Abstract
We describe two cases of eumycetoma in the legs. The infections could not be adequately diagnosed by classical mycology, but the causative agents were successfully identified as Madurella mycetomatis by species-specific PCR and DNA sequencing.
CASE REPORT
Case 1.
A 39-year-old man, born in and still living in Mali, was first seen on 2 December 2000 at Saint Joseph Hospital in Paris, France. The patient presented with a fistulated infection of the left ankle, showing multiple draining sinuses. Radiographs identified a lytic lesion in the distal part of the fibula. The patient had a history of excision of an abscess containing black grains near the ankle joint 3 or 4 years ago in Mali. At that time, he received co-trimoxazole for several months without apparent improvement. The abscess, which appeared to be a mycetoma lesion, did not heal. Apparently, either the excision was incomplete, or the patient developed a reinfection. One week after admission to the hospital, the patient underwent massive excision of soft tissues and infected bone. Numerous black grains were seen in the surgical specimens, which were hard in consistency and irregular in size and shape. Direct microscopic examination of the grains revealed fungal hyphae with some swollen cells. Prior to culture, the grains were washed in sterile water and then seeded directly onto Sabouraud dextrose agar and chocolate blood agar and incubated at 30 and 37°C. The culture grew better at 37°C. After 24 h of incubation, Streptococcus pyogenes (group A) was isolated, and 4 or 5 days later, a slow-growing fungus producing brown, diffusing pigments was recovered. The fungus showed more rapid growth on chocolate agar. To enhance sporulation, the fungus was subcultured on soil extract agar and incubated at 25°C for 8 weeks. The culture remained sterile: no spores or conidia were seen (Fig. 1A). After 8 weeks of incubation, some swollen chlamydospore-like cells were observed. The isolated fungus could not be accurately identified to the species level. No additional pathological investigation was performed for this patient. The case was diagnosed as eumycetoma and managed with several surgical debridements combined with chemotherapy. Three weeks after the initial excision, muscle flap coverage and skin grafting were performed. The patient received oral itraconazole and intravenous amoxicillin for 6 weeks, followed by oral amoxicillin and itraconazole for 14 weeks, after which the medical treatment was stopped. No evidence of recurrence of infection was observed at the last follow-up (after 2 months). Since then, the patient has not returned for follow-up. However, in an indirect contact with a cousin of the patient revealed that in May 2003 the wound was still draining.
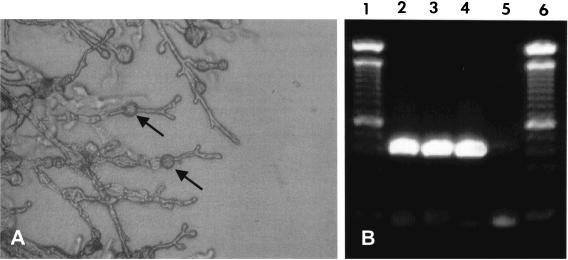
FIG. 1.
(A) Direct microscopy of 8-week-old cultures of M. mycetomatis (isolate P-1) on soil extract agar. Only vegetative hyphae with some chlamydospore-like cells (arrows) were seen. (B) M. mycetomatis-specific PCR for species identification using primers 26.1A and 28.3A (1). Lanes 1 and 6 contain a 100-bp molecular size marker. Lanes 2 to 5 show the PCR amplicons of P-1, P2, positive control, and negative control, respectively. Both P-1 and P-2 were positive.
Case 2.
A 45-year-old man, born in Mali and living in Paris, France, for more than 20 years but traveling to Mali regularly, presented at the Orthopedic Department of the Croix Saint Simon Hospital on 3 January 2001. The patient was complaining of rheumatic arthritis in the left knee joint. During examination, the orthopedic surgeon discovered a large subcutaneous indolent mass in the inner part of the right knee (Fig. 2A). There were no sinuses and consequently no discharge. Magnetic resonance images showed a heterogeneous soft tissue mass without any bone or joint involvement (Fig. 2B). The subcutaneous mass initiated gradually and had grown slowly over the past 3 years. The patient did not recall any trauma, puncture, or wound at the site of the infection. Complete surgical excision was performed, and the entire tissue biopsied was sent for microbiological and pathological examination (Fig. 2C). Direct examination and cultures were done in the microbiology laboratory of Croix Saint Simon Hospital, while pathological examinations were done by Michel Forest, Cochin Hospital, Paris, France. The lesion was well encapsulated, and it appeared to have been excised completely. The bone and the knee joint were healthy and intact. The lesion measured more than 15 cm in length and consisted of dense fibrous tissue containing multiple cavities full of black grains and bloody pus (Fig. 2E and F). The black grains had a hard consistency and different shapes and sizes. Microscopically, the grains showed hyphae and swollen cells. Pure filamentous fungus was isolated from the grains. Culturing was done as described for the first case. The fungus also showed better growth at 37°C than at 30°C. Optimal growth was obtained on chocolate agar after 3 or 4 days (Fig. 2D). The fungus was subcultured on soil extract agar for identification for 2 to 3 weeks, but again the fungus remained sterile, and its accurate identification was impossible. To avoid recurrence, the patient received oral itraconazole (400 mg two times a day) immediately after surgery. Due to digestive problems, the dose was reduced to 300 mg (two times a day), and this therapy was applied for 3 months. The patient was seen at the end of treatment, 1 month later, and again after 1 year. He was cured completely, and in June 2003 there were no signs of recurrence.
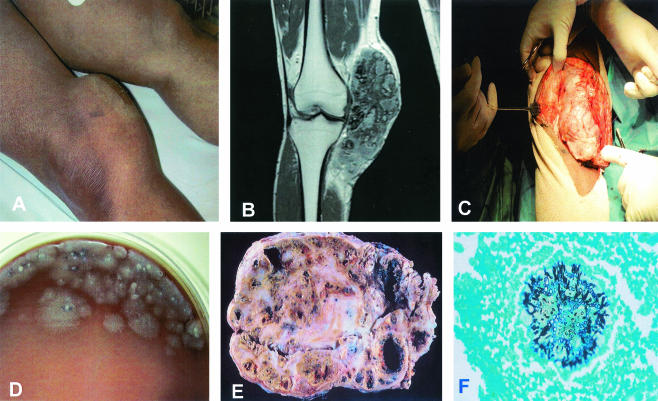
FIG. 2.
(A) Massive subcutaneous mass involving the inner part of the right knee. (B) T1-weighted magnetic resonance image after gadolinium injection, showing a large heterogeneous soft tissue mass. Bone and knee joint are healthy and not involved. (C) During surgical excision, the lesion appeared to be well capsulated, and it was excised completely. Note the massive lesion, which measures about 16 cm. (D) Four-day-old culture of the black grains on chocolate blood agar. The fungus starts presenting as white mycelium and later become olivaceous or brown. (E) The lesion consisted of dense fibrous tissues containing multiple cavities full of thick exudates containing pus, blood, and grains. (F) Gomeri-Grocott staining showing a fungal grain surrounded by inflammatory cells. One can clearly see the fungal hyphae growing toward the periphery of the grain.
Tropical mycoses are frequently imported into Western countries with increasing numbers of immigrants. This challenges the medical microbiological laboratory, since correct diagnosis of agents of mycetoma is usually difficult, although more specific culture media have been described for certain species of fungi (7, 8, 12). It is, however, hard to anticipate which medium to use, and the efficacy of these media is not unchallenged (unpublished observations). de Hoog et al. (6), for example, reviewed eight cases imported into The Netherlands, of which only one, Actinomadura madurae, belonged to a commonly known agent of mycetoma. The remaining isolates either belonged to very rare species, such as Phialophora cyanescens, now known as Cylindrocarpon cyanescens (13), or were not identified at all. In our patients, although the infections were correctly diagnosed from the clinical prospective as being eumycetoma, the identification of the causative organisms remained a problem. For this reason, the two fungal isolates were sent to the Department of Medical Microbiology and Infectious Diseases, Erasmus MC, Rotterdam, The Netherlands, for identification. The two isolates were coded P-1 and P-2 and subcultured on Sabouraud dextrose agar and incubated at 37°C for 3 weeks. Identification was attempted on the basis of the keys provided by de Hoog et al. (S. de Hoog, D. Adelmann, A. Ahmed, and A. van Belkum, submitted for publication). The thalli were built of regularly septate hyphae without clamp connections or fruit bodies. Both cultures had remained sterile, were a dark brownish color, and produced black diffusing pigments. Morphologically, the cultures were similar to the known agent of eumycetoma, Madurella mycetomatis.
Three-week-old cultures, incubated at 37°C, were subjected to DNA isolation. A previously identified clinical isolate of M. mycetomatis was included as a positive control. Approximately 1 g of fungal material was excised from the agar and transferred into 6 ml of a 0.9% NaCl solution. The material was homogenized by sonification for 2 min at 30 μ (Soniprep 150; Beun de Ronde, Abcoude, The Netherlands) to fully disrupt the mycelium. Five hundred microliters of this suspension was taken, and DNA was isolated by the DNA extraction method of Boom et al. (3). The extracted DNA was tested directly by M. mycetomatis species-specific PCR as has been described before (1). The PCR mixture without template DNA was used as a negative control. The PCR was performed in a model 60 thermocycler (Biomed, Theres, Germany). The PCR products were analyzed by electrophoresis on 1% agarose gels. The specific 424-bp fragments were seen for both fungal isolates, which exactly matched the positive control. No amplicons were seen in the negative-control lane (Fig. 1B).
PCR primer sequences were based on the internal transcribed spacer (ITS) of M. mycetomatis ribosomal genes and were previously shown to react only with M. mycetomatis DNA (1). The resulting PCR products differ between M. mycetomatis and the remaining agents of black grain mycetoma, including Madurella grisea (1). Based on this PCR result, the two isolates were presumptively identified as M. mycetomatis. In order to have further confirmation and to study the phylogenetic relationship with other M. mycetomatis isolates from different geographical origins, the two isolates were subjected to ITS sequencing using primers ITS1 and ITS4 (11). Sequencing reactions were performed in 10-μl volumes containing 15 to 50 ng of fungal DNA, 4 pmol of sequencing primer, and 4 μl of BigDye Mix (Applied Biosystems, Nieuwerkerk aan de IJssel, The Netherlands). The DNA products were analyzed on an ABI Prism 310 genetic analyzer (Applied Biosystems).The sequences were adjusted using SeqMan software (DNAStar, Madison, Wis.) and aligned using BioNumerics (Applied Maths, Kortrijk, Belgium). The sequences were directly compared in a local research database at the Centraalbureau voor Schimmelcultures (CBS). The P-1 and P-2 sequences showed >99% similarity with those of M. mycetomatis reference strains CBS 247.48, CBS 110087, the neotype strain of M. mycetomatis (CBS 109801, isolated from Sudan) and with three other clinical isolates from Sudan (7). Thus, PCR and sequencing confirmed that both causative agents were M. mycetomatis, belonging to the order of the Sordariales in the class Ascomycetes (1, 7).
M. mycetomatis is the most prevalent etiological agent of black grain eumycetoma (10), which is a chronic granulomatous infection of subcutaneous and deep tissues (2). In some areas where M. mycetomatis is endemic, it has been held responsible for more than 70% of all mycetoma infections, although such studies have been done without molecular verification of the identity (2). The two patients were clinically diagnosed as suffering from eumycetoma, and both were treated with a combination of antimycotic agents and surgery. Unfortunately, the first patient is still not cured. Since fungal mycetoma is always difficult to treat and the chance of recurrence of the infection is high, especially when only surgery is performed, follow-up is important (2, 10). The second patient was better at follow-up. The treatment was stopped rather early, but since his lesion was well capsulated, the patient was seen after 1.5 years. The knee was then unaffected.
Both patients were originally from Mali, which is located in the area of the African continent where mycetoma is endemic. Our patients were living in France, but their clinical history showed that they most likely acquired their infection in Mali. The most recent report of the disease in Mali, dating back to 1996 (10), claimed that eumycetoma was caused mainly by M. mycetomatis, but this was not confirmed by molecular methods (9).
Most cases of mycetoma that are imported into Western countries are still not easily diagnosed. The clinical picture, comprising the presence of a subcutaneous mass, sinuses, and discharge containing grains, is diagnostic for mycetoma. However, a large number of etiological agents are able to cause infection with this appearance. In addition, these microorganisms show poor morphological differentiation and usually present as sterile hyphae. Although phialidic conidiogenesis has been described for M. mycetomatis (4, 5), we have not been able to specifically induce this type of fungal differentiation. Classical identification remains therefore difficult (6, 7). Although phenotypic tests are considered a valuable first-stage identification for M. mycetomatis, molecular methods have now enabled even more definite identification (12) and allow the performance of detailed studies on the incidence of M. mycetomatis and phylogenetically similar agents. Last but not least, the application of these molecular tests directly to clinical aspirates from madura lesions could significantly reduce the duration of the diagnostic procedure.
REFERENCES
- 1.Ahmed, A. O., M. M. Mukhtar, M. Kools-Sijmons, A. H. Fahal, S. de Hoog, B. G. van den Ende, E. E. Zijlstra, H. Verbrugh, E. S. Abugroun, A. M. Elhassan, and A. van Belkum. 1999. Development of a species-specific PCR-restriction fragment length polymorphism analysis procedure for identification of Madurella mycetomatis. J. Clin. Microbiol. 37:3175-3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boiron, P., R. Locci, M. Goodfellow, S. A. Gumaa, K. Isik, B. Kim, M. M. McNeil, M. C. Salinas-Carmona, and H. Shojaei. 1998. Nocardia, nocardiosis and mycetoma. Med. Mycol. 36:26-37. [PubMed] [Google Scholar]
- 3.Boom, R., C. J. Sol, M. M. Salimans, C. L. Jansen, P. M. Wertheim-van Dillen, and J. van der Noordaa. 1990. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 28:495-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borelli, D. 1957. Madurella mycetomi: fialides, fialospores. Inoculation al raton. Bol. Lab. Clin. 2:1-8. [Google Scholar]
- 5.Borelli, D. 1962. Medios caseros para micologia. Arch. Venez. Med. Trop. Parasitol. 4:301-310. [Google Scholar]
- 6.de Hoog, G. S., A. Buiting, C. S. Tan, A. B. Stroebel, C. Ketterings, E. J. de Boer, B. Naafs, R. Brimicombe, M. K. Nohlmans-Paulssen, G. T. Fabius, et al. 1993. Diagnostic problems with imported cases of mycetoma in The Netherlands. Mycoses 36:81-87. [DOI] [PubMed] [Google Scholar]
- 7.de Hoog, G. S., J. Guarro, J. Gene, and M. J. Figueras. 2000. Atlas of clinical fungi, 2nd ed. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands.
- 8.MacKinnon, J. E. 1954. A contribution to the study of the causal organisms of maduromycosis. Trans. R. Soc. Trop. Med. Hyg. 48:470-480. [DOI] [PubMed] [Google Scholar]
- 9.Mahe, A., M. Develoux, C. Lienhardt, S. Keita, and P. Bobin. 1996. Mycetomas in Mali: causative agents and geographic distribution. Am. J. Trop. Med. Hyg. 54:77-79. [DOI] [PubMed] [Google Scholar]
- 10.McGinnis, M. R. 1996. Mycetoma. Dermatol. Clin. 14:97-104. [DOI] [PubMed] [Google Scholar]
- 11.White, T. J., T. Burns, S. Lee, and J. Taylor. 1996. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p. 315-322. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White (ed.), PCR protocols: a guide to method and applications. Academic Press, San Diego, Calif.
- 12.Yera, H., M. E. Bougnoux, C. Jeanrot, M. T. Baixench, G. De Pinieux, and J. Dupouy-Camet. 2003. Mycetoma of the foot caused by Fusarium solani: identification of the etiologic agent by DNA sequencing. J. Clin. Microbiol. 41:1805-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zoutman, D. E., and L. Sigler. 1991. Mycetoma of the foot caused by Cylindrocarpon destructans. J. Clin. Microbiol. 29:1855-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]