Abstract
This report describes the C-to-B capsular switching in four Neisseria meningitidis strains belonging to the electrophoretic type 37 (ET-37) complex. In particular, one strain belonged to the new sequence type 1860, which was first detected in the year 2000 in Italy and is now frequently isolated. The presence of switched serogroup B strains deserves special attention if they prove as able to spread as their serogroup C progenitors belonging to the hypervirulent ET-37 complex.
In Neisseria meningitidis, the horizontal transfer of siaD genes encoding polysialyltransferases has been shown to result in capsular serogroup switching in vitro and in vivo (2, 4, 8, 15). Capsule switching may be an important virulence mechanism of meningococci and other encapsulated bacterial pathogens (7, 15), with significant implications for the use of specific polysaccharide vaccines. Serogroup B and C meningococci, isolated from areas where disease is endemic and from outbreaks in developed countries, belong mainly to the electrophoretic type 5 (ET-5) complex, ET-37 complex, and A4 cluster (11).
This report describes the molecular characteristics of four serogroup B strains responsible for invasive meningococcal disease in Italy. All four strains belonged to the ET-37 complex and showed porin antigens (PorA and PorB) 2a:P1.2, 2a:P1.5,2, 2a:P1.5, and 2b:P1.5, which are usually detected in serogroup C isolates but are quite uncommon in serogroup B strains in Italy. The B:2a:P1.2 strain was isolated in the year 1994, whereas the other three strains were detected during the year 2002. No other strains with these phenotypes have ever been detected in Italy among the more than 600 clinical strains typed within the National Surveillance Program of Bacterial Meningitis since 1994.
None of the four strains were from case-cluster or epidemiologically related patients. In Italy, a situation of low endemicity (0.4 case per 100,000 inhabitants) was characterized by the predominance of serogroup B and the presence of a large number of different phenotypes (12).
The four group B meningococci were analyzed by molecular techniques to provide evidence of a capsular switching from serogroup C to serogroup B.
In particular, multilocus sequence typing (MLST) (10), porA variable region (VR) typing (5, 13), and pulsed-field gel electrophoresis (PFGE) (6) were performed in parallel with a sample of 25 serogroup C strains from our reference laboratory collection (13 representatives of serotype C:2b and 12 representatives of C:2a, with the different serosubtypes P1.5, P1.5,2, and P1.2). All isolates were serogrouped and serosubtyped as previously described (12).
MLST.
All 12 strains examined with the C:2a phenotype belonged to the sequence type 11 (ST11)/ET-37 complex, while the 13 C:2b isolates belonged to two different STs, the ST8/A4 cluster and the ST1860/ET-37 complex.
The first C:2b:P1.5/ST1860 strains were isolated in Italy during the year 2000 after a cluster of three cases among young adults, one of whom came from Argentina. These isolates are single-locus mutants of ST11 (gdh), whereas they differ for as many as five loci (aroE, fumC, gdh, pdhC, and pgm) when compared to the ST8 strains found among the majority of the C:2b meningococci isolated before the year 2000 in Italy. This new ST isolated among the Italian C:2b strains has been deposited in the Neisseria MLST database (http://neisseria.org/nm/typing/mlst/).
The three B:2a meningococci examined in this study belong to ST11, whereas the B:2b strain, isolated in the year 2002, belongs to ST1860.
PorA VR typing.
For PorA typing, only two regions of the porA gene, which include VR1, VR2, and VR3, were sequenced by following the procedures already described (13). To assign VR sequences to families and variants, the deduced amino acid sequences of VR1 and VR2 were submitted to the N. meningitidis PorA variable region database (http://neisseria.org/nm/typing/pora/).
All four serogroup B strains showed the same combination of variants as the serogroup C strain examined, in particular, variants 5, 2, and 36b for VR1, VR2, and VR3, respectively.
PFGE.
The infrequent-cutting restriction enzyme NheI (6) was used in PFGE to demonstrate the direct clonal origin of these four serogroup B strains from the serogroup C meningococci examined as controls.
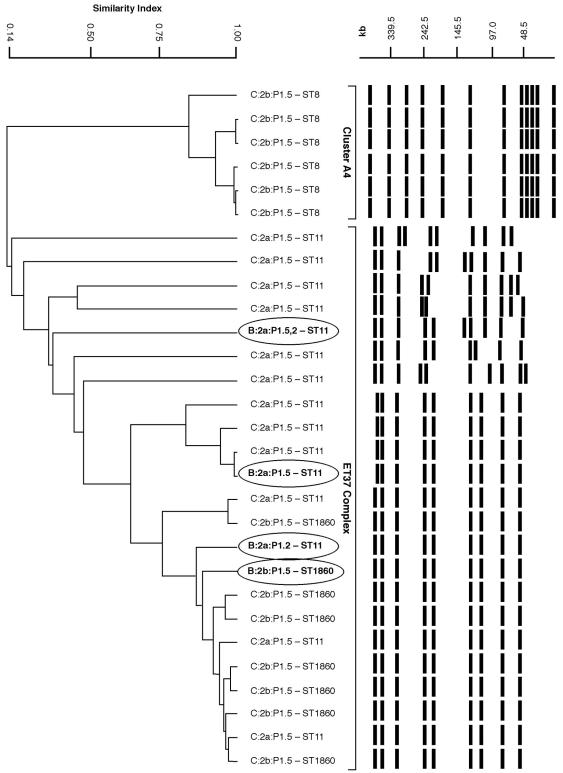
The fingerprints, together with the dendrogram of similarity (Fig. 1), showed that the four serogroup B strains clustered with the serogroup C strains belonging to the ET-37 complex and were either closely related (differing by one to three bands) or indistinguishable from strain patterns of the A4 cluster. Serotype 2a/ET-37 strains belonged to a single clone, while the 2b strains were divided into two PFGE clones corresponding to the two different STs (ST8 and ST1860). The fingerprints of strains belonging to ST1860, either C:2b or B:2b, were closely related to those of ST11/ET-37, thus emphasizing their genetic similarity.
FIG. 1.
PFGE patterns of 29 N. meningitidis strains. On the left is a dendrogram of similarity from cluster analysis generated by unweighted pair group method analysis, obtained by using Diversity Database software version 2 (Bio-Rad). On the right are PFGE-digitized profiles for each phenotype and corresponding ST. The four strains exhibiting capsule switch are indicated in boldface type and circled.
IS1655.
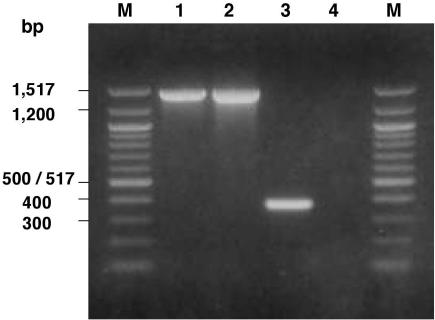
In order to acquire additional information on the relatedness between the ST1860/ET-37 and ST11/ET-37 strains, they were further analyzed for the presence of the insertion sequence (IS) 1655 in the orf2 gene. As recently demonstrated by Claus et al. (3), this IS is present only in ET-37 strains and may differentiate them from those belonging to the A4 cluster. In fact, Fig. 2 shows the presence of an amplicon of the expected size (about 1,460 bp) only between the ST11/ET-37 (lane 1) and ST1860/ET-37 (lane 2) strains.
FIG. 2.
PCR results for the presence of IS1655 in the orf2 gene of the N. meningitidis strains examined. Lane M, molecular size markers, 100-bp DNA ladder (New England Biolabs). Lanes 1 and 2 show the presence of the amplicon of about 1,460 bp in one serogroup C strain representative of the ST11/ET-37 complex and in the B:2b:P1.5 switched ST1860/ET-37 strain, respectively. Lane 3 shows a shorter amplicon of about 390 bp in a strain representative of ST8/A4, which lacks the IS1655. Lane 4 shows the absence of the amplicon in a strain representative of ST44/lineage III.
The molecular techniques described in this report confirm the capsule replacement in the four serogroup B meningococci. In particular, switching occurred in three B:2a:P1.5,2 strains, belonging to the ST11/ET-37 complex already described by others (9, 14) as able to switch the capsule, and in one B:2b:P1.5 strain with ST1860, detected for the first time in this study. Alcalá et al. (1) reported that C:2b:P1.5 meningococci isolated in Spain from 1992 to 1999 belong to the ST11/ET-37 complex. In Italy, C:2b:P1.5 meningococci belonging to the ET-37 complex appeared only recently, since the first strain was isolated in the year 2000 and exhibited the new ST1860. All the C:2b:P1.5 strains isolated in previous years belonged instead to the ST8/A4 cluster.
MLST analysis of all C:2b:P1.5 strains isolated between 2000 and 2002 showed that ST1860/ET-37 accounted for about 50% of the total C:2b strains isolated in the country (data not shown). Therefore, it seems that this new ST is gradually displacing the ST8/A4 cluster and that it is responsible for invasive disease mainly in teenagers and young adults. The detection of the switched strains belonging to the ST1860/ET-37 complex deserves special attention to verify if this B variant is also as able to spread as its serogroup C progenitor.
Capsule switching has already been reported in Canada and in Spain after mass vaccination campaigns (2, 8, 14) and in the Czech Republic during an epidemic situation (9). Since in Italy the incidence of invasive meningococcal disease is low and no mass vaccination has been introduced, the presence of capsule-switched strains may be more easily attributable to a selection for competition between meningococcal genotypes in the nasopharynx than to vaccine-induced protective immunity.
However, the emergence of a natural escape mutant for the capsule, within the ET-37 complex known to harbor hypervirulent strains, should be carefully monitored in the future for its impact on the pattern of disease in Italy and elsewhere.
Acknowledgments
We thank the microbiologists of hospital laboratories participating in the Italian National Surveillance of Bacterial Meningitis for isolating the strains and sending them to the reference laboratory of the Istituto Superiore di Sanitá (Rome).
This work was partially funded by Ministero della Salute (Rome), Programma per la Ricerca Finalizzata, 2002. This study made use of the Neisseria Multilocus Sequence Typing website (http://neisseria.mlst.net) developed by Man-Suen Chan and maintained at the University of Oxford. The development of this site is funded by the Wellcome Trust.
REFERENCES
- 1.Alcalá, B., C. Salcedo, L. Arreaza, S. Barron, L. de la Fuente, and J. A. Vazquez. 2002. The epidemic wave of meningococcal disease in Spain in 1996-1997: probably a consequence of strain displacement. J. Med. Microbiol. 51:1102-1106. [DOI] [PubMed] [Google Scholar]
- 2.Alcalá, B., L. Arreaza, C. Salcedo, M. J. Uria, L. de la Fuente, and J. A. Vazquez. 2002. Capsule switching among C:2b:P1.2,5 meningococcal epidemic strains after mass immunization campaign, Spain. Emerg. Infect. Dis. 8:1512-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Claus, H., J. Stoevesandt, M. Frosch, and U. Vogel. 2001. Genetic isolation of meningococci of the electrophoretic type 37 complex. J. Bacteriol. 183:2570-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frosch, M., and T. E. Meyer. 1992. Transformation-mediated exchange of virulence determinants by co-cultivation of pathogenic Neisseriae. FEMS Microbiol. Lett. 79:345-349. [DOI] [PubMed] [Google Scholar]
- 5.Gorla, M. C. O., A. P. S. Lemos, C. T. Sacchi, J. C. de Moraes, and L. G. Milagres. 2003. Comparison of PorA VR types and porA promoter sequence from Neisseria meningitidis B isolated from non-immunised children and vaccine failures immunised with a serogroup B outer membrane protein vaccine. Vaccine 21:2871-2876. [DOI] [PubMed] [Google Scholar]
- 6.Hartstein, A., C. Phelps, and A. Lemonte. 1995. Typing of sequential bacterial isolates by pulsed-field gel electrophoresis. Diagn. Microbiol. Infect. Dis. 22:309-314. [DOI] [PubMed] [Google Scholar]
- 7.Kelly, T., J. P. Dillard, and J. Yother. 1994. Effect of genetic switching of capsular type on virulence of Streptococcus pneumoniae. Infect. Immun. 62:1813-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kertesz, D. A., M. B. Coulthart, J. A. Ryan, W. M. Johnson, and F. E. Ashton. 1998. Serogroup B, electrophoretic type 15 Neisseria meningitidis in Canada. J. Infect. Dis. 177:1754-1757. [DOI] [PubMed] [Google Scholar]
- 9.Kriz, P., D. Giorgini, M. Musilek, M. Larribe, and M. K. Taha. 1999. Microevolution through DNA exchange among strains of Neisseria meningitidis during an outbreak in the Czech Republic. Res. Microbiol. 150:273-280. [DOI] [PubMed] [Google Scholar]
- 10.Maiden, M. C. J., J. A. Bygraves, E. Feil, G. Morelli, J. E. Russell, R. Urwin, Q. Zhang, J. Zhou, K. Zurth, D. A. Caugant, I. M. Feavers, M. Achtman, and B. G. Spratt. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. USA 95:3140-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maiden, M. C. J. 2002. Population structure of Neisseria meningitidis, p. 150-169. In C. Ferreiros, M. T. Criado, and J. Vázquez (ed.), Emerging strategies in the fight against meningitis: molecular and cellular aspects. Horizon Scientific Press, Wymondham, United Kingdom.
- 12.Mastrantonio, P., P. Stefanelli, C. Fazio, T. Sofia, A. Neri, G. La Rosa, C. Marianelli, M. Muscillo, M. G. Caporali, and S. Salmaso. 2003. Serotype distribution, antibiotic susceptibility, and genetic relatedness of Neisseria meningitidis strains recently isolated in Italy. Clin. Infect. Dis. 36:422-428. [DOI] [PubMed] [Google Scholar]
- 13.Mölling, P., M. Unemo, A. Bäckman, and P. Olcén. 2000. Genosubtyping by sequencing group A, B and C meningococci; a tool for epidemiological studies of epidemics, clusters and sporadic cases. APMIS 108:509-516. [PubMed] [Google Scholar]
- 14.Pérez-Trallero, E., D. Vicente, M. Montes, and R. Cisterna. 2002. Positive effect of meningococcal C vaccination on serogroup replacement in Neisseria meningitidis. Lancet 360:953. [DOI] [PubMed] [Google Scholar]
- 15.Swartley, J. S., A. A. Marfin, S. Edupuganti, L. J. Liu, P. Cieslak, B. Perkins, J. D. Wenger, and D. S. Stephens. 1997. Capsule switching of Neisseria meningitidis. Proc. Natl. Acad. Sci. USA 94:271-276. [DOI] [PMC free article] [PubMed] [Google Scholar]