneuronal inclusions, shows a parallel with α-synuclein, the major protein component of Lewy bodies (Jellinger 2008). Like CD3, α-synuclein plays a major role in neurodevelopment, particularly development of the forebrain, as well as synaptic plasticity, and its alteration with age and disease is considered a major pathogenic factor in the progression of Parkinson's disease (Lee and Trojanowski 2006). Thus, a common process appears to be emerging in Parkinson's disease pathogenesis, that is, an aberrant return of neurodevelopmental and synaptic processes, associated with progressive neurodegeneration. This also parallels other studies from our laboratory, indicating aberrant re-entry of neurons into the cell cycle during neurodegenerative processes (Chou et al. 2001; McShea et al. 2007; Previll et al. 2007; Zhu et al. 2008; Lee et al. 2009), cycling neuroblasts being critical to neurodevelopment. While the implications of these general processes in the treatment of Parkinson's disease and Lewy body dementia remain to be elucidated, further studies exploring the parallels between aberrant neurodevelopment and neurodegeneration are warranted and may lead to novel therapeutic opportunities (Woods et al. 2007).
In conclusion, in this study we demonstrated a striking CD3 immunoreactivity in Lewy bodies and Lewy neurites in subjects with Parkinson's disease and Lewy body dementia. This is the first description of this antigen outside of the hematopoietic system in humans, and raises interesting possibilities in terms of the pathogenesis of neurodegenerative diseases.
Acknowledgments
Work in the authors' laboratories is supported by the National Institutes of Health (XWZ, HGL, MAS) and the Michael J. Fox Foundation (SGC).
References
- Abbas AK, Lohr J, Knoechel B. Balancing autoaggressive and protective T cell responses. J Autoimmun. 2007;28:59–61. doi: 10.1016/j.jaut.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barco A, Patterson S, Alarcon JM, Gromova P, Mata-Roig M, Morozov A, Kandel ER. Gene expression profiling of facilitated L-LTP in VP16-CREB mice reveals that BDNF is critical for the maintenance of LTP and its synaptic capture. Neuron. 2005;48:123–137. doi: 10.1016/j.neuron.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Baudouin SJ, Angibaud J, Loussouarn G, Bonnamain V, Matsuura A, Kinebuchi M, Naveilhan P, Boudin H. The signaling adaptor protein CD3zeta is a negative regulator of dendrite development in young neurons. Mol Biol Cell. 2008;19:2444–2456. doi: 10.1091/mbc.E07-09-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger LM, Shatz CJ. Immune signalling in neural development, synaptic plasticity and disease. Nat Rev Neurosci. 2004;5:521–531. doi: 10.1038/nrn1428. [DOI] [PubMed] [Google Scholar]
- Chou TT, Trojanowski JQ, Lee VM. p38 mitogen-activated protein kinase-independent induction of gadd45 expression in nerve growth factor-induced apoptosis in medulloblastomas. J Biol Chem. 2001;276:41120–41127. doi: 10.1074/jbc.M102832200. [DOI] [PubMed] [Google Scholar]
- Fink CC, Bayer KU, Myers JW, Ferrell JE, Jr, Schulman H, Meyer T. Selective regulation of neurite extension and synapse formation by the beta but not the alpha isoform of CaMKII. Neuron. 2003;39:283–297. doi: 10.1016/s0896-6273(03)00428-8. [DOI] [PubMed] [Google Scholar]
- Huh GS, Boulanger LM, Du H, Riquelme PA, Brotz TM, Shatz CJ. Functional requirement for class I MHC in CNS development and plasticity. Science. 2000;290:2155–2159. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger KA. Neuropathological aspects of Alzheimer disease, Parkinson disease and frontotemporal dementia. Neurodegener Dis. 2008;5:118–121. doi: 10.1159/000113679. [DOI] [PubMed] [Google Scholar]
- Kuhns MS, Davis MM, Garcia KC. Deconstructing the form and function of the TCR/CD3 complex. Immunity. 2006;24:133–139. doi: 10.1016/j.immuni.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Lanier LL. On guard--activating NK cell receptors. Nat Immunol. 2001;2:23–27. doi: 10.1038/83130. [DOI] [PubMed] [Google Scholar]
- Lee HG, Casadesus G, Nunomura A, Zhu X, Castellani RJ, Richardson SL, Perry G, Felsher DW, Petersen RB, Smith MA. The neuronal expression of MYC causes a neurodegenerative phenotype in a novel transgenic mouse. Am J Pathol. 2009;174:891–897. doi: 10.2353/ajpath.2009.080583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee VM, Trojanowski JQ. Mechanisms of Parkinson's disease linked to pathological alpha-synuclein: new targets for drug discovery. Neuron. 2006;52:33–38. doi: 10.1016/j.neuron.2006.09.026. [DOI] [PubMed] [Google Scholar]
- Lohmann C, Myhr KL, Wong RO. Transmitter-evoked local calcium release stabilizes developing dendrites. Nature. 2002;418:177–181. doi: 10.1038/nature00850. [DOI] [PubMed] [Google Scholar]
- Malissen M, Gillet A, Rocha B, Trucy J, Vivier E, Boyer C, Kontgen F, Brun N, Mazza G, Spanopoulou E, et al. T cell development in mice lacking the CD3-zeta/eta gene. Embo J. 1993;12:4347–4355. doi: 10.1002/j.1460-2075.1993.tb06119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McShea A, Lee HG, Petersen RB, Casadesus G, Vincent I, Linford NJ, Funk JO, Shapiro RA, Smith MA. Neuronal cell cycle re-entry mediates Alzheimer disease-type changes. Biochim Biophys Acta. 2007;1772:467–472. doi: 10.1016/j.bbadis.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Pitcher LA, van Oers NS. T-cell receptor signal transmission: who gives an ITAM? Trends Immunol. 2003;24:554–560. doi: 10.1016/j.it.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Polleux F, Morrow T, Ghosh A. Semaphorin 3A is a chemoattractant for cortical apical dendrites. Nature. 2000;404:567–573. doi: 10.1038/35007001. [DOI] [PubMed] [Google Scholar]
- Previll LA, Crosby ME, Castellani RJ, Bowser R, Perry G, Smith MA, Zhu X. Increased expression of p130 in Alzheimer disease. Neurochem Res. 2007;32:639–644. doi: 10.1007/s11064-006-9146-3. [DOI] [PubMed] [Google Scholar]
- Smith MA, Kutty RK, Richey PL, Yan SD, Stern D, Chader GJ, Wiggert B, Petersen RB, Perry G. Heme oxygenase-1 is associated with the neurofibrillary pathology of Alzheimer's disease. Am J Pathol. 1994a;145:42–47. [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Taneda S, Richey PL, Miyata S, Yan SD, Stern D, Sayre LM, Monnier VM, Perry G. Advanced Maillard reaction end products are associated with Alzheimer disease pathology. Proc Natl Acad Sci U S A. 1994b;91:5710–5714. doi: 10.1073/pnas.91.12.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberger LA. Immunocytochemistry. New York: Wiley; 1986. [Google Scholar]
- Woods J, Snape M, Smith MA. The cell cycle hypothesis of Alzheimer's disease: Suggestions for drug development. Biochim Biophys Acta. 2007;1772:503–508. doi: 10.1016/j.bbadis.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Zhan Y, Brown C, Maynard E, Anshelevich A, Ni W, Ho IC, Oettgen P. Ets-1 is a critical regulator of Ang II-mediated vascular inflammation and remodeling. J Clin Invest. 2005;115:2508–2516. doi: 10.1172/JCI24403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Siedlak SL, Smith MA, Perry G, Chen SG. LRRK2 protein is a component of Lewy bodies. Ann Neurol. 2006;60:617–618. doi: 10.1002/ana.20928. author reply 618-619. [DOI] [PubMed] [Google Scholar]
- Zhu X, Siedlak SL, Wang Y, Perry G, Castellani RJ, Cohen ML, Smith MA. Neuronal binucleation in Alzheimer disease hippocampus. Neuropathol Appl Neurobiol. 2008;34:457–465. doi: 10.1111/j.1365-2990.2007.00908.x. [DOI] [PubMed] [Google Scholar]


