Abstract
Face perception remains one of the most intensively researched areas in psychology and allied disciplines, and there has been much debate regarding the early origins and experiential determinants of face processing. This article reviews studies, the majority of which have appeared in the past decade, that discuss possible mechanisms underlying face perception at birth and document the prominent role of experience in shaping infants’ face-processing abilities. In the first months of life, infants develop a preference for female and own-race faces and become better able to recognize and categorize own-race and own-species faces. This perceptual narrowing and shaping of the “face space” forms a foundation for later face expertise in childhood and adulthood and testifies to the remarkable plasticity of the developing visual system.
Keywords: infancy, face perception, neural plasticity, own-race effect, own-species effect, gender preferences, perceptual narrowing
Faces are perhaps the most prominent visual stimuli in infants’ lives, and throughout their development, they will encounter a great many. Because of its importance, face perception remains one of the most intensively researched areas in psychology and allied disciplines. Faces are multidimensional stimuli, and they provide rich sources of visual information with social significance. This information can be transient (e.g., face states, such as emotions and expression) or more permanent and durable (e.g., face traits, which include attractiveness, gender, race, and species).
One theoretical framework for understanding face perception is the concept of “face space,” introduced by Valentine (1991; Valentine & Endo, 1992), who proposed that faces are encoded and stored as individual points in a theoretical multidimensional space defined by the dimensions that serve to discriminate faces. These dimensions include, among others, familiarity, identity, prototypicality, distinctiveness, inversion, race, age, and species. Valentine has used this model to derive testable predictions, and the concept of face space has been used by innumerable authors as an effective heuristic or metaphor to capture the multidimensional nature of the representation of facial information. It has been further suggested that the face-space model can be beneficially applied to face processing in infancy (e.g., Hayden, Bhatt, Joseph, & Tanaka, 2007; Humphreys & Johnson, 2007; Sangrigoli & de Schonen, 2004a, 2004b). Recent research has documented some of the ways in which, over the course of the 1st year, infants’ face space becomes narrowed and attuned to the faces they see most often, and we review this work, which has key implications for the adaptive significance of face-processing ability and for understanding the role of experience in developing gender-, race-, and species-specific face expertise.
FACE PERCEPTION: THE INITIAL REPRESENTATION
Humans are born with a predisposition or bias to attend to faces: Just a few minutes after birth, newborns will visually track a face-like schematic stimulus more than they will track a stimulus with the features scrambled (Goren, Sarty, & Wu, 1975; Johnson, Dziurawiec, Ellis, & Morton, 1991). However, the nature of this attentional preference is still an open and debated question. In particular, there are two broad views that have been expressed. The first view is that newborn face perception is driven by functional properties of the newborn visual system that direct attention to structural properties of visual stimuli that happen to be found in faces but have nothing to do with faces per se; the second view is that infants are born with a representational bias that is face specific. Although we will discuss both of these views in turn, it is important to note that we are not claiming that there is an innate preference for faces in newborns, given that a considerable amount of development takes place in the prenatal period and that demonstrating that something is innate is almost impossible in humans.
The human face moves, is three-dimensional, has areas of high contrast, and presents different visual images (e.g., changes of expression, changes of viewpoint such as frontal or profile), and contains both internal and external features. Each of these stimulus properties is not face specific and, individually, they will attract newborns’ visual attention: Accordingly it has been suggested that since they are each inherently attractive and found in combination in faces, there is no need to posit a face-specific bias to account for newborns’ attraction to faces (Slater, 1993). The claim that newborns’ face preference may derive from functional properties of the visual system that a priori have nothing to do with faces has recently found support in a series of studies that demonstrate the roles of top-heaviness (Cassia, Turati, & Simion, 2004; Simion, Valenza, Cassia, Turati, & Umilta, 2002; Turati, Simion, Milani, & Umilta, 2002) and congruency (Cassia, Valenza, Simion, & Leo, 2008). With respect to top-heaviness, newborns show a reliable preference for stimuli that have more elements in the upper part than in the lower part. Congruency can be defined as “the presence of a congruent or corresponding relationship between the shape and the orientation of the bounded area delimiting the pattern and the spatial disposition of the included features” (Cassia et al., 2008, p. 808). With the example of congruency illustrated in Figure 1, newborns prefer the congruent pattern on the left to the noncongruent pattern on the right (Cassia et al., 2008, Experiment 3). Cassia et al. (2008, p. 818) summarize their findings by suggesting that “facedness is the emerging product of at least two different perceptual properties— top-heaviness and congruency—each of which is capable to elicit newborns’ preferences when embedded in nonfacelike stimuli (which)… are thought to derive from basic constraints on the newborns’ visual system.”
Figure 1.

Two of the patterns presented to newborns by Cassia et al. (2008): face-like congruent (left) and face-like noncongruent (right).
Others have argued that newborns’ face preference is driven by a specific bias toward the face geometry, stating, for example, that “there does seem to be some representational bias… that the neonate brings to the learning situation for faces” (Karmiloff-Smith, 1996, p. 3). Johnson and Morton (1991) have argued that infants are born with some information about the structure of faces. This information is in the form of a face-detecting subcortical device they call CONSPEC (short for conspecifics), which comprises just three dark patches in a triangle, corresponding to eyes and mouth, and which serves to direct the newborn infant’s visual attention to faces. An alternative view is that an early-appearing representation bias that is specific to faces may be more elaborate than a preference for three dots or patches. This view is supported by two sets of findings.
The first set involves the imitation of facial gestures. Newborn (and older) infants will imitate a variety of facial gestures they see an adult model performing (Meltzoff & Moore, 1977, 1983) and, minutes after birth, will even imitate facial gestures produced by the first face they have seen (Reissland, 1988). Several possible mechanisms that might underlie neonatal imitation have been put forward. One is an active intermodal matching mechanism (e.g., Meltzoff & Moore, 1997), in which babies match what they see but cannot feel (the other person’s facial gestures) with what they can feel but cannot see (their own face). This idea is linked to the suggestion that imitation serves a social identity function (Meltzoff & Moore, 2002) and that infants have the neural machinery to code others as “like me” (Meltzoff, 2004; but see Jones, 2007). An alternative, or perhaps complementary, view, based on neurophysiological findings, is that the observation of facial gestures results in the resonance of “mirror neurons” (i.e., neurons that fire both when a particular behavior is enacted and when it is observed being enacted by another), which subsequently gives rise to a replication (i.e., imitation) of the observed gestures (Ferrari et al., 2006; Jackson, Meltzoff, & Decety, 2006; Lepage & Théret, 2007; Molenberghs, Cunnington, & Mattingley, 2009; Rizzolatti & Graighero, 2004).
The second set of findings suggesting that facial biases go beyond the idea of CONSPEC involves the preference for attractive faces. For thousands of years of recorded history, humans have been attracted to, and beguiled by, attractive faces. A commonly held view has been that this attraction reflects arbitrary standards of beauty that emerge as a result of experience and cultural norms—in other words, “beauty is in the eye of the beholder.” This view is challenged by the finding that preferences for attractive faces are present in very early infancy. When infants whose age averaged less than 3 days old were presented with pairs of female faces that, as judged by adults, were similar in all stimulus properties except attractiveness, the infants showed a consistent preference for the more attractive face in each pair (Slater et al., 1998). In newborns, the effect is orientation specific in that it is found with upright, but not inverted, faces and is driven by attention to internal features (Slater, Bremner, et al., 2000; Slater, Quinn, Hayes, & Brown, 2000).
There have been several interpretations of the attractiveness effect. One is in terms of prototype formation. When several faces of the same gender, ethnicity, and age are averaged or morphed, the resulting average or prototype is always perceived as attractive, and it has been suggested that infants prefer attractive or prototypical faces because they are easier to classify as a face (Langlois & Roggman, 1990). Support for this interpretation as it applies to newborn preferences is given by the finding that the ability to form a facial prototype is present in newborns (Walton & Bower, 1993) and that attractiveness preferences have not been found in 15-min-old newborns (Kalakanis, 1998, cited in Ramsey-Rennels & Langlois, 2007). Thus, older newborns’ preference for attractive faces could result from rapid prototype formation based on the faces the infants have seen since birth.
Although it is possible that the attractiveness preference may result from a face-specific representation that is present at birth, an alternative view is that it may result from general properties of nervous systems that are neither specific to human faces nor to humans. Support for this interpretation is given by the finding that 3- to 4-month-old human infants prefer attractive over unattractive (as judged by adults) domestic cat and wild cat (tiger) faces (Quinn, Kelly, Lee, Pascalis, & Slater, 2008), as well as by the finding that chickens prefer beautiful human faces (Ghirlanda, Jansson, & Enquist, 2002).
The debate about whether newborns’ preference for faces results from domain-general nonspecific perceptual properties or from representational biases that are face-specific allows for a plausible alternative interpretation. It may be that both general properties of nervous systems and face-specific biases contribute to ensure that faces attract newborns’ visual attention. Nevertheless, it is clear that the newborn face preference is guided by low-spatial frequency information, and a strong case has been made that it relies primarily on a subcortical route (Johnson, 2005).
There is increasing neurological and behavioral evidence suggesting that face perception is an experience-expectant process and that infant representation of faces becomes more specific or narrowed with development and particularly attuned to the types of faces that infants most often encounter (Nelson, 2003; Simion, Leo, Turati, Valenza, & Dalla Barba, 2007). There are also data to suggest that the general mechanisms that underlie newborns’ attraction to faces are different from those that attract 3-month-olds, and there is some degree of specificity of cortical processing of faces as early as 3 months of age (Cassia, Kuefner, Westerlund, & Nelson, 2006; Turati, Valenza, Leo, & Simion, 2005).
BECOMING A NATIVE FACE PROCESSOR
It is well documented that infants’ speech perception becomes exquisitely attuned to their native language in the second half of their 1st year of life, just before they produce their first meaningful word. Until the age of around 6 months, infants can discriminate subtle phonetic differences that distinguish speech sounds in both their native language and unfamiliar languages, whereas by 12 months of age, infants have lost this capacity for unfamiliar languages and have become particularly attuned to the phonetic variations in their native language: They have become “native language specialists” or “native listeners” (Hollich & Houston, 2007; Kuhl et al., 2006; Nazzi, Jusczyk, & Johnson, 2000; Werker, 1989).
In recent years, evidence has emerged that, with infants’ increasing exposure to the faces they encounter most frequently, their face-processing system, or face space, undergoes a similar process of perceptual narrowing and tuning over the 1st year of life, and that this perceptual tuning involves considerable neural plasticity. Experience has an early impact: Within the newborn period, infants come to prefer their mother’s face (Bushnell, Sai, & Mullin, 1989; Pascalis, de Schonen, Morton, Deruelle, & Fabre-Grenet, 1995), and there is evidence for some degree of perceptual and cortical specialization in infants’ processing of faces by the age of 3 months (de Haan, Pascalis, & Johnson, 2002; Halit, Csibra, Volein, & Johnson, 2004; Humphreys & Johnson, 2007). Recent investigations that have focused on how infants respond to gender, race, and species information in faces have produced evidence that illustrates this perceptual tuning over the first 3 months.
By 3 months of age, infants who have a female as their primary caregiver (the vast majority of them!) prefer to look at female faces when they are shown paired with male faces. In addition, when 3-month-olds reared by a female caregiver are shown a set of female faces, they will subsequently prefer a novel female face that is paired with one of those shown previously; however, when shown a set of male faces, they will not exhibit a novelty preference for a new male face over a familiarized one (even though they are able to discriminate between the individual male faces; Quinn, Yahr, Kuhn, Slater, & Pascalis, 2002). This pattern of results indicates that the infants represent a category of female faces as individual exemplars, whereas the male faces are represented at the summary, category level (i.e., male). The infants have become, in some small way, “female experts” (Quinn et al., 2002; Ramsey, Langlois, & Marti, 2005). The role of experience in inducing this effect is confirmed by the complementary finding that infants reared with a male as their primary caregiver look more at male faces than at female faces (Quinn et al., 2002). Interestingly, this early female preference interacts with the other-race effect (ORE; see next), as it is found with own-race but not other-race faces (Quinn, Uttley, et al., 2008; examples of the faces presented are given in Figure 2).
Figure 2.

Pairs of the Chinese and Caucasian faces presented to infants by Quinn, Uttley, et al. (2008).
A similar finding of attunement to the category of faces that is most often encountered is seen with the ORE, which is the well-established finding that individuals discriminate more easily between faces of their own race than between faces of other races (“Why do they all look the same?”; Rhodes, Locke, Ewing, & Evangelista, 2009). The ORE has its origins in early infancy. When shown own-race faces paired with other-race faces, newborn infants demonstrated no spontaneous preference for faces from their own ethnic group, a further confirmation that the initial face space is broadly based. However, at age 3 months, infants showed a significant looking preference for own-race faces, a finding that applies to Caucasian, African, and Chinese infants (Bar-Haim, Ziv, Lamy, & Hodes, 2006; Kelly, Liu, et al., 2007; Kelly et al., 2005; see Figure 3 for faces shown by Kelly et al., 2005). The ORE is readily modified at this age, and short-term familiarization with just a few exemplars of another race group is sufficient to reduce the ORE (Sangrigoli & de Schonen, 2004b). In a similar vein, a study by Bar-Haim et al. (2006) found that infants raised in a cross-race environment did not show the effect. Specifically, Ethiopian-Israeli 3-month-olds who had experienced intensive exposure to both Caucasian and African noncaregiver faces looked equally at faces of both races when tested.
Figure 3.

Examples of the Caucasian, Middle Eastern, African, and Asian faces shown by Kelly et al. (2005).
These nascent origins of the ORE become more finely tuned as infancy progresses: Despite showing a preference for looking at own-race faces, Caucasian 3-month-olds were able to discriminate between individual faces within their own facial group and within three other-race groups (African, Middle Eastern, and Chinese; Kelly, Quinn, et al., 2007). However, after extensive continued experience with own-race faces and limited experience with other-race faces, their discrimination by age 9 months was restricted to own-race faces. This effect has been found both with Caucasian infants who have had little exposure to Asian faces and with Chinese infants who have had little exposure to Caucasian faces (Kelly, Quinn, et al., 2007; Kelly et al., 2009). Although Caucasian 9-month-olds discriminated only between own-race faces, they nevertheless retained the ability to form discrete categories of Caucasian and Asian faces, each of which excluded instances of the other (Anzures, Quinn, Pascalis, Slater, & Lee, 2010). The fact that the same-race faces were discriminated suggests that they were represented through categorization (i.e., the formation of distinct groups, each composed of similar yet distinguishable exemplars), whereas the lack of discrimination for other-race faces suggests that they were represented through categorical perception (i.e., the formation of distinct groups composed of similar exemplars that are difficult to discriminate). This pattern of results, in turn, implies that by 9 months of age, same- and other-race faces are represented by different category structures and, hence, occupy different locations in the hypothetical face space. In particular, the representation of same-race faces includes subordinate information about the individual identities of the faces, whereas the representation of other-race faces remains at the summary, category level and does not contain exemplar-individuating information.
A phenomenon parallel to the ORE is the other-species effect (OSE). In one study, for example, 6-month-olds were adept at discriminating between the faces of a monkey species (Macaca fascicularis; see Figure 4), whereas 9-month-olds showed evidence of discrimination only between individuals of their own species, as did adults (Pascalis, de Haan, & Nelson, 2002). But like the ORE, the OSE is readily modified: Infants who, beginning at age 6 months, were regularly exposed to different individual same-species monkey faces over a 3-month period no longer showed the OSE at age 9 months (Pascalis et al., 2005).
Figure 4.
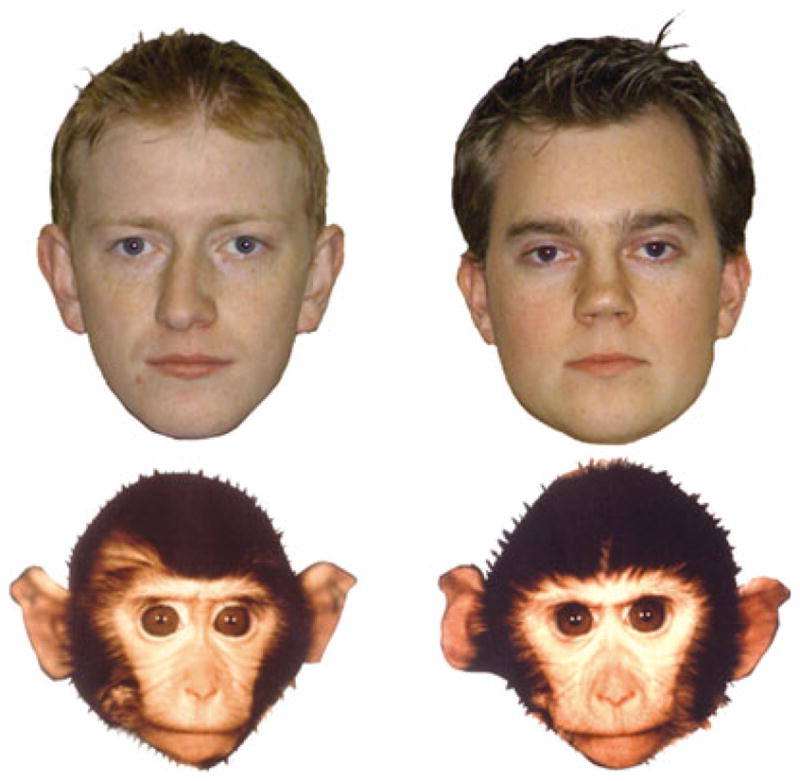
By 9 months of age, human infants showed evidence of discrimination only between individuals of their own species, as did adults (Pascalis et al., 2002).
CONCLUSIONS
Collectively, these findings demonstrate that newborn infants enter the world with a tendency to orient toward, and attend to, faces. However face specific this bias may be, it is clearly broadly based, not human specific, and is possibly governed by subcortical structures (Johnson, 2005). The preference for attractive faces is robust beyond the newborn period: Older infants prefer attractive stimulus faces of all kinds—infant, adult, male, female, and those of different races (Langlois, Ritter, Roggman, & Vaughn, 1991; Langlois et al., 1987; Samuels, Butterworth, Roberts, Graupner, & Hole, 1994; Samuels & Ewy, 1985; van Duuren, Kendell-Scott, & Stark, 2003). And this preference persists throughout life: “There are few more pleasurable sights than a beautiful face” (Rhodes, 2006, p. 200).
Facial input from the infant’s environment shapes the face-processing system early in infancy in different ways, resulting in visual preferences for gender and for own-race faces, and better recognition accuracy with own-race and own-species faces. With respect to gender, the findings from Quinn et al. (2002), who had reported that the preference for female faces reared by female caregivers was reversed in a small sample of infants reared by male caregivers, suggest that it is the experience with the primary caregiver’s face that determines the preference. However, these preferences are readily modified, and same-sex recognition advantages are found in childhood and adolescence (Ge et al., 2008).
In the case of race (ORE), the previously mentioned findings of Bar-Haim et al. (2006) on the absence of ORE in Ethiopian infants born in Israel and exposed to both Caucasian and African noncaregivers suggest that the noncaregiver faces experienced most frequently direct infant preferences for same-versus other-race faces. It seems likely that similar experiences account for the OSE. This perceptual narrowing effect in face perception for the ORE and OSE, which parallels the native language effect, suggests a more general change in neural networks involved in early perception and cognition (Johnson & Munakata, 2005; Sangrigoli, Pallier, Argenti, Ventureyra, & de Schonen, 2005; Scott, Pascalis, & Nelson, 2007).
This review has focused on changes in face processing in the 1st year of life. Of course, there is considerable flexibility thereafter. With respect to the ORE and OSE, both effects are reversible in childhood and adulthood. Regarding the ORE, for example, Korean adults who were adopted by French families between the ages of 3 and 9 years were better able to identify Caucasian faces than Asian faces (Sangrigoli et al., 2005). There is also evidence that early experience with infant faces (i.e., by having a younger sibling), and presumably other face categories, can save the processing system from the loss of plasticity that might otherwise take place for these categories between childhood and adolescence (Cassia, Kuefner, Picozzi, & Vescova, 2009). Additionally, the ORE is reduced or absent in adults who were given practice individuating other-race faces (Rhodes et al., 2009; Tanaka & Pierce, 2009). Regarding OSE, adults who specialize in species or breeds (e.g., bird-watchers, zookeepers, dog show judges) become expert at distinguishing between difference species or individuating individuals (Diamond & Carey, 1986). One of the factors that may account for the flexibility observed for language and face processing during childhood is that the synaptic pruning that follows the exuberant synaptogenesis of early childhood is gradual and not complete until late childhood, leaving some dormant connections ready to be reactivated (Scott et al., 2007). However, the face-processing system in adults also possesses a certain degree of modifiability, allowing it to adapt to new sets of stimuli (e.g., Gauthier, Tarr, Anderson, Skudlarski, & Gore, 1999; Gauthier, Williams, Tarr, & Tanaka, 1998).
The finding that perception of facial attractiveness, gender, other races, and other species appears to undergo different developmental trajectories in early infancy has considerable theoretical importance in that it confirms the multidimensionality of the face space from a very early age. Future research will shed further light on the amounts and types of experience, age of acquisition of experience, and plasticity of face processing throughout development. Nevertheless, the fact that the face space is malleable in infancy, yet remains flexible in childhood and probably throughout life, testifies to the remarkable plasticity of both the developing and the mature visual system.
References
- Anzures G, Quinn PC, Pascalis O, Slater A, Lee K. Categorization, categorical perception, and asymmetry in infants’ perception of face race. Developmental Science. 2010;13:553–564. doi: 10.1111/j.1467-7687.2009.00900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y, Ziv T, Lamy D, Hodes RM. Nature and nurture in own-race face processing. PsychologicalScience. 2006;17:159–163. doi: 10.1111/j.1467-9280.2006.01679.x. [DOI] [PubMed] [Google Scholar]
- Bushnell IWR, Sai F, Mullin JT. Neonatal recognition of the mother’s face. British Journal of Developmental Psychology. 1989;7:3–15. [Google Scholar]
- Cassia VM, Kuefner D, Picozzi M, Vescova E. Early experience predicts later plasticity for face processing: Evidence for the reactivation of dormant effects. Psychological Science. 2009;20:853–859. doi: 10.1111/j.1467-9280.2009.02376.x. [DOI] [PubMed] [Google Scholar]
- Cassia VM, Kuefner D, Westerlund A, Nelson CA. A behavioural and ERP investigation of 3-month-olds’ face preferences. Neuropsychologia. 2006;44:2113–2125. doi: 10.1016/j.neuropsychologia.2005.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassia VM, Turati C, Simion F. Can a nonspecific bias toward top-heavy patterns explain newborns’ face preference? Psychological Science. 2004;15:379–383. doi: 10.1111/j.0956-7976.2004.00688.x. [DOI] [PubMed] [Google Scholar]
- Cassia VM, Valenza E, Simion F, Leo I. Congruency as a nonspecific perceptual property contributing to newborns’ face preference. Child Development. 2008;79:807–820. doi: 10.1111/j.1467-8624.2008.01160.x. [DOI] [PubMed] [Google Scholar]
- de Haan M, Pascalis O, Johnson MH. Specialization of neural mechanisms underlying face recognition in human infants. Journal of Cognitive Neuroscience. 2002;14:199–209. doi: 10.1162/089892902317236849. [DOI] [PubMed] [Google Scholar]
- Diamond R, Carey S. Why faces are and are not special: An effect of expertise. Journal of Experimental Psychology—General. 1986;115:107–117. doi: 10.1037//0096-3445.115.2.107. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, Visalberghi E, Paukner A, Fogassi L, Ruggiero A, Suomi SJ. Neonatal imitation in rhesus macaques. PLoS Biology. 2006;4:1501–1508. doi: 10.1371/journal.pbio.0040302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier I, Tarr MJ, Anderson AW, Skudlarski P, Gore JC. Activation of the middle fusiform “face area” increases with expertise in recognizing novel objects. Nature Neuroscience. 1999;2:568–573. doi: 10.1038/9224. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Williams P, Tarr MJ, Tanaka J. Training “Greeble” experts: A framework for studying expert object recognition processes. Vision Research. 1998;38:2401–2428. doi: 10.1016/s0042-6989(97)00442-2. [DOI] [PubMed] [Google Scholar]
- Ge L, Anzures G, Wang Z, Kelly DJ, Pascalis O, Quinn PC, et al. An inner face advantage in children’s recognition of familiar peers. Journal of Experimental Child Psychology. 2008;101:124–136. doi: 10.1016/j.jecp.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghirlanda S, Jansson L, Enquist M. Chickens prefer beautiful humans. Human Nature—An Interdisciplinary Biosocial Perspective. 2002;13:383–389. doi: 10.1007/s12110-002-1021-6. [DOI] [PubMed] [Google Scholar]
- Goren CC, Sarty M, Wu PYK. Visual following and pattern discrimination of face-like stimuli by newborn infants. Pediatrics. 1975;56:544–549. [PubMed] [Google Scholar]
- Halit H, Csibra G, Volein A, Johnson MH. Face-sensitive cortical processing in early infancy. Journal of Child Psychology and Psychiatry. 2004;45:1228–1234. doi: 10.1111/j.1469-7610.2004.00321.x. [DOI] [PubMed] [Google Scholar]
- Hayden A, Bhatt RS, Joseph JE, Tanaka JW. The other-race effect in infancy: Evidence using a morphing technique. Infancy. 2007;12:95–104. doi: 10.1111/j.1532-7078.2007.tb00235.x. [DOI] [PubMed] [Google Scholar]
- Hollich GJ, Houston DM. Language development: From speech perception to first words. In: Slater A, Lewis M, editors. Introduction to infant development. Oxford, UK: Oxford University Press; 2007. pp. 170–188. [Google Scholar]
- Humphreys K, Johnson MH. The development of “face-space” in infancy. Visual Cognition. 2007;15:578–598. [Google Scholar]
- Jackson PL, Meltzoff AN, Decety J. Neural circuits involved in imitation and perspective-taking. Neuroimage. 2006;31:429–439. doi: 10.1016/j.neuroimage.2005.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH. Subcortical face processing. Nature Reviews Neuroscience. 2005;6:766–774. doi: 10.1038/nrn1766. [DOI] [PubMed] [Google Scholar]
- Johnson MH, Dziurawiec S, Ellis H, Morton J. Newborns’ preferential tracking of face-like stimuli and its subsequent decline. Cognition. 1991;40:1–19. doi: 10.1016/0010-0277(91)90045-6. [DOI] [PubMed] [Google Scholar]
- Johnson MH, Morton J. Conspec and Conlern—A 2-process theory of infant face recognition. Psychological Review. 1991;98:164–181. doi: 10.1037/0033-295x.98.2.164. [DOI] [PubMed] [Google Scholar]
- Johnson MH, Munakata Y. Processes of change in brain and cognitive development. Trends in Cognitive Science. 2005;9:152–158. doi: 10.1016/j.tics.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Jones S. Imitation in infancy: The development of mimicry. Psychological Science. 2007;18:593–599. doi: 10.1111/j.1467-9280.2007.01945.x. [DOI] [PubMed] [Google Scholar]
- Karmiloff-Smith A. The connectionist infant: Would Piaget turn in his grave? In: Slater A, Muir D, editors. SRCD Newsletter. 1996. Fall. pp. 1–3.pp. 10 [Google Scholar]
- Kelly DJ, Liu S, Ge L, Quinn PC, Slater AM, Lee K, et al. Cross-race preferences for same-race faces extend beyond the African versus Caucasian contrast in 3-month-old infants. Infancy. 2007;11:87–95. doi: 10.1080/15250000709336871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DJ, Liu S, Lee K, Quinn PC, Pascalis O, Slater AM, et al. Development of the other-race effect in infancy: Evidence towards universality? Journal of Experimental Child Psychology. 2009;104:105–114. doi: 10.1016/j.jecp.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DJ, Quinn PC, Slater AM, Lee K, Ge L, Pascalis O. The other-race effect develops during infancy: Evidence of perceptual narrowing. Psychological Science. 2007;18:1084–1089. doi: 10.1111/j.1467-9280.2007.02029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DJ, Quinn PC, Slater AM, Lee K, Gibson A, Smith M, et al. Three-month-olds, but not newborns, prefer own-race faces. Developmental Science. 2005;8:F31–F36. doi: 10.1111/j.1467-7687.2005.0434a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl PK, Stevens E, Hayashi A, Deguchi T, Kiritani S, Iverson P. Infants show a facilitative effect for native language phonetic perception between 6 and 12 months. Developmental Science. 2006;9:F13–F21. doi: 10.1111/j.1467-7687.2006.00468.x. [DOI] [PubMed] [Google Scholar]
- Langlois JH, Ritter JM, Roggman LA, Vaughn LS. Facial diversity and infant preferences for attractive faces. Developmental Psychology. 1991;27:79–84. doi: 10.1037//0012-1649.35.3.848. [DOI] [PubMed] [Google Scholar]
- Langlois J, Roggman LA. Attractive faces are only average. Psychological Science. 1990;1:115–121. [Google Scholar]
- Langlois JH, Roggman LA, Casey RJ, Ritter JM, Rieser-Danner LA, Jenkins VY. Infant preferences for attractive faces: Rudiments of a stereotype? Developmental Psychology. 1987;23:363–369. [Google Scholar]
- Lepage JF, Théret H. The mirror neuron system: Grasping others’ actions from birth? Developmental Science. 2007;10:513–529. doi: 10.1111/j.1467-7687.2007.00631.x. [DOI] [PubMed] [Google Scholar]
- Meltzoff AN. The case for developmental cognitive science: Theories of people and things. In: Bremner G, Slater A, editors. Theories of infant development. Oxford, UK: Blackwell; 2004. pp. 145–173. [Google Scholar]
- Meltzoff AN, Moore MK. Imitation of facial and manual gestures by human neonates. Science. 1977;198:75–78. doi: 10.1126/science.198.4312.75. [DOI] [PubMed] [Google Scholar]
- Meltzoff AN, Moore MK. Newborn infants imitate adult facial gestures. Child Development. 1983;54:702–709. [PubMed] [Google Scholar]
- Meltzoff AN, Moore MK. Explaining facial imitation: A theoretical model. Early Development and Parenting. 1997;6:179–192. doi: 10.1002/(SICI)1099-0917(199709/12)6:3/4<179::AID-EDP157>3.0.CO;2-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff AN, Moore MK. Imitation, memory, and the representation of persons. Infant Behavior and Development. 2002;25:39–61. doi: 10.1016/0163-6383(94)90024-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenberghs P, Cunnington R, Mattingley JB. Is the mirror neuron system involved in imitation? A short review and meta-analysis. Neuroscience and Biobehavioral Reviews. 2009;33:975–980. doi: 10.1016/j.neubiorev.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Nazzi T, Jusczyk P, Johnson EK. Language discrimination by English-learning 5-month-olds: Effects of rhythm and familiarity. Journal of Memory and Language. 2000;43:1–19. [Google Scholar]
- Nelson CA. The development of face recognition reflects an experience-expectant and activity-dependent process. In: Pascalis O, Slater A, editors. The development of face processing in infancy and early childhood. New York: Nova Science; 2003. pp. 79–97. [Google Scholar]
- Pascalis O, de Haan M, Nelson CA. Is face processing species-specific during the first year of life? Science. 2002;292:1321–1323. doi: 10.1126/science.1070223. [DOI] [PubMed] [Google Scholar]
- Pascalis O, de Schonen S, Morton J, Deruelle C, Fabre-Grenet M. Mother’s face recognition by neonates—A replication and extension. Infant Behavior and Development. 1995;18:79–85. [Google Scholar]
- Pascalis O, Scott LS, Kelly DJ, Shannon RW, Nicholson E, Coleman M, et al. Plasticity of face processing in infancy. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:5297–5300. doi: 10.1073/pnas.0406627102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn PC, Kelly DJ, Lee K, Pascalis O, Slater AM. Preference for attractive faces in human infants extends beyond conspecifics. Developmental Science. 2008;11:76–83. doi: 10.1111/j.1467-7687.2007.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn PC, Uttley L, Lee K, Gibson A, Smith M, Slater AM, et al. Infant preference for female faces occurs for same-but not other-race faces. Journal of Neuropsychology. 2008;2:15–26. doi: 10.1348/174866407x231029. [DOI] [PubMed] [Google Scholar]
- Quinn PC, Yahr J, Kuhn A, Slater AM, Pascalis O. Representation of the gender of human faces by infants: A preference for female. Perception. 2002;31:1109–1121. doi: 10.1068/p3331. [DOI] [PubMed] [Google Scholar]
- Ramsey JL, Langlois JH, Marti NC. Infant categorization of faces: Ladies first. Developmental Review. 2005;25:212–246. [Google Scholar]
- Ramsey-Rennels JL, Langlois JH. How infants perceive and process faces. In: Slater A, Lewis M, editors. Introduction to infant development. Oxford, UK: Oxford University Press; 2007. pp. 191–232. [Google Scholar]
- Reissland N. Neonatal imitation in the 1st hour of life—Observations in rural Nepal. Developmental Psychology. 1988;24:464–469. [Google Scholar]
- Rhodes G. The evolutionary psychology of facial beauty. Annual Review of Psychology. 2006;57:199–226. doi: 10.1146/annurev.psych.57.102904.190208. [DOI] [PubMed] [Google Scholar]
- Rhodes G, Locke V, Ewing L, Evangelista E. Race coding and the other-race effect in face recognition. Perception. 2009;38:232–241. doi: 10.1068/p6110. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Graighero L. The mirror-neuron system. Annual Review of Neuroscience. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Samuels CA, Butterworth G, Roberts T, Graupner L, Hole G. Babies prefer attractiveness to symmetry. Perception. 1994;23:823–831. doi: 10.1068/p230823. [DOI] [PubMed] [Google Scholar]
- Samuels CA, Ewy R. Aesthetic perception of faces during infancy. British Journal of Developmental Psychology. 1985;3:221–228. [Google Scholar]
- Sangrigoli S, de Schonen S. Effect of visual experience on face processing: A developmental study of inversion and inversion and non-native effects. Developmental Science. 2004a;7:75–87. doi: 10.1111/j.1467-7687.2004.00324.x. [DOI] [PubMed] [Google Scholar]
- Sangrigoli S, de Schonen S. Recognition of own-race and other-race faces by three-month-olds. Journal of Child Psychology and Psychiatry. 2004b;45:1219–1227. doi: 10.1111/j.1469-7610.2004.00319.x. [DOI] [PubMed] [Google Scholar]
- Sangrigoli S, Pallier C, Argenti AM, Ventureyra VAG, de Schonen S. Reversibility of the other-race effect in face recognition during childhood. Psychological Science. 2005;16:440–444. doi: 10.1111/j.0956-7976.2005.01554.x. [DOI] [PubMed] [Google Scholar]
- Scott LS, Pascalis O, Nelson CA. A domain general theory of the development of perceptual discrimination. Current Directions in Psychological Science. 2007;16:197–201. doi: 10.1111/j.1467-8721.2007.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simion F, Leo I, Turati C, Valenza E, Dalla Barba B. How face specialization emerges in the first months of life. Progress in Brain Research. 2007;164:169–185. doi: 10.1016/S0079-6123(07)64009-6. [DOI] [PubMed] [Google Scholar]
- Simion F, Valenza E, Cassia VM, Turati C, Umilta C. Newborns’ preference for up-down asymmetrical configurations. Developmental Science. 2002;5:427–434. [Google Scholar]
- Slater AM. Visual perceptual abilities at birth: Implications for face perception. In: de Boysson-Bardies B, de Schonen S, Jusczyk P, McNeilage P, Morton J, editors. Developmental neurocognition: Speech and face processing in the first year of life. Dordrecht, Netherlands: Kluwer Academic; 1993. pp. 125–134. [Google Scholar]
- Slater A, Bremner JG, Johnson SP, Sherwood P, Hayes RA, Brown E. Newborn infants’ preference for attractive faces: The role of internal and external facial features. Infancy. 2000;1:265–274. doi: 10.1207/S15327078IN0102_8. [DOI] [PubMed] [Google Scholar]
- Slater A, Quinn PC, Hayes RA, Brown E. The role of facial orientation in newborn infants’ preference for attractive faces. Developmental Science. 2000;3:181–185. [Google Scholar]
- Slater A, von der Schulenberg C, Brown E, Badenoch M, Butterworth G, Parsons S, et al. Newborn infants prefer attractive faces. Infant Behavior and Development. 1998;21:345–354. [Google Scholar]
- Tanaka JW, Pierce LJ. The neural plasticity of other-race face recognition. Cognitive Affective and Behavioral Neuroscience. 2009;9:122–131. doi: 10.3758/CABN.9.1.122. [DOI] [PubMed] [Google Scholar]
- Turati C, Simion F, Milani I, Umilta C. Newborn preference for faces: What is crucial? Developmental Psychology. 2002;38:875–882. [PubMed] [Google Scholar]
- Turati C, Valenza E, Leo I, Simion F. Three-month-olds’ visual preference for faces and its underlying visual processing mechanisms. Journal of Experimental Child Psychology. 2005;90:255–273. doi: 10.1016/j.jecp.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Valentine T. A unified account of the effects of distinctiveness, inversion, and race in face recognition. Quarterly Journal of Experimental Psychology Section A—Human Experimental Psychology. 1991;43:161–204. doi: 10.1080/14640749108400966. [DOI] [PubMed] [Google Scholar]
- Valentine T, Endo M. Towards an exemplar model of face processing—The effects of race and distinctiveness. Quarterly Journal of Experimental Psychology Section A—Human Experimental Psychology. 1992;44:671–703. doi: 10.1080/14640749208401305. [DOI] [PubMed] [Google Scholar]
- van Duuren M, Kendell-Scott L, Stark N. Early aesthetic choices: Infant preferences for attractive premature infant faces. International Journal of Behavioral Development. 2003;27:212–219. [Google Scholar]
- Walton GE, Bower TGR. Newborns form prototypes in less than 1 minute. Psychological Science. 1993;4:203–205. [Google Scholar]
- Werker JF. Becoming a native listener. American Scientist. 1989;77:54–59. [Google Scholar]


