Abstract
Obesity increases cardiovascular morbidity and mortality in part by inducing hypertension. One factor linking excess fat mass to cardiovascular diseases may be the sympathetic cardiovascular actions of leptin. Initial studies of leptin showed it regulates appetite and enhances energy expenditure by activating sympathetic nerve activity (SNA) to thermogenic brown adipose tissue. Further study, however, demonstrated leptin also causes sympathetic excitation to the kidney that, in turn, increases arterial pressure. In animal studies, elevating circulating leptin levels increased arterial pressure. Moreover, mice with diet-induced obesity have a preserved arterial pressure response to leptin despite the resistance to the metabolic action of leptin and these mice have elevated baseline arterial pressure. Conversely, severely obese, but leptin-deficient, mice and humans display low sympathetic tone and decreased blood pressure. Together, these findings demonstrate that leptin plays a physiological role in maintaining sympathetic tone and blood pressure, and further suggest that hyperleptinemia may contribute to the elevated blood pressure associated with obesity. Consistent with this selectivity in leptin resistance, mounting evidence suggests that the sympathetic nervous system subserving different tissues is differentially controlled by leptin. For instance, different molecular signaling mechanisms are engaged by the leptin receptor to control the regional sympathetic nerve activity. Understanding the mechanisms by which leptin controls the sympathetic nervous system will provide insight into the cardiovascular complications of obesity.
Keywords: Leptin, sympathetic nerve activity, obesity, hypertension
INTRODUCTION
Obesity is a serious health problem, particularly in the United States. Clinical and animal studies have confirmed a strong relationship between obesity and hypertension [1,2]. The sympathetic nervous system (SNS) has been implicated in the pathophysiology of obesity-induced hypertension [3].
The discovery of leptin in 1994 has allowed a tremendous advance in the neurobiology of energy homeostasis and obesity. Leptin is an adipocyte-derived hormone that circulates in proportion to body fat [4]. This hormone is considered as a critical signal that feeds back to inform the central nervous system about the status of peripheral energy reserves [5–7]. In addition to its effects on food intake and energy expenditure, leptin action has been shown to influence several other functions including the neuroendocrine and reproductive functions, insulin secretion, sympathetic nervous system and blood pressure [8].
REGIONAL SYMPATHETIC NERVE ACTIVATION TO LEPTIN
The SNS is an important regulatory mechanism of both metabolic and cardiovascular functions [9]. Consistent with its role in the regulation of energy expenditure, leptin was found to increase norepinephrine turnover in thermogenic brown adipose tissue (BAT) [10]. Using multifiber recording of regional SNA, intravenous administration of leptin in Sprague-Dawley rats caused a significant and dose-dependent increase in SNA to BAT [11]. Unexpectedly, leptin caused sympathoactivation to other beds not usually considered thermogenic, such as the kidney, hindlimb, and adrenal gland [11]. Satoh et al. [12] investigated the effect of leptin on circulating catecholamines and found that leptin administration caused a significant and dose-dependent increase in plasma concentration of norepinephrine and epinephrine.
Regional sympathetic nerve activation to leptin respond non-uniformly to baroreflex activation and hypothermia [13,14]. Leptin-induced increases in renal SNA can be suppressed by baroreflex activation, suggesting that the increase in renal SNA subserves circulatory functions. In contrast, leptin-induced BAT sympathoactivation was not prevented by baroreflex activation, suggesting the recruitment of sympathetic fibers to BAT serve thermogenic or metabolic, rather than circulatory functions [13]. The effect of leptin on regional SNA response to hypothermia differs also between sympathetic fibers that serve circulatory or thermogenic functions. Leptin, at a low dose that does not alter baseline SNA, acutely enhances sympathetic outflow to BAT in response to hypothermia in lean rats. This effect is specific for thermogenic SNA because leptin does not affect the response of renal SNA to hypothermia [14]. The differential effect of leptin on the responses of renal and BAT SNA to the different stimuli indicate that leptin controls SNS in a tissue-specific manner, likely through different signaling pathways in the central nervous system.
BLOOD PRESSURE EFFECTS OF LEPTIN
Because of the key role of the SNS in controlling renal function, a determinant of blood pressure [15], renal sympathetic activation in response to leptin was expected to elevate blood pressure. In an acute study Dunbar et al. [16] showed that leptin causes a slow but progressive increase in arterial pressure. Leptin infusion for 12 days increased arterial pressure and heart rate, despite a decrease in body weight that would be expected to decrease arterial pressure [17]. Leptin-induced increases in arterial pressure are probably caused by the hormone’s central action because intracerebroventricular (ICV) administration of leptin mimics the effects of systemic administration [18]. The substantial dose-dependent increase in heart rate and the greater response to air-jet stress observed in leptin-treated rats, give support for centrally-mediated activation of the SNS [18]. Moreover, blocking the adrenergic system inhibits the pressor response to leptin [19].
Further evidence for the pressor effects of leptin derives from studies of transgenic mice overexpressing leptin in the liver [20]. These mice had 10-fold increases in plasma leptin and decreased body weight. Despite the decreased body weight which would be expected to lower arterial pressure, the transgenic mice overexpressing leptin had significantly higher arterial pressure than non-transgenic littermates. The transgenic mice also had increased urinary excretion of norepinephrine. The increase in arterial pressure was normalized after alpha-adrenergic or ganglionic blockade, again demonstrating the importance of the SNS in the pressor effects of leptin.
More insight into the role of leptin in arterial pressure control, were obtained by studying the obese, leptin-deficient ob/ob mice [21]. Despite body weights nearly twice as high as their lean controls, the leptin-deficient ob/ob mice had lower arterial pressure. Aizawa-Abe et al. [20] subsequently reported that administration (i.e. restoration) of leptin to ob/ob mice increased systolic blood pressure by as much as 25 mmHg, despite decreases in food intake and body weight. Leptin deficiency in human is also associated with hypotension and an absence of risk factors for cardiovascular diseases despite the severe obesity [22]. In contrast to leptin-deficient ob/ob mice, the hyperleptinemic agouti yellow obese mice have elevated arterial pressure [21]. The elevated arterial pressure in the agouti mice is dependent on the elevated levels of leptin [20]. Altogether, these findings demonstrate that leptin contributes physiologically to the regulation of arterial pressure with pathophysiological implications for obesity-associated hypertension.
SELECTIVE LEPTIN RESISTANCE IN OBESITY
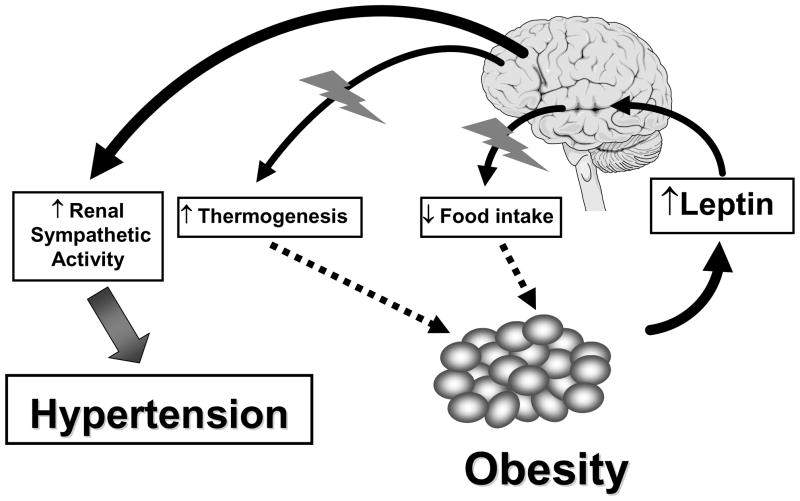
Common human obesity is associated with circulating hyperleptinemia, suggesting that these subjects have resistance to the anorectic and weight-reducing effects of leptin [23]. Under these circumstances, in order for leptin to have a role in obesity-related hypertension, leptin resistance has to be selective, with preservation of renal/cardiovascular sympathetic responses to leptin. We have demonstrated that in two murine models of obesity, leptin resistance is indeed selective, with preservation of the effects of leptin on renal SNA (Figure 1). The anorexic and weight-reducing effects of leptin were attenuated in agouti obese mice compared to lean littermates. However, the increase in renal SNA in response to leptin was identical in both lean and obese mice [24,25].
Figure 1.
The concept of selective leptin resistance holds that there is resistance to the metabolic (appetite- and weight-reducing) actions of leptin, but preservation of the cardiovascular sympatho-excitatory actions of leptin. This phenomenon might explain how hyperleptinemia could be accompanied by increased adiposity leading to obesity, but still contribute to sympathetic overactivity and hypertension because of preservation of the sympathetic actions of leptin to some organs involved in the blood pressure regulation such as the kidney.
This phenomenon of selective leptin resistance is also observed in an acquired model of obesity, namely mice with diet-induced obesity (DIO) [26]. We found that DIO mice also have preserved renal sympathetic activation to leptin despite the resistance to the anorectic and weight-reducing effect of leptin. Of note, preservation of the regional SNA responses to leptin is not uniform, but is instead specific to the kidney. Indeed, while renal sympathetic activation to leptin was preserved, the BAT SNA response to leptin was significantly attenuated in DIO mice [26]. These data support the concept that leptin regulates regional sympathetic outflow to peripheral tissues in a highly differential way.
In DIO mice that had been fed a high-fat diet, arterial pressure also responded to leptin, as chronic leptin treatment significantly increased arterial pressure [26]. Leptin caused comparable increases in arterial pressure in both the DIO (~10 mmHg) and lean mice (~11 mm Hg). These novel findings demonstrate a preserved renal SNA response to leptin does, in fact, translate into a preserved arterial pressure response; this enhances the potential pathophysiologic significance of the selectivity observed in the leptin resistance phenomenon.
This concept of selective leptin resistance may represent an important pathophysiologic mechanism that explains leptin’s role in obesity-associated hypertension (Figure 1). Accumulating data suggest that in obese humans, leptin has a pathological role in causing hypertension and sympathetic overactivity. Overactivity of the SNS is a common feature of obesity in humans. Indeed, study of regional sympathetic activity in obese humans using norepinephrine spillover has demonstrated that obesity is associated with increased SNA to the kidney [27]. In addition, there is a positive correlation between plasma leptin levels and blood pressure. The existence of a strong correlation between leptin plasma concentration and renal SNA across a broad range of leptin values in men of widely differing adiposity [28,29] indicate that leptin may be the main cause of the hypertension and sympathetic activation that is associated with obesity, not only in animal models, but also in humans.
ROLE OF THE ARCAUTE NUCLEUS IN THE SYMPATHETIC AND CARDIOVASCULAR RESPONSES TO LEPTIN
Leptin decreases appetite and increases energy expenditure by activating leptin receptors on specific neurons in the brain. These neurons lie in regions of the brain that are known to control energy homeostasis and autonomic function [5]. The leptin receptor is enriched in the arcuate nucleus of the hypothalamus [6]. Consistent with this, lesioning the arcuate nucleus virtually abolished the increase in SNA to kidney during intravenous administration of leptin [30]. To test further the role of this arcuate nucleus in the sympathetic and cardiovascular effects of leptin, we assessed the sympathetic and arterial pressure responses to intra-arcuate injection of leptin in rats [31]. Microinjecting leptin (500 ng) into the arcuate nucleus induced sympathetic activation to the kidney and increased arterial pressure. These responses were similar to those evoked by ICV leptin [31]. These results indicate that the sympathetic and cardiovascular effects of leptin might be evoked by the action of this hormone in the arcuate nucleus of the hypothalamus.
LEPTIN RECEPTOR SIGNALING
Alternative splicing of a single transcript encoded by the leptin receptor gene produces six different isoforms of the leptin receptor. While the extracellular domain is conserved across all the isoforms of the receptor, the length of the intracellular domains varies. The ObRb form with a long 302-residue intracellular domain appears to mediate most of the biological effects of leptin [32]. The db/db mouse lacks the ObRb form of the receptor, resulting from a premature stop codon at the 3′-end of the leptin receptor transcript [33]. In contrast, the obese Zucker rat has defects in all forms of the leptin receptor due to an amino acid substitution (glutamine 269 proline) in the extracellular domain [34,35]. Of note, neuron-specific expression of ObRb completely rescued the obesity in db/db mice [36]. On the other hand, neuronal deletion of ObRb leads to obesity in mice [37,38]. These data provides direct evidence that leptin exerts its effect via interaction with neuronal ObRb in the central nervous system.
Evidence for the role of the leptin receptor in the SNA responses to leptin derives from the observation that the leptin receptor-deficient obese Zucker rats have an absent sympathetic response to leptin [11]. However, it was not clear which form of the leptin receptor was involved because this mutation in leptin receptor observed in the obese Zucker rat results in glutamine 269-to-proline 269 amino acid substitution in the extracellular domain common to all known isoforms of the leptin receptor.
To test whether the ObRb is mediating the sympathetic nerve response to leptin, we compared the renal SNA response to leptin between control and db/db mice [39]. In control mice, ICV administration of graded doses of leptin caused a significant and dose-dependent increase in renal SNA. In contrast, the db/db mice exhibited markedly blunted renal SNA response to ICV administration of leptin. Our data demonstrate that leptin-induced sympathetic activation to the kidney is mediated by the ObRb, the long form of the leptin receptor.
There are three primary intracellular signaling pathways that emanate from ObRb. Each pathway is activated by phosphorylation of specific tyrosine residues in ObRb: 1) from Tyr985 of ObRb which signals mitogen activated protein (MAP) kinase; and 2) from Tyr1138 of ObRb which signals STAT3; and 3) from Jak2 tyrosine phosphorylation sites. In addition, Jak2 tyrosine phosphorylation during ObRb stimulation mediates some signals independently of tyrosine phosphorylation sites on ObRb (e.g. a portion of MAP kinase activation, insulin receptor substrate (IRS) phosphorylation that activate PI3 kinase) [40].
Phosphorylation of Tyr1138 recruits STAT3 to the ObRb/Jak2 complex, resulting in tyrosine phosphorylation and subsequent nuclear translocation of STAT3 to mediate transcriptional regulation. STAT3 mediates the transcription of the socs3 gene (which encodes the SH2 domain-containing feedback inhibitor, suppressor of cytokine signaling, SOCS-3), as well as other genes. SOCS3 binds to Tyr985 of ObRb to mediate inhibition of ObRb-STAT3 signaling.
The STAT pathway was the first signaling mechanism associated with the leptin receptor [41]. While in vitro studies have shown that leptin receptors signal through different STAT molecules, in vivo studies have demonstrated that, in the hypothalamus, this occurs mainly through activation of STAT3. Myers and colleagues have performed experiments demonstrating that this pathway is essential for leptin regulation of energy homeostasis but not for the control of reproductive function, growth, or glucose homeostasis [42]. To investigate the contribution of STAT3 signaling to leptin action in vivo, these investigators replaced the gene encoding the leptin receptor in mice with an allele coding for a replacement of Tyr1138 in ObRb with a serine residue (ObRb(S1138)) that specifically disrupts ObRb-STAT3 signaling but preserves other leptin mechanisms. Specific disruption of the ObRb-STAT3 pathway in mice results in obesity and hyperphagia attributable to the impaired effect of leptin on the melanocortin system. However, these mice remain fertile and less diabetic than the db/db mice, perhaps because of the preserved action of leptin on neuropeptide Y–containing neurons [42]. These data demonstrate that activation of STAT3 involves selective leptin action.
The phosphorylation of Tyr985 creates a binding site for the COOH-terminal SH2 domain of the tyrosine phosphatase, Shp2. Recruitment of SH2 domain results in its tyrosine phosphorylation and recruitment of GRB2, the first step in the canonical p21ras-MAP kinase signaling pathway that involves MAP kinase activation. Whereas Tyr985 mediates most MAP kinase stimulation during ObRb signaling, a small amount of MAP kinase activity occurs independently of ObRb phosphorylation, presumably via tyrosine phosphorylation sites on Jak2 [40,43].
Leptin and PI3 kinase signaling
Although several forms of PI3 kinases exist in higher organisms, the class Ia enzyme is thought to be the most relevant for the regulatory events in cell physiology and pathophysiology [44]. This class consists of a catalytic subunit (p100α, p110β or p110δ) which form a dimeric complex with the p85 regulatory subunit. Activated PI3 kinase converts phosphatidylinositol-4,5-phosphate (PIP2) to phosphatidylinositol-3,4,5-phosphate (PIP3) which modulates several downstream pathways including Akt. Accumulation of PIP3 is antagonized by the lipid phosphatase Pten (phosphatase and tensin homologue deleted on chromosome 10) which dephosphorylate the 3-position of the inositol ring of PIP3.
By engaging Jak2, the leptin receptor is able to stimulate IRSs which, in turn, activate PI3 kinase through an association to its regulatory subunit [45]. PI3 kinase is a crucial signaling pathway for the control of appetite by leptin. Indeed, the effect of leptin on food intake is reversed by blockade of this enzyme. This pathway appears to be involved in the modulation of neuronal firing rate [45] and gene transcription [47]. Schwartz and colleagues demonstrated that pharmacological inhibition of PI3 kinase reversed the decrease in npy and agrp genes expression induced by leptin [48].
To study the contribution of PI3 kinase signaling to leptin action, we recently developed a mouse model with enhanced PIP3 levels specifically in cells which express ObRb [49]. Overactivation of the PI3 kinase pathway was mimicked by deletion of the PIP3 phosphatase Pten (PtenΔObRbmice). To determine the functional effect of ObRb-specific Pten deficiency on activation of the PI3 kinase pathway in the hypothalamus, leptin’s ability to activate Akt (phospho-Akt) in hypothalamic neurons was compared between PtenΔObRb and control mice. Leptin activation of Akt in the hypothalamus of PtenΔObRb mice was increased when compared to controls. In contrast, basal and leptin-stimulated phospho-STAT3 formation in the hypothalamus was unchanged in PtenΔObRb mice. To assess the impact of activated PIP3-dependent signaling in leptin-responsive hypothalamic neurons on energy homeostasis, we measured body weight of control and PtenΔObRb mice. PtenΔObRb mice have lower body weight as compared to their control littermates. To investigate whether overactivated PI3 kinase signaling in ObRb-expressing neurons and subsequent leanness in PtenΔObRb mice depend on leptin, we next analyzed the impact of ObRb-specific Pten ablation in the leptin-deficient ob/ob (ob/ob-PtenΔObRb) mice [49]. Of interest, obesity of ob/ob-PtenΔObRb mice was unaltered as compared to ob/ob mice indicating that the changes we observe in PtenΔObRb mice are leptin-dependent [49]. Altogether, these data indicate that ObRb-specific inactivation of the Pten gene selectively enhances activation of the PI3 kinase pathway in ObRb-expressing neurons of PtenΔObRb mice.
Using pharmacologic blockade, we also obtained evidence that PI3 kinase mediates the renal SNA response to leptin [50]. In mice, we compared renal SNA response to leptin before and after ICV administration of PI3 kinase inhibitors. Both LY294002 and wortmannin markedly attenuated the increase in renal SNA induced by leptin. These inhibitors did not affect the sympathoactivation to stimulation of the melanocortin system suggesting that their inhibitory effect on the responses to leptin was a specific effect [50]. In a subsequent study in rat, we found that the role of PI3 kinase in leptin-induced sympathoactivation was specific to the kidney as pretreatment with LY294002 prevented the effect of leptin on renal SNA without altering the SNA responses to other beds including BAT, hindlimb, and adrenal gland [51]. Finally, we demonstrated the critical role of PI3 kinase in mediating the renal sympathetic nerve activation to leptin in obesity [52]. Indeed, we found that pre-treatment with PI3 kinase inhibitor, LY294002, blocked the renal SNA response to leptin in DIO as well as agouti obese mice.
Leptin and MAP kinase signaling
The leptin receptor activates MAP kinase [41,53]. As mentioned above, this occurs through different mechanisms, including Shp2 and direct phosphorylation by Jak2. Recently, we obtained evidence implicating the p42/44 isoform of MAP kinase as a signaling pathway of the hypothalamic leptin receptor [51]. We found that ICV administration of leptin (10 μg) activated the p42/44, but not the p38, isoform of MAP kinase in the mediobasal hypothalamus [51].
We also obtained evidence that this enzyme is crucial for the catabolic effect of leptin as blockade of hypothalamic p42/44 MAP kinase with specific pharmacologic inhibitors (PD98059 and U0126) reversed the weight-reducing effects of leptin [51]. Of interest, inhibitors of p42/44 MAP kinase such as PD98059 also prevented the effects of leptin on SNA to thermogenic BAT, but not to the kidney, hindlimb or adrenal gland. MAP kinase is, therefore, not involved in the control of renal SNA by leptin. The inability of MAP kinase inhibition to block the renal sympathetic response to leptin demonstrates that leptin effects on renal SNA are exclusively mediated by PI3 kinase.
MELANOCORTIN 4 RECEPTORS ARE KEY DOWNSTREAM MEDIATORS OF SYMPATHETIC ACTION OF LEPTIN
To investigate the role of the melanocortin-4 receptor (MC-4R), a downstream pathway in leptin signaling, in the renal sympathetic response to leptin, we compared the effect of leptin on renal SNA between the wild type mice and obese MC-4R knockout mice [39]. ICV administration of leptin increased renal SNA in the wild type mice, but leptin had no significant effect on renal SNA in the homozygous MC-4R knockout mice. Heterozygous MC-4R knockout mice had an intermediate renal SNA response to leptin.
Baseline arterial pressure did not differ between the obese MC-4R knockout mice and their wild type controls when measured in an anesthetized state [39]. In line with our data, John Hall’s group used radiotelemetry to shown normal arterial pressure in the obese MC-4R knockout mice [54]. In a subsequent study, this team has shown that long-term administration of leptin caused no significant increase in arterial pressure in the MC-4R knockout mice [55]. These data demonstrate that leptin relies on the renal SNA to increase arterial pressure because lack of renal SNA response to leptin is associated with the absence of hypertension despite obesity and loss of arterial pressure response to leptin. Consistent with the rodent data, a recent study demonstrated that human subjects that carry a mutation in the MC-4R are protected against the autonomic dysfunction and hypertension that are commonly associated with obesity [56].
CONCLUSIONS
In the recent years, significant advance in understanding the pathophysiological role of leptin in obesity-induced cardiovascular diseases have been made. Insights into the sympathetic and cardiovascular actions of leptin and the recognition of selective leptin resistance have contributed greatly to our understanding of the cardiovascular and metabolic complications of obesity and the metabolic syndrome.
The divergent signaling capacities of the leptin receptor allow leptin to control separate physiological processes. Although STAT3 has emerged as a primary intracellular signaling pathway of the leptin receptor, there is growing recognition that other intracellular pathways associated with the leptin receptor are important for the regulation of physiological functions by leptin. Understanding the molecular mechanisms by which leptin controls the sympathetic nervous system involved in the regulation of the cardiovascular function will offer the tantalizing possibility that it may be possible to selectively interfere with potentially deleterious renal and cardiovascular sympathetic action of leptin.
References
- 1.Montani JP, Antic V, Yang Z, Dulloo A. Pathways from obesity to hypertension: from the perspective of a vicious triangle. Int J Obes Relat Metab Disord. 2002;26 (Suppl 2):S28–S38. doi: 10.1038/sj.ijo.0802125. [DOI] [PubMed] [Google Scholar]
- 2.Rahmouni K, Correia ML, Haynes WG, Mark AL. Obesity-associated hypertension: new insights into mechanisms. Hypertension. 2005;45:9–14. doi: 10.1161/01.HYP.0000151325.83008.b4. [DOI] [PubMed] [Google Scholar]
- 3.Esler M, Straznicky N, Eikelis N, Masuo K, Lambert G, Lambert E. Mechanisms of sympathetic activation in obesity-related hypertension. Hypertension. 2006;48:787–96. doi: 10.1161/01.HYP.0000242642.42177.49. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 5.Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22:221–32. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–71. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 7.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–95. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 8.Flier JS. Obesity wars: molecular progress confronts an expanding epidemic. Cell. 2004;116:337–350. doi: 10.1016/s0092-8674(03)01081-x. [DOI] [PubMed] [Google Scholar]
- 9.Dulloo AG. A sympathetic defense against obesity. Science. 2002;297:780–1. doi: 10.1126/science.1074923. [DOI] [PubMed] [Google Scholar]
- 10.Collins S, Kuhn CM, Petro AE, Swick AG, Chrunyk BA, Surwit RS. Role of leptin in fat regulation. Nature. 1996;380:677. doi: 10.1038/380677a0. [DOI] [PubMed] [Google Scholar]
- 11.Haynes WG, Morgan DA, Walsh SA, Mark AL, Sivitz WI. Receptor-mediated regional sympathetic nerve activation by leptin. J Clin Invest. 1997;100:270–78. doi: 10.1172/JCI119532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Satoh N, Ogawa Y, Katsuura G, et al. Sympathetic activation of leptin via the ventromedial hypothalamus: leptin-induced increase in catecholamine secretion. Diabetes. 1999;48:1787–93. doi: 10.2337/diabetes.48.9.1787. [DOI] [PubMed] [Google Scholar]
- 13.Hausberg M, Morgan DA, Chapleau MA, Sivitz WI, Mark AL, Haynes WG. Differential modulation of leptin-induced sympathoexcitation by baroreflex activation. J Hypertens. 2002;20:1633–41. doi: 10.1097/00004872-200208000-00027. [DOI] [PubMed] [Google Scholar]
- 14.Hausberg M, Morgan DA, Mitchell JL, Sivitz WI, Mark AL, Haynes WG. Leptin potentiates thermogenic sympathetic responses to hypothermia: a receptor-mediated effect. Diabetes. 2002;51:2434–40. doi: 10.2337/diabetes.51.8.2434. [DOI] [PubMed] [Google Scholar]
- 15.DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev. 1997;77:75–197. doi: 10.1152/physrev.1997.77.1.75. [DOI] [PubMed] [Google Scholar]
- 16.Dunbar JC, Hu Y, Lu H. Intracerebroventricular leptin increases lumbar and renal sympathetic nerve activity and blood pressure in normal rats. Diabetes. 1997;46:2040–3. doi: 10.2337/diab.46.12.2040. [DOI] [PubMed] [Google Scholar]
- 17.Shek EW, Brands MW, Hall JE. Chronic leptin infusion increases arterial pressure. Hypertension. 1998;31:409–14. doi: 10.1161/01.hyp.31.1.409. [DOI] [PubMed] [Google Scholar]
- 18.Correia ML, Morgan DA, Sivitz WI, Mark AL, Haynes WG. Leptin acts in the central nervous system to produce dose-dependent changes in arterial pressure. Hypertension. 2001;37:936–42. doi: 10.1161/01.hyp.37.3.936. [DOI] [PubMed] [Google Scholar]
- 19.Carlyle M, Jones OB, Kuo JJ, Hall JE. Chronic cardiovascular and renal actions of leptin: role of adrenergic activity. Hypertension. 2002;39:496–501. doi: 10.1161/hy0202.104398. [DOI] [PubMed] [Google Scholar]
- 20.Aizawa-Abe M, Ogawa Y, Masuzaki H, et al. Pathophysiological role of leptin in obesity-related hypertension. J Clin Invest. 2000;105:1243–52. doi: 10.1172/JCI8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mark AL, Shaffer RA, Correia ML, Morgan DA, Sigmund CD, Haynes WG. Contrasting blood pressure effects of obesity in leptin-deficient ob/ob mice and agouti yellow obese mice. J Hypertens. 1999;17:1949–53. doi: 10.1097/00004872-199917121-00026. [DOI] [PubMed] [Google Scholar]
- 22.Ozata M, Ozdemir IC, Licinio J. Human leptin deficiency caused by a missense mutation: Multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab. 1999;84:3686–95. doi: 10.1210/jcem.84.10.5999. [DOI] [PubMed] [Google Scholar]
- 23.Considine RV, Sinha MK, Heiman ML, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–5. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 24.Correia ML, Haynes WG, Rahmouni K, Morgan DA, Sivitz WI, Mark AL. The concept of selective leptin resistance: evidence from agouti yellow obese mice. Diabetes. 2002;51:439–42. doi: 10.2337/diabetes.51.2.439. [DOI] [PubMed] [Google Scholar]
- 25.Rahmouni K, Haynes WG, Morgan DA, Mark AL. Selective resistance to central neural administration of leptin in agouti obese mice. Hypertension. 2002;39:486–90. doi: 10.1161/hy0202.102836. [DOI] [PubMed] [Google Scholar]
- 26.Rahmouni K, Morgan DA, Morgan GM, Mark AL, Haynes WG. Role of selective leptin resistance in diet-induced obesity hypertension. Diabetes. 2005;54:2012–18. doi: 10.2337/diabetes.54.7.2012. [DOI] [PubMed] [Google Scholar]
- 27.Vaz M, Jennings G, Turner A, Cox H, Lambert G, Esler M. Regional sympathetic nervous activity and oxygen consumption in obese normotensive human subjects. Circulation. 1997;96:3423–9. doi: 10.1161/01.cir.96.10.3423. [DOI] [PubMed] [Google Scholar]
- 28.Barba G, Russo O, Siani A, et al. Plasma leptin and blood pressure in men: Graded association independent of body mass and fat pattern. Obes Res. 2003;11:160–6. doi: 10.1038/oby.2003.25. [DOI] [PubMed] [Google Scholar]
- 29.Eikelis N, Schlaich M, Aggarwal A, Kaye D, Esler M. Interactions between leptin and the human sympathetic nervous system. Hypertension. 2003;41:1072–9. doi: 10.1161/01.HYP.0000066289.17754.49. [DOI] [PubMed] [Google Scholar]
- 30.Haynes WG. Interaction between leptin and sympathetic nervous system in hypertension. Curr Hypertens Rep. 2000;2:311–8. doi: 10.1007/s11906-000-0015-1. [DOI] [PubMed] [Google Scholar]
- 31.Rahmouni K, Morgan DA. Hypothalamic arcuate nucleus mediates the sympathetic and arterial pressure responses to leptin. Hypertension. 2007;49:647–52. doi: 10.1161/01.HYP.0000254827.59792.b2. [DOI] [PubMed] [Google Scholar]
- 32.Tartaglia LA. The leptin receptor. J Biol Chem. 1997;272:6093–6. doi: 10.1074/jbc.272.10.6093. [DOI] [PubMed] [Google Scholar]
- 33.Chen H, Charlat O, Tartaglia LA, et al. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491–5. doi: 10.1016/s0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- 34.Iida M, Murakami T, Ishida K, Mizuno A, Kuwajima M, Shima K. Substitution at codon 269 (glutamine --> proline) of the leptin receptor (OB-R) cDNA is the only mutation found in the Zucker fatty (fa/fa) rat. Biochem Biophys Res Commun. 1996;224:597–604. doi: 10.1006/bbrc.1996.1070. [DOI] [PubMed] [Google Scholar]
- 35.Phillips MS, Liu Q, Hammond HA, et al. Leptin receptor missense mutation in the fatty Zucker rat. Nat Genet. 1996;13:18–19. doi: 10.1038/ng0596-18. [DOI] [PubMed] [Google Scholar]
- 36.de Luca C, Kowalski TJ, Zhang Y, et al. Complete rescue of obesity, diabetes, and infertility in db/db mice by neuron-specific LEPR-B transgenes. J Clin Invest. 2005;115:3484–93. doi: 10.1172/JCI24059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen P, Zhao C, Cai X, et al. Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest. 2001;108:1113–21. doi: 10.1172/JCI13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McMinn JE, Liu SM, Liu H, et al. Neuronal deletion of Lepr elicits diabesity in mice without affecting cold tolerance or fertility. Am J Physiol Endocrinol Metab. 2005;289:E403–11. doi: 10.1152/ajpendo.00535.2004. [DOI] [PubMed] [Google Scholar]
- 39.Rahmouni K, Haynes WG, Morgan DA, Mark AL. Role of melanocortin-4 receptors in mediating renal sympathoactivation to leptin and insulin. J Neurosci. 2003;23:5998–6004. doi: 10.1523/JNEUROSCI.23-14-05998.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Myers MG. Leptin receptor signaling and the regulation of mammalian physiology. Recent Prog Horm Res. 2004;59:287–304. doi: 10.1210/rp.59.1.287. [DOI] [PubMed] [Google Scholar]
- 41.Vaisse C, Halaas JL, Horvath CM, Darnell JE, Jr, Stoffel M, Friedman JM. Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat Genet. 1996;14:95–7. doi: 10.1038/ng0996-95. [DOI] [PubMed] [Google Scholar]
- 42.Bates SH, Stearns WH, Dundon TA, et al. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–9. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- 43.Banks AS, Davis SM, Bates SH, Myers MG. Activation of downstream signals by the long form of the leptin receptor. J Biol Chem. 2000;275:14563–72. doi: 10.1074/jbc.275.19.14563. [DOI] [PubMed] [Google Scholar]
- 44.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–19. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 45.Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers MG, Jr, Schwartz MW. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature. 2001;413:794–5. doi: 10.1038/35101657. [DOI] [PubMed] [Google Scholar]
- 46.Niswender KD, Schwartz MW. Insulin and leptin revisited: adiposity signals with overlapping physiological and intracellular signaling capabilities. Front Neuroendocrinol. 2003;24:1–10. doi: 10.1016/s0091-3022(02)00105-x. [DOI] [PubMed] [Google Scholar]
- 47.Zhao AZ, Huan JN, Gupta S, Pal R, Sahu A. A phosphatidylinositol 3-kinase phosphodiesterase 3B-cyclic AMP pathway in hypothalamic action of leptin on feeding. Nat Neurosci. 2002;5:727–8. doi: 10.1038/nn885. [DOI] [PubMed] [Google Scholar]
- 48.Morrison CD, Morton GJ, Niswender KD, Gelling RW, Schwartz MW. Leptin inhibits hypothalamic Npy and Agrp gene expression via a mechanism that requires phosphatidylinositol 3-OH-kinase signaling. Am J Physiol Endocrinol Metab. 2005;289:E1051–7. doi: 10.1152/ajpendo.00094.2005. [DOI] [PubMed] [Google Scholar]
- 49.Plum L, Rother E, Munzberg H, et al. Enhanced leptin-stimulated Pi3k activation in the CNS promotes white adipose tissue transdifferentiation. Cell Metab. 2007;6:431–45. doi: 10.1016/j.cmet.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 50.Rahmouni K, Haynes WG, Morgan DA, Mark AL. Intracellular mechanisms involved in leptin regulation of sympathetic outflow. Hypertension. 2003;41:763–7. doi: 10.1161/01.HYP.0000048342.54392.40. [DOI] [PubMed] [Google Scholar]
- 51.Rahmouni K, Sigmund CD, Haynes WG, Mark AL. Hypothalamic ERK Mediates the Anorectic and Thermogenic Sympathetic Effects of Leptin. Diabetes. 2009;58:536–42. doi: 10.2337/db08-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morgan DA, Thedens DR, Weiss R, Rahmouni K. Mechanisms mediating renal sympathetic activation to leptin in obesity. Am J Physiol-Reg Integ Comp Physiol. 2008;295:R1730–6. doi: 10.1152/ajpregu.90324.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bjorbaek C, Buchholz RM, Davis SM, et al. Divergent roles of SHP-2 in ERK activation by leptin receptors. J Biol Chem. 2001;276:4747–55. doi: 10.1074/jbc.M007439200. [DOI] [PubMed] [Google Scholar]
- 54.Tallam LS, Stec DE, Willis MA, da Silva AA, Hall JE. Melanocortin-4 receptor-deficient mice are not hypertensive or salt-sensitive despite obesity, hyperinsulinemia, and hyperleptinemia. Hypertension. 2005;46:326–32. doi: 10.1161/01.HYP.0000175474.99326.bf. [DOI] [PubMed] [Google Scholar]
- 55.Tallam LS, da Silva AA, Hall JE. Melanocortin-4 receptor mediates chronic cardiovascular and metabolic actions of leptin. Hypertension. 2006;48:58–64. doi: 10.1161/01.HYP.0000227966.36744.d9. [DOI] [PubMed] [Google Scholar]
- 56.Greenfield JR, Miller JW, Keogh JM, et al. Modulation of Blood Pressure by Central Melanocortinergic Pathways. N Engl J Med. 2009;360:44–52. doi: 10.1056/NEJMoa0803085. [DOI] [PubMed] [Google Scholar]