Abstract
Rationale and Objective
31P magnetization saturation transfer (MST) experiment is the most widely used method to study ATP metabolism kinetics. However, its lengthy data acquisition time greatly limits the wide biomedical applications in vivo, especially for studies requiring high spatial and temporal resolutions. We aim to develop a novel superfast MST method that can accurately quantify ATP production rate constants (kf) through creatine kinase (CK) or ATP synthase (ATPase) with two spectra.
Methods and Results
The T1nom (T1 nominal) method utilizes a correction factor to compensate the partially relaxed MST experiments, thus allowing measurement of enzyme kinetics with an arbitrary repetition time and flip angle, which consequently reduces the data acquisition time of a transmurally differentiated CK kf measurement by 91% as compared to the conventional method with spatial localization. The novel T1nom method is validated theoretically with numerical simulation, and further verified with in vivo swine hearts, as well as CK and ATPase activities in rat brain at 9.4 Tesla. Importantly, the in vivo data from swine hearts demonstrate for the first time, that within an observation window of 30 minutes, the inhibition of CK activity by iodoacetamide does not limit LV chamber contractile function.
Conclusions
A novel MST method for superfast examination of enzyme kinetics in vivo has been developed and verified theoretically and experimentally. In the in vivo normal heart, redundant multiple supporting systems of myocardial ATP production, transportation, and utilization exist, such that inhibition of one mechanism, does not impair the normal LV contractile performance.
Keywords: heart, metabolism, magnetic resonance spectroscopy, ATP, phosphates
INTRODUCTION
The adenosine triphosphate (ATP) metabolism in a living organ is characterized by a chemical exchange network among phosphocreatine (PCr), ATP, and inorganic phosphate (Pi), which is largely controlled by the enzymes creatine kinase (CK, catalyzing PCr↔ATP) and ATP synthase (ATPase, catalyzing Pi↔ATP): 1, 2
where kf and kr are the pseudo-first-order forward and reverse rate constants for CK and ATPase reactions. Under most in vivo circumstances, a steady-state condition is established, resulting in equal forward and reverse fluxes for both CK and ATPase reactions 1, 2. Therefore, the kinetics of PCr↔ATP↔Pi chemical exchange can be characterized by two forward pseudo-first-order rate constants (kf,CK for PCr→ATP and kf,ATPase for Pi→ATP), and studied by 31P magnetization saturation transfer (MST) experiment where ATPγ resonance is selectively saturated 1, 2.
The exchange rates of CK and ATPase reactions have been extensively studied on various organs, such as heart, brain, and skeletal muscle 3-5. Previous studies have suggested that the kinetics of the PCr↔ATP↔Pi exchange network may be associated with the pathological status of the organ. For example, significantly lowered ATP production rates via CK have been observed in association with various heart diseases in both large animal models 4, 5 and patients 6-8. The cerebral ATP metabolic rate through ATPase has been demonstrated to be tightly coupled to brain activity level in a rat model 9. In addition, the CK activity in the visual cortex of human brain was increased during visual simulation 10. In contrast, in heart it was found that CK forward flux rate was independent from the increase of cardiac workloads in response to catecholamine stimulations 5.
In order to compensate the lengthy data acquisition time imposed by conventional MST technique, Dr. Bottomley et al. proposed a four-angle saturation transfer (FAST) method, allowing rapid in vivo measurement of CK reaction rates with four short-repetition time (TR) spectra 11. This method was later employed by Dr. Weiss et al. in patients to examine the myocardial CK reaction kinetics 11. We have recently reported an improved MST method for measuring CK kinetics with as few as three spectra 12, the method focused on minimizing the saturation time by optimizing the pre-saturation delay, which resulted in a significant reduction of repetition time.
In the present study, we demonstrate a novel steady-state MST method (T1nom) for performing extremely rapid measurements of CK and ATPase kinetics with arbitrary repetition time and flip angle (FA). The accurate quantification of kf under such partial relaxation conditions requires only two spectra. The T1nom method is theoretically validated based on numerical simulation of modified Bloch-McConnell equations that govern the evolution of spin magnetizations during MST experiment. In addition, an optimization strategy for finding the best acquisition parameter range (TR and FA) used in T1nom method is provided. The new method is verified experimentally with in vivo measurements of: 1) kf,CK on swine heart model during the process of CK inhibition by iodoacetamide (IAA) infusion; and 2) both kf,CK and kf,ATPase on rat brain model at rest condition. Finally, the T1nom method was employed to measure the myocardial CK forward rate constant with transmural differentiation, demonstrating a reduction of data acquisition time by 91% as compared to a similar study using conventional saturation transfer method 13.
THEORY
kf calculation of conventional steady-state MST experiment
The evolution of spin magnetizations in the coupled CK and ATPase reactions can be characterized by the modified Bloch-McConnell equations 14, 15 shown as below:
| [1] |
| [2] |
| [3] |
When ATPγ is selectively saturated as applied in MST experiments, Equations [1] to [3] change to:
| [4] |
| [5] |
| [6] |
Equation [4] and [5] are mathematically equivalent, therefore CK and ATPase reactions are treated together using the same equations in the following discussion. The extent of the reduction of PCr and Pi magnetizations in response to ATPγ saturation is proportional to the forward rate constants:
| [7] |
where Mss and M0 represent the fully relaxed magnetizations with and without saturation on ATPγ and T1int is the intrinsic longitudinal relaxation time constant. kf calculation using Equation [7] is called conventional steady-state MST experiment, which requires measurement of two fully relaxed spectra (M0 and Mss) 14, 15. The validity of steady-state MST experiment has been confirmed by the fact that the intrinsic T1 is constant among subjects with different physiological and pathologic conditions 1, 4, 5, 9, 16-18 and even, in some case, among different species 19. The reliable intrinsic T1 can be measured using conventional progressive MST experiment, which employs multiple data acquisitions with progressively prolonged saturation time on ATPγ and thus extremely time consuming (Online Supplemental Materials).
T1nom method for extremely rapid kf measurement and quantification
The conventional steady-state MST experiment is inefficient in terms of signal to noise (SNR) per unit acquisition time due to full relaxation prerequisite for both M0 and Mss measurements. In addition, full relaxation requirement results in very long TR since the T1s of the 31P metabolites are characteristically long 20, which leads to a prohibitively lengthy total acquisition time for studies requiring higher spatial or temporal discrimination.
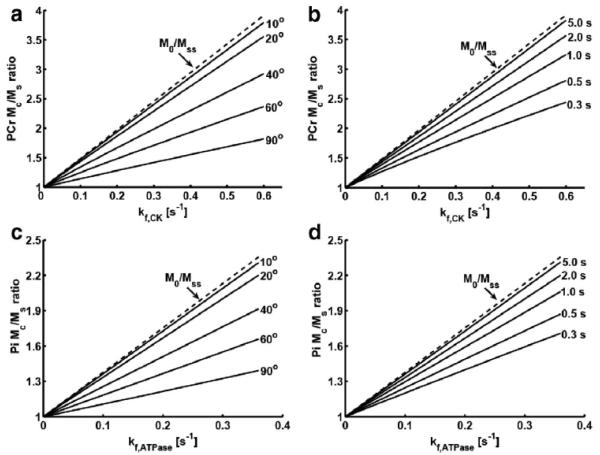
Preferably such experiments should be performed with a short TR and an appropriate FA to maximize the SNR per unit acquisition time. The pulse sequence employed is illustrated in Figure 1. For the reason of simplicity, we chose to utilize the same TR and FA for both saturated and control spectra. Two new steady-state measurements would be obtained from spectra obtained without (Mc) and with (Ms) saturation on ATPγ as compared to M0 and Mss in conventional steady-state MST experiment (Figure 2). In this case, Equation [7] no longer holds for kf calculation due to extra saturation factor from partial relaxation. The new relationship between kf value and the extent of magnetization reduction in response to ATPγ saturation can be elucidated by numerical simulation with various kf values and acquisition parameters (Figure 3). The simulation results suggest an approximately linear relationship between Mc/Ms ratio and kf values under various acquisition conditions. Therefore, based on a simple linear regression, Equation [7] can be re-formulated into the following equation for kf quantification under partial relaxation conditions:
| [8] |
where β is the intercept (usually within ±5% of 1) and T1nom is the slope of the line obtained by linear regression of the simulated Mc/Ms vs kf plot. Equation [8] is similar to the following equation which is the rearrangement of Equation [7] (dashed lines in Figure 3):
| [9] |
Equation [8] indicates that, the partial relaxation effects can be largely accounted for by one empirical parameter T1nom (means nominal T1 in contrast to intrinsic T1 as in Equation [9]). In general, T1nom is a function of both spin system parameters (T1int and pool size ratios of metabolites, such as PCr/ATP or Pi/ATP ratio), and acquisition parameters (TR and FA) and it approaches to T1int as TR increases and/or FA decreases:
| [10] |
There is no general analytical expression for Equation [10], however, the value of T1nom can be obtained with linear regression of simulated Mc/Ms vs kf plot based on Equation [1] to [6]. In practice T1nom and β can be empirically determined for specific experimental setup, and then kf value can be readily calculated with Mc and Ms measurements according to Equation [8].
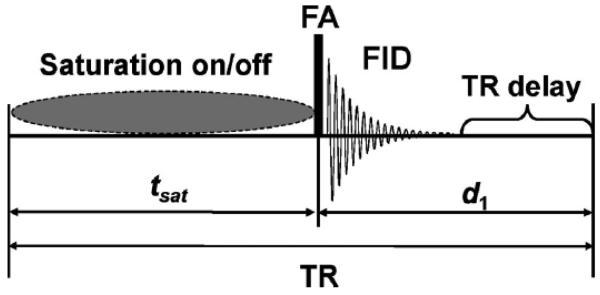
Figure 1.
Schematic view of pulse sequence used in steady-state magnetization saturation transfer experiment. For control spectrum, the saturation pulse train is replaced by a simple delay (or changed to a symmetrically opposite frequency for correcting spillover effect) such that the repetition time (TR) for both measurements would be same. Arbitrary flip angle (FA) for excitation is achieved by BIR4 pulse 21.
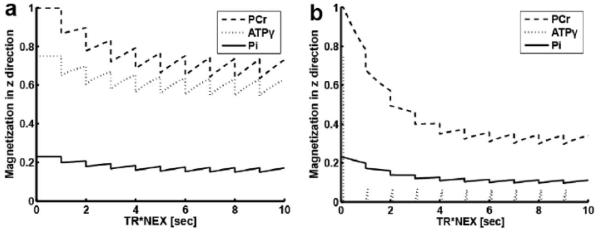
Figure 2.
Numerical simulation of Equations [1] to [6] using human brain data at 7 Tesla 16, demonstrating new steady-state magnetizations (Mc (panel a) and Ms (panel b)) under partial relaxation condition. Spin system parameters: pool size ratio of PCr : ATPγ : Pi = 1 : 0.75 : 0.23; intrinsic T1 for PCr, ATPγ and Pi are 4.86, 1.35 and 3.77 sec. Chemical exchange parameters: kf,CK = 0.3 sec−1; kf,ATPase = 0.18 sec−1. Acquisition parameters: TR= 1 sec, FA = 30°, d1 = 0.1 sec.
Figure 3.
Simulated Mc/Ms ratio vs kf plot for CK (a, b) and ATPase (c, d) reactions of human brain at 7 Tesla under various flip angles (a and c, TR = 1 sec) and repetition times (b and d, FA = 30°). Spin system parameters: pool size ratio of metabolites (PCr : ATPγ : Pi) = 1 : 0.75 : 0.23; intrinsic T1 for PCr, ATPγ and Pi are 4.86, 1.35 and 3.77 sec. Chemical exchange parameters: kf,CK = 0~0.6 sec−1; kf,ATPase = 0~0.36 sec−1. Acquisition parameter: d1 = 0.1 sec.
Optimization strategy for T1nom method
T1nom method allows kf calculation with arbitrary repetition time and flip angle. However, the best experimental condition (optimal TR and FA) remains unclear. Here we provide an optimization strategy to generate the best TR/FA range for T1nom-based kf measurement and quantification. The goal of optimization is to have the smallest relative kf calculation error for a given data acquisition time. Three types of kf error have been considered in this section. Analytical expression for each type is provided followed by a demonstration of parameter optimization using human brain studies at 7 Tesla 16.
Type 1 error: Mc/Ms vs kf nonlinearity
Mc/Ms vs kf plot in Figure 3 are not perfectly straight lines. The relative kf calculation error due to non-linearity is defined as below:
| [11] |
where kfreal and kfcal stand for actual kf and kf calculated from Equation [8], respectively. The deviation due to nonlinearity is acquisition condition dependent, thus the optimization can be performed such that the type 1 error is minimized.
Type 2 error: Spectral SNR
kf calculation is based on two measurements from control (Mc) and saturated (Ms) spectra (Equation [8]), each of which is subject to sampling error due to finite spectral SNR. The measurement error of each spectrum would in turn contribute to the final kf calculation error following error propagation theory.
Assuming a constant total acquisition time (t) and intrinsic scanner noise level (σ), the final kf relative error due to spectral SNR can be expressed as:
| [12] |
Equation [12] (see deduction in Online Supplemental Materials) takes into account both the SNR of each spectrum (Mc and Ms) and the sensitivity level of kf calculation towards spectral errors (T1nom value). A normalized type 2 error (KSNR) can be introduced from Equation [12]:
| [13] |
Due to lack of extra information on MR system performance or total data acquisition time, the optimization strategy is based on minimizing KSNR level.
Type 3 error: Flip angle inaccuracy
Flip angle can vary spatially due to B1 field inhomogeneity, especially in the case of surface coil and ultra-high magnetic field. Such variation can be greatly minimized by using adiabatic pulses (such as BIR4 pulse as employed in the present study 21). Therefore, the accuracy of kf calculation based on T1nom method would be affected by flip angle variation. The relative kf calculation error due to flip angle inaccuracy can be expressed by the following equation (See detailed deduction in Online Supplemental Materials):
| [14] |
Kflip is a non-dimensional parameter that characterizes the sensitivity level of kf error due to flip angle error, i.e., a smaller absolute Kflip value means the kf calculation is more robust against flip angle variation. The negative sign in Equation [14] indicates that an underestimation of flip angle would result in overestimation of kf and vice versa. The optimization strategy hereby is to find the acquisition conditions that lead to a Kflip value below an arbitrary level.
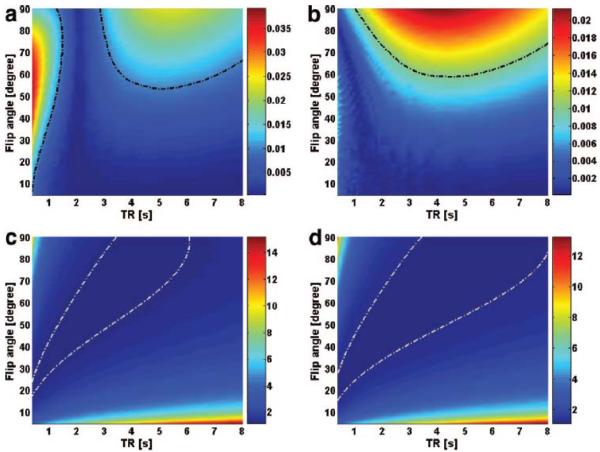
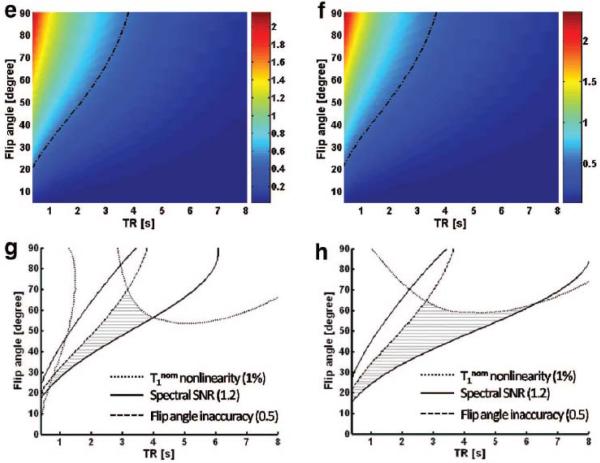
As a demonstration, numerical simulation for each type of kf error (Equations [11], [13] and [14]) have been carried out based on human brain data at 7 Tesla 16 and the results are shown in Figure 4a-f. By setting arbitrary cutoff criteria for each type of kf error, the overall optimized TR and FA range for the T1nom experiment can be obtained (Figure 4g-h, shadowed regions).
Figure 4.
Numerical simulation of three types of kf error for CK (a, c, e) and ATPase (b, d, f) reactions: T1nom nonlinearity (a, b), spectral SNR (c, d) and flip angle inaccuracy (e, f). Parameters used for numerical simulation were the same as in Figure 3. The dash-dotted lines (0.01 for a and b; 1.2 for c and d, 0.5 for e and f) represent the criteria used in optimization strategy for choosing the best TR and FA ranges. The best TR and FA ranges that satisfy all the criteria are shown in panel g (CK) and h (ATPase) labeled with shadow.
MATERIALS AND METHODS
All experiments were performed in accordance with the animal use guidelines of the University of Minnesota, and the experimental protocol was approved by the University of Minnesota Research Animal Resources Committee. The investigation conformed to the “Guide for the care and use of laboratory animals” published by the National Institutes of Health (NIH publication No 85-23.).
In vivo swine heart studies
Validation of T1nom method was performed with a creatine kinase inhibition experiment by iodoacetamide (IAA), an irreversible CK inhibitor 22. Young female Yorkshire swine (~30 kg, n=8) were employed for the study. Iodoacetamide solution (450 mmol/L) was administrated (1 mL/kg/hr iv), and a complete CK activity inhibition (as evidenced by M0,PCr = Mss,PCr) was usually achieved with a total dose of 0.45 mmol/kg iv. Infusion was paused every 10 min, and steady-state MST experiments were performed in both fully and partially relaxed conditions, with interleaved acquisition. Dummy scans were employed to enforce steady state for MST experiments with partial relaxation. 5 more pigs received an extra catecholamine intervention (dopamine/dobutamine, each of 10 μg/kg/min iv) after complete inhibition of CK. Details of the open-chest surgery preparation and 31P MRS have been described previously 5 and are included in the Online Supplemental Materials.
T1nom method was further employed to measure myocardial CK activity with transmural differentiation on female Yorkshire pigs (~40 kg, n=4). The spatially localized measurement was achieved with one dimensional chemical shift imaging (1D-CSI) sequence. Detailed methods are included in the Online Supplemental Materials.
To examine whether the LV contractile function can be maintained when the CK system is completely inhibited, additional 6 swine were employed for the cardiac MRI study on a clinical 1.5 Tesla scanner. LV chamber function was measured throughout the process of CK inhibition via iodoacetamide infusion at both basal and high cardiac workload conditions. Detailed cardiac MRI methods are included in the Online Supplemental Materials.
In vivo rat brain studies
Male Sprague-Dawley rats (n=5) were employed for brain studies. Details of rat preparation as well as MRS data acquisition have been published previously (Online Supplemental Materials) 9.
RESULTS
Cardiovascular Physiologic Studies using a Swine Model
31P MR spectroscopy data
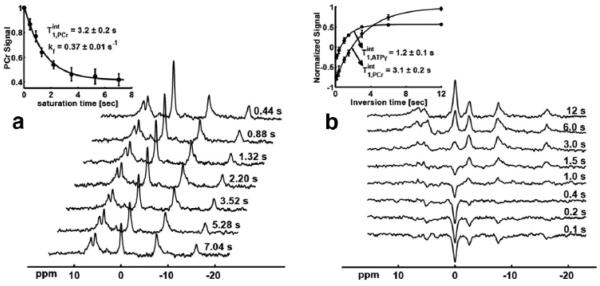
Intrinsic T1 measurements before and after complete CK inhibition yielded the same results for PCr (3.2±0.2 vs 3.1±0.2 s, p=NS, see in Figure 5), suggesting that T1int value is independent of CK activity and thus it is feasible to apply T1nom method to calculate the CK activity based on a constant T1int value.
Figure 5.
Measurement of intrinsic T1 using (a) progressive magnetization saturation transfer before CK inhibition and (b) inversion recovery after CK inhibition. NEX=8 for each spectrum. Calculation of intrinsic T1 is performed by fitting the PCr or ATPγ signals to an exponential relaxation model (shown in figure insets). Error bar represents the standard error from 4 independent pigs.
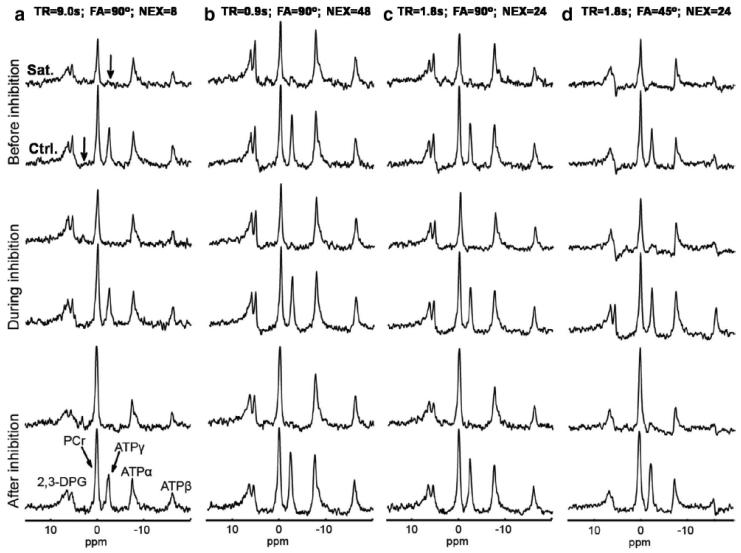
Figure 6 illustrates the representative spectra from steady-state MST experiments with various acquisition conditions throughout the CK inhibition process. ATPγ saturation was achieved by BISTRO saturation pulse train 23, which has been shown to have negligible spillover effects on the neighboring PCr peak 12. As CK gets completely inhibited (top to bottom), the PCr magnetization in saturated spectra (Sat.) all approaches to that of control spectra (Ctrl.) regardless of acquisition conditions, in agreement with Equation [8] that when kf equals 0, Mc/Ms ratio equals 1.
Figure 6.
Representative spectra from steady-state MST experiments before, during and after CK inhibition. Each steady-state MST experiment consisted of two spectra, with saturation pulse set at ATPγ frequency (Sat.) or symmetrically opposite site (Ctrl.), as indicated by the bold arrows. Spectra from different columns (a-d) were acquired with different acquisition parameters as indicated by the legend on top. Condition a utilized full relaxation, representing the conventional method whereas all the others utilized partial relaxation, representing the T1nom method. Condition d represents an optimized condition according to the optimization strategy (similar to figure 4g).
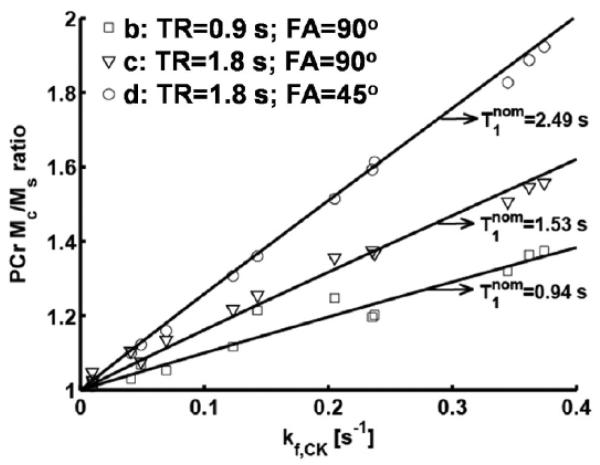
PCr signals measured with partially relaxed conditions (Figure 6b-d) throughout the CK inhibition process were quantified, and the ratio of PCr signals in control and saturated spectra was plotted against the CK kf value as measured by conventional steady-state MST experiments (Figure 7). The plot indicates a linear relationship between PCr signal ratios and kf values, with a slope depending on the acquisition parameters. Also included in Figure 7 (solid lines) are the simulation results with the same parameters as utilized by the experiment. The experimental results matched the simulation, indicating the validity of T1nom method. Notably, the steady-state MST experiments in condition d produced the least kf measurement error as compared to conditions b and c, consistent with the prediction based on the simulation results using the optimization strategy.
Figure 7.
Quantification of steady-state MST results from the MRS data used in Figure 6. PCr signal ratio from conditions b-d were plotted against kf value which was calculated according to Equation [7] from condition a. The solid lines were generated from simulation with the same parameters as in conditions b-d.
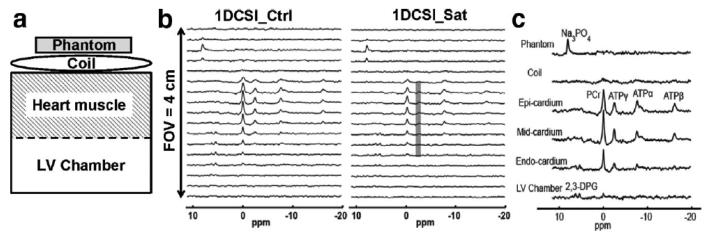
Figure 8 illustrates a typical set of transmurally differentiated measurement of creatine kinase forward flux rate constant (kf,CK) using T1nom method in combination of 1D-CSI sequence. The 1D-CSI spectra (Figure 8, panel b) displayed a typical “column” along the phase encoding direction perpendicular to the surface coil plane, as demonstrated by the minimal overlap of the characteristic resonances representing different depths away from the surface coil. Namely, signals are from compounds of: localization phantom (Na3PO4), coil, myocardium characterized by high levels of PCr and ATP, and erythrocytes from the LV cavity blood characterized by 2,3-DPG peaks. The particular setup generated a T1nom of 1.8 sec, which was utilized for kf calculation according to Equation [8]. Panel c illustrates the reconstructed spectra demonstrating the spatially localized kf measurements from the subepi- and the subendo- layers of LV anterior wall. Based on 4 swine studies, the corresponding kf values are 0.36±0.03 sec−1 and 0.40±0.03 sec−1 for subepi- and subendo- myocardial layers, respectively.
Figure 8.
Transmural measurement of creatine kinase forward flux rate constant (kf,CK) using T1nom method in combination of 1D-CSI sequence. Panel a, schematic view of the experimental setup. A phantom tube (200 μL 0.5 mol/L Na3PO4 in water) was sutured onto the surface coil as a spatial reference. Panel b, representative 1D-CSI spectra with (1DCSI_Sat, saturation pulse indicated by grey band) and without (1DCSI_Ctrl) saturation on ATPγ resonance for transmural measurement of kf,CK. Total data acquisition time is 13.6 min with a spatial resolution of 2.4 mm. Acquisition parameters are: field of view (FOV)=40 mm, phase encoding steps=17, TR=3 sec, FA=90°, NEX=8. Panel c, reconstructed transmural spectra from 1D-CSI data for actual kf calculation. A 9-term Fourier series windowing algorithm 32 was employed for the spectra reconstruction in order to increase the spectral signal-to-noise ratio. The reconstructed voxel can be arbitrarily shifted. T1nom is calculated to be 1.8 sec based on the acquisition parameters. The kf,CK values from 4 pig studies are 0.36±0.03 sec−1 and 0.40±0.03 sec−1 for the epi- and the endo- layers of left ventricle, respectively.
Hemodynamic, myocardial energetics and MRI data in response to CK inhibition
The hemodynamic and myocardial energetic data in response to CK inhibition via iodoacetamide infusion are summarized in Online Table II and III, respectively. Iodoacetamide infusion significantly increased the heart rate (p<0.05 vs baseline). However, within an observation window of 30 min, both the LV systolic pressure (LVSP) and the high energy phosphate PCr/ATP ratio are maintained despite of complete inhibition of CK activity. In respond to catecholamine stimulation, both the heart rate and LV systolic pressure increased significantly (Online Table III, p<0.05 vs IAA).
The LV contractile functions measured by cardiac MRI during baseline and high cardiac workload states with or without creatine kinase inhibition are summarized in the Online Supplemental Materials. Representative movies of LV short-axis cine imaging on one heart are also included in the Online Supplemental Materials. The LV contractile functions in terms of ejection fraction and systolic thickening fraction were not impaired during CK inhibition. Moreover, despite of CK inhibition, the heart can respond to catecholamine stimulation with an increased ejection fraction as non-inhibited hearts do (p<0.05 vs IAA, Online Figure I).
Taken together, these data demonstrate that LV contractile performance is maintained when the ATP production rate via CK is inhibited, suggesting existence of multiple and redundant ATP production systems in supporting the chemical energy need of the contractile apparatus.
In vivo Rat Brain Studies
The non-invasive T1nom method is further verified on rat brain at 9.4 Tesla with measurements of the CK and ATPase activities at rest condition (Online Figure II). There is no statistically significant difference between the kf values measured by conventional (TR=9 sec, FA=90°) and T1nom (TR=3 sec, FA=45°) methods (kf,CK: 0.26±0.04 sec−1 vs 0.24±0.03 sec−1, p=NS; kf,ATPase: 0.17±0.06 sec−1 vs 0.15±0.08 sec−1, p=NS).
DISCUSSIONS
The present work demonstrated a novel and simple method (T1nom) to quantify kf under partial relaxation conditions, allowing steady-state MST experiments to be performed with arbitrary repetition time and flip angle. The T1nom method features with extremely fast kf measurement yet simple linear algorithm (Equation [8]) for quantification. In addition, the optimization strategy would significantly enhance the performance of T1nom method by minimizing the final kf errors. By necessity, the T1nom method together with the optimization strategy can greatly facilitate the in vivo enzyme kinetic studies that demand high spatial and temporal resolution.
Versatility of T1nom method
The linear relationship between Mc/Ms ratio and kf is well maintained throughout a large range of simulated acquisition parameters (Figure 3). More extensive simulation suggested that this linear relationship holds in general regardless of pool size ratio or intrinsic T1 values, suggesting T1nom method a versatile tool for kinetic studies independent of experimental setup.
In the present study, the T1nom method is theoretically demonstrated based on the human brain study at 7 Tesla (three-site exchange model, PCr↔ATP↔Pi) and further experimentally verified on an in vivo swine heart model for measuring myocardial CK forward reaction rate constant at 9.4 Tesla (two-site model, PCr↔ATP). The two-site model is preferably employed for myocardial bioenergetic studies since the Pi resonance is largely overlapped by the 2,3-diphosphoglycerate peaks from blood and thus difficult to quantify unless spatial localization is employed 24. When applied to the two-site exchange model, ATP↔Pi reaction (corresponding to Equations [3] and [6]) was ignored during the numerical simulation process (Mc/Ms vs kf, Figure 3) for finding the T1nom value. Therefore, the T1nom method-based kf calculation is readily applicable to both two- and three-site models as supported by the good agreement between experimental and simulation results shown in Figure 7 and 8.
Validity of the methodology
The T1nom method can be considered as an improved version of conventional steady-state MST technique. The extensive previous studies on CK and ATPase kinetics have suggested that the intrinsic T1 is constant among subjects regardless of physiological and pathologic conditions 1, 4, 5, 9, 16-18. This is consistent with the observation in the present study that T1,PCrint is a constant among subjects and independent of reaction rate change throughout CK inhibition process. The Intrinsic T1 (T1int) characterizes the relaxation process of a spin population to re-establish the thermal equilibrium distribution (spin-lattice relaxation) 25. Therefore, in a defined magnetic field of a given organ of interest, the T1int of a compound is a constant, which should only reflect its characteristic molecular tumbling rate 25. However, the reported T1int value does vary due to different magnetic fields, species, organs, pulse and pulse sequences, and acquisition parameters. Therefore, it is always recommended to be cautious when using the T1int value from literature. In a rare case where a biological system has no prior report of its T1int, a direct T1int measurement of a few healthy subjects should be performed prior to the application of T1nom method.
The T1nom method is highly robust to the variation of pool size ratio of metabolites, such as PCr/ATP ratio for CK reaction and Pi/ATP ratio for ATPase reaction. For the acquisition parameters within the optimized region as shown in Figure 4g-h, the relative kf measurement error due to a variation of pool size ratios of metabolite is less than 1/8 of the variation level itself, i.e., a change of PCr/ATP ratio of 40% would result only a 5% of kf measurement error using the T1nom method. Finally, in case of large change of pool size ratios of metabolites, an iteration approach can be employed to correct for the originally assumed pool size ratio based on Mc measurement (Online Figure III). The iteration approach is based on the assumption that the change in pool size ratio is proportionally reflected in the magnetization (Mc) ratio measured in control spectra as long as the intrinsic T1s in the two statuses are the same:
| [15] |
Numerical simulation suggested a correction precision of >95% for Equation [15] with wide range of parameters (CK study of human brain at 7 Tesla 16, kf,CK=0.15~0.6 sec−1, TR=0.4~8 sec, FA=5~90°). Equation [15] is useful for obtaining the metabolites’ pool size ratio in the absence of fully relaxed measurements, which is highly valuable since the pool size ratio such as PCr/ATP has been widely accepted as a useful index for the bioenergetic status 26, 27. Therefore, based on Equation [8] and [15], a complete energetic study of both pool size ratio of metabolites and enzyme activity level can be performed without fully relaxed measurements.
Enhanced performance from optimization strategy
The performance of kf measurement using the T1nom method would be greatly enhanced by the optimization strategy, which is based on the kf error analysis to generate the best acquisition parameter range (TR and FA, that are most relevant to the longitudinal relaxation processing).
Type 1 error as defined by Equation [11] represents the accuracy of kf calculation using the T1nom method. As shown in Figure 4a-b, the type 1 error for human brain studies at 7 Tesla is below 1% for most acquisition conditions. Similar type 1 error levels were observed from numerical simulations with parameters that are characteristic of heart and skeletal muscle. Those simulation results again demonstrated the versatility of T1nom method for measuring enzyme kinetics on various organs.
The type 2 error specifically addresses the spectral SNR issue. For MR experiment with partial relaxation, the spectral SNR per unit acquisition time would be maximized if the flip angle is chosen at the Ernst angle that is determined by TR and spin’s longitudinal relaxation time. When chemical exchange is involved, the Ernst angle also depends on the reaction rate. Therefore, the Ernst angle for control and saturated spectrum would be different. However, applying different flip angles for Mc and Ms measurements would render the spectrum comparison less intuitive and the kf calculation more prone to flip angle inaccuracy. In current approach, instead, both spectra are acquired with a same flip angle that is globally optimized according to error propagation theory (Equation [13]). Since the acquisition parameters are identical for both Mc and Ms spectra, any measurement error due to flip angle variation would be cancelled out in Equation [8] for kf calculation and the only residual effect would be the change of T1nom value, which is taken into account as the type 3 error. Even though none of the Mc or Ms measurements is acquired exactly at its Ernst angle, the overall performance from this globally optimized flip angle is still substantially better than the conventional steady-state MST. Taking human brain studies at 7 Tesla for instance (same parameter as used in Figure 4), the T1nom method can easily achieve a level of <1% type 1 error. The same type 1 error level would require a TR of 16 sec for the conventional MST methods (99% full relaxation, FA=90°). Should such an experiment be performed under an optimized condition using the T1nom method (e.g. TR=2 sec, FA=45°), an 88% reduction of total acquisition time could be achieved assuming a same number of signal averaging (NEX).
Type 3 error deals with the residual effects of flip angle inaccuracy on the final kf calculation error. As demonstrated in Figure 4e-f, the spin system becomes more robust against flip angle variation as flip angle decreases or TR increases. This result is consistent with the previous simulation results showing that T1nom approaches to T1int as flip angle decreases or TR increases (Figure 3). Therefore, based on the analysis of type 3 error (Figure 4e-f) we can compensate the impact of flip angle variation to an arbitrary level at an expense of reduced SNR per unit time. This is advantageous over some other rapid saturation transfer methods which employ multiple flip angles for calculating the kf and thus more vulnerable to flip angle variation, such as FAST method 11.
The superior performance of the T1nom method is demonstrated by the transmurally differentiated measurement of kf,CK (Figure 8). The total data acquisition time using T1nom method in combination of 1D-CSI sequence (2 sets of spectra, 17 phase encoding steps, NEX=8, TR=3 sec) is 13.6 minutes. In contrast, a similar transmurally differentiated kf,CK measurement performed by Robitaille PM et al. using a conventional saturation transfer method took 153.6 min (8 sets of spectra, 18 phase encoding steps, NEX=8 and TR=8 sec) to accomplish the data acquisition 13. Therefore, the present study demonstrates that using T1nom method results in a reduction of data acquisition time by 91.2% as compared to the conventional saturation transfer method.
LV contractile function in relation to CK inhibition in the in vivo heart
Upon inhibition of creatine kinase via iodoacetamide infusion, the LV function and systemic hemodynamic did not change within an observation window of 30 min (Online Tables), the high energy phosphate PCr/ATP ratio was preserved (Online Table III), and the inorganic phosphate (Pi) level did not increase (Figure 6). Collectively, the present study demonstrate for the first time that a normal LV chamber contractile function can be maintained in the presence of complete inhibition of CK activity in normal in vivo heart under basal and high cardiac workstates. This finding is surprising, and raises a significant question of what a significant role CK plays in the cascade of ATP production, transportation and utilization. In the normal in vivo heart, the ATP production rate via creatine kinase exceeds that of the mitochondria ATPase by an order of magnitude 4. Therefore, it is possible that a small amount of residual CK activity may be sufficient to support normal LV function in a relatively short term. In the present study, it is possible that a residual undetectable creatine kinase activity of 5% (or less) remained at 30 minutes after the IAA infusion initiation. Based on the signal to noise ratio (Figure 6), a 5% of residual CK activity could be at noise level. This small fraction of residual creatine kinase-derived ATP, along with other ATP sources, could be sufficient in supporting cardiac contractile function in the short term.
The severity of CK kinetics change is related to the chronic cardiac pathologic changes such as severity of the LV hypertrophy or LV dysfunction 4-8. However, the mechanisms of these relationships are still unknown. The results of the present study demonstrate that an acute severe inhibition of ATP production rate via CK does not impair the LV regional or global contractile function. The finding of normal LV chamber function with complete inhibition of CK activity in the present study is in agreement with the previous observation using engineered mice, which demonstrated a normal growth and LV chamber function in mice with double knockout of the muscle and mitochondrial isoforms (M/MtCK-/- mice) 28. Taken together, these data suggest that in normal heart under in vivo conditions, redundant supporting systems exist to maintain an important organ function. These data also suggest that in the failing hearts that are usually severely hypertrophied, the redundant supporting systems such as CK, mitochondrial electron transport system and ATPase, may all be impaired. Consequently, the severity of the alterations of each of these systems is related to the severity of the LV dysfunction such as being observed earlier 6, 7, 29-31.
In summary, we have demonstrated a novel steady-state magnetization saturation transfer method (T1nom) together with an optimization strategy that allows accurate kf quantification under partially relaxed acquisition conditions. The new method features an unprecedented fast kf measurement yet simple linear algorithm for quantification. This method enables broad applications for in vivo enzyme kinetic studies that require high spatial or temporal resolution. Using this novel NMR methods and an established swine model, these data demonstrate that acute inhibition of CK activity does not limit LV chamber function in the in vivo heart.
Supplementary Material
Non-standard Abbreviations and Acronyms
| Symbols | Actual physical meaning |
|---|---|
| d1 | The total time in MST experiment when ATPγ is not saturated |
| FA | Flip angle of excitation pulse. |
| kf | Pseudo-first-order forward rate constant of enzyme reactions |
| kr | Pseudo-first-order reverse rate constant of enzyme reactions |
| Kflip | Normalized relative kf error due to flip angle inaccuracy |
| KSNR | Normalized relative kf error due to spectral SNR |
| M0 | Steady-state magnetization in control spectrum obtained under fully relaxed condition |
| Mc | Steady-state magnetization in control spectrum obtained under partially relaxed condition |
| Mss | Steady-state magnetization in saturated spectrum obtained under fully relaxed condition |
| Ms | Steady-state magnetization in saturated spectrum obtained under partially relaxed condition |
| NEX | Number of excitations for signal averaging |
| t | Total scan time (equation [13]) |
| tsat | The duration of saturation pulse in the MST experiment |
| T1app | Apparent longitudinal relaxation time when ATPγ is continuously saturated |
| T1int | Intrinsic longitudinal (spin-lattice) relaxation time constant |
| T1mix | Approximate longitudinal relaxation time constant when chemical exchange is involved |
| T1nom | Nominal T1, defined as the slope of linear regression of simulated Mc/Ms vs kf data curves (equation [8]) |
| TR | Repetition time of MST experiment. |
| β | Intercept of linear regression of simulated Mc/Ms vs kf data curves (equation [8]) |
| σ | Intrinsic MR system noise level (equation [13]) |
Detailed descriptions about different types of T1 are included in the Online Supplemental Materials.
Acknowledgments
SOURCES OF FUNDING These works were supported by National Institutes of Health grants: HL50470, HL 67828, HL 95077, HL 100407, NS041262, NS057560, NS070839, P41 RR008079, P30 NS057091; and Keck Foundation.
Footnotes
DISCLOSURES None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bittl JA, Ingwall JS. Reaction rates of creatine kinase and ATP synthesis in the isolated rat heart. A 31P NMR magnetization transfer study. J Biol Chem. 1985;260:3512–3517. [PubMed] [Google Scholar]
- 2.Ugurbil K. Magnetization-transfer measurements of individual rate constants in the presence of multiple reactions. J Magn Reson. 1985;64:207–219. [Google Scholar]
- 3.Ingwall JS, Kramer MF, Fifer MA, Lorell BH, Shemin R, Grossman W, Allen PD. The creatine kinase system in normal and diseased human myocardium. N Engl J Med. 1985;313:1050–1054. doi: 10.1056/NEJM198510243131704. [DOI] [PubMed] [Google Scholar]
- 4.Murakami Y, Zhang J, Eijgelshoven MH, Chen W, Carlyle WC, Zhang Y, Gong G, Bache RJ. Myocardial creatine kinase kinetics in hearts with postinfarction left ventricular remodeling. Am J physiol. 1999;276:H892–900. doi: 10.1152/ajpheart.1999.276.3.H892. [DOI] [PubMed] [Google Scholar]
- 5.Ye Y, Wang C, Zhang J, Cho YK, Gong G, Murakami Y, Bache RJ. Myocardial creatine kinase kinetics and isoform expression in hearts with severe LV hypertrophy. Am J Physiol Heart Circ Physiol. 2001;281:H376–386. doi: 10.1152/ajpheart.2001.281.1.H376. [DOI] [PubMed] [Google Scholar]
- 6.Weiss RG, Gerstenblith G, Bottomley PA. ATP flux through creatine kinase in the normal, stressed, and failing human heart. Proc Natl Acad Sci U S A. 2005;102:808–813. doi: 10.1073/pnas.0408962102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith CS, Bottomley PA, Schulman SP, Gerstenblith G, Weiss RG. Altered creatine kinase adenosine triphosphate kinetics in failing hypertrophied human myocardium. Circulation. 2006;114:1151–1158. doi: 10.1161/CIRCULATIONAHA.106.613646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ingwall JS, Weiss RG. Is the failing heart energy starved? On using chemical energy to support cardiac function. Circ Res. 2004;95:135–145. doi: 10.1161/01.RES.0000137170.41939.d9. [DOI] [PubMed] [Google Scholar]
- 9.Du F, Zhu XH, Zhang Y, Friedman M, Zhang N, Ugurbil K, Chen W. Tightly coupled brain activity and cerebral ATP metabolic rate. Proc Natl Acad Sci U S A. 2008;105:6409–6414. doi: 10.1073/pnas.0710766105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen W, Zhu XH, Adriany G, Ugurbil K. Increase of creatine kinase activity in the visual cortex of human brain during visual stimulation: a 31P magnetization transfer study. Magn Reson Med. 1997;38:551–557. doi: 10.1002/mrm.1910380408. [DOI] [PubMed] [Google Scholar]
- 11.Bottomley PA, Ouwerkerk R, Lee RF, Weiss RG. Four-angle saturation transfer (FAST) method for measuring creatine kinase reaction rates in vivo. Magn Reson Med. 2002;47:850–863. doi: 10.1002/mrm.10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiong Q, Li Q, Mansoor A, Jameel MN, Du F, Chen W, Zhang J. Novel strategy for measuring creatine kinase reaction rate in the in vivo heart. Am J Physiol Heart Circ Physiol. 2009;297:H1010–1019. doi: 10.1152/ajpheart.01195.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robitaille PM, Abduljalil A, Rath D, Zhang H, Hamlin RL. Transmural saturation transfer analysis of the creatine kinase system in the mammalian heart. Magn Reson Med. 1993;30:4–10. doi: 10.1002/mrm.1910300103. [DOI] [PubMed] [Google Scholar]
- 14.Bloch F. Nuclear Induction. Phys Rev. 1946;70:460–474. [Google Scholar]
- 15.McConnell HM. Reaction rates by nuclear magnetic resonance. J Chem Phys. 1958;28:430–431. [Google Scholar]
- 16.Du F, Zhu XH, Qiao H, Zhang X, Chen W. Efficient in vivo 31P magnetization transfer approach for noninvasively determining multiple kinetic parameters and metabolic fluxes of ATP metabolism in the human brain. Magn Reson Med. 2007;57:103–114. doi: 10.1002/mrm.21107. [DOI] [PubMed] [Google Scholar]
- 17.Bittl JA, Balschi JA, Ingwall JS. Effects of norepinephrine infusion on myocardial high-energy phosphate content and turnover in the living rat. J Clin Invest. 1987;79:1852–1859. doi: 10.1172/JCI113027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lei H, Ugurbil K, Chen W. Measurement of unidirectional Pi to ATP flux in human visual cortex at 7 T by using in vivo 31P magnetic resonance spectroscopy. Proc Natl Acad Sci U S A. 2003;100:14409–14414. doi: 10.1073/pnas.2332656100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedrich J, Nascimben L, Liao R, Ingwall JS. Phosphocreatine T1 measurements with and without exchange in the heart. Magn Reson Med. 1993;30:45–50. doi: 10.1002/mrm.1910300108. [DOI] [PubMed] [Google Scholar]
- 20.Bottomley PA, Ouwerkerk R. Optimum flip-angles for exciting NMR with uncertain T1 values. Magn Reson Med. 1994;32:137–141. doi: 10.1002/mrm.1910320120. [DOI] [PubMed] [Google Scholar]
- 21.Garwood M, Ke Y. Symmetric pulses to induce arbitrary flip angles with compensation for RF inhomogeneity and resonance offsets. J Magn Reson. 1991;94:511–525. [Google Scholar]
- 22.Tian R, Ingwall JS. Energetic basis for reduced contractile reserve in isolated rat hearts. Am J physiol. 1996;270:H1207–1216. doi: 10.1152/ajpheart.1996.270.4.H1207. [DOI] [PubMed] [Google Scholar]
- 23.de Graaf RA, Luo Y, Garwood M, Nicolay K. B1-insensitive, single-shot localization and water suppression. J Magn Reson B. 1996;113:35–45. doi: 10.1006/jmrb.1996.0152. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J, Duncker DJ, Xu Y, Zhang Y, Path G, Merkle H, Hendrich K, From AH, Bache RJ, Ugurbil K. Transmural bioenergetic responses of normal myocardium to high workstates. Am J physiol. 1995;268:H1891–1905. doi: 10.1152/ajpheart.1995.268.5.H1891. [DOI] [PubMed] [Google Scholar]
- 25.Gadian DG. Nuclear magnetic resonance and its applications to living systems. Oxford University Press (Clarendon); Oxford: 1982. p. 109. [Google Scholar]
- 26.Zhang J, Wilke N, Wang Y, Zhang Y, Wang C, Eijgelshoven MH, Cho YK, Murakami Y, Ugurbil K, Bache RJ, From AH. Functional and bioenergetic consequences of postinfarction left ventricular remodeling in a new porcine model. MRI and 31 P-MRS study. Circulation. 1996;94:1089–1100. doi: 10.1161/01.cir.94.5.1089. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J, Toher C, Erhard M, Zhang Y, Ugurbil K, Bache RJ, Lange T, Homans DC. Relationships between myocardial bioenergetic and left ventricular function in hearts with volume-overload hypertrophy. Circulation. 1997;96:334–343. doi: 10.1161/01.cir.96.1.334. [DOI] [PubMed] [Google Scholar]
- 28.Saupe KW, Spindler M, Tian R, Ingwall JS. Impaired cardiac energetics in mice lacking muscle-specific isoenzymes of creatine kinase. Circ Res. 1998;82:898–907. doi: 10.1161/01.res.82.8.898. [DOI] [PubMed] [Google Scholar]
- 29.Liu J, Wang C, Murakami Y, Gong G, Ishibashi Y, Prody C, Ochiai K, Bache RJ, Godinot C, Zhang J. Mitochondrial ATPase and high-energy phosphates in failing hearts. Am J Physiol Heart Circ Physiol. 2001;281:H1319–1326. doi: 10.1152/ajpheart.2001.281.3.H1319. [DOI] [PubMed] [Google Scholar]
- 30.Ye Y, Gong G, Ochiai K, Liu J, Zhang J. High-energy phosphate metabolism and creatine kinase in failing hearts: a new porcine model. Circulation. 2001;103:1570–1576. doi: 10.1161/01.cir.103.11.1570. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, Merkle H, Hendrich K, Garwood M, From AH, Ugurbil K, Bache RJ. Bioenergetic abnormalities associated with severe left ventricular hypertrophy. J Clin Invest. 1993;92:993–1003. doi: 10.1172/JCI116676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garwood M, Schleich T, Ross BD, Matson GB, Winters WD. A modified rotating frame experiment based on a Fourier series window function. Application to in vivo spatially localized NMR spectroscopy. J Magn Reson. 1985;65:239–251. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.