Abstract
Background
We sought to estimate the prevalence and incidence of hepatitis C virus (HCV) infection among Aboriginal young people who use drugs and to identify risk factors associated with HCV infection in this population.
Methods
The Cedar Project is a longitudinal study involving Aboriginal young people living in Vancouver and Prince George, British Columbia. Eligibility criteria include age from 14 to 30 years and self-reported use (smoking or injection) of illicit drugs (e.g., crystal methamphetamine, crack cocaine, heroin or other opiates, and cocaine) at least once in the month before enrolment. At each visit, participants completed a detailed questionnaire administered by an Aboriginal interviewer. For this analysis, we included information for 512 participants who were recruited between September 2003 and April 2005.
Results
Among the 512 participants, the prevalence of HCV infection was 34.8% (95% confidence interval [CI] 30.6%–38.9%); the rates were similar in Prince George and Vancouver (34.5% and 35.0% respectively, p = 0.37). Among those who reported the use of injection drugs at baseline (n = 286), the prevalence of HCV infection was 59.4% (95% CI 53.8%–65.1%); the rate in this group was slightly higher in Prince George than in Vancouver (62.4% v. 57.1% respectively, p = 0.37). The prevalence was 3.5% among participants who reported smoking drugs (n = 226). In the multivariate logistic regression analysis, factors significantly associated with HCV infection among participants who used injection drugs included daily injection of opiates (adjusted odds ratio [OR] 2.7, 95% CI 1.0–7.4), reuse of syringes (adjusted OR 2.4, 95% CI 1.3–4.4), having at least 1 parent who attended residential school (adjusted OR 1.9, 95% CI 1.1–3.4), female sex (adjusted OR 1.9, 95% CI 1.1–3.4) and duration of injection drug use (per year) (adjusted OR 1.4, 95% CI 1.3–1.5). The crude incidence rate of HCV infection was 10.6% and the incidence density estimate was 9.9 per 100 person-years in this cohort.
Interpretation
The prevalence of HCV infection was elevated among Aboriginal young people living in Prince George and Vancouver who use drugs. Culturally based prevention, treatment and harm-reduction programs are urgently needed in this population.
Little is known about the extent of the hepatitis C (HCV) epidemic among Aboriginal people in North America.1,2 In Canada, the reasons for this include limited surveillance data, underreporting of HCV infection and inconsistent documentation of ethnicity between the provinces.3 As a result, national surveillance data can offer only a minimum estimate of the number of infected people. Regrettably, alarming trends have already emerged. The Public Health Agency of Canada estimates that the prevalence of HCV infection is 0.8% in the general population of Canada and that it is 7-fold higher among Aboriginal people than among non-Aboriginal people.3 Estimates also suggest that the number of new cases is 2.5 times higher among Aboriginal people than in the general population.4 These data, however, represent Aboriginal people who live in urban areas and may not be generalizable to the entire Aboriginal population. A recent analysis conducted by Health Canada’s First Nations, Inuit and Aboriginal Health Branch found that Status Indians represented 4% of the population of the province of British Columbia but accounted for 10% of cases of HCV infection reported in 2001.4 Other data indicate that the prevalence of HCV infection in Aboriginal populations in different regions of Canada ranges between 0.4% and 29.3%.1
Indigenous people’s vulnerability to HCV infection and other infectious diseases is becoming increasingly apparent worldwide. A better understanding of the factors and processes that cause drug-related harm among Aboriginal young people is urgently required. Aboriginal scholars have long suggested that any discussions related to addictions and concomitant vulnerability to infectious disease must be framed within the context of the legacy of colonization, including the residential school system, which removed more than 100 000 Aboriginal children from their families between 1874 and 19865-7 in an attempt to assimilate and Christianize the youngest generations of Aboriginal people in the absence of their parents and leaders.8 Although some factors unique to the transmission of infectious disease among young people who use injection drugs are known, studies addressing sex- and drug-related harm and the impact of the residential school system among Aboriginal young people are lacking, particularly in smaller cities and in reserve communities.9
Aboriginal leaders and HIV/AIDS service providers are concerned about the impact of the HIV and HCV epidemics, not only in small and large cities but in rural settings as well.10 We conducted this study to estimate the prevalence and incidence of HCV infection among Aboriginal young people who use drugs and reside in Prince George, a forestry and mining town in the northern interior of British Columbia, and in Vancouver. In addition, we sought to identify demographic and behavioural factors associated with HCV infection in this population.
Methods
Study design and population
We followed the guidelines provided in the Canadian Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans11 in the development and conduct of this study. We paid particular attention to section 6.0, which pertains to research involving Aboriginal subjects. Our First Nations collaborators, including Aboriginal HIV/AIDS service organizations, were involved in the conception, design and implementation of the Cedar Project. They also reviewed the results of this analysis and approved this manuscript for publication. The study design was approved by the University of British Columbia/Providence Health Care Research Ethics Board.
The Cedar Project is an ongoing prospective cohort study involving Aboriginal young people who use drugs and live in Prince George and Vancouver, with target recruitments of 300 participants in each city. Eligibility criteria include age from 14 to 30 years and use (smoking or injection) of illicit drugs (e.g., crystal methamphetamine, crack cocaine, heroin or other opiates, and cocaine) in the month before enrolment. Participants were eligible to participate if they had been residing in the greater Vancouver or Prince George regions and provided written informed consent. Nonrandom samples of participants were recruited from both cities through a variety of methods, including referral by health care providers, community outreach, participant referral and word of mouth.Based on 2001 Census data, there are an estimated 14 080 Aboriginal people between 15 and 34 years of age residing in the Northern Health Authority (Prince George) and 7675 in the Vancouver Coastal Health Authority (Vancouver).
Data collection and laboratory testing
All participants met with a single study coordinator, who explained procedures, sought informed consent and confirmed study eligibility. Venous blood samples were drawn and tested for HIV and HCV antibodies. At enrolment, participants completed an interviewer-administered questionnaire to elicit sociodemographic data and data on their use (smoking or injection) of illicit drugs, injection practices, sexual risk behaviours and use of health care services. Various sections of the Vancouver Injection Drug User Study (VIDUS) questionnaire were tested and included in our baseline questionnaire.12 Aboriginal study personnel conducted all of the interviews; interviewers were blinded to the HIV and HCV status of the participants.
We used algorithms for HCV serologic testing similar to those used in other studies conducted in this region.13 In brief, AxSYM HCV version 3.0 (Abbott Laboratories, Chicago, Ill.) was used to screen all plasma samples. Negative samples did not undergo further testing. All positive samples underwent further testing with the recombinant Ortho HCV 3.0 ELISA test system (Ortho Clinical Diagnostic Inc., Rochester, NY). Samples that tested positive with both assays were classified as positive. Samples that tested positive by the AxSYM HCV test and negative by the Ortho HCV test were classified as negative.
All eligible participants had private interviews, including pre- and post-test counselling with trained nurses. Participants were requested to return for their HCV test result, at which time referral for HIV/AIDS and hepatitis C care was provided if requested. In addition, subjects were referred to clinics for immunization against hepatitis A and B.
Participants were given a small stipend at each study visit as compensation for their time and to facilitate transportation.
Estimation of HCV prevalence and incidence
To estimate the prevalence of HCV infection, we used a cohort of 512 participants who were recruited between September 2003 and April 2005, had completed their baseline interview during this period and had an HCV antibody test result from this visit.
To estimate the incidence of HCV infection, we used a cohort of 198 participants who completed their enrolment visit between September 2003 and October 2004, were HCV-negative at enrolment and completed at least 1 follow-up HCV antibody test during the observation period (September 2003 to April 2005). The event of interest in this analysis was time to HCV infection. For participants with new infection, we calculated time to HCV infection as the duration (in months) from the date of enrolment to the date of the first positive antibody test result. Follow-up time for event-free participants was calculated as the duration from enrolment to the date of their most recent negative antibody test result. We determined estimates of HCV incidence using crude rates and incidence density methods. To assess the strength of association between factors of interest and HCV infection, we calculated both unadjusted and adjusted odds ratios (ORs) and 95% confidence intervals (CIs). The low number of HCV seroconversions (n = 21) during the study period did not permit a thorough analysis of risk factors for incident infection.
Because of the known association between HCV infection and parenteral drug use, we stratified prevalence rates of HCV infection by injection and noninjection drug use. We restricted analyses of risk factors to participants who reported injection of drugs. Variables of interest included age, incarceration and housing (stable v. unstable). Participants considered to have stable housing were those living in their own house or apartment. Unstable housing was defined as living arrangements that included single-room occupancy hotels, transitional living arrangements (“couch surfing”) and homelessness. Risky injection practices included borrowing and lending of previously used syringes. Drug use behaviours included frequent injection, type of drug, bingeing behaviour and overdose experience. As in previous reports, we defined frequent injection as injection of cocaine, heroin or “speedballs” (cocaine and heroin combined) once or more per day. We defined bingeing as periods when drugs were injected more frequently than usual. Risky sexual behaviours in the 6 months before enrolment and in the 6 months before each follow-up visit included having an HCV-positive sexual partner and exchanging money or drugs for sex.
Statistical analysis
We analyzed bivariate categorical data using the Pearson χ2 test. We used the Fisher exact test to analyze bivariate categorical data when 25% or more of the expected cell frequencies in a contingency table were less than 5. We compared numeric variables (e.g., age at enrolment, age at first injection) between HCV-positive and HCV-negative participants using the Wilcoxon rank-sum test. We used multivariate logistic regression analysis to model the independent association of demographic variables and behavioural risk factors with HCV infection. Variables that were at least marginally significant in unadjusted analyses (p < 0.10) were included in the multivariate logistic regression model. We calculated unadjusted ORs and 95% CIs using logistic regression analysis. All reported p values are 2-sided.
Results
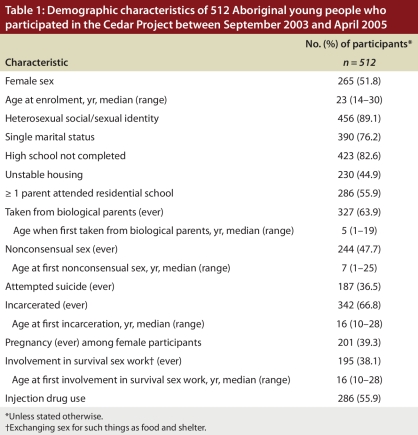
The demographic characteristics of the 512 participants are summarized in Table 1. A total of 178 participants were HCV positive at enrolment, for a prevalence of 34.8% (95% CI 30.6%–38.9%). The prevalence was similar in both locations (34.5% in Prince George and 35.0% in Vancouver, p = 0.37). Of the 286 participants at baseline who reported using injection drugs, 170 were HCV positive, for a prevalence of 59.4% (95% CI 53.8%–65.1%); 38% of these HCV-positive participants reported that they had been injecting drugs for 2 years or less. The prevalence among those who used injection drugs was higher in Prince George than in Vancouver (62.4% v. 57.1%); this difference was not statistically significant (p = 0.37). The prevalence among the 226 participants at baseline who reported noninjection use of drugs was 3.5%.
Table 1.
Demographic characteristics of 512 Aboriginal young people who participated in the Cedar Project between September 2003 and April 2005
A total of 199 participants who were HCV negative at enrolment were followed for a median of 11 months. These participants contributed 212.7 person-years of observation (data not shown). During the observation period, 21 new infections were recorded in this cohort, for a crude incidence rate of 10.6% and an incidence density estimate of 9.9 per 100 person-years. The incidence of HCV infection was higher in Prince George than in Vancouver (12.9 v. 7.5 per 100 person-years); this difference was not statistically significant (p > 0.10). The incidence density was 22.6 per 100 person-years among the participants who reported injection drug use (n = 71) and 6.7 per 100 person-years among those who reported noninjection drug use (n = 128). Of the 21 participants with new HCV infection, 14 (67%) reported use of injection drugs at enrolment. Of the 7 (33%) who reported noninjection drug use at enrolment, all 7 reported use of injection drugs at 1 or more follow-up visits.
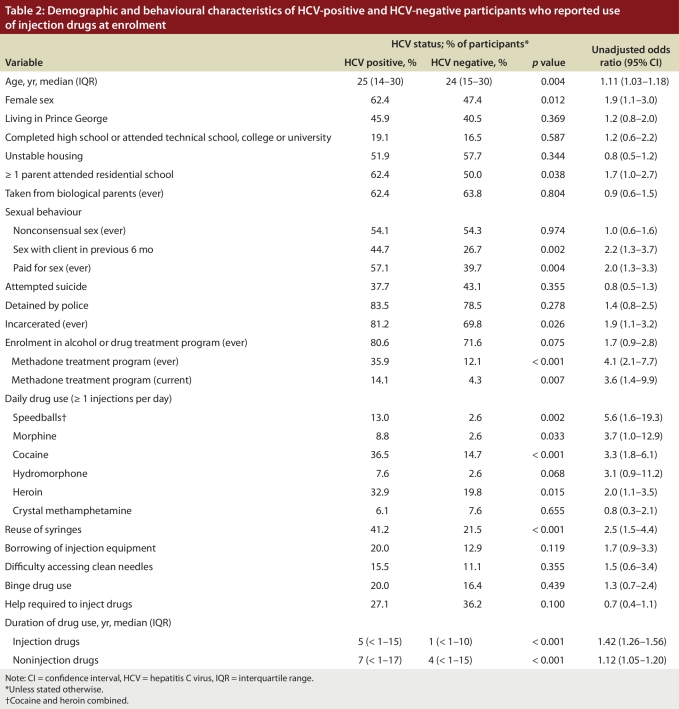
In the univariate analysis, we found that HCV infection at enrolment among the participants who reported use of injection drugs was significantly associated with daily injection of drugs (Table 2): speedballs (unadjusted OR 5.6, 95% CI 1.6–19.3), morphine (unadjusted OR 3.7, 95% CI 1.0–12.9), cocaine (unadjusted OR 3.3, 95% CI 1.8–6.1), hydromorphone (unadjusted OR 3.1, 95% CI 0.9–11.2) and heroin (unadjusted OR 2.0, 95% CI 1.1–3.5). HCV infection at enrolment was also significantly associated with female sex, having at least 1 parent who attended residential school, involvement in sex work, incarceration, current or past methadone treatment and reuse of syringes. Other significant risk factors were duration of drug use and age (Table 2): participants who were HCV positive at enrolment reported significantly longer periods of drug use (injection drugs, unadjusted OR 1.42 [95% CI 1.26–1.56]; noninjection drugs, unadjusted OR 1.12 [95% CI 1.05–1.20) and were older (unadjusted OR 1.11, 95% CI 1.03–1.18) than the HCV-negative participants.
Table 2.
Demographic and behavioural characteristics of HCV-positive and HCV-negative participants who reported use of injection drugs at enrolment
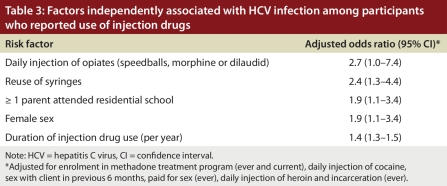
In the multivariate analysis, we combined responses for daily use of some opiates (morphine, hydromorphone or speedballs) into 1 variable (i.e., daily injection of opiates) because the numbers were small for both HCV-positive and HCV-negative participants.We found that the following risk factors were independently associated with HCV infection in the multivariate analysis: daily injection of opiates (adjusted OR 2.7, 95% CI 1.0–7.4), reuse of syringes (adjusted OR 2.4, 95% CI 1.3–4.4), having at least 1 parent who attended residential school (adjusted OR 1.9, 95% CI 1.1–3.4), female sex (adjusted OR 1.9, 95% CI 1.1–3.4) and duration of injection drug use (adjusted OR 1.4, 95% CI 1.3–1.5) (Table 3). Estimates of risk for the variables in this multivariate model were similar after adjustment for geographic location.
Table 3.
Factors independently associated with HCV infection among participants who reported use of injection drugs
Interpretation
In this group of Aboriginal young people who use injection drugs in Prince George and Vancouver, HCV infection spread to levels above 55%, and the incidence rate of HCV infection was 10.6%. These findings were unexpected and reflect how rapidly this virus can spread even in the early period just after injection drug use begins.13-16 Aboriginal and non-Aboriginal scholars agree that the relation between the cumulative effects of historical and current trauma are directly related to the HIV epidemic among indigenous peoples in North America. The prevalence of HCV infection among the Aboriginal young people who reported use of injection drugs in our study (59%) was higher than the prevalence in a previous study involving young people who were using injection drugs in Vancouver (46%).17 Furthermore, the prevalence of HCV infection among the Aboriginal young people in our study who live in the northern community of Prince George mirrored the prevalence among young people using injection drugs in Vancouver’s Downtown Eastside. Given that Vancouver has consistently been described as an epicentre of the HIV and HCV epidemics in British Columbia and in Canada since the 1990s, these findings indicate that the faces of these epidemics are changing.
We found that daily injection of opiates, reuse of syringes, having at least 1 parent who attended residential school, female sex and duration of injection drug use were independent risk factors for HCV infection at baseline. The strong association between frequent use of opiates and HCV prevalence is a relatively new finding in this setting. A persistent risk factor for blood-borne infection in Vancouver’s Downtown Eastside is frequent injection of cocaine.16,17 The fact that young people in our study reported injecting a wide array of opiates (morphine, hydromorphone and speedballs) along with cocaine on a daily basis and that daily opiate use, and not cocaine use, was associated with HCV seropositivity warrants further investigation. Methadone maintenance therapy is the primary treatment of opiate addiction in Canada and has proven effective in preventing HIV infection.18 Unfortunately, the evidence supporting its effectiveness in reducing HCV incidence is less substantial.19,20 In this study, methadone maintenance therapy was not associated with a reduction of risk for HCV infection. Despite this lack of evidence, methadone maintenance therapy continues to be recommended, in part because it helps to reduce the number of daily opiate injections and because injection episodes are presumed to be safer when they occur. Increased efforts must be made to determine the barriers to receiving such treatment for Aboriginal young people, particularly in smaller city centres.
We found that reusing injection equipment was another significant risk factor for HCV infection in our study. Because of previously demonstrated associations between other high-risk behaviours related to accessibility of clean syringes,18 we compared participants in our study who reported reusing syringes during the 6 months before enrolment with those who did not reuse injection equipment. Indeed, participants who reported reusing their syringes were significantly more likely to report borrowing and sharing injection equipment, bingeing with injection drugs and requiring assistance injecting during that period.
In recent years the federal government and Aboriginal communities across Canada have been debating the relative toll of the cumulative and intergenerational effects of historical trauma related to the Indian residential school system.19 The forced removal of children from their homes and placement in boarding schools is considered by many researchers, based on testimony given by family and community, to be directly responsible for poor health outcomes among survivors, including the abuse of drugs and alcohol.20 Currently, there are an estimated 80 000 survivors of the residential school system alive in Canada, of whom 35 000 are in British Columbia.21 As affected communities raise their children and grandchildren, the intergenerational effects of abuse and familial fragmentation become evident.22,23 Several Aboriginal HIV/AIDS service providers have suggested that drug use is just one way that people deal with the complex effects of poverty, despair, discrimination, loss of language and traditional territories and the erosion of culture.24 Previous research has identified a bivariate relation between having a parent who attended residential school and involvement in the child welfare system with sexual abuse among Aboriginal young people who use drugs in British Columbia.25 The mechanism of effect between having a parent who attended residential school and increased risk of HCV infection should be the subject of further study.
We found that female sex was significantly associated with HCV infection, even after we adjusted for demographic and behavioural risk factors. Although the majority of these infections can be attributed to injection-related risk behaviours, many young Aboriginal women are intimately involved with men who are usually older and who use injection drugs themselves.26,27 Indeed, research has demonstrated that, for women who rely on their partners for drug acquisition, preparation and injection, the distribution of power and control in sexual and injection relationships often lies with drug-injecting men who control the money and the drugs.28,29 Combined, these factors lead to an increased likelihood of unsafe sex and of the female partner being “second on the needle.”12
Our study has several limitations. We used self-reported behavioural data obtained from a non-probability sample of individuals. Consequently, there is potential for recall bias, socially desirable reporting and misclassification of exposure variables. Responses to questions concerning drug use and sexual behaviours may have been influenced by the participants’ knowledge of their HCV antibody status. In addition, recall problems may exist with reporting of past traumatic life events (e.g., being taken from biological parents, whether parents attended residential school, and sexual abuse or nonconsensual sex). The effect of memory on our estimates of risk for these factors is difficult to assess. Nondifferential misclassification of an exposure variable and the resultant bias can lead to a relative risk estimate that may be either toward or away from the null hypothesis.30 Furthermore, our estimate of the incidence of HCV infection may have been affected by differential drop-out. We found that participants who were lost to follow-up were younger and more frequent drug users than those who completed follow-up visits. Therefore, our estimates of incidence may be lower than their true values.
Despite these limitations, we believe these data provide important epidemiologic information about the prevalence and incidence of HCV infection in a high-risk Aboriginal population. In the absence of postexposure prophylaxis for HCV infection and an effective vaccine against HCV, primary prevention programs must focus on safer injection practices and reducing the number of people who initiate injection drug use. The high baseline prevalence rates in our study underscore the importance of carefully examining the effectiveness of current harm reduction initiatives in both rural and urban settings. Culturally based prevention, treatment and harm-reduction programs are urgently needed for young people who use injection drugs. Acknowledging the intergenerational trauma related to the residential school system may be one way that Aboriginal leadership and addiction specialists can begin to mitigate the potential impact of the HCV epidemic in their communities.
Acknowledgments
We are indebted to the study participants for their continued participation in the Cedar Project. We thank the members of the Cedar Project Partnership (the Prince George Friendship Centre, Carrier Sekani Family Services, Positive Living North, Red Road Aboriginal HIV/AIDS Network, Central Interior Native Health, Vancouver Native Health Society, Healing Our Spirit, and Q'wemtsin Health Society) for their conviction and for holding us accountable to the voices of Aboriginal young people. We also thank our study staff and elder advisory for their continued conviction and contributions.
Biographies
Kevin JP Craib is at the School of Population and Public Health, University of British Columbia, Vancouver, B.C.
Patricia M Spittal is at the School of Population and Public Health, University of British Columbia, B.C.
Sheetal H Patel is at the School of Population and Public Health, University of British Columbia, B.C.
Wayne M Christian is chief, Splats’in/Secwepemc First Nation, B.C.
Akm Moniruzzaman is at the School of Population and Public Health, University of British Columbia, B.C.
Margo E Pearce is at the School of Population and Public Health, University of British Columbia, B.C.
Lou Demerais is executive director, Vancouver Native Health Society, Vancouver, B.C.
Christopher Sherlock is at St. Paul’s Hospital Virology Lab, University of British Columbia, B.C.
Martin T Schechter is at the School of Population and Public Health, University of British Columbia, B.C.
The Cedar Project Partnership is composed of the Prince George Friendship Centre, Carrier Sekani Family Services, Positive Living North, Red Road Aboriginal HIV/AIDS Network, Central Interior Native Health, Vancouver Native Health Society, Healing Our Spirit, and Q'wemtsin Health Society, in B.C., Canada.
Footnotes
Competing interests: None declared.
Funding source: The study was supported by a grant from the Institute for Aboriginal Peoples Health of the Canadian Institutes of Health Research (CIHR), the Status of Women Canada and the Providence Healthcare Research Institute. Dr. Spittal is the recipient of the CIHR New Investigator Career Award. Dr. Schechter holds a Canada Research Chair in HIV/AIDS and Urban Population Health. Dr. Moniruzzaman is supported by a CIHR doctoral research award.
Contributors: All of the authors contributed substantially to the conception and design of the study, the analysis and interpretation of data, and the drafting and revision of the manuscript. All of the authors approved the final version of the manuscript.
References
- 1.Riben P, Bailey G, Hudson S, McColloch K, Dignan T, Martin D. Hepatitis C in Canada’s first nations and Inuit populations: an unknown burden. Can J Public Health. 2000;91(Suppl 1):S16–S18. [PubMed] [Google Scholar]
- 2.McMahon Brian J, Hennessy Thomas W, Christensen Carol, Bruden Dana, Sullivan Daniel G, Homan Chriss, Deubner Heike, Bruce Michael G, Livingston Stephen, Williams James, Gretch David R. Epidemiology and risk factors for hepatitis C in Alaska Natives. Hepatology. 2004;39(2):325–332. doi: 10.1002/hep.20046. [DOI] [PubMed] [Google Scholar]
- 3.Zou S, Tepper M, Giulivi A. Current status of hepatitis C in Canada. Can J Public Health. 2000;91(Suppl 1):S10–S16. doi: 10.1007/BF03405100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dawar M, Buxton J. Rate of reporting of hepatitis C infections in the Status Indian population of British Columbia. Paper presented at: 2nd Canadian Conference on Hepatitis C; 2004 Mar. 27–30; Vancouver, BC. 2001. [Google Scholar]
- 5.Gagné M. The role of dependency and colonialism in generating trauma in First Nations citizens. In: Danieli Y, editor. International handbook of multigenerational legacies of trauma. New York: Plenum Press; 1998. pp. 355–372. [Google Scholar]
- 6.Miller JR. Shinwauk’s vision. Toronto: University of Toronto Press; 1996. [Google Scholar]
- 7.Royal Commission on Aboriginal Peoples. Report of the Royal Commission on Aboriginal Peoples. Ottawa: The Commission; 1996. [Google Scholar]
- 8.Christian Wayne M, Spittal Patricia M. The Cedar Project: acknowledging the pain of our children. Lancet. 2008;372(9644):1132–1133. doi: 10.1016/S0140-6736(08)61460-9. [DOI] [PubMed] [Google Scholar]
- 9.Vernon I. Killing us quietly: Native Americans and HIV/AIDS. Lincoln (NB): University of Nebraska Press; 2001. [Google Scholar]
- 10.O’Neil J. Research on HIV/AIDS in Aboriginal people: a background paper. Ottawa: Medical Services Branch, Health Canada; 1998. pp. 4–41. [Google Scholar]
- 11.Canadian Institutes of Health Research, Natural Sciences and Engineering Research Council of Canada, Social Sciences and Humanities Research Council of Canada. Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans. 1998 (with 2000, 2002 and 2005 amendments). http://www.pre.ethics.gc.ca/eng/policy-politique/tcps-eptc.
- 12.Spittal Patricia M, Craib Kevin J P, Wood Evan, Laliberté Nancy, Li Kathy, Tyndall Mark W, O'Shaughnessy Michael V, Schechter Martin T. Risk factors for elevated HIV incidence rates among female injection drug users in Vancouver. CMAJ. 2002;166(7):894–899. http://www.cmaj.ca/cgi/pmidlookup?view=long&pmid=11949985. [PMC free article] [PubMed] [Google Scholar]
- 13.Patrick D M, Tyndall M W, Cornelisse P G, Li K, Sherlock C H, Rekart M L, Strathdee S A, Currie S L, Schechter M T, O'Shaughnessy M V. Incidence of hepatitis C virus infection among injection drug users during an outbreak of HIV infection. CMAJ. 2001;165(7):889–895. http://www.cmaj.ca/cgi/pmidlookup?view=long&pmid=11599327. [PMC free article] [PubMed] [Google Scholar]
- 14.Hahn Judith A, Page-Shafer Kimberly, Lum Paula J, Bourgois Philippe, Stein Ellen, Evans Jennifer L, Busch Michael P, Tobler Leslie H, Phelps Bruce, Moss Andrew R. Hepatitis C virus seroconversion among young injection drug users: relationships and risks. J Infect Dis. 2002 Nov 04;186(11):1558–1564. doi: 10.1086/345554. [DOI] [PubMed] [Google Scholar]
- 15.Thorpe Lorna E, Ouellet Lawrence J, Hershow Ronald, Bailey Susan L, Williams Ian T, Williamson John, Monterroso Edgar R, Garfein Richard S. Risk of hepatitis C virus infection among young adult injection drug users who share injection equipment. Am J Epidemiol. 2002;155(7):645–653. doi: 10.1093/aje/155.7.645. http://aje.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=11914192. [DOI] [PubMed] [Google Scholar]
- 16.Novelli Laura A, Sherman Susan G, Havens Jennifer R, Strathdee Steffanie A, Sapun Marcella. Circumstances surrounding the first injection experience and their association with future syringe sharing behaviors in young urban injection drug users. Drug Alcohol Depend. 2005;77(3):303–309. doi: 10.1016/j.drugalcdep.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 17.Miller Cari L, Johnston Caitlin, Spittal Patricia M, Li Kathy, Laliberté Nancy, Montaner Julio S G, Schechter Martin T. Opportunities for prevention: hepatitis C prevalence and incidence in a cohort of young injection drug users. Hepatology. 2002;36(3):737–742. doi: 10.1053/jhep.2002.35065. [DOI] [PubMed] [Google Scholar]
- 18.Wood Evan, Tyndall Mark W, Spittal Patricia M, Li Kathy, Hogg Robert S, O'Shaughnessy Michael V, Schechter Martin T. Needle exchange and difficulty with needle access during an ongoing HIV epidemic. Int J Drug Policy. 2002;13:95–102. doi: 10.1016/S0955-3959(02)00008-7. [DOI] [Google Scholar]
- 19.Fournier Suzanne, Crey Ernie. Stolen from our embrace: the abduction of First Nations children and the restoration of Aboriginal communities. Vancouver: Douglas and McIntyre; 1997. [Google Scholar]
- 20.Adelson Naomi. The embodiment of inequity: health disparities in aboriginal Canada. Can J Public Health. 2005;96(Suppl 2):S45–S61. doi: 10.1007/BF03403702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aboriginal Healing Foundation. The healing has begun: an operational update from the Aboriginal Healing Foundation. Ottawa: The Foundation; 2002. http://www.ahf.ca/publications/residential-schools-resources. [Google Scholar]
- 22.Simoni Jane M, Sehgal Shalini, Walters Karina L. Triangle of risk: urban American Indian women's sexual trauma, injection drug use, and HIV sexual risk behaviors. AIDS Behav. 2004;8(1):33–45. doi: 10.1023/b:aibe.0000017524.40093.6b. [DOI] [PubMed] [Google Scholar]
- 23.Simons Ronald L, Whitbeck Les B. Sexual abuse as precursor to prostitution and victimization among adolescent and adult homeless adult women. J Fam Issues. 1991;12(3):361–379. [Google Scholar]
- 24.BC Aboriginal AIDS Task Force. The red road: pathways to wholeness. An Aboriginal strategy for HIV and AIDS in BC. Victoria (BC): Aboriginal Health Division, Ministry of Health; 1999. http://www.hls.gov.bc.ca/publications/year/1999/red-road.pdf. [Google Scholar]
- 25.Pearce Margo E, Christian Wayne M, Patterson Katharina, Norris Kat, Moniruzzaman Akm, Craib Kevin J P, Schechter Martin T, Spittal Patricia M Cedar Project Partnership. The Cedar Project: historical trauma, sexual abuse and HIV risk among young Aboriginal people who use injection and non-injection drugs in two Canadian cities. Soc Sci Med. 2008;66(11):2185–2194. doi: 10.1016/j.socscimed.2008.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evans Jennifer L, Hahn Judith A, Page-Shafer Kimberly, Lum Paula J, Stein Ellen S, Davidson Peter J, Moss Andrew R. Gender differences in sexual and injection risk behavior among young active injection drug users in San Francisco (the UFO Study. J Urban Health. 2003;80(1):137–146. doi: 10.1093/jurban/jtg137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fox Rena K, Currie Sue L, Evans Jennifer, Wright Teresa L, Tobler Leslie, Phelps Bruce, Busch Michael P, Page-Shafer Kimberly A. Hepatitis C virus infection among prisoners in the California state correctional system. Clin Infect Dis. 2005 Jun 09;41(2):177–186. doi: 10.1086/430913. [DOI] [PubMed] [Google Scholar]
- 28.Miller Cari L, Spittal Patricia M, LaLiberte Nancy, Li Kathy, Tyndall Mark W, O'Shaughnessy Michael V, Schechter Martin T. Females experiencing sexual and drug vulnerabilities are at elevated risk for HIV infection among youth who use injection drugs. J Acquir Immune Defic Syndr. 2002;30(3):335–341. doi: 10.1097/00126334-200207010-00010. [DOI] [PubMed] [Google Scholar]
- 29.Doherty M C, Garfein R S, Monterroso E, Brown D, Vlahov D. Correlates of HIV infection among young adult short-term injection drug users. AIDS. 2000;14(6):717–726. doi: 10.1097/00002030-200004140-00011. [DOI] [PubMed] [Google Scholar]
- 30.Fung K Y, Howe G R. Methodological issues in case-control studies. III--The effect of joint misclassification of risk factors and confounding factors upon estimation and power. Int J Epidemiol. 1984;13(3):366–370. doi: 10.1093/ije/13.3.366. [DOI] [PubMed] [Google Scholar]