Abstract
Background
Triiodothyronine levels decrease in infants and children after cardiopulmonary bypass. We tested the primary hypothesis that triiodothyronine (T3) repletion is safe in this population and produces improvements in postoperative clinical outcome.
Methods and Results
The TRICC study was a prospective, multicenter, double-blind, randomized, placebo-controlled trial in children younger than 2 years old undergoing heart surgery with cardiopulmonary bypass. Enrollment was stratified by surgical diagnosis. Time to extubation (TTE) was the primary outcome. Patients received intravenous T3 as Triostat (n=98) or placebo (n=95), and data were analyzed using Cox proportional hazards. Overall, TTE was similar between groups. There were no differences in adverse event rates, including arrhythmia. Prespecified analyses showed a significant interaction between age and treatment (P=0.0012). For patients younger than 5 months, the hazard ratio (chance of extubation) for Triostat was 1.72. (P=0.0216). Placebo median TTE was 98 hours with 95% confidence interval (CI) of 71 to 142 compared to Triostat TTE at 55 hours with CI of 44 to 92. TTE shortening corresponded to a reduction in inotropic agent use and improvement in cardiac function. For children 5 months of age, or older, Triostat produced a significant delay in median TTE: 16 hours (CI, 7–22) for placebo and 20 hours (CI, 16–45) for Triostat and (hazard ratio, 0.60; P=0.0220).
Conclusions
T3 supplementation is safe. Analyses using age stratification indicate that T3 supplementation provides clinical advantages in patients younger than 5 months and no benefit for those older than 5 months.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00027417.
Keywords: cardiopulmonary bypass, congenital heart surgery, thyroid hormone
Cardiopulmonary bypass (CPB) induces marked and persistent depression of circulating thyroid hormone levels in adults and children.1,2 Depression of thyroid hormone levels contributes to postoperative morbidity in adults undergoing CPB. Thus, thyroid hormone repletion in the form of triiodothyronine (T3) during and after CPB is an intuitive solution that has been the subject of several investigations.2,3 Controlled randomized studies demonstrate that parental T3 repletion during coronary bypass surgery in adults improves postoperative ventricular function.2–4 The clinical response to T3 repletion observed in these studies has been variable. Mullis-Jansson et al4 showed that T3 reduces the use of inotropic agents and mechanical devices, and it decreases the incidence of myocardial ischemia. Klemperer et al2 did not demonstrate a T3 influence on these clinical outcome parameters but did show a reduced rate of atrial fibrillation.3 Infants and young children undergoing cardiac surgery for congenital heart defects demonstrate more profound and persistent decreases in thyroid hormone levels after CPB than those that occur in adults.1,5 In this immature population, postoperative abnormalities in hemodynamic parameters are associated with depression of thyroid hormone levels.1,6 Accordingly, several investigators have conducted randomized clinical trials with relatively small subject numbers (between 28 and 42 total subjects per study) to explore the utility of thyroid hormone supplementation in children undergoing CPB7–9 or to evaluate pharmacokinetics.10 The authors of those studies acknowledged some clinical design issues, which hamper interpretation of the results. These include the use of surrogate end points and inadequate power.7–10 Furthermore, their published reports often lacked details regarding adverse events.7–10 Bettendorf et al7 showed some elevation in the Therapeutic Interventional Scoring System score and cardiac index in patients receiving T3 infusion. Chowdhury et al8 showed in a small randomized study that treating patients with a defined nadir in perioperative T3 levels increased the Therapeutic Interventional Scoring System score and lowered inotropic score. Mackie et al9 showed that T3 infusion improved composite clinical score and time to negative fluid balance in neonates undergoing aortic arch reconstruction.
Although well-powered, randomized, clinical drug trials are considered the gold standard for scientific evidence, these studies are rarely performed in children during and immediately after cardiac surgery.11 For instance, of the primary inotropic agents and vasodilators commonly used in support of this population, only milrinone has been subjected to careful evaluation in a placebo-controlled trial.12 The primary objectives of the TRICC (triiodothyronine supplementation in infants and children undergoing cardiopulmonary bypass) clinical trial were to provide essential safety and efficacy data regarding the use of intravenous T3 in infants and young children undergoing CPB.13
Study Design and Statistical Methods
The TRICC study was a prospective, multicenter, double-blind, randomized, placebo-controlled trial to evaluate the effects of T3 supplementation after CPB in children younger than age 2 years. We tested the primary hypothesis that T3 (liothyronine, Triostat; King Pharmaceuticals) repletion is safe and, compared to placebo, produces significant improvements in postoperative clinical outcome parameters. The primary efficacy analysis evaluated the effect of T3 supplementation on time from aortic cross-clamp removal to extubation (TTE). Studies of children undergoing surgery for congenital heart disease conducted at single centers with uniform hospital utilization have shown that lower TTE results in overall reduced hospital stay and cost.14 Accordingly, TTE is considered an important clinical end point and benchmark in pediatric cardiac surgical series, so it was chosen as an objective outcome measure for this study.
We developed a complex stratification design to accommodate the heterogeneity of pathology inherent in a population of children undergoing surgery for congenital heart disease. Patients were randomized to Triostat or placebo groups within 1 of 9 diagnostic surgical management categories to provide balanced subject numbers between treatment groups for each classification (Table 1). Each diagnostic category was defined to include patients with similar characteristics within a narrow age range. The primary analysis was performed using Cox proportional hazards, including terms for diagnostic category and external factors that delayed weaning. The target sample size (total consented N=98 per treatment group) was predicted to provide statistical power of >80% to identify a treatment effect corresponding to a hazard ratio of 1.55 with the alpha level at 0.05 (2-sided).
Table 1.
Baseline Characteristics by Treatment Group According to Diagnostic and Risk Strata
| Placebo Total (N=95) | Triostat Total (N=98) | |
|---|---|---|
| Gender | ||
| Female | 43 (45.3%) | 42 (42.9%) |
| Male | 52 (54.7%) | 56 (57.1%) |
| Age (mo) | ||
| N | 95 | 98 |
| Mean | 5.8 | 5.3 |
| SD | 5.3 | 4.6 |
| Median | 4.6 | 5.4 |
| 25%, 75% | 0.8, 7.8 | 1.1, 7.3 |
| Min, Max | 0.1, 23.2 | 0.0, 21.2 |
| High-risk | ||
| Total | 31 | 31 |
| Infant CoA | 2 (2.1%) | 2 (2.0%) |
| TGA | 14 (14.7%) | 14 (14.3%) |
| HLV | 5 (5.3%) | 5 (5.1%) |
| TA PVD | 3 (3.2%) | 4 (4.1%) |
| Other high-risk | 7 (7.4%) | 6 (5.1%) |
| Low-risk | ||
| Total | 64 | 67 |
| Isolated VSD | 21 (22.1%) | 21 (21.4%) |
| TOF | 17 (17.9%) | 17 (17.3%) |
| CAVC | 15 (15.8%) | 16 (16.3%) |
| Other low-risk | 7 (7.4%) | 7 (7.1%) |
| SVC-PA | 4 (4.2%) | 6 (6.1%) |
Baseline characteristics for the total population within surgical diagnostic categories are shown. There are no significant differences between Triostat and placebo cohorts for any group. The first 5 diagnostic groups are high-risk with Aristotle scores >10; the next 5 are lower-risk with score Aristotle score <10.
CAVC indicates complete atrioventricular valve repair; CoA, neonatal coarctation repair; HLV, hypoplastic left ventricle with Norwood; SVC-PA, superior vena cava to pulmonary artery shunt; TAPVD, repair of total anomalous pulmonary venous drainage and other high risk operations; TGA, transposition of great arteries with arterial switch; TOF, tetralogy of Fallot; VSD, isolated ventricular septal defect.
Patients younger than age 2 years were enrolled and no quota was required for each diagnostic category. Before unblinding, the 9 diagnostic categories were collapsed into 2 risk classifications based on the basic Aristotle score with age adjustments (high-risk ≥10 or low-risk <10).15 Patient characteristics and adverse events were compared using the χ2 test or Fisher exact test as appropriate. Continuous characteristics and outcomes were compared using the t test, Wilcoxon test, or analysis of variance for repeated measures. All confidence intervals (CI) in this manuscript are 95% CIs. No adjustments were made for multiple testing, and all results except TTE for the entire study population should be considered secondary. Patients were included in analyses if they received at least 1 dose of study drug. Analyses controlled for stratification variables used a binary term (high risk/low risk) because there were so few patients in some of the categories. An additional binary term to indicate that external factors had caused delays in weaning was included as specified by the protocol. The distributions of TTE were compared across treatment groups using Cox proportional hazards. Time zero was defined as the time of cross-clamp removal and spanned until to the first of death, extubation, or 7 days. Patients who died were censored at the earliest of 7 days or death, and patients who were still intubated at 7 days after cross-clamp removal were censored at 7 days. The half-life for T3 was previously estimated at 16 hours for infants.6 Censoring at 7 days approximated half-life times 10 from the final dosage administered in this protocol. Therefore, T3 effect would be reduced after 7 days, and prolonged intubation likely would be caused by factors not subject to T3 regulation. As a post hoc sensitivity analysis, the same analysis for time to extubation was performed with censoring at 30 days. Medians and 95% CI for TTE are based on the Kaplan–Meier estimate to account for censoring. Age was identified as a potential effect modifier before the study, and a Cox proportional hazards model including terms for age and the age-by-treatment interaction was used.
Enrollment was conducted between August 2001 and August 2007. Institutional Review Boards at all participating centers approved this study. A data-monitoring committee conducted interim safety analyses.
Study Procedures
All treating physicians were blinded to study treatment. All intensive care treating physicians agreed to terminate mechanical ventilation based on patient-related parameters and to minimize nonpatient-related delays. Clinical practice regarding use of inotropic agents was not specified in the protocol. Randomization was performed centrally by the Investigative Drug Service at Seattle Children’s Hospital. Randomization was blocked to maintain equal numbers divided between Triostat and placebo groups at individual centers. Syringes containing either Triostat or equivalent volume saline were prepared by the pharmacist and labeled as study drug. The blinded study drug was administered intravenously over the course of 10 minutes as a bolus of 0.4 µg/kg immediately before CPB, 0.4 µg/kg on the release of the aortic cross-clamp, and then 0.2 µg/kg at intervals of 3, 6, and 9 hours after cross-clamp release.
Blood samples for total T3 levels were drawn before the first infusion and 1, 6, 12, 24, and 72 hours after cross-clamp removal. Samples for T3 levels were forwarded to a central laboratory for processing and analyses (North Shore University Hospital).
Echocardiograms for cardiac functional assessment were performed at 6, 24, and 72 hours after cross-clamp removal. Ejection fraction was derived using 2-dimensional echocardiography Simpson biplane method. The systemic myocardial performance index was obtained using pulsed Doppler, as described by Tei et al,16 and relates inversely to myocardial function. Echocardiograms from the first enrolled patients at each center and random subsequent patients from each center were reviewed at Seattle Children’s Hospital to assure study consistency. Inotropic score was calculated over 6-hour periods for the first 24 hours by the commonly used algorithm in pediatric intensive cares studies: dopamine+dobutamine+(epinephrine×100)+(milrinone×10) (dosages are expressed in micrograms per kilogram per minute).17 This algorithm assigns a score of 0 for patients not receiving an inotropic agent. All adverse events were recorded up to 30 days after receiving the last dose of study medication.
Results
Patient Characteristics
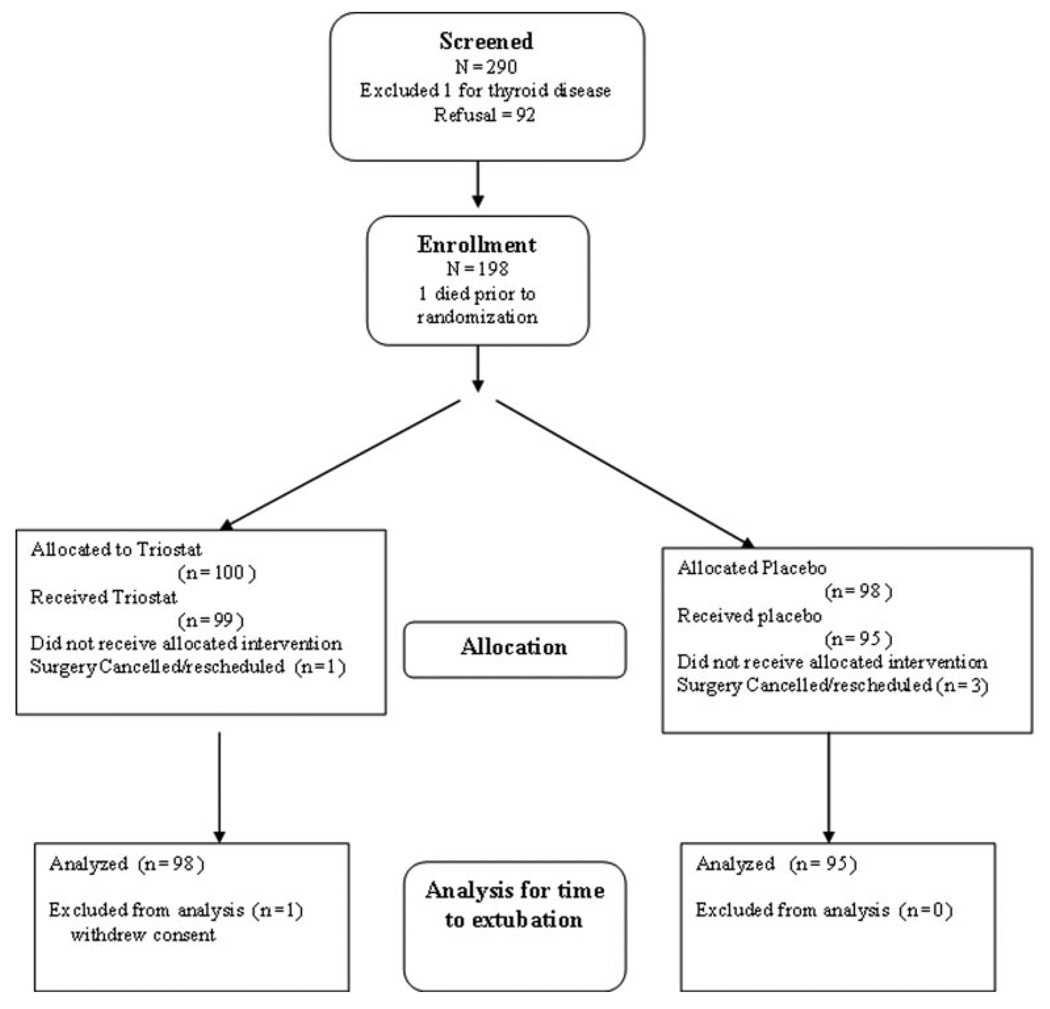
One hundred ninety-eight patients were randomized and 193 patients were included in analyses (Figure 1). Of the 5 not included, 3 patients in the placebo group and 1 in the Triostat group did not receive study drug because surgery was cancelled or rescheduled because of operating room factors. One patient in the Triostat group was withdrawn at the request of the parents. Three patients had surgical mishaps or anatomic conditions unrelated to the study, which substantially effected extubation time. Per protocol, a binary variable was added to all statistical models to control for the delay in weaning for these subjects.
Figure 1.
Flow diagram for patient enrollment and protocol completion.
Patient demographics including diagnosis, Aristotle score, and age are shown in Table 1. Randomizations within the stratified categories and according to risk were nearly identical between placebo and Triostat groups. Potential confounders such as mean cardiopulmonary bypass time, aortic cross-clamp time, remaining open chest after surgery, day of the week, and study center are also shown in Table 2. There were no significant differences between groups for these parameters. There was an imbalance in time of day, with proportionally more Triostat patients who underwent operation in the morning than those in the placebo group.
Table 2.
Surgical Variables in Treatment Cohorts
| Triostat (N=98) | Placebo (N=95) | |
|---|---|---|
| Morning surgery* | ||
| No | 16 (16.3%) | 28 (29.5%) |
| Yes | 82 (83.7%) | 67 (70.5%) |
| Day of wk | ||
| Monday | 15 (15.3%) | 17 (17.9%) |
| Tuesday | 31 (31.6%) | 24 (25.3%) |
| Wednesday | 16 (16.3%) | 22 (23.2%) |
| Thursday | 26 (26.5%) | 26 (27.4%) |
| Friday | 10 (10.2%) | 6 (6.3%) |
| Center | ||
| Site 1 | 15 (15.3%) | 11 (11.6%) |
| Site 5 | 11 (11.2%) | 15 (15.8%) |
| Sites 2, 3, 4 | 16 (16.3%) | 19 (20.0%) |
| Site 0 | 56 (57.1%) | 50 (52.6%) |
| Open chest | ||
| No | 87 (88.8%) | 84 (88.4%) |
| Yes | 11 (11.2%) | 11 (11.6%) |
| Bypass time (hr) | ||
| N | 98 | 95 |
| Mean (SE) | 1.809 (0.113) | 1.802 (0.112) |
| SD | 1.12 | 1.089 |
| Median | 1.617 | 1.583 |
| Cross-clamp time (hr) | ||
| N | 93 | 90 |
| Mean (SE) | 1.080 (0.061) | 1.070 (0.054) |
| SD | 0.592 | 0.51 |
| Median | 0.917 | 1.025 |
Comparisons between Triostat and Placebo are not significant, except for morning surgery (*P=0.03).
TTE
One hundred fifty-nine of 193 patients were extubated within 7 days. We found that treatment assignment did not reduce TTE (hazard ratio, 0.96; P=0.8170) using Cox proportional hazards for the entire randomized population (n=193). Post hoc analyses performed with censoring after 30 days showed similar results (Supplemental Table I available online at http://circ.ahajournals.org/cgi/content/full/CIRCULATIONAHA.109.926394/DCI). Aristotle classification was significantly related to TTE, and the hazard ratio (chance of extubation) associated with the low risk cohort was 2.74 (P<0.0001) relative to the high-risk cohort. However, analyses by Cox proportional hazards showed no significant interaction between treatment and risk category (P=0.2496). Therefore, the influence of Triostat on TTE does not differ by risk category within this cohort. Imbalanced covariates (morning vs afternoon surgery) listed in Table 2 did not affect the results.
Cardiac Function and Inotropic Use
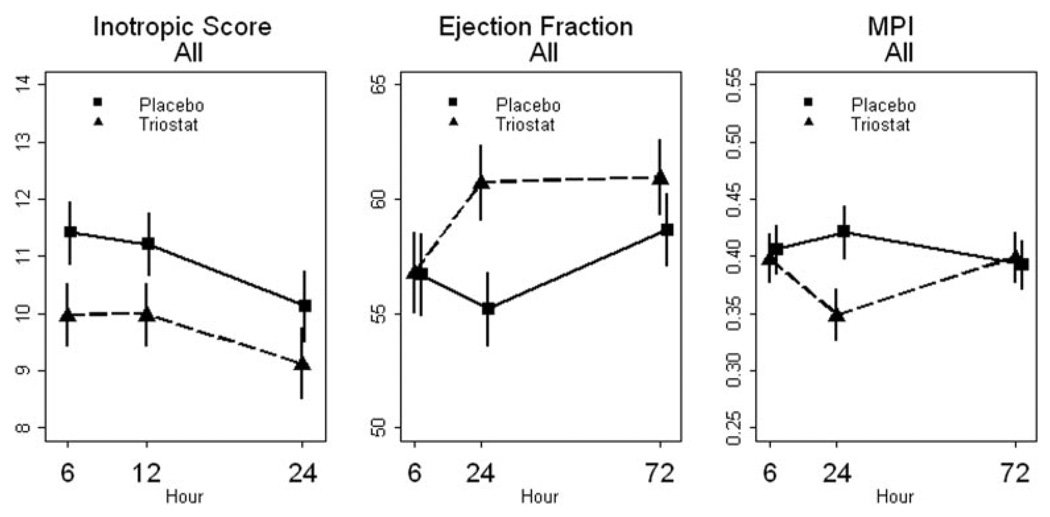
There were no significant differences between Triostat and placebo cohorts for heart rate, mean arterial blood pressure, or mean arterial blood pressure times heart rate over the first 24 hours. Overall inotropic use by center is shown in the Supplemental Table II (available online at http://circ.ahajournals.org/cgi/content/full/CIRCULATIONAHA.109.926394/DCI). The inotropic scores were not significantly different between Triostat and placebo groups (Figure 2 and Table 3).
Figure 2.
Inotropic score and cardiac function parameters for the study population (see Table 2 for analysis of variance results). Data shown as mean±standard error of the mean. Scale does not start at 0. Results for statistical analyses are shown in Table 3.
Table 3.
Type I Error Probabilities for Inotropic Score and Echocardiographic Data
| Effect | IS | MPI | EF |
|---|---|---|---|
| All | |||
| Treatment | 0.2083 | 0.1807 | 0.1902 |
| Time | 0.0016 | 0.2221 | 0.1141 |
| Treatment*time | 0.643 | 0.0513 | 0.0113 |
| <5 mo | |||
| Treatment | 0.03 | 0.689 | 0.6353 |
| Time | 0.3648 | 0.8326 | 0.0898 |
| Treatment*time | 0.2079 | 0.0246 | 0.0271 |
| ≥5 mo | |||
| Treatment | 0.4955 | 0.0401 | 0.1226 |
| Time | <0.0001 | 0.0531 | 0.6076 |
| Treatment*time | 0.0467 | 0.8399 | 0.2296 |
Values are derived from analysis of variance analyses of inotropic score and echocardiographic data (see Figure 5).
EF indicates ejection fraction; IS, inotropic score; MPI, myocardial performance index.
Echocardiographic Parameters
Statistical analyses (analysis of variance) for the cardiac functional indices were complicated by convergence of values from the 2 groups at 72 hours (Figure 2, Table 3). Treatment–time interaction for ejection fraction was significant when comparing the entire placebo and Triostat cohorts. Because of the T3 dosing schedule, these time–treatment interactions are not unexpected. We had relatively few (<10%) missing data points for the 6-hour and 24-hour time points and (<20%) for the 72 hours, which might have affected the analyses of echocardiographic data. There was no systematic dropout, and missing points were caused by random events such as availability of instrumentation for performing echocardiograms or lack of sufficient ultrasound window in particular patients.
Safety
The rates of all adverse events, serious adverse events, and death or mechanical circulatory life support were similar between the treatment groups (details provided in Supplemental Table III available online at http://circ.ahajournals.org/cgi/content/full/CIRCULATIONAHA.109.926394/DCI) for the entire cohort and for the age categories. There were a total of 8 deaths among study participants—3 in the Triostat group and 5 in the placebo group. One death occurred >30 days after surgery. A previous study had reported 2 patients with tachyarrhythmia in the T3 treatment arm only of a randomized study.9 However, the incidence of clinically significant tachyarrhythmia requiring intervention was similar between the 2 groups. Junctional ectopic tachycardia occurred in 7.3% with placebo and in 8.1% with Triostat (P=0.84) and, respectively, supraventricular tachycardia 2.1% vs 3.0% (P=1.0). Ventricular tachycardia occurred in 1 patient in the placebo group only. Study drug was discontinued for 1 patient, who received placebo, at the request of the operating surgeon because of a tachyarrhythmia. There were no significant differences in adverse events between the age subgroups.
Age Analysis
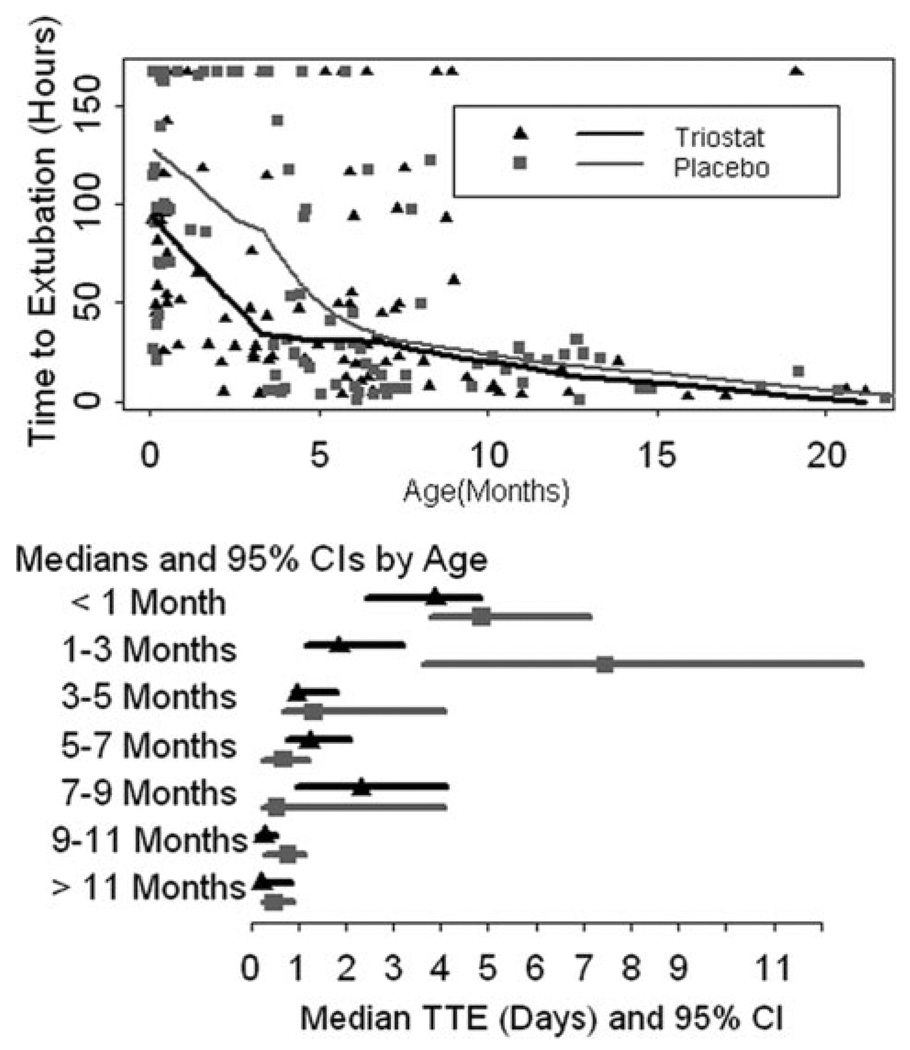
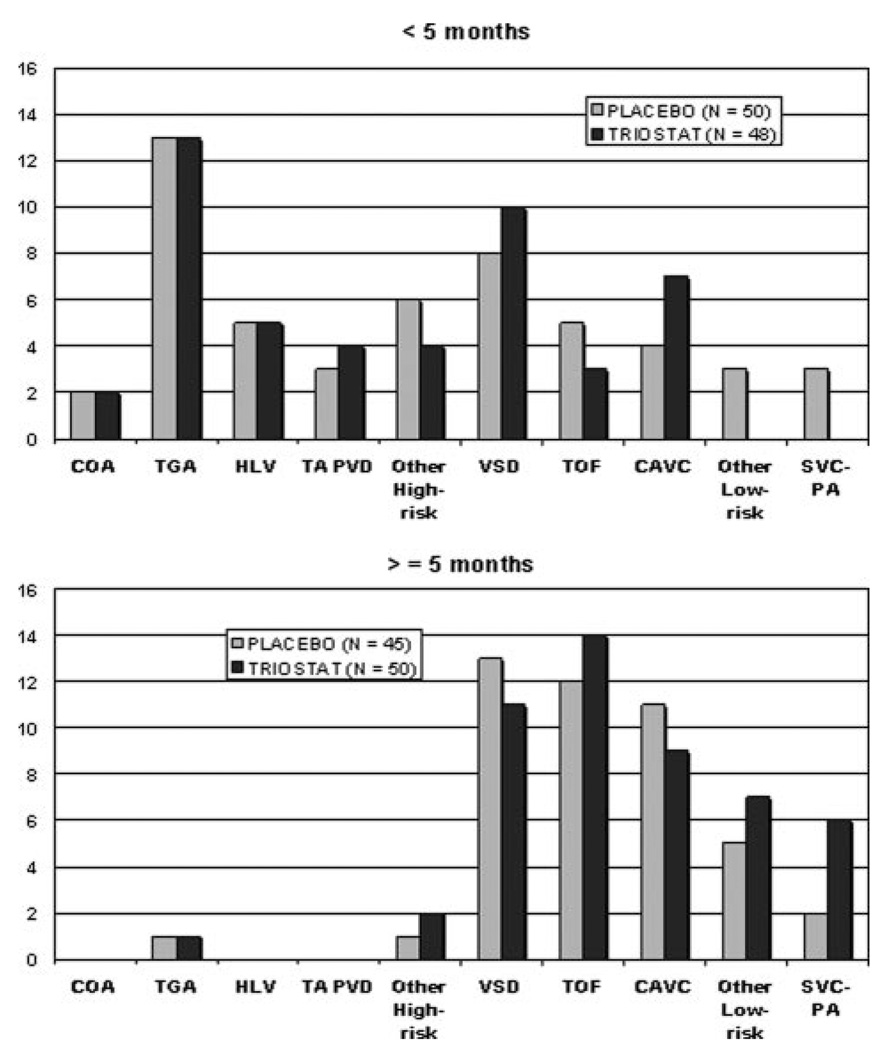
For the secondary analyses of TTE adjusted for age, we identified a significant interaction between age and treatment (P=0.0012). Adjusting the primary Cox proportional hazards model for age showed a hazard ratio of 1.7 (CI, 1.04 –2.78; P=0.0039) for the youngest patients. These results indicated that age was an important factor in determining treatment effect but did not indicate a specific age group in which the effect was the strongest. Further regression diagnostics, based solely on the placebo group, showed that age has a threshold effect on TTE (Figure 3). TTE vs age regression curves for treatment and placebo groups begin to converge between 4 and 5 months of age. The regression curves confirmed the results of the Cox proportional hazards model indicating age influence on treatment, but they also showed this effect substantially diminishing as patients aged older than 5 months. Based on these regression diagnostics, we separated the cohort into 2 groups: younger than 5 months of age and age 5 months or older. The median age for the whole population was 4.7 months, so this grouping produced nearly equal numbers in the groups younger than 5 months old and 5 months old or older groups. Within each age group cohort, treatment assignment was balanced (Figure 4). The heterogeneity of diagnoses and the differences between the 2 age groups are illustrated in Figure 4. Demographics for each age group are shown in Table 4.
Figure 3.
Relationship between time to extubation and age in months (top). Smoothed regression lines for placebo and Triostat groups converge just after 5 months of age. Median time to extubation with confidence intervals is shown by age groups (bottom).
Figure 4.
Subject numbers for each surgical diagnostic category. Abbreviations are listed in Table 1.
Table 4.
Demographic and Surgical Variables by Age Group
| Triostat, <5 mo (N=48) |
Placebo, <5 mo (N=50) |
Triostat, ≥5 mo (N=50) |
Placebo, ≥5 mo (N=45) |
|
|---|---|---|---|---|
| Gender | ||||
| Female | 20 (41.7%) | 21 (42.0%) | 22 (44.0%) | 22 (48.9%) |
| Male | 28 (58.3%) | 29 (58.0%) | 28 (56.0%) | 23 (51.1%) |
| Age (mo) | ||||
| Mean (SE) | 1.7 (0.22) | 1.9 (0.25) | 8.7 (0.55) | 10.0 (0.70) |
| SD | 1.5 | 1.8 | 3.9 | 4.7 |
| Median | 1.0 | 1.3 | 7.3 | 8.0 |
| Morning surgery* | ||||
| No | 7 (14.6%) | 20 (40.0%) | 9 (18.0%) | 8 (17.8%) |
| Yes | 41 (85.4%) | 30 (60.0%) | 41 (82.0%) | 37 (82.2%) |
| Day of wk | ||||
| Monday | 10 (20.8%) | 10 (20.0%) | 5 (10.0%) | 7 (15.6%) |
| Tuesday | 10 (20.8%) | 13 (26.0%) | 21 (42.0%) | 11 (24.4%) |
| Wednesday | 11 (22.9%) | 12 (24.0%) | 5 (10.0%) | 10 (22.2%) |
| Thursday | 9 (18.8%) | 11 (22.0%) | 17 (34.0%) | 15 (33.3%) |
| Friday | 8 (16.7%) | 4 (8.0%) | 2 (4.0%) | 2 (4.4%) |
| Pooled center | ||||
| Site 1 | 12 (25.0%) | 7 (14.0%) | 3 (6.0%) | 4 (8.9%) |
| Site 5 | 2 (4.2%) | 6 (12.0%) | 9 (18.0%) | 9 (20.0%) |
| Site 2, 3, 4 | 7 (14.6%) | 9 (18.0%) | 9 (18.0%) | 10 (22.2%) |
| Site 0 | 27 (56.3%) | 28 (56.0%) | 29 (58.0%) | 22 (48.9%) |
| Open chest | ||||
| No | 37 (77.1%) | 39 (78.0%) | 50 (100.0%) | 45 (100.0%) |
| Yes | 11 (22.9%) | 11 (22.0%) | 0 (0.0%) | 0 (0.0%) |
| Bypass time (hr) | ||||
| Mean (SE) | 2.175 (0.181) | 2.104 (0.180) | 1.458 (0.120) | 1.465 (0.106) |
| SD | 1.255 | 1.272 | 0.846 | 0.713 |
| Median | 1.95 | 1.933 | 1.167 | 1.333 |
| Cross-clamp time (hr) | ||||
| N | 48 | 48 | 45 | 42 |
| Mean (SE) | 1.178 (0.089) | 1.159 (0.070) | 0.976 (0.082) | 0.968 (0.081) |
| SD | 0.618 | 0.486 | 0.551 | 0.524 |
| Median | 1.05 | 1.283 | 0.783 | 0.858 |
Comparisons between Triostat and placebo are not significant, except for morning surgery (*P=0.005).
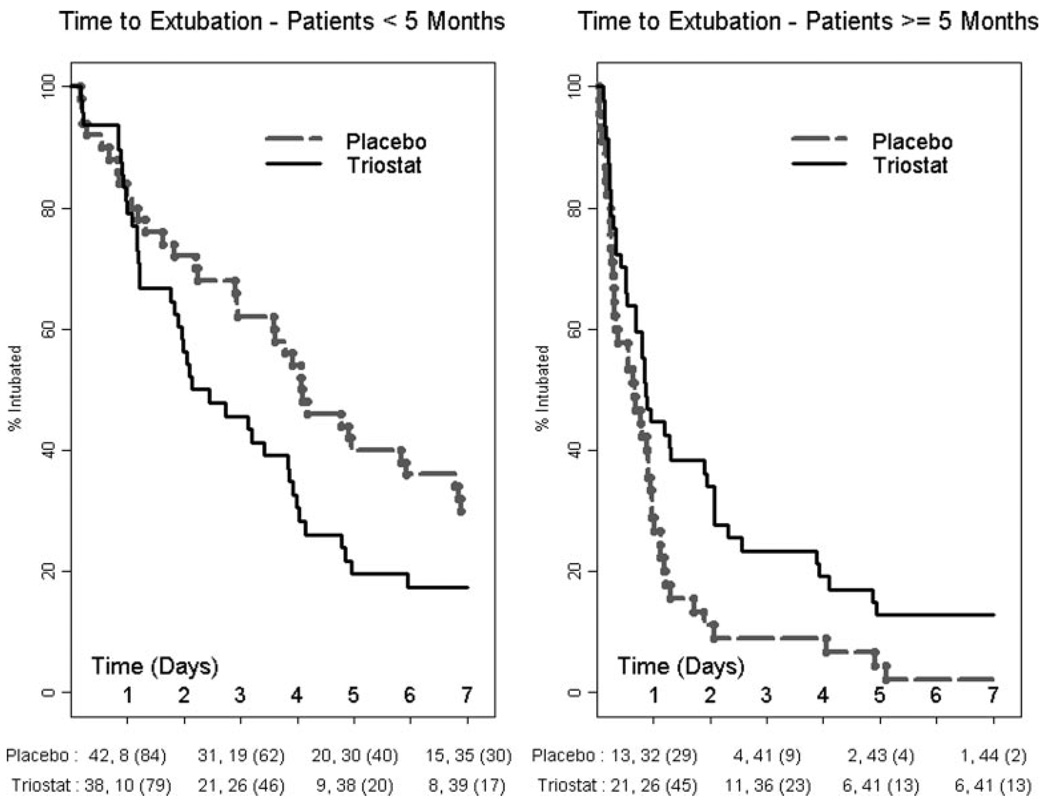
Comparison between the age groups using Cox proportional hazards showed that infants older than 5 months were extubated sooner than patients younger than 5 months (hazard ratio, 1.76 for older compared to younger patients; P=0.0054). For patients younger than 5 months of age, randomization to Triostat resulted in significant shortening in TTE. The placebo group median TTE was 98 hours (95% CI, 71–142). The Triostat group median TTE was 55 hours (95% CI, 44–92). The hazard ratio among patients younger than 5 months was 1.72 (P=0.0216). For patients 5 months of age or older, randomization to Triostat resulted in small but significant delay in median TTE. The median TTE in hours was 16 (95% CI, 7–22) for placebo and 20 (95% CI, 16–45) for Triostat, and the hazard ratio was 0.60 (P=0.0220). The Kaplan–Meier curves for these populations are shown in Figure 5.
Figure 5.
Kaplan–Meier curves for subjects younger than 5 months age and subjects aged 5 months and older. Statistics by Cox proportional hazards: hazard ratio for the Triostat group among patients younger than 5 months was 1.72 (P=0.0216), and for those older than 5 months hazard ratio was 0.60 (P=0.0220). Below the graph are number at risk and number of extubations (% intubated).
As noted for the entire cohort, there were no significant differences for most of the potential confounders between placebo and Triostat within the age groups (Table 4). Distribution between morning and afternoon surgeries was imbalanced with proportionally more Triostat patients undergoing morning surgery. However, this difference had no impact on time to extubation results. Post hoc analyses with censoring at 30 days did not change the results appreciably (Supplemental Table I).
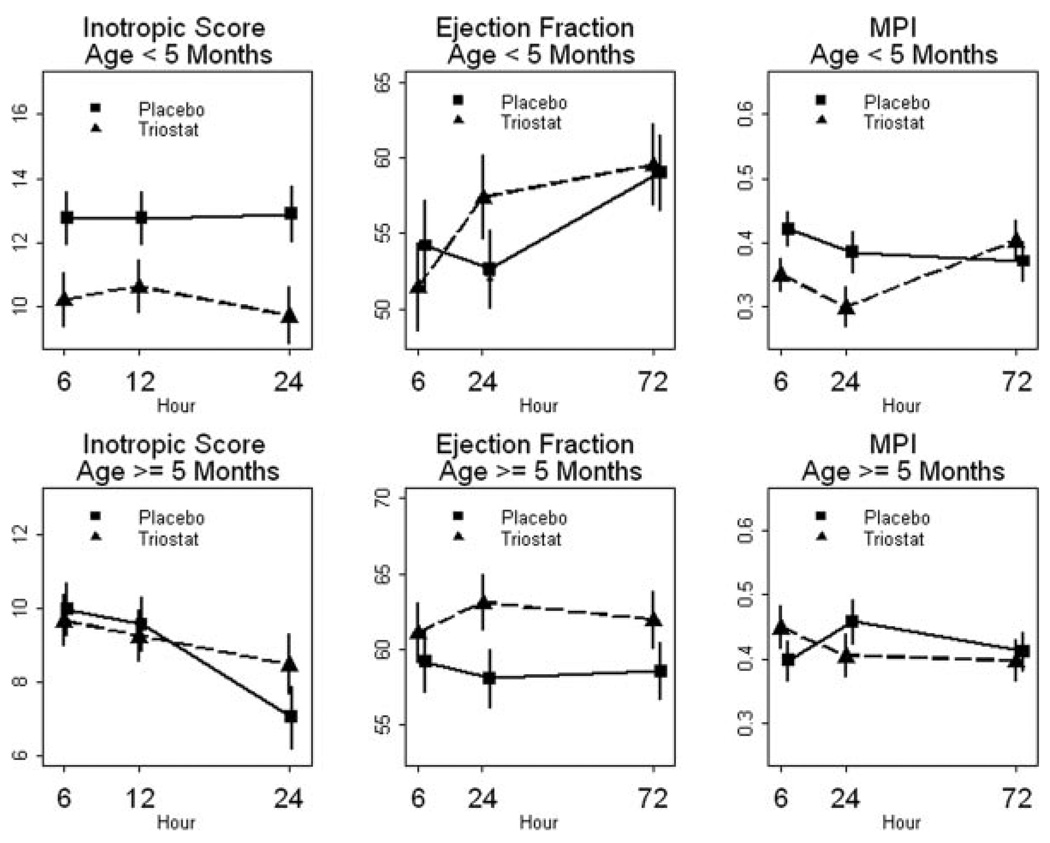
T3 supplementation substantially reduced inotropic agent utilization in the patients younger than 5 months old (analysis of variance for treatment effect, P=0.03). Mean inotropic score was reduced by as much as 23% at 24 hours (Figure 6). There were no significant differences between Triostat and placebo groups for the patients older than 5 months. We also identified a significant time–treatment interaction for ejection fraction and myocardial performance index for the age group younger than 5 months old (Figure 6, Table 2). The maximal difference between treatment groups for these parameters occurred at 24 hours, with some convergence at 72 hours.
Figure 6.
Inotropic score and cardiac function data. Statistics from analysis of variance are shown in Table 2. Data shown as mean±standard error of the mean. Scale does not start at 0. Results for statistical analyses are shown in Table 3.
Total T3 Concentrations
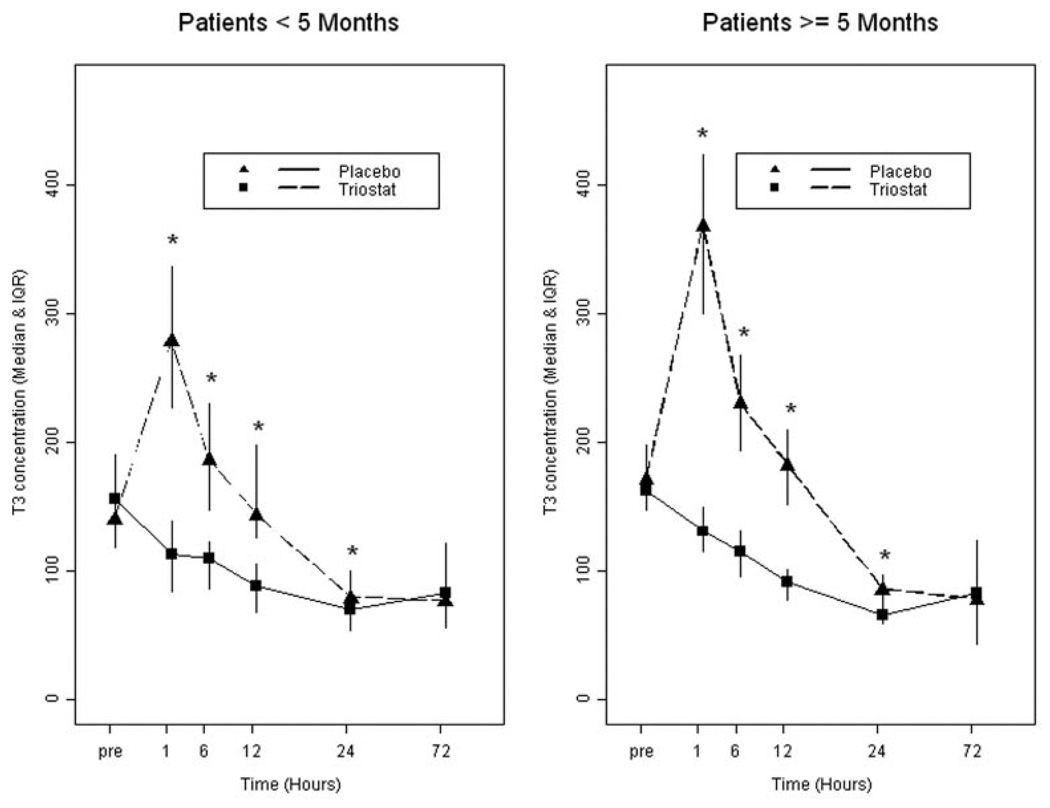
T3 supplementation increased circulating total T3 levels above those in the placebo group for at least 24 hours after cross-clamp removal (P<0.001) for the entire cohort and for the age groups. Plasma levels for the 2 age groups are shown in Figure 7. Normal values are 95 to 220 ng/dL.18 Among patients in the placebo group, there were no differences in baseline T3 level by age (older than 5 months vs younger than 5 months). There were no significant differences between treatment groups in number of patients with results below the normal range (Triostat, n=3; placebo, n=1). For Triostat patients, the median change from baseline T3 was significantly higher in older patients for hours 1, 24, and 72. At hour 6, the difference approached significance. The greatest difference was at 1 hour, when the median change among older patients was +203 ng/dL compared to 133 ng/dL in younger patients (P=0.0096). These differences occurred despite standard dosing per weight.
Figure 7.
Total serum triiodothyronine levels in ng/dL showing median and intraquartile range. The median (50th percentile) and the intraquartile range (25th–75th percentile) are displayed. Probability values are from the Wilcoxon test. Normal values are 95 to 220 ng/dL (see text for reference). *P<0.001 compared to placebo for individual time point.
Discussion
This investigation represents one of the largest clinical trials reported in children undergoing cardiopulmonary bypass.7–9 We did not identify a T3 treatment effect on the primary end point, TTE, for the entire study population. Prespecified secondary analyses confirmed the well-known influence of age on TTE and, more importantly, revealed the interaction between age and T3 supplementation effect. Several intraoperative factors, such as cardiopulmonary bypass time, contribute to the impact of age on TTE. Postoperative practice strategies, which execute very early extubation for many older infants and children after surgery for congenital heart disease,19 are used much less frequently in young infants.14 Rapid extubation in the older cohort developed increasing acceptance during the conduct of the study and was not anticipated during the original trial design and power analyses. Thus, the relatively short extubation times in older patients as illustrated in Figures 3 and 5 likely limited our ability to detect a significant treatment effect for the whole study population.
Further secondary analyses indicated that the T3 treatment effect on TTE reversed direction as age increased past 5 months. Accordingly, we believe that the post hoc delineation of the cohorts into 2 age subgroups was justified by the regression analyses (Figure 3). The separation into these 2 cohorts also corresponds to pediatric cardiology clinical practice in many tertiary centers where most patients younger than 5 months undergo surgery for medical necessity and elective surgeries are delayed until after 5 to 6 months of age.20 We designed the stratification to determine the influence of risk, assessed by Aristotle score, on treatment effect. The results indicate that preoperative risk was not a significant factor. The complex nature of our stratification design in a multicenter trial precluded a priori randomization for additional factors such as graded urgency of surgery. Furthermore, a subjective analysis based on medical necessity could not be performed post hoc without substantial bias, but this dichotomization might be used in design of future studies.
The phenomenon, whereby T3 poses benefit for age younger than 5 months but some detriment for those aged 5 months and older, suggests that the difference in response according to age has a physiological basis. Thyroid hormone elicits multiple affects on the cardiovascular system and other organ systems. Therefore, we can only speculate on the mechanisms responsible for these developmental differences. Our data indicate that T3 levels in the Triostat group return to levels near those in the placebo group by 24 hours. One might question how the temporary elevation impacts clinical parameters several hours or days after declination. T3 exerts action through both nongenomic and genomic mechanisms.21,22 The nongenomic mechanisms likely occur at the cell membrane and are immediate. These actions include rapid T3-mediated stimulation of Na+ influx, which indirectly increases intracellular Ca2+ via Na+-Ca2+ exchange, leading to positive inotropic effects.23 However, the genomic mechanisms involve T3 transport across membranes, binding to thyroid nuclear receptors and modulation of transcription and protein translation. Although our previous study showed that T3 rapidly initiates transcription of some genes in infants,24 the ultimate result of this transactivation may not be apparent for several hours or days.21 Delay in some T3-mediated actions may explain, in part, the lack of treatment benefit in the patients older than 5 months, particularly because most are extubated by 24 hours. Conceivably, the relatively minor but highly significant prolongation of TTE in this age group with T3 supplementation reflects the dominance of nongenomic action relative to the genomic soon after receiving the loading doses. The significant decrease in inotropic agent use in the treated patients younger than 5 months old not only supports the validity of our TTE results but also suggests that T3 alters adrenoreceptor sensitivity in an age-dependent manner. The existence of an interaction between T3 and the inotropic agents, particularly the β-adrenergic agonists, is plausible and has been suggested by studies in cells or hearts ex vivo.25 However, evidence from most studies performed in vivo does not support a role for T3 enhancement in myocardial adrenoreceptor sensitivity.26,27 More likely, the reduction in inotropic support represents the treating physician response to improved hemodynamics such as improved cardiac function and lower systemic vascular resistance, which are well-documented effects of thyroid hormone therapy28 and implicated by echocardiographic data in our study.
Importantly, T3 repletion appears safe, and this study should obviate the perception of risk generated by symptoms in patients with hyperthyroid states. Previous studies included either scattered reports of various adverse events, often not assigned to a specific treatment group, or no safety assessment at all.6,8,9 Isolated cases of tachyarrhythmia were reported in patients receiving T3.6,9 Our current study corroborated data from large studies in adults showing no increased risk of postoperative rhythm disorders.3,4
In summary, this controlled randomized study showed that time to extubation among children younger than 5 months of age recovering from cardiac surgery was reduced by T3 supplementation during and after cardiopulmonary bypass. The clinical response was accompanied by an improvement in cardiac function and a decrease in inotropic support. Placebo-controlled trials in infants undergoing cardiac procedures are rare, and conduct of these trials encounters multiple challenges. The results of this study emphasize the importance importance of appropriate age or diagnostic stratification in this population. The data in our study are somewhat limited by lack of previous specification in age stratification. However, the data strongly support performance of another trial in the patients younger than 5 months of age.
Supplementary Material
Acknowledgments
The following TRICC investigators participated in the enrollment of patients, data collection, study coordination, laboratory core, or central randomization: Seattle Children’s Hospital—L. Permut, M. Lewin, A. Cesan, M.B. Lee, C. Fearneyhough, K. Rodriquez, K. Gama, and E. Mano; University of California San Francisco—T.A. Tacy and A. Azakie; Oakland Children’s Hospital—J. Simon; Children’s Hospital of Los Angeles—G. Kung; Sutter Medical Center—T. Donnel; Texas Children’s Hospital—R. Pignatelli; North Shore University Hospital—I. Klein; Data Monitoring Committee, University of Washington—R. Knopp and F. Kim; Seattle Children’s Hospital—B. Hardy and M. Cohen; and independent medical monitor, Mayo Clinic—H. Burkhardt.
Sources of Funding
This research was supported by a grant to Dr Portman from the U.S. Food and Drug Administration Orphan Product Development Program (R01 FD-R-1971-01) and the University of Washington Clinical and Translational Science Award grant 1ULI RR025014-01 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Drug was supplied by King Pharmaceutical.
Footnotes
Reprints: Information about reprints can be found online at http://www.lww.com/reprints
Presented at the 2009 American Heart Association meeting in Orlando, Fla, November 14–18, 2009.
The online Data Supplement can be found with this article at http://circ.ahajournals.org/cgi/content/full/CIRCULATIONAHA.109.926394/DCI.
Disclosures
None.
References
- 1.Bettendorf M, Schmidt KG, Tiefenbacher U, Grulich-Henn J, Heinrich UE, Schonberg DK. Transient secondary hypothyroidism in children after cardiac surgery. Pediatr Res. 1997;41:375–379. doi: 10.1203/00006450-199703000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Klemperer JD, Klein I, Gomez M, Helm RE, Ojamaa K, Thomas SJ, Isom OW, Krieger K. Thyroid hormone treatment after coronary-artery bypass surgery. N Engl J Med. 1995;333:1522–1527. doi: 10.1056/NEJM199512073332302. [DOI] [PubMed] [Google Scholar]
- 3.Klemperer JD, Klein IL, Ojamaa K, Helm RE, Gomez M, Isom OW, Krieger KH. Triiodothyronine therapy lowers the incidence of atrial fibrillation after cardiac operations. Ann Thorac Surg. 1996;61:1323–1327. doi: 10.1016/0003-4975(96)00102-6. discussion 1328–1329. [DOI] [PubMed] [Google Scholar]
- 4.Mullis-Jansson SL, Argenziano M, Corwin S, Homma S, Weinberg AD, Williams M, Rose EA, Smith CR. A randomized double-blind study of the effect of triiodothyronine on cardiac function and morbidity after coronary bypass surgery. J Thorac Cardiovasc Surg. 1999;117:1128–1134. doi: 10.1016/s0022-5223(99)70249-7. [DOI] [PubMed] [Google Scholar]
- 5.Plumpton K, Haas NA. Identifying infants at risk of marked thyroid suppression post-cardiopulmonary bypass. Intensive Care Med. 2005;31:581–587. doi: 10.1007/s00134-004-2549-1. [DOI] [PubMed] [Google Scholar]
- 6.Portman MA, Fearneyhough C, Ning X, Duncan B, Rosenthal G, Lupinetti F. Triiodothyronine repletion in infants during cardiopulmonary bypass for congenital heart surgery. J Thorac Cardiovasc Surg. 2000;120:604–608. doi: 10.1067/mtc.2000.108900. [DOI] [PubMed] [Google Scholar]
- 7.Bettendorf M, Schmidt KG, Grulich-Henn J, Ulmer HE, Heinrich UE. Tri-iodothyronine treatment in children after cardiac surgery: a double-blind, randomised, placebo-controlled study. Lancet. 2000;356:529–534. doi: 10.1016/S0140-6736(00)02576-9. [DOI] [PubMed] [Google Scholar]
- 8.Chowdhury D, Ojamaa K, Parnell VA, McMahon C, Sison CP, Klein I. A prospective randomized clinical study of thyroid hormone treatment after operations for complex congenital heart disease. J Thorac Cardiovasc Surg. 2001;122:1023–1025. doi: 10.1067/mtc.2001.116192. [DOI] [PubMed] [Google Scholar]
- 9.Mackie AS, Booth KL, Newburger JW, Gauvreau K, Huang SA, Laussen PC, DiNardo JA, del Nido PJ, Mayer JE, Jr, Jonas RA, McGrath E, Elder J, Roth SJ. A randomized, double-blind, placebo-controlled pilot trial of triiodothyronine in neonatal heart surgery. J Thorac Cardiovasc Surg. 2005;130:810–816. doi: 10.1016/j.jtcvs.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 10.Mainwaring RD, Capparelli E, Schell K, Acosta M, Nelson JC. Pharmacokinetic Evaluation of Triiodothyronine Supplementation in Children After Modified Fontan Procedure. Circulation. 2000;101:1423–1429. doi: 10.1161/01.cir.101.12.1423. [DOI] [PubMed] [Google Scholar]
- 11.Gidding SS. The importance of randomized controlled trials in pediatric cardiology. JAMA. 2007;298:1214–1216. doi: 10.1001/jama.298.10.1214. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman TM, Wernovsky G, Atz AM, Kulik TJ, Nelson DP, Chang AC, Bailey JM, Akbary A, Kocsis JF, Kaczmarek R, Spray TL, Wessel DL. Efficacy and safety of milrinone in preventing low cardiac output syndrome in infants and children after corrective surgery for congenital heart disease. Circulation. 2003;107:996–1002. doi: 10.1161/01.cir.0000051365.81920.28. [DOI] [PubMed] [Google Scholar]
- 13.Portman MA, Fearneyhough C, Karl TR, Tong E, Seidel K, Mott A, Cohen G, Tacy T, Lewin M, Permut L, Schlater M, Azakie A. The Triiodothyronine for Infants and Children Undergoing Cardiopulmonary Bypass (TRICC) study: design and rationale. Am Heart J. 2004;148:393–398. doi: 10.1016/j.ahj.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 14.Heinle JS, Diaz LK, Fox LS. Early extubation after cardiac operations in neonates and young infants. J Thorac Cardiovasc Surg. 1997;114:413–418. doi: 10.1016/S0022-5223(97)70187-9. [DOI] [PubMed] [Google Scholar]
- 15.Lacour-Gayet F, Clarke D, Jacobs J, Comas J, Daebritz S, Daenen W, Gaynor W, Hamilton L, Jacobs M, Maruszsewski B, Pozzi M, Spray T, Stellin G, Tchervenkov C, Mavroudis C. The Aristotle score: a complexity-adjusted method to evaluate surgical results. Eur J Cardiothorac Surg. 2004;25:911–924. doi: 10.1016/j.ejcts.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 16.Tei C, Nishimura RA, Seward JB, Tajik AJ. Noninvasive Doppler-derived myocardial performance index: correlation with simultaneous measurements of cardiac catheterization measurements. J Am Soc Echocardiogr. 1997;10:169–178. doi: 10.1016/s0894-7317(97)70090-7. [DOI] [PubMed] [Google Scholar]
- 17.Wernovsky G, Wypij D, Jonas RA, Mayer JE, Jr, Hanley FL, Hickey PR, Walsh AZ, Chang AC, Castaneda AR, Newburger JW. Postoperative course and hemodynamic profile after the arterial switch operation in neonates and infants. A comparison of low-flow cardiopulmonary bypass and circulatory arrest. Circulation. 1995;92:2226–2235. doi: 10.1161/01.cir.92.8.2226. [DOI] [PubMed] [Google Scholar]
- 18.Kratzsch J, Schubert G, Pulzer F, Pfaeffle R, Koerner A, Dietz A, Rauh M, Kiess W, Thiery J. Reference intervals for TSH and thyroid hormones are mainly affected by age, body mass index and number of blood leucocytes, but hardly by gender and thyroid autoantibodies during the first decades of life. Clin Biochem. 2008;41:1091–1098. doi: 10.1016/j.clinbiochem.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Morales DL, Carberry KE, Heinle JS, McKenzie ED, Fraser CD, Jr, Diaz LK. Extubation in the operating room after Fontan’s procedure: effect on practice and outcomes. Ann Thorac Surg. 2008;86:576–581. doi: 10.1016/j.athoracsur.2008.02.010. discussion 581–572. [DOI] [PubMed] [Google Scholar]
- 20.Chang RK, Chen AY, Klitzner TS. Factors associated with age at operation for children with congenital heart disease. Pediatrics. 2000;105:1073–1081. doi: 10.1542/peds.105.5.1073. [DOI] [PubMed] [Google Scholar]
- 21.Danzi S, Dubon P, Klein I. Effect of serum triiodothyronine on regulation of cardiac gene expression: role of histone acetylation. Am J Physiol Heart Circ Physiol. 2005;289:H1506–H1511. doi: 10.1152/ajpheart.00182.2005. [DOI] [PubMed] [Google Scholar]
- 22.Hyyti OM, Ning XH, Buroker NE, Ge M, Portman MA. Thyroid hormone controls myocardial substrate metabolism through nuclear receptor-mediated and rapid posttranscriptional mechanisms. Am J Physiol Endocrinol Metab. 2006;290:E372–E379. doi: 10.1152/ajpendo.00288.2005. [DOI] [PubMed] [Google Scholar]
- 23.Wang YG, Dedkova EN, Fiening JP, Ojamaa K, Blatter LA, Lipsius SL. Acute exposure to thyroid hormone increases Na+ current and intracellular Ca2+ in cat atrial myocytes. J Physiol. 2003;546:491–499. doi: 10.1113/jphysiol.2002.032847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Danzi S, Klein I, Portman MA. Effect of triiodothyronine on gene transcription during cardiopulmonary bypass in infants with ventricular septal defect. Am J Cardiol. 2005;95:787–789. doi: 10.1016/j.amjcard.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 25.Novotny J, Bourova L, Malkova O, Svoboda P, Kolar F. G proteins, beta-adrenoreceptors and beta-adrenergic responsiveness in immature and adult rat ventricular myocardium: influence of neonatal hypo- and hyperthyroidism. J Mol Cell Cardiol. 1999;31:761–772. doi: 10.1006/jmcc.1998.0913. [DOI] [PubMed] [Google Scholar]
- 26.Hoit BD, Khoury SF, Shao Y, Gabel M, Liggett SB, Walsh RA. Effects of thyroid hormone on cardiac beta-adrenergic responsiveness in conscious baboons. Circulation. 1997;96:592–598. doi: 10.1161/01.cir.96.2.592. [DOI] [PubMed] [Google Scholar]
- 27.Portman MA, Qian K, Krueger JJ, Ning XH. Direct Action of T3 on Phosphorylation Potential in the Sheep Heart in vivo. Am J Physiol Heart Circ Physiol. 2005;288:2484–2490. doi: 10.1152/ajpheart.00848.2004. [DOI] [PubMed] [Google Scholar]
- 28.Doin FL, Borges Mda R, Campos O, de Camargo Carvalho AC, de Paola AA, Paiva MG, Abucham J, Moises VA. Effect of central hypothyroidism on Doppler-derived myocardial performance index. J Am Soc Echocardiogr. 2004;17:622–629. doi: 10.1016/j.echo.2004.03.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.