Abstract
Enterovirus (EV) detection by a new commercially available reverse transcription (RT)-PCR assay (Penter RT-PCR test) was compared with EV isolation from cell cultures and with EV detection by an in-house RT-PCR assay. Of the 54 cerebrospinal fluid specimens collected during a summer outbreak of aseptic meningitis, 52% were positive by cell culture versus 76% by in-house RT-PCR assay and 80% by the new RT-PCR test (52 versus 76 versus 80%; P = 0.003). This new reliable EV RNA detection test is suitable for clinical diagnosis of EV-related meningitis and may improve the management of EV-related neurological syndromes.
Enteroviruses (EVs) of the Picornaviridae family (64 distinct serotypes) are etiological causes of neurological syndromes in infants and adults (9, 14). Aseptic meningitis is the most common EV-related central nervous system syndrome and may be difficult to distinguish from meningitis caused by herpesviruses or bacteria, particularly in young infants and children (9, 10). When classical cell culture techniques are used, attempts to isolate EVs from cerebrospinal fluid (CSF) samples are frequently unsuccessful because of the low viral titers in clinical specimens and because some serotypes grow poorly in cell culture (3, 4). Therefore, in-house PCR techniques for the detection of the EV genome have been introduced, allowing sensitive and rapid identification of EV-infected CSF samples (1, 11, 12, 17) and improving the management of young infants with meningitis (8). However, the application of in-house EV PCR assays to the clinical diagnosis of EV-related neurological syndromes is actually limited by the time-consuming methods and the lack of standardization of procedures (6, 13). In this study, a new commercially available and well-standardized reverse transcription (RT)-PCR test (Penter RT-PCR) (2) was compared with cell culture and with an in-house reference RT-PCR assay for the detection of EV in CSF of patients with aseptic meningitis.
Fifty-four archived CSF specimens taken from 49 children (mean age, 6 years; range, 10 days to 12 years) and 5 adults (mean age, 29 years; range, 19 to 33 years) hospitalized at the university hospital of Reims (Reims, France) for meningitis syndrome during a summer outbreak of aseptic meningitis (May to November 2001) were retrospectively selected. CSF samples were included in this study providing they revealed a typical profile of aseptic meningitis with elevated levels of protein (>0.45 g/liter) and/or nuclear cells (>6 cells/mm3), normal glucose levels, negative Gram stain results, and negative bacterial and fungi cultures (15). Virus isolation had been prospectively performed for each CSF sample on human diploid fibroblasts (MRC-5) and rhesus monkey kidney cells (MA-104) as previously described (7). EV isolates were typed by the standard method of virus neutralization (Lim-Banyesch-Melnick immune serum pools) (4). After testing of CSF by viral culture, aliquots of CSF were stored at −80°C for later use in detecting enteroviral RNA by RT-PCR.
The new EV Penter RT-PCR test (new-generation EV consensus kit; Argène Biosoft, Varhiles, France) utilized several pairs of stair primers selected for annealing in the 5′ untranslated region, which is highly conserved in the EV group (2), and was used according to the manufacturer's instructions. Briefly, viral RNA was extracted from 140 μl of CSF sample by a rapid-extraction protocol (viral RNA minikit; Qiagen, Courtaboeuf, France) and eluted in a final volume of 40 μl of diethyl pyrocarbonate sterile water. A 10-μl aliquot of this material was added to 40 μl of a master mix prepared using buffers and enzymes provided by the manufacturer (Argène Biosoft). RT of target RNA and amplification of cDNA occur in a single-tube reaction following the amplification program described by the manufacturer (Argène Biosoft). An internal control provided by the manufacturer was tested with a duplicate sample after RNA extraction to check the absence of RT-PCR inhibitors in each specimen tested. After chemical denaturation, the single-stranded PCR products obtained from samples and internal controls were separately hybridized by two different biotinylated probes and detected with a streptavidin-peroxidase system on a microtiter plate (2). Results were scored as positive when the optical density (OD) value at 450 nm was above a cutoff value calculated for each serial assay by following the recommendations of the manufacturer (Argène Biosoft) (2).
In the second part of this study, the 54 selected CSF specimens were also analyzed by a reference in-house nested RT-PCR assay by using primers previously described by Zoll et al. (17) and a previously described protocol (1). Chi-square tests were performed using the software InsStad 2.00 (GraphPad Software, San Diego, Calif.).
The specificity of the Penter RT-PCR system used for the detection of EV genomes in CSF was demonstrated in a previously published study (2). The sensitivity of the new one-step Penter RT-PCR test was estimated to correspond to 0.05 50% tissue culture infectious doses of poliovirus strain Sabin 2 per 140 ml, according to the protocol previously described by Andréoletti et al. (1; data not shown). Moreover, the results obtained by testing the 2003 (fourth) EV proficiency panel from European Quality Control for Molecular Diagnosis showed that the new Penter test was capable of detecting 35 copies of EV genomes per one-step-RT-PCR tube (data not shown).
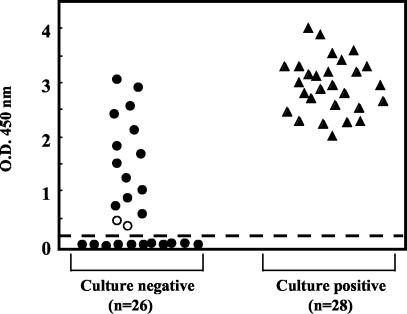
Of the 54 CSF specimens selected, 28 (52%) were positive for EV isolation from cell culture whereas 41 (76%) were positive by the in-house RT-PCR assay and 43 (80%) were positive by the new Penter RT-PCR test (52 versus 76 versus 80%; P = 0.003). The 28 EV strains isolated from CSF samples were tested by virus neutralization assays resulting in the identification of the following viral types: echovirus 30 (13 strains), echovirus 6 (9 strains), and echovirus 13 (6 strains). EV RNA was detected by the Penter RT-PCR assay in all CSF samples from which an EV strain was recovered by cell culture (Table 1). The OD values obtained by testing these CSF samples with the EV Penter RT-PCR assay ranged from 2.01 to 4.00 (mean value ± standard deviation, 2.95 ± 0.51), whereas the mean OD value corresponding to the cutoff below which results were interpreted as negative was 0.13 (standard deviation, ±0.05) (Fig. 1). All of these 28 CSF samples positive by both EV cell culture and EV Penter RT-PCR were positive by the in-house RT-PCR assay (Table 1). Twenty-six of the selected CSF samples were negative for EV isolation from cell cultures. EV RNA was detected by the Penter RT-PCR assay in 15 (58%) of these 26 CSF samples, whereas EV genomes were detected in 13 (50%) of the same samples by the in-house RT-PCR assay (58 versus 50%; P > 0.5) (Table 1). Low OD values (0.43 and 0.47) were observed for the two samples for which results of the two RT-PCR methods were discrepant, whereas the 13 CSF samples positive by both Penter RT-PCR and in-house RT-PCR assays showed OD values ranging from 0.61 to 3.1 (Fig. 1). The two discrepant samples were positive upon repetition of the whole Penter RT-PCR procedure with a new aliquot of CSF, whereas they remained negative by the in-house RT-PCR assay, demonstrating an absence of false-positive results. The positive amplification of the internal control in all tested CSF specimens allowed us to certify the absence of false-negative results with the Penter RT-PCR assay.
TABLE 1.
Comparison of rates of detection of EV in CSF specimens of patients with aseptic meningitis by viral cell culture, in-house reference RT-PCR, and the new Penter RT-PCR assay
FIG. 1.
Distribution of optical density values obtained by detection of EV RNA in 54 CSF samples from patients with aseptic meningitis. RNA detection was by a new one-step PCR method (Penter RT-PCR) with colorimetric microwell detection assay. The dashed line represents the mean OD value (mean OD ± standard deviation, 0.13 ± 0.05) below which the assay was interpreted as negative (cutoff value). •, OD values obtained from EV culture-negative CSF specimens (n = 26); ○, OD values obtained from CSF samples negative by cell culture and in-house RT-PCR assay (n = 2); ▴, OD values obtained from EV culture-positive CSF samples (n = 28).
Evaluation of a commercially available EV PCR assay is a prerequisite for further standardization of qualitative EV genome detection in CSF samples of patients with neurological syndromes (6, 13). In the present study, we evaluated a new commercially available RT-PCR assay which utilizes the principle of stair primers located in the 5′ uncoding region to amplify EV genomes (5), detects the EV amplicons in a microwell colorimetric assay within 10 h, and demonstrates the absence of PCR inhibitors by using a positive control tested with a duplicate sample after RNA extraction. This new Penter RT-PCR method has been evaluated in a recent study that demonstrated its ability to recognize all 64 prototypes of human EV strains but not parechovirus 1 and 2 (2). In this study, we used a modified version of this EV amplification method that allows a one-tube RT-PCR amplification of EV genomes.
In the present study, this new one-step RT-PCR assay (Penter RT-PCR) was compared with cell culture and with a previously described in-house RT-PCR assay for the detection of EV in CSF of patients with aseptic meningitis. Our data showed that the Penter RT-PCR assay was more sensitive than viral cultures and than the in-house RT-PCR assay (Table 1). With the exception of two CSF specimens, all the samples positive by Penter RT-PCR were confirmed as positive by the in-house reference RT-PCR (Table 1), thus excluding the possibility of false-positive RT-PCR results. For all the CSF samples negative by the Penter RT-PCR assay, the positive amplification of the internal control in a duplicate reaction allowed us to exclude the presence of RT-PCR inhibitors and therefore the possibility of false-negative results. We observed that results for two of the culture-negative CSF specimens were discrepant by Penter and in-house RT-PCR assays (Table 1). These discrepancies may be explained by the fact that the Penter RT-PCR test utilizes several pairs of stair primers, increasing the chance of amplifying EV target RNA present in low titers in CSF specimens (5). This hypothesis is supported by the low OD values obtained by the Penter RT-PCR assay for these two CSF specimens (Fig. 1).
In the present study, around 58% of the patients with samples negative for EV isolation from culture were found by the Penter RT-PCR to have EV RNA in their CSF. Prior studies comparing cell culture and PCR assays for EV detection have demonstrated that the use of PCR techniques significantly increases the rates of detection of EV in CSF samples of patients with aseptic meningitis or other neurological syndromes (6, 7, 16). The low rate of EV detection by cell culture compared with results obtained by RT-PCR assays may be explained either by low viral loads in CSF, by the presence of infectious EVs that grow poorly or not at all in classical cell cultures, or by the presence of replication-defective or antibody-neutralized viruses unable to propagate in cell culture (3, 4, 12).
In conclusion, our findings indicated that the Penter RT-PCR assay was more sensitive than viral cultures and than the in-house RT-PCR assay for the detection of EV RNA in CSF specimens of patients with aseptic meningitis, suggesting that this new EV RT-PCR test is suitable for use in the clinical diagnosis of EV-related meningitis. The use of this reliable EV RNA detection assay would improve the management of the infant and adult patients with EV-related neurological syndromes by promoting rapid discharge of patients and reducing unnecessary diagnostic and therapeutic interventions.
Acknowledgments
We thank Martine Joannès, Côme Baranger, and Nicolas Raoux, Argène Biosoft, France, for their valuable collaboration and constant technical support in the present study and for providing us with kits. We thank Bruno Lina and Geneviève Billaud, French reference laboratory for EVs, for providing us with several EV strains that were used as positive controls for in-house RT-PCR assays.
This work was supported by a local grant from University Champagne Ardenne (medicine faculty EA-3309).
REFERENCES
- 1.Andréoletti, L., D. Hober, S. Belaich, P. E. Lobert, A. Dewilde, and P. Wattré. 1996. Rapid detection of enterovirus in clinical specimens using PCR and microwell capture hybridization assay. J. Virol. Methods 62:1-10. [DOI] [PubMed] [Google Scholar]
- 2.Bourlet, T., V. Caro, S. Minjolle, I. Jusselin, B. Pozzetto, R. Crainic, and R. Colimon. 2003. New PCR test that recognizes all human prototypes of enterovirus: application for clinical diagnosis. J. Clin. Microbiol. 41:1750-1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chonmaitree, T., C. Ford, C. Sanders, and H. Lucia. 1988. Comparison of cell cultures for rapid isolation of enteroviruses. J. Clin. Microbiol. 26:2576-2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chonmaitree, T., C. D. Baldwin, and H. Lucia. 1989. Role of the virology laboratory in diagnosis and management of patients with central nervous system disease. Clin. Microbiol. Rev. 2:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colimon, R., S. Minjolle, P. Andre, C. T. de la Pintiere, A. Ruffault, C. Michelet, and F. Cartier. 1996. New type of primers (stair primers) for PCR amplification of the variable V3 region of the human immunodeficiency virus. J. Virol. Methods 58:7-19. [DOI] [PubMed] [Google Scholar]
- 6.Lina, B., B. Pozzetto, L. Andreoletti, E. Beguier, T. Bourlet, E. Dussaix, L. Grangeot-Keros, B. Gratacap-Cavallier, C. Henquell, M. C. Legrand-Quillien, A. Novillo, P. Palmer, J. Petitjean, K. Sandres, P. Dubreuil, H. Fleury, F. Freymuth, I. Leparc-Goffart, D. Hober, J. Izopet, H. Kopecka, Y. Lazizi, H. Lafeuille, P. Lebon, and M. Aymard. 1996. Multicenter evaluating of a commercially available PCR assay for diagnosing enterovirus infection in a panel of cerebrospinal fluid specimens. J. Clin. Microbiol. 34:3002-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pozo, F., I. Casas, A. Tenorio, G. Trallero, and J. M. Echevarria. 1998. Evaluation of a commercially available reverse transcription PCR assay for the diagnosis of enteroviral infection in archival and prospectively collected cerebrospinal fluid specimens. J. Clin. Microbiol. 36:1741-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramers, C., G. Billman, M. Hartin, S. Ho, and M. H. Sawyer. 2000. Impact of a diagnostic cerebrospinal fluid enterovirus polymerase chain reaction test on management of patients. JAMA 283:2680-2685. [DOI] [PubMed] [Google Scholar]
- 9.Rotbart, H. A. 1995. Enteroviral infections of the central nervous system. Clin. Infect. Dis. 20:971-981. [DOI] [PubMed] [Google Scholar]
- 10.Rotbart, H. A. 2000. Viral meningitis. Semin. Neurol. 20:277-292. [DOI] [PubMed] [Google Scholar]
- 11.Rotbart, H. A., M. H. Sawyer, S. Fast, C. Lewinsk, N. Murphy, E. Keyser, J. Spadoro, S. Kao, and S. Loeffelholz. 1994. Diagnosis of enteroviral meningitis by using PCR with a colorimetric microwell detection assay. J. Clin. Microbiol. 32:2590-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rotbart, H. A., and J. Romero. 1995. Laboratory diagnosis of enteroviral infections, p. 401-418. In H. A. Rotbart (ed.), Human enterovirus infections. American Society for Microbiology, Washington, D.C.
- 13.Smalling, T. W., S. E. Sefers, H. Li, and Y.-W. Tang. 2002. Molecular approaches to detecting herpes simplex virus and enteroviruses in the central nervous system. J. Clin. Microbiol. 40:2317-2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Regenmortel, M. H. J., C. M. Fauquet, D. H. L. Bishop, E. B. Carstens, M. K. Estes, S. M. Lemon, J. Maniloff, M. A. Mayo, D. J. McGeoch, C. R. Pringle, and R. B. Wicker. 2000. Virus taxonomy: classification and nomenclature of viruses. Seventh report of the International Committee on Taxonomy of Viruses, p. 657-677. Academic Press, New York, N.Y.
- 15.Wong, M., B. L. Schlaggar, R. S. Buller, J. A. Storch, and M. Landt. 2000. Cerebrospinal fluid protein concentration in pediatric patients. Arch. Pediatr. Adolesc. Med. 154:827-831. [DOI] [PubMed] [Google Scholar]
- 16.Yerly, S., A. Gervaix, V. Simonet, M. Callisch, L. Perrin, and W. Wunderli. 1996. Rapid and sensitive detection of enteroviruses in specimens from patients with aseptic meningitis. J. Clin. Microbiol. 34:199-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zoll, G. J., W. J. Melchers, H. Kopecka, G. Jambroes, H. J. Van der Poel, and J. M. Galama. 1992. General primer-mediated polymerase chain reaction for detection of enteroviruses: application for diagnostic routine and persistent infections. J. Clin. Microbiol. 30:160-165. [DOI] [PMC free article] [PubMed] [Google Scholar]