Abstract
Vitamin D is classically recognized for its role in calcium homeostasis and skeletal metabolism. Over the last few decades, vitamin D deficiency has increased in prevalence in adults and children. Potential extraskeletal effects of vitamin D have been under investigation for several diseases. Several cross-sectional studies have associated lower vitamin D status with decreased lung function. This finding has prompted investigators to examine the association of vitamin D deficiency with several chronic lung diseases. One major focus has been the link between maternal vitamin D status and childhood asthma. Vitamin D deficiency has also been associated with increased risk of respiratory infection from influenza A and Mycobacterium tuberculosis. Other chronic respiratory diseases associated with vitamin D deficiency include cystic fibrosis, interstitial lung disease, and chronic obstructive pulmonary disease. This review will examine the current clinical literature and potential mechanisms of vitamin D in various pulmonary diseases.
Introduction
Vitamin D is a seco-steroid hormone important in bone mineralization and calcium homeostasis. Recently, research has found that vitamin D may play a role in multiple chronic diseases such as cancer, autoimmune diseases, infections, and cardiovascular disorders (1, 2). Vitamin D may also have a role in several diseases involving the respiratory system. Higher vitamin D concentrations, assessed by 25-hydroxyvitamin D [25(OH)D],7 have been associated with better lung function as measured by forced expiratory volume in 1 s (FEV1) in a large cross-sectional study of the U.S. population in the NHANES III. (3) Although the precise connection between vitamin D status and lung function is unclear at this point, the mechanism by which vitamin D improves lung function may be through its action on regulating inflammation (4–6), inducing antimicrobial peptides (7), and/or its action on muscle (8, 9).
There have been numerous studies looking at vitamin D status in association with various lung diseases focusing on asthma, chronic obstructive pulmonary disease (COPD), cystic fibrosis (CF), and respiratory infections. These studies have demonstrated a high prevalence of vitamin D deficiency in their participants (10–19). Furthermore, several studies have reported that lower maternal vitamin D status during pregnancy or during early childhood increases the risk of asthma and wheezing in the offspring and later childhood, respectively (20–24). Vitamin D deficiency has been associated with lower lung function in COPD and CF patients (3, 13, 16, 17, 25). Other studies have shown an association between vitamin D deficiency and infections such as Mycobacterium tuberculosis (TB) (14, 15, 26–33) and upper respiratory tract infections (18, 19, 34–36). The principal limitation is that the majority of these studies have been cross-sectional by design, with only a limited number of prospective randomized clinical trials. The purpose of this review is to examine the current evidence for a protective role of vitamin D in the following lung diseases: asthma, CF, interstitial lung disease (ILD), COPD, and respiratory infections.
Physiology of vitamin D
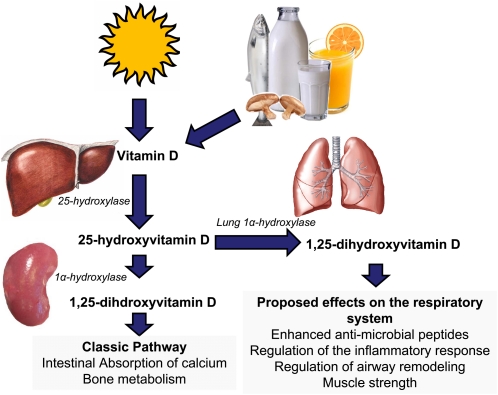
Cholecalciferol (vitamin D3) is synthesized upon exposure of the skin to UVB (290–315 nm), resulting in the conversion of endogenous 7-dehydrocholesterol to previtamin D3, which isomerizes to vitamin D3. After entering the circulation, it is transported by the vitamin D binding protein or albumin. Vitamin D3 is hydroxylated in the liver by 25-hydroxylase to its major circulating metabolite, 25(OH)D3, which is converted to the biologically active form of vitamin D, 1,25 dihydroxyvitamin D3 [1,25(OH)2D3], in the kidney and other tissues by the 1α-hydroxylase (37) (Fig. 1).
Figure 1.
Vitamin D is produced in skin upon exposure to UVB radiation from the sun or from limited dietary sources such as fish and irradiated mushrooms and fortified foods such as milk and orange juice. Vitamin D (D3 from skin only and D2 or D3 from dietary sources) enters the circulation and is hydroxylated in the 25- position by the 25-hydroxylase to form its major circulating form, 25(OH)D, which has a circulating half-life of ∼3 wk. The 25(OH)D then circulates to the kidney and is hydroxylated in the 1-position by the 1α-hydroxylase to form the hormonal form of vitamin D, 1,25(OH)2D. Other tissues such as the epithelial lining of the lung and immune cells in the lung also possess the 1α-hydroxylase to produce local concentrations of 1,25(OH)2D. Proposed extrarenal effects of 1,25(OH)2D produced by epithelial and lung cells include increasing antimicrobial peptide production, regulation of the inflammatory response, and airway remodeling. Vitamin D may also play a role in respiratory muscle function.
The prevalence of vitamin D deficiency has been increasing in the general population in recent decades. The majority of circulating 25(OH)D is derived from sun exposure, with a limited dietary contribution. The increased prevalence of vitamin D deficiency is attributed to sun avoidance, indoor lifestyle, use of sunscreen, and decreased intake of vitamin D-containing foods (1). Because vitamin D is sequestered in adipose tissue, the increasing prevalence of obesity also increases the prevalence of vitamin D deficiency (1).
Asthma
Epidemiology of vitamin D deficiency in asthma
Recent epidemiologic data suggest an association between vitamin D deficiency and asthma (20, 38). Asthma is a disorder characterized by varying and recurring symptoms of airflow obstruction and bronchial hyper-responsiveness in the setting of inflammation (39). Vitamin D deficiency has been found to increase the risk of severe asthma exacerbation defined as the need for emergency department evaluation or hospitalization (40). Analysis of the NHANES III data reported that patients with asthma and vitamin D deficiency [(25(OH)D < 10 μg/L] had higher rates of recent upper respiratory tract infections compared to those with serum 25(OH)D concentrations > 30 μg/L (59 vs. 22%; P < 0.001) (18). Several other cross-sectional studies conducted in adults and children have found that vitamin D deficiency was associated with lower lung function, wheezing, and asthma control (Table 1).
Table 1.
Summary of clinical studies examining vitamin D status and asthma
| Investigator | Population studied | Vitamin D status assessed by | Effects of vitamin D in population studied |
| Pregnancy | |||
| Erkkola (23) | Prospective cohort of pregnant mothers and their children in Finland | Maternal diet (FFQ) | Maternal vitamin D intake resulted in a lower risk of asthma in children at 5 y old |
| Camargo (20) | Prospective cohort of pregnant mothers and their children in USA | Maternal diet (FFQ) | Maternal intake of vitamin D reduced the risk of wheezing in children at 3 y old |
| Miyake (21) | Prospective cohort of Japanese pregnant women and their children | Maternal diet (FFQ) | Maternal intake of vitamin D at >25% percentile lowered the risk of wheezing in children by 16–24 mo old |
| Devereux (22) | Case-control study of pregnant women and their children | Maternal diet (FFQ) | Highest quintile for maternal intake of vitamin D compared to lowest quintile lowered risk of wheezing in children at 5 y old |
| Gale (51) | Prospective cohort of pregnant women and their children in the UK | Maternal 25(OH)D | Maternal 25(OH)D >30 ng/ml increased the risk of asthma in children at 9 y old compared to <12 ng/ml |
| Childhood | |||
| Hypponen (50) | Prospective cohort of children starting at age 1 y and followed for 30 y | Not assessed | Vitamin D supplementation was associated with a nonsignificant increase in risk of asthma at 31 y old |
| Brehm (10) | Cross-sectional study of Costa Rican children | 25(OH)D | Vitamin D insufficiency (<30 μg/L) in 6- to 14-y-old children was associated with an increase in rate of any hospitalization, any use of anti inflammatory medications, and increased airway responsiveness |
| Chinellato (11) | Cross-sectional study of Italian children 5–11 y old | 25(OH)D | Decreased 25(OH)D was associated with a reduction in asthma control |
| Freishtat (41) | Cross-sectional case control study of children 6–20 y old with and without asthma in USA | 25(OH)D | Vitamin D deficiency more common in asthmatics |
| Searing (45) | Cross-sectional study of children with asthma | 25(OH)D | Vitamin D status [25(OH)D] inversely correlated with increased corticosteroid usage and positively correlated with FEV1 and FEV1/FVC |
| Brehm (40) | Children with mild to moderate asthma randomized to 1 of 4 controller medications | 25(OH)D | Vitamin D insufficiency (<30 μg/L) was associated with an increased rate of severe asthma exacerbation |
| Hughes (44) | Retrospective cohort of Australian adults 18–61 y old | 25(OH)D | Vitamin D status [25(OH)D] was not associated with history of childhood asthma |
| Camargo (49) | Prospective cohort of newborns | Cord blood 25(OH)D | 25(OH)D cord blood concentrations were inversely associated with risk of respiratory infections and wheezing, but not with asthma by 5 y old |
| Chinellato (46) | Cross-sectional study of Italian children with intermittent asthma | 25(OH)D | 25(OH)D was inversely correlated with impairment in lung function and increased bronchoconstriction to exercise |
| Adult | |||
| Sutherland (24) | Cross-sectional study of adults with asthma | 25(OH)D | Low 25(OH)D concentrations were associated with impaired lung function, increased airway hyper-responsiveness |
| Li (42) | Cross-sectional study of Chinese adults 18 y or older with a new diagnosis of asthma | 25(OH)D | Vitamin D deficiency was present in the Chinese population and had a positive association with lung function |
| Devereux (43) | Case-control study of Scottish adults 18–50 y old with mild to moderate asthma | 25(OH)D | Vitamin D status was not associated with asthma and Scottish adults |
Vitamin D status and effects on asthmatic control
A cross-sectional study on 616 asthmatic Costa Rican children found that higher serum 25(OH)D concentrations were associated with a reduction in the need for antiinflammatory medications and hospitalization during the previous year (10). However, several studies from other countries have demonstrated mixed results in the association of vitamin D deficiency and asthma (Table 1) (11, 41–44). Sutherland et al. (24) reported that in patients with asthma, higher serum 25(OH)D concentrations were associated with higher FEV1. Higher serum 25(OH)D concentrations were also associated with reduced airway hyper-responsiveness and improved in vitro responsiveness to glucocorticoids (24). Others have demonstrated an inverse correlation between serum 25(OH)D concentrations and the amount of steroid medication prescribed to asthmatic patients (45) and a positive correlation with FEV1 (42), FEV1% predicted (42), and FEV1/forced vital capacity (FVC). (42, 45) Searing et al. (45) also reported a decreased inflammatory response in the combination dexamethasone and vitamin D treatment group compared to the dexamethasone treatment group alone by in vitro analysis of MKP-1 and IL-10 levels. This suggests that vitamin D may potentiate the effects of steroids in asthmatic patients. The childhood asthma management program study randomized children with mild to moderate persistent asthma to commonly used controller medications. They found that vitamin D-deficient children were at increased risk for a severe exacerbation defined as a hospitalization or emergency department visit (40). Finally, Chinellato et al. (46) reported higher prevalence of exercise induced bronchoconstriction in asthmatic children who were vitamin D deficient.
Mechanisms of vitamin D in the pathophysiology of asthma
The effects of vitamin D deficiency on asthma pathophysiology are not completely understood. Many researchers have focused on the potential of vitamin D to dampen the inflammatory immune response in patients with asthma, although other mechanisms may be involved. Studies conducted in a murine model of asthma found that the hormonal form of vitamin D shifted the T-regulatory lymphocyte response from a T helper cell 1 to a less inflammatory T helper cell 2 dominant phenotype (4, 5).
In vivo studies suggest that vitamin D increases the production of IL-10, an antiinflammatory cytokine involved in the pathogenesis of asthma, from T cells in both steroid-sensitive and -resistant asthma patients (6). In contrast, Hypponen et al. (47) demonstrated a U-shaped relationship between serum 25(OH)D and IgE concentrations in adults from the UK. This finding may suggest increased risk of allergic disease such as asthma with low or high serum concentrations of 25(OH)D. The current working hypothesis is that vitamin D potentially reduces the inflammatory response in asthma, leading to a decrease in the amount of antiinflammatory medications such as glucocorticoids that are used by patients.
Vitamin D may also regulate matrix metalloproteinases (MMP) that are involved in airway remodeling. Song et al. (48) found that pretreatment of human airway smooth muscle cells with 1,25(OH)2D3 decreased the in vitro production of MMP-9 and a disintegrin and metalloprotease 33 (ADAM33) when these cells were exposed to serum from asthmatic patients. This inhibitory effect on MMP-9 and ADAM33 may indicate that vitamin D sufficiency prevents further airway narrowing in asthmatic patients. In summary, vitamin D may have a beneficial role in the pathology of asthma by shifting the Th1 and Th2 balance, reducing inflammation, regulating MMP, and reducing airway remodeling.
Vitamin D status in pregnancy and early childhood
Studies of ∼5000 pregnant women from the United States, Finland, Scotland, and Japan demonstrated an inverse association between maternal intake of vitamin D and the rate of wheezing in their offspring (20–23) (Table 1). In children, a vitamin D-deficient diet was associated with a decreased response to bronchodilators (22), increased incidence of allergic rhinitis (23), and increased incidence of asthma (23). Other studies have found that maternal diets poor in vitamin D lead to an increased risk of reactive airways in the offspring (20–23). An additional study has reported that the cord-blood 25(OH)D concentrations in children were inversely associated with risk of wheezing at 15 mo, 3 y, and 5 y, yet no association was observed between cord-blood 25(OH)D concentrations and incidence of asthma in children at 5 y (49).
In a prospective cohort study, Hypponen et al. (50) reported that children receiving vitamin D supplementation (2000 IU/d) in the first year of life had a nonsignificant increase risk of developing asthma (P = 0.08). A limitation of this study was that vitamin D status was not confirmed by serum testing of 25(OH)D. A similar study from the UK of 596 pregnant women demonstrated that higher serum 25(OH)D concentrations during pregnancy conferred increased risk of asthma in their offspring by 9 y of age (51). The major limitation of this study was a dropout rate of ∼60% at 9 y (51, 52). These seemingly conflicting findings will be further examined in a large, randomized, multi-center trial, the Vitamin D Antenatal Asthma Reduction Trial, which will test the hypothesis that vitamin D supplementation can prevent or reduce asthma, wheezing, and other allergic illnesses (53).
CF
Epidemiology
CF is the most common inherited respiratory disorder in the Western world. The CF transmembrane conductance regulator mutation leads to thick secretions that are retained in the airways, preventing adequate bacterial killing by antibacterial factors from the lining of the lung, excessive inflammation, and eventual respiratory failure (54). As the median lifespan of CF patients has reached almost 40 y, the effect of the high prevalence of vitamin D deficiency has become more apparent (55, 56). The causes of vitamin D deficiency in CF are: inadequate intake of vitamin D, diminished body fat, pancreatic exocrine insufficiency causing vitamin D malabsorption, limited sun exposure (57), and decreased serum vitamin D binding protein (58). At large CF centers, >90% of the patients have 25(OH)D concentrations < 30 μg/L (59, 60).
Mechanisms of vitamin D in the pathophysiology of CF
There is a limited understanding of the extraskeletal functions of vitamin D such as the production of antimicrobial peptides in CF. These peptides, cathelicidins and defensins, play a role in the innate host defenses against airway pathogens. LL-37, a cathelicidin, is cleaved from the full-length hCAP18 protein and is the only cathelicidin present in humans. The LL-37 antimicrobial peptide has antimicrobial activity against Gram-positive, Gram-negative bacteria, fungi, and some viruses (7). In vitro studies have demonstrated that 1,25(OH)2D3 is necessary for pathogens to induce cathelicidin mRNA expression in both normal and CF bronchial epithelial cells (61). Vitamin D status has been associated with higher levels of circulating LL-37 in septic patients (62). Given the potential of vitamin D to induce antimicrobial peptides, optimizing vitamin D status may decrease the frequency of pulmonary exacerbations in CF patients. However, no studies published to date have tested this hypothesis.
Vitamin D status and clinical studies in CF
Numerous studies have demonstrated a correlation between decreased bone mineral density in CF and decreased FEV1% predicted (16, 63–67) (Table 2). Low bone mineral density places these patients at increased risk for fractures of thoracic vertebrae and ribs, leading to an ineffective cough and/or impairment in airway clearance (58). Two other retrospective studies found a positive correlation between serum 25(OH)D concentrations and lung function indicators FEV1% predicted (16) and FEV1 (17). Another study in Scandinavian CF patients demonstrated that IgG levels were inversely correlated with serum 25(OH)D concentrations, suggesting there is an association between vitamin D status and the degree of inflammation in CF patients (67). Future randomized trials will attempt to determine how vitamin D effects lung function, reduces the rate of CF exacerbations, and improve bacterial clearance during pulmonary exacerbations (68).
Table 2.
Summary of clinical studies examining vitamin D status in participants with CF, ILD, and COPD
| Investigator | Population studied | Vitamin D status assessed by | Effects of vitamin D in population |
| CF | |||
| Wolfenden (16) | Retrospective study of adults with CF | 25(OH)D | 25(OH)D concentrations were positively associated with FEV1% predicted |
| Stephenson (17) | Retrospective study of adults with CF | 25(OH)D | Trend for higher 25(OH)D positively associated with higher FEV1 (P = 0.06) |
| Pincikova (67) | Cross-sectional study of adults and children with CF | 25(OH)D | 25(OH)D positively correlated with FEV1 and inversely with IgG |
| ILD | |||
| Hagaman (12) | Cross-sectional study of single-center ILD clinic | 25(OH)D | High prevalence of vitamin D insufficiency in ILD clinic, specifically those with connective tissue–related ILD |
| COPD | |||
| Janssens (13) | Cross-sectional study of subjects with COPD who were former smokers | 25(OH)D | 25(OH)D concentrations positively correlated with severity of COPD and FEV1 |
| Ferrari (25) | Cross-sectional study of adults with COPD | 25(OH)D | 25(OH)D concentrations correlated with diminished FEV1 and exercise capacity |
| de Batlle (104) | Cross-sectional study of hospitalized adults with COPD in Spain | Diet (FFQ) | Decreased vitamin D intake in COPD patients admitted to the hospital |
| Miscellaneous | |||
| Forli (8) | Norwegian patients with advanced lung disease | 25(OH)D | Vitamin D deficiency in advanced disease may be associated with muscle weakness |
Vitamin D status and ILD
ILD is a heterogeneous set of disorders that is characterized by damage of the lung parenchyma (69) and has been ineffectively treated with corticosteroids (70). Recently, vitamin D status has been associated with the severity of ILD (Table 2). Olson et al. (71) found the highest mortality in the IPF population was in the winter months, even after accounting for infectious etiologies, suggesting a potential link between vitamin D and ILD. Mascitelli et al. (72) speculated that increased mortality due to infection in the winter months may due to vitamin D deficiency. Hagaman et al. (12) reported that ILD patients in Cincinnati had a high prevalence of vitamin D deficiency [38% of patients had 25(OHOD < 20 μg/L]. Those with connective tissue disease ILD were more likely to have diminished 25(OH)D concentrations compared to the other forms of ILD (deficient 52 vs. 20%; P < 0.0001). Most importantly, the connective tissue disease-ILD group with reduced levels of 25(OH)D had a clinically significant decline in their lung function [FVC, P = 0.015; diffusing capacity of the lung for carbon monoxide (DLCO), P = 0.004].
The pathophysiology of vitamin D deficiency in ILD has been studied by Ramirez et al. (73) using a murine model. They demonstrated that 1,25(OH2)D3 inhibits TGFβ1 stimulation of profibrotic phenotypes in lung fibroblasts and epithelial cells. In summary, there is early epidemiologic evidence demonstrating an association between vitamin D deficiency and ILD; however, the mechanism by which vitamin D may be protective in ILD remains unclear and not well studied in vitro or in clinical studies.
COPD
By 2020 COPD may become the 3rd leading cause of death worldwide (74). COPD is a lung disease associated with significant and progressive irreversible airflow obstruction (75). Recently, a number of studies have shown an association between vitamin D deficiency and severity of COPD (13, 25). Lower vitamin D status in COPD may be due to diminished production of pre-vitamin D3 associated with skin aging caused by smoking and limited UVB exposure (1, 76).
Studies have shown that the degree of vitamin D deficiency correlates with the severity of the disease as measured by the reduction of FEV1 (3, 13, 25) (Table 2). The difference in FEV1 between the highest and lowest quintiles of serum 25(OH)D was greater in those with a diagnosis of chronic bronchitis (248 mL) or emphysema (344 mL) than in the other participants. When evaluating the interaction between emphysema and chronic bronchitis in regards to serum 25(OH)D concentrations, the results were not significant (3). Ferrari et al. (25) also demonstrated that the maximal exercise capacity and carbon monoxide transfer in the single breath method were both positively correlated with serum 25(OH)D concentrations (r = 0.247, P < 0.05; and r = 0.496, P < 0.001). Impairment of exercise capacity in COPD may be related to a reduction in muscle strength (8); however, it is unclear whether vitamin D may play a role in exercise capacity. One longitudinal study of current smokers with mild to moderate COPD evaluated vitamin D status in participants with rapid and slow lung function decline over a 6-y period. It found no difference in the serum 25(OH)D concentrations of the rapid decliners compared to the slow decliners (25.0 and 25.9 μg/L, respectively; P = 0.54) (77). Patients with COPD are at risk for vitamin D deficiency; however, it remains to be seen if correction of vitamin D deficiency leads to a slower decline in lung function and improvement in exercise capacity.
Respiratory infections
The effects of vitamin D on respiratory infections have been studied in a variety disease processes ranging from TB to upper respiratory tract infections. One of the mechanisms by which vitamin D improves recovery from infection appears to be by enhancing innate immunity by upregulation of antimicrobial peptides, as discussed earlier.
TB
TB is a global epidemic, with an incidence of 8.9–9.9 million cases in 2008 (78). Before the etiologic cause of TB was determined in 1903 by Robert Koch, cod liver oil and sun exposure, both sources of vitamin D, were commonly used to treat patients infected with TB (79, 80).
Several studies across ethnic backgrounds have demonstrated a positive association between prevalence of TB and vitamin D deficiency (14, 15, 26–33, 81, 82) (Table 3). However, two smaller studies did not find an association between vitamin D status and risk of TB infection (83, 84). A recent meta-analysis of trials involving TB patients demonstrated that participants with TB had significantly lower serum 25(OH)D concentrations compared to matched controls (0.68; 95% CI = 0.43–0.93) (85).
Table 3.
Summary of clinical studies examining vitamin D status and supplementation with vitamin D in participants with TB infection
| Investigator | Population studied | Vitamin D status assessed by | Effects of vitamin D in population studied |
| Randomized trials | |||
| Martineau (86) | Double-blinded, randomized trial of adult patients with TB exposure | 25(OH)D | Single dose of vitamin D enhanced antimycobacterial immunity in patients with TB exposure |
| Morcos (87) | Small (n = 24) trial of vitamin D in children with new diagnosis of TB randomized to traditional treatment vs. traditional treatment and vitamin D | Serum 1,25 dihydroxyvitamin D | Vitamin D supplementation resulted in clinical improvement |
| Wejse (90) | Randomized trial of vitamin D vs. placebo in Guinea-Bussau with TB | 25(OH)D | Randomized trial did not show any difference between placebo and vitamin D group in regards to mortality or improvement in clinical outcomes. However, 25(OH)D did not differ between vitamin D and placebo. |
| Nursyam (88) | Randomized trial of vitamin D or placebo in Indonesian TB patients | 25(OH)D | Participants treated with vitamin D had higher rate of sputum conversion at 8 wk compared to placebo |
| Martineau (89) | TB patients randomized to vitamin D supplementation or placebo at 14, 28, and 42 d | 25(OH)D | Sputum conversion rate at 8 wk did not differ between the vitamin D and placebo groups; however, those with the tt genotype of the TaqI polymorphism had a higher percentage of sputum conversion vs. placebo |
| Observational studies | |||
| Fielding (105) | Case series of 7 adults with pulmonary TB treated with vitamin D | None | Cavitations reduced in size in 6 of 7 patients |
| Case-control studies | |||
| Ho-Pham (81) | Case-control study of adults with TB from Vietnam compared to healthy controls | 25(OH)D | Vitamin D deficiency was more prevalent in adult men with TB but not in adult women with TB |
| Nielsen (26) | Case-control study of Greenlander patients with TB compared to healthy controls | 25(OH)D | Vitamin D insufficiency [25(OH)D < 30 ng/mL] and elevated 25(OH)D > 56 μg/L were associated with TB infection |
| Wilkinson (14) | Case-control study of Asians with TB and healthy controls in the UK | 25(OH)D | Severe vitamin D deficiency associated with a high rate of TB in this population |
| Gibney (29) | Case-control study of African immigrants with TB in Australia compared to healthy controls | 25(OH)D | 25(OH)D concentrations were inversely associated with risk of TB |
| Sasidharan (31) | Case-control study of hospitalized adults and children with TB compared to healthy controls | 25(OH)D | 25(OH)D concentrations were lower in the participants with TB compared to the control group |
| Grange (83) | Case-control study comparing Indonesian patients 18–50 y old with smear-positive TB compared with healthy controls | 25(OH)D | There was no difference in vitamin D status between the TB patients and the control group |
| Davies (15) | Case-control of adults with culture-positive TB and healthy controls | 25(OH)D | TB patients had lower 25(OH)D than did the healthy controls |
| Davies (32) | Prospective case-control study predicted of patients with TB in Kenya and healthy controls | 25(OH)D | TB patients had lower 25(OH)D than did the healthy controls |
| Davies (33) | Prospective case-control study of patients with TB and healthy controls in Thailand | 25(OH)D | TB patients had lower 25(OH)D than did the healthy controls |
| Chan (84) | Case-control study of TB patients and healthy controls in China | 25(OH)D | There was no difference in vitamin D status between the TB patients and the control group |
| Wejse (82) | Unmatched case-control study of West African patients with TB and healthy controls | 25(OH)D | Vitamin D deficiency was not more common among West African TB patients than controls |
| Retrospective case series | |||
| Williams (27) | Retrospective case series study of children attending TB clinic in the UK | 25(OH)D | 86% of children with latent or active TB had low 25(OH)D (<30 μg/L) |
| Ustianowski (28) | Retrospective case series study of UK patients with TB | 25(OH)D | Vitamin D deficiency was common in all ethnic groups with the exception of white Europeans and Chinese/South East Asians |
| Retrospective study | |||
| Yamshchikov (30) | Southeastern U.S. patients with active TB | 25(OH)D | 86% of participants had low 25(OH)D <30 μg/L |
Early clinical trials have been conducted to test whether vitamin D therapy improves TB outcomes. One study demonstrated that participants who were exposed to TB and who were treated with a single dose of 100,000 IU of vitamin D2 had increased in vitro activity of Mycobacterium bovis bacille Calmette-Guerin-lux luminescence compared to the healthy controls at 24 h (0.57 vs. 0.71; 95% CI = 0.01–0.25; P = 0.03), suggesting an enhanced innate immune response (86). Another study by Morcos et al. (87) found that children diagnosed with TB and treated with conventional therapy plus vitamin D compared to placebo for 2 mo did have clinical improvement characterized by less febrile episodes, resolution of cachexia, and reduction in lymph node enlargement. However, there was no evidence of radiographic improvement, which may be related to the short follow-up. Nursyam et al. (88) demonstrated that pulmonary TB patients treated with conventional TB therapy plus 100,000 IU of vitamin D daily had a significantly higher sputum conversion rate at 6 wk compared to TB patients treated with conventional TB therapy alone. A similar trial reported no difference in sputum conversion rate in participants who received 100,000 IU of cholecalciferol or placebo on 3 different occasions at 14, 28, and 42 d. However, the sputum conversion rate was statistically higher only in those in the vitamin D group with the tt genotype TaqI vitamin D receptor polymorphism (89). In contrast, an African study, in which participants received traditional therapy plus 100,000 IU of vitamin D at baseline and 5 and 8 mo or placebo, did not show any effect of vitamin D therapy (90). However, serum 25(OH)D concentrations in the vitamin D group did not differ from the placebo group, raising questions about the sufficiency and frequency of the vitamin D dosing regimen (90). Overall, these initial clinical associations and early trials suggest that vitamin D may be beneficial as an adjunctive treatment to the traditional therapy in patients with TB; however, additional randomized trials need to be conducted to clarify the role of vitamin D.
Upper respiratory infections
Ecological studies have suggested that an environmental factor such as vitamin D could explain the seasonality of influenza (91–95). This has been observed in several epidemiologic studies (3 case controls, 1 observational, 1, prospective cohort, and 1 cross sectional) showing an association between vitamin D deficiency and increased risk of respiratory infection (18, 19, 34–36, 49) (Table 4). An NHANES III analysis was the largest of these studies (∼18,000 patients) and demonstrated an association between vitamin D deficiency and upper respiratory tract infections (18). Three smaller case control studies did not reveal an association between vitamin D deficiency and respiratory infections (96–98). However, 95% of the participants in the acute lower respiratory tract infection group received some form of vitamin D supplementation, thereby increasing the serum 25(OH)D in the placebo group (96). An additional possibility is that the association of vitamin D deficiency with increased respiratory infection occurs only in those patients with the most severe pulmonary illnesses (35). Approximately 50% of the patients admitted to the pediatric intensive care unit with respiratory illness were vitamin D deficient (<20 μg/L) compared to only 20% on the general medical floor (OR = 8.23; 95% CI = 1.4–48; P = 0.02) (97). The inconsistencies in these reports illustrate the need to evaluate the effects of vitamin D on respiratory infection with randomized control trials.
Table 4.
Summary of clinical studies examining vitamin D status and supplementation with vitamin D in participants with lung infection1
| Investigator | Population studied | Vitamin D status assessed by | Effects of Vitamin D in population studied |
| Randomized trials | |||
| Urashima (100) | Randomized controlled trial of vitamin D in children in Japan | 25(OH)D | Vitamin D3 supplementation in winter significantly reduced the incidence of nasal swab confirmed influenza A |
| Aloia (99, 106) | Post hoc analysis of African American postmenopausal women randomized to placebo or vitamin D | 25(OH)D | Vitamin D supplementation reduced the rate of self-reported colds of flu |
| Manaseki-Holland (101) | Randomized controlled trial of children with pneumonia treated with vitamin D or placebo | None | Treatment with vitamin D3 was associated with fewer repeat episodes of pneumonia |
| Avenell (102) | Randomized controlled trial of vitamin D, calcium, both, or placebo in adults | 25(OH)D | Vitamin D supplementation did not reduce infection |
| Li-Ng (103) | Randomized controlled trial of vitamin D or placebo in adults for 12 wk | 25(OH)D | Vitamin D supplementation did not reduce infection |
| Observational studies | |||
| Laaksi (34) | Cohort study of Finnish men in the military | 25(OH)D | Men with low 25(OH)D concentrations missed more work from infections than those without infections |
| Ginde (18) | Cross-sectional study of U.S. population (NHANES III) | 25(OH)D | URTI were associated with lower 25(OH)D concentrations |
| Case-control study | |||
| Rehman (98) | Case-control study evaluating children with subclinical rickets and recurrent respiratory infections who were given vitamin D and calcium compared to healthy controls | None | No difference in rate of infections between the 2 groups |
| Wayse (35) | Indian children aged ≤5 y hospitalized with ALRI | 25(OH)D | ALRI were associated with vitamin D deficiency [25(OH)D < 9 μg/L] |
| Karatekin (36) | Turkish neonates with ALRI hospitalized in intensive care unit | 25(OH)D | Vitamin D–deficient neonates were at increased risk for ALRI |
| McNallly (97) | Canadian children aged ≤5 y hospitalized for ALRI and controls | 25(OH)D | No difference in vitamin D status in the control group and the ALRI group |
| Roth (96) | Canadian children < 25 mo of age with bronchiolitis and controls | 25(OH)D | No difference in vitamin D status in the control group and the ALRI group |
| Roth (19) | Rural Bangladesh children <23 mo of age with ALRI and controls | 25(OH)D | Lower 25(OH)D was associated with increased risk of ALRI |
ALRI, acute lower respiratory infection; URTI, upper respiratory tract infection.
There are only a few published randomized controlled trials evaluating the effects of vitamin D on infectious outcomes. In a post hoc analysis of women participating in a vitamin D trial for osteoporosis, Aloia et al. (99) found improved upper respiratory tract symptoms in participants receiving vitamin D. A study by Urashima et al. (100) determined that wintertime vitamin D supplementation decreased the risk of influenza A infection diagnosed by nasal swab in school age children. Similarly, a trial conducted in Afghanistan reported a decreased risk of recurrent pneumonia with a single dose of 100,000 IU of vitamin D over the next 3 mo (101). Other trials evaluating the effects of vitamin D supplementation on reducing the rate of respiratory illness did not demonstrate a significant decrease in the occurrence of respiratory illnesses (Table 3). These studies may not have had achieved adequate serum 25(OH)D concentrations or the dosage of vitamin D was too small to be effective (102, 103). At this time, further studies need to be conducted to determine if higher doses of vitamin D are able to prevent and/or treat respiratory infections.
Conclusion
At this time, there is a considerable amount of evidence that implicates vitamin D as a factor associated with various chronic lung diseases. The question remains whether vitamin D deficiency contributes to the etiology of lung disease or if vitamin D deficiency is simply a manifestation of the lung disease and/or its treatment. As research in this field unfolds, vitamin D supplementation will need to be evaluated in larger trials focused on specific respiratory diseases. More research is needed studying the potential mechanisms by which vitamin D may be protective in respiratory diseases. Currently, there are 26 trials listed on clinicaltrials.gov that involve vitamin D and various lung diseases. These future randomized prospective controlled trials will shed light on the true benefits of vitamin D and potential mechanisms in both preventing and treating chronic lung diseases.
Acknowledgments
JDF and VT analyzed data; JDF, RG, and VT wrote the paper. VT had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Supported by NIH K23 AR054334 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, NIH T32DK007734 from The National Institute of Diabetes and Digestive and Kidney Diseases, NIH 5UL1RR025008 from the National Center for Research Resources, and by a Cystic Fibrosis Foundation Center Grant to the Emory Cystic Fibrosis Center.
Author disclosures: J. D. Finklea, R. E. Grossmann, and V. Tangpricha, no conflicts of interest.
Abbreviations used: CF, cystic fibrosis; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; FEV1/FVC, forced expiratory volume in 1 s/forced vital capacity; ILD, interstitial lung disease; MMP, matrix metalloproteinase; 25(OH)D, 25-hydroxyvitamin D; 25(OH)D3, 25-hydroxyvitamin D3; 1,25(OH)2D3, 1,25 dihydroxyvitamin D3; TB, Mycobacterium tuberculosis.
Literature Cited
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81 [DOI] [PubMed] [Google Scholar]
- 2.Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080S–6S [DOI] [PubMed] [Google Scholar]
- 3.Black PN, Scragg R. Relationship between serum 25-hydroxyvitamin d and pulmonary function in the third national health and nutrition examination survey. Chest. 2005;128:3792–8 [DOI] [PubMed] [Google Scholar]
- 4.Searing DA, Leung DY. Vitamin D in atopic dermatitis, asthma and allergic diseases. Immunol Allergy Clin North Am. 2010;30:397–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahon BD, Wittke A, Weaver V, Cantorna MT. The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. J Cell Biochem. 2003;89:922–32 [DOI] [PubMed] [Google Scholar]
- 6.Xystrakis E, Kusumakar S, Boswell S, Peek E, Urry Z, Richards DF, Adikibi T, Pridgeon C, Dallman M, et al. Reversing the defective induction of IL-10-secreting regulatory T cells in glucocorticoid-resistant asthma patients. J Clin Invest. 2006;116:146–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Smet K, Contreras R. Human antimicrobial peptides: defensins, cathelicidins and histatins. Biotechnol Lett. 2005;27:1337–47 [DOI] [PubMed] [Google Scholar]
- 8.Forli L, Bjortuft O, Boe J. Vitamin D status in relation to nutritional depletion and muscle function in patients with advanced pulmonary disease. Exp Lung Res. 2009;35:524–38 [DOI] [PubMed] [Google Scholar]
- 9.Hopkinson NS, Li KW, Kehoe A, Humphries SE, Roughton M, Moxham J, Montgomery H, Polkey MI. Vitamin D receptor genotypes influence quadriceps strength in chronic obstructive pulmonary disease. Am J Clin Nutr. 2008;87:385–90 [DOI] [PubMed] [Google Scholar]
- 10.Brehm JM, Celedon JC, Soto-Quiros ME, Avila L, Hunninghake GM, Forno E, Laskey D, Sylvia JS, Hollis BW, et al. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am J Respir Crit Care Med. 2009;179:765–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chinellato I, Piazza M, Sandri M, Peroni D, Piacentini G, Boner AL. Vitamin D serum levels and markers of asthma control in Italian children. J Pediatr 2011;158:437–41 [DOI] [PubMed] [Google Scholar]
- 12.Hagaman JT, Panos RJ, McCormack FX, Thakar CV, Wikenheiser-Brokamp KA, Shipley RT, Kinder BW. Vitamin D deficiency and reduced lung function in connective tissue-associated interstitial lung diseases. Chest 2011;139:353–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janssens W, Bouillon R, Claes B, Carremans C, Lehouck A, Buysschaert I, Coolen J, Mathieu C, Decramer M, et al. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax. 2010;65:215–20 [DOI] [PubMed] [Google Scholar]
- 14.Wilkinson RJ, Llewelyn M, Toossi Z, Patel P, Pasvol G, Lalvani A, Wright D, Latif M, Davidson RN. Influence of vitamin D deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: a case-control study. Lancet. 2000;355:618–21 [DOI] [PubMed] [Google Scholar]
- 15.Davies PD, Brown RC, Woodhead JS. Serum concentrations of vitamin D metabolites in untreated tuberculosis. Thorax. 1985;40:187–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolfenden LL, Judd SE, Shah R, Sanyal R, Ziegler TR, Tangpricha V. Vitamin D and bone health in adults with cystic fibrosis. Clin Endocrinol (Oxf). 2008;69:374–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stephenson A, Brotherwood M, Robert R, Atenafu E, Corey M, Tullis E. Cholecalciferol significantly increases 25-hydroxyvitamin D concentrations in adults with cystic fibrosis. Am J Clin Nutr. 2007;85:1307–11 [DOI] [PubMed] [Google Scholar]
- 18.Ginde AA, Mansbach JM, Camargo CA., Jr Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2009;169:384–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roth DE, Shah R, Black RE, Baqui AH. Vitamin D status and acute lower respiratory infection in early childhood in Sylhet, Bangladesh. Acta Paediatr. 2010;99:389–93 [DOI] [PubMed] [Google Scholar]
- 20.Camargo CA, Jr, Rifas-Shiman SL, Litonjua AA, Rich-Edwards JW, Weiss ST, Gold DR, Kleinman K, Gillman MW. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. Am J Clin Nutr. 2007;85:788–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyake Y, Sasaki S, Tanaka K, Hirota Y. Dairy food, calcium and vitamin D intake in pregnancy, and wheeze and eczema in infants. Eur Respir J. 2010;35:1228–34 [DOI] [PubMed] [Google Scholar]
- 22.Devereux G, Litonjua AA, Turner SW, Craig LC, McNeill G, Martindale S, Helms PJ, Seaton A, Weiss ST. Maternal vitamin D intake during pregnancy and early childhood wheezing. Am J Clin Nutr. 2007;85:853–9 [DOI] [PubMed] [Google Scholar]
- 23.Erkkola M, Kaila M, Nwaru BI, Kronberg-Kippila C, Ahonen S, Nevalainen J, Veijola R, Pekkanen J, Ilonen J, et al. Maternal vitamin D intake during pregnancy is inversely associated with asthma and allergic rhinitis in 5-year-old children. Clin Exp Allergy. 2009;39:875–82 [DOI] [PubMed] [Google Scholar]
- 24.Sutherland ER, Goleva E, Jackson LP, Stevens AD, Leung DY. Vitamin D levels, lung function, and steroid response in adult asthma. Am J Respir Crit Care Med. 2010;181:699–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrari M, Schenk K, Papadopoulou C, Ferrari P, Carbonare LD, Bertoldo F. Serum 25-hydroxy vitamin D and exercise capacity in COPD. Thorax. Epub 2010 Oct 30 [DOI] [PubMed] [Google Scholar]
- 26.Nielsen NO, Skifte T, Andersson M, Wohlfahrt J, Soborg B, Koch A, Melbye M, Ladefoged K. Both high and low serum vitamin D concentrations are associated with tuberculosis: a case-control study in Greenland. Br J Nutr. 2010;104:1487–91 [DOI] [PubMed] [Google Scholar]
- 27.Williams B, Williams AJ, Anderson ST. Vitamin D deficiency and insufficiency in children with tuberculosis. Pediatr Infect Dis J. 2008;27:941–2 [DOI] [PubMed] [Google Scholar]
- 28.Ustianowski A, Shaffer R, Collin S, Wilkinson RJ, Davidson RN. Prevalence and associations of vitamin D deficiency in foreign-born persons with tuberculosis in London. J Infect. 2005;50:432–7 [DOI] [PubMed] [Google Scholar]
- 29.Gibney KB, MacGregor L, Leder K, Torresi J, Marshall C, Ebeling PR, Biggs BA. Vitamin D deficiency is associated with tuberculosis and latent tuberculosis infection in immigrants from sub-Saharan Africa. Clin Infect Dis. 2008;46:443–6 [DOI] [PubMed] [Google Scholar]
- 30.Yamshchikov AV, Kurbatova EV, Kumari M, Blumberg HM, Ziegler TR, Ray SM, Tangpricha V. Vitamin D status and antimicrobial peptide cathelicidin (LL-37) concentrations in patients with active pulmonary tuberculosis. Am J Clin Nutr. 2010;92:603–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sasidharan PK, Rajeev E, Vijayakumari V. Tuberculosis and vitamin D deficiency. J Assoc Physicians India. 2002;50:554–8 [PubMed] [Google Scholar]
- 32.Davies PD, Church HA, Brown RC, Woodhead JS. Raised serum calcium in tuberculosis patients in Africa. Eur J Respir Dis. 1987;71:341–4 [PubMed] [Google Scholar]
- 33.Davies PD, Church HA, Bovornkitti S, Charumilind A, Byrachandra S. Altered vitamin D homeostasis in tuberculosis. Int Med Thailand. 1988;4:45–7 [Google Scholar]
- 34.Laaksi I, Ruohola JP, Tuohimaa P, Auvinen A, Haataja R, Pihlajamaki H, Ylikomi T. An association of serum vitamin D concentrations < 40 nmol/L with acute respiratory tract infection in young Finnish men. Am J Clin Nutr. 2007;86:714–7 [DOI] [PubMed] [Google Scholar]
- 35.Wayse V, Yousafzai A, Mogale K, Filteau S. Association of subclinical vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. Eur J Clin Nutr. 2004;58:563–7 [DOI] [PubMed] [Google Scholar]
- 36.Karatekin G, Kaya A, Salihoglu O, Balci H, Nuhoglu A. Association of subclinical vitamin D deficiency in newborns with acute lower respiratory infection and their mothers. Eur J Clin Nutr. 2009;63:473–7 [DOI] [PubMed] [Google Scholar]
- 37.Sandhu MS, Casale TB. The role of vitamin D in asthma. Ann Allergy Asthma Immunol 2010;105:191–199; quiz 200–192, 217 [DOI] [PubMed] [Google Scholar]
- 38.Ginde AA, Mansbach JM, Camargo CA., Jr Vitamin D, respiratory infections, and asthma. Curr Allergy Asthma Rep. 2009;9:81–7 [DOI] [PubMed] [Google Scholar]
- 39.National Asthma Education and Prevention Program: Expert panel report III: guidelines for the diagnosis and management of asthma. Bethesda, MD: National Heart, Lung, and Blood Institute, 2007. NIH Publication No. 08-4051 [cited 2010 Nov 19]. Available from:www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm Respir Crit Care Med. 1998;157:1892–9 [Google Scholar]
- 40.Brehm JM, Schuemann B, Fuhlbrigge AL, Hollis BW, Strunk RC, Zeiger RS, Weiss ST, Litonjua AA. Serum vitamin D levels and severe asthma exacerbations in the Childhood Asthma Management Program study. J Allergy Clin Immunol 2010;126:52–58 e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freishtat RJ, Iqbal SF, Pillai DK, Klein CJ, Ryan LM, Benton AS, Teach SJ. High prevalence of vitamin D deficiency among inner-city African American youth with asthma in Washington, DC. J Pediatr. 2010;156:948–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li F, Peng M, Jiang L, Sun Q, Zhang K, Lian F, Litonjua AA, Gao J, Gao X. Vitamin D deficiency is associated with decreased lung function in Chinese adults with asthma. Respiration. Epub 2010 Dec 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Devereux G, Wilson A, Avenell A, McNeill G, Fraser WD. A case-control study of vitamin D status and asthma in adults. Allergy. 2010;65:666–7 [DOI] [PubMed] [Google Scholar]
- 44.Hughes AM, Lucas RM, Ponsonby AL, Chapman C, Coulthard A, Dear K, Dwyer T, Kilpatrick TJ, McMichael AJ, et al. The role of latitude, ultraviolet radiation exposure and vitamin D in childhood asthma and hayfever: an Australian multicenter study. Pediatr Allergy Immunol. Epub 2010 Sep 30 [DOI] [PubMed] [Google Scholar]
- 45.Searing DA, Zhang Y, Murphy JR, Hauk PJ, Goleva E, Leung DY. Decreased serum vitamin D levels in children with asthma are associated with increased corticosteroid use. J Allergy Clin Immunol. 2010;125:995–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chinellato I, Piazza M, Sandri M, Peroni DG, Cardinale F, Piacentini GL, Boner AL. Vitamin D serum levels and exercise-induced bronchoconstriction in children with asthma. Eur Respir J. Epub 2010 Nov 11 [DOI] [PubMed] [Google Scholar]
- 47.Hypponen E, Berry DJ, Wjst M, Power C. Serum 25-hydroxyvitamin D and IgE: a significant but nonlinear relationship. Allergy. 2009;64:613–20 [DOI] [PubMed] [Google Scholar]
- 48.Song Y, Qi H, Wu C. Effect of 1,25-(OH)2D3 (a vitamin D analogue) on passively sensitized human airway smooth muscle cells. Respirology. 2007;12:486–94 [DOI] [PubMed] [Google Scholar]
- 49.Camargo CA, Jr, Ingham T, Wickens K, Thadhani R, Silvers KM, Epton MJ, Town GI, Pattemore PK, Espinola JA, et al. Cord-blood 25-hydroxyvitamin D levels and risk of respiratory infection, wheezing, and asthma. Pediatrics 2011;127:e180–7 [DOI] [PubMed] [Google Scholar]
- 50.Hypponen E, Sovio U, Wjst M, Patel S, Pekkanen J, Hartikainen AL, Jarvelinb MR. Infant vitamin D supplementation and allergic conditions in adulthood: northern Finland birth cohort 1966. Ann N Y Acad Sci. 2004;1037:84–95 [DOI] [PubMed] [Google Scholar]
- 51.Gale CR, Robinson SM, Harvey NC, Javaid MK, Jiang B, Martyn CN, Godfrey KM, Cooper C. Maternal vitamin D status during pregnancy and child outcomes. Eur J Clin Nutr. 2008;62:68–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gilbert CR, Arum SM, Smith CM. Vitamin D deficiency and chronic lung disease. Can Respir J. 2009;16:75–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.VDAART VDAART Index Page [cited 2011 March]. Available from: http://clinicaltrials.gov/ct2/show/NCT00920621
- 54.O'Sullivan BP, Freedman SD. Cystic fibrosis. Lancet. 2009;373:1891–904 [DOI] [PubMed] [Google Scholar]
- 55.Brennan AL, Geddes DM, Gyi KM, Baker EH. Clinical importance of cystic fibrosis-related diabetes. J Cyst Fibros. 2004;3:209–22 [DOI] [PubMed] [Google Scholar]
- 56.Cystic Fibrosis Foundation National Patient Registry Annual Data Report for 2009. Bethesda, MD: Cystic Fibrosis Foundation; 2010 [Google Scholar]
- 57.Chandra P, Wolfenden LL, Ziegler TR, Tian J, Luo M, Stecenko AA, Chen TC, Holick MF, Tangpricha V. Treatment of vitamin D deficiency with UV light in patients with malabsorption syndromes: a case series. Photodermatol Photoimmunol Photomed. 2007;23:179–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hall WB, Sparks AA, Aris RM. Vitamin d deficiency in cystic fibrosis. Int J Endocrinol. 2010;2010:218691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rovner AJ, Stallings VA, Schall JI, Leonard MB, Zemel BS. Vitamin D insufficiency in children, adolescents, and young adults with cystic fibrosis despite routine oral supplementation. Am J Clin Nutr. 2007;86:1694–9 [DOI] [PubMed] [Google Scholar]
- 60.Boyle MP, Noschese ML, Watts SL, Davis ME, Stenner SE, Lechtzin N. Failure of high-dose ergocalciferol to correct vitamin D deficiency in adults with cystic fibrosis. Am J Respir Crit Care Med. 2005;172:212–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yim S, Dhawan P, Ragunath C, Christakos S, Diamond G. Induction of cathelicidin in normal and CF bronchial epithelial cells by 1,25-dihydroxyvitamin D(3). J Cyst Fibros. 2007;6:403–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jeng L, Yamshchikov AV, Judd SE, Blumberg HM, Martin GS, Ziegler TR, Tangpricha V. Alterations in vitamin D status and anti-microbial peptide levels in patients in the intensive care unit with sepsis. J Transl Med. 2009;7:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Elkin SL, Fairney A, Burnett S, Kemp M, Kyd P, Burgess J, Compston JE, Hodson ME. Vertebral deformities and low bone mineral density in adults with cystic fibrosis: a cross-sectional study. Osteoporos Int. 2001;12:366–72 [DOI] [PubMed] [Google Scholar]
- 64.Haworth CS, Selby PL, Webb AK, Dodd ME, Musson H, McL Niven R, Economou G, Horrocks AW, Freemont AJ, et al. Low bone mineral density in adults with cystic fibrosis. Thorax. 1999;54:961–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Henderson RC, Madsen CD. Bone density in children and adolescents with cystic fibrosis. J Pediatr. 1996;128:28–34 [DOI] [PubMed] [Google Scholar]
- 66.Haworth CS, Selby PL, Horrocks AW, Mawer EB, Adams JE, Webb AK. A prospective study of change in bone mineral density over one year in adults with cystic fibrosis. Thorax. 2002;57:719–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pincikova T, Nilsson K, Moen IE, Karpati F, Fluge G, Hollsing A, Knudsen PK, Lindblad A, Mared L, Pressler T, et al. Inverse relation between vitamin D and serum total immunoglobulin G in the Scandinavian Cystic Fibrosis Nutritional Study. Eur J Clin Nutr. 2011;65:102–9 [DOI] [PubMed] [Google Scholar]
- 68.National Institutes of Health Trial no. NCT00788138 [cited 2011 March 18]. Available from: http://clinicaltrials.gov
- 69.Raghu G, Brown KK. Interstitial lung disease: clinical evaluation and keys to an accurate diagnosis. Clin Chest Med. 2004;25:409–19 [DOI] [PubMed] [Google Scholar]
- 70.Kim R, Meyer KC. Therapies for interstitial lung disease: past, present and future. Ther Adv Respir Dis. 2008;2:319–38 [DOI] [PubMed] [Google Scholar]
- 71.Olson AL, Swigris JJ, Raghu G, Brown KK. Seasonal variation: mortality from pulmonary fibrosis is greatest in the winter. Chest. 2009;136:16–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mascitelli L, Pezzetta F, Goldstein MR. Vitamin D and mortality from pulmonary fibrosis. Chest. 2010;137:495–6 [DOI] [PubMed] [Google Scholar]
- 73.Ramirez AM, Wongtrakool C, Welch T, Steinmeyer A, Zugel U, Roman J. Vitamin D inhibition of pro-fibrotic effects of transforming growth factor beta1 in lung fibroblasts and epithelial cells. J Steroid Biochem Mol Biol. 2010;118:142–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chatila WM, Wynkoop WA, Vance G, Criner GJ. Smoking patterns in African Americans and whites with advanced COPD. Chest. 2004;125:15–21 [DOI] [PubMed] [Google Scholar]
- 75.Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–46 [DOI] [PubMed] [Google Scholar]
- 76.Janssens W, Lehouck A, Carremans C, Bouillon R, Mathieu C, Decramer M. Vitamin D beyond bones in chronic obstructive pulmonary disease: time to act. Am J Respir Crit Care Med. 2009;179:630–6 [DOI] [PubMed] [Google Scholar]
- 77.Kunisaki KM, Niewoehner DE, Singh RJ, Connett JE. Vitamin D status and longitudinal lung function decline in the Lung Health Study. Eur Respir J. 2011;37:238–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.WHO Global tuberculosis control. A short update to the 2009 report. Geneva: WHO; 2010 [Google Scholar]
- 79.Chocano-Bedoya P, Ronnenberg AG. Vitamin D and tuberculosis. Nutr Rev. 2009;67:289–93 [DOI] [PubMed] [Google Scholar]
- 80.Martineau AR, Honecker FU, Wilkinson RJ, Griffiths CJ. Vitamin D in the treatment of pulmonary tuberculosis. J Steroid Biochem Mol Biol. 2007;103:793–8 [DOI] [PubMed] [Google Scholar]
- 81.Ho-Pham LT, Nguyen ND, Nguyen TT, Nguyen DH, Bui PK, Nguyen VN, Nguyen TV. Association between vitamin D insufficiency and tuberculosis in a vietnamese population. BMC Infect Dis. 2010;10:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wejse C, Olesen R, Rabna P, Kaestel P, Gustafson P, Aaby P, Andersen PL, Glerup H, Sodemann M. Serum 25-hydroxyvitamin D in a West African population of tuberculosis patients and unmatched healthy controls. Am J Clin Nutr. 2007;86:1376–83 [DOI] [PubMed] [Google Scholar]
- 83.Grange JM, Davies PD, Brown RC, Woodhead JS, Kardjito T. A study of vitamin D levels in Indonesian patients with untreated pulmonary tuberculosis. Tubercle. 1985;66:187–91 [DOI] [PubMed] [Google Scholar]
- 84.Chan TY, Poon P, Pang J, Swaminathan R, Chan CH, Nisar M, Williams CS, Davies PD. A study of calcium and vitamin D metabolism in Chinese patients with pulmonary tuberculosis. J Trop Med Hyg. 1994;97:26–30 [PubMed] [Google Scholar]
- 85.Nnoaham KE, Clarke A. Low serum vitamin D levels and tuberculosis: a systematic review and meta-analysis. Int J Epidemiol. 2008;37:113–9 [DOI] [PubMed] [Google Scholar]
- 86.Martineau AR, Wilkinson RJ, Wilkinson KA, Newton SM, Kampmann B, Hall BM, Packe GE, Davidson RN, Eldridge SM, et al. A single dose of vitamin D enhances immunity to mycobacteria. Am J Respir Crit Care Med. 2007;176:208–13 [DOI] [PubMed] [Google Scholar]
- 87.Morcos MM, Gabr AA, Samuel S, Kamel M, el Baz M, el Beshry M, Michail RR. Vitamin D administration to tuberculous children and its value. Boll Chim Farm. 1998;137:157–64 [PubMed] [Google Scholar]
- 88.Nursyam EW, Amin Z, Rumende CM. The effect of vitamin D as supplementary treatment in patients with moderately advanced pulmonary tuberculous lesion. Acta Med Indones. 2006;38:3–5 [PubMed] [Google Scholar]
- 89.Martineau AR, Timms PM, Bothamley GH, Hanifa Y, Islam K, Claxton AP, Packe GE, Moore-Gillon JC, Darmalingam M, et al. High-dose vitamin D3 during intensive-phase antimicrobial treatment of pulmonary tuberculosis: a double-blind randomised controlled trial. Lancet. 2011;377:242–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wejse C, Gomes VF, Rabna P, Gustafson P, Aaby P, Lisse IM, Andersen PL, Glerup H, Sodemann M. Vitamin D as supplementary treatment for tuberculosis: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2009;179:843–50 [DOI] [PubMed] [Google Scholar]
- 91.Faumuina R, Bilbao J, Aspy CB, Mold JW. Is vitamin D deficiency associated with a greater likelihood of contracting influenza? J Okla State Med Assoc. 2010;103:118–9 [PubMed] [Google Scholar]
- 92.Hope-Simpson RE. The role of season in the epidemiology of influenza. J Hyg (Lond). 1981;86:35–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.White AN, Ng V, Spain CV, Johnson CC, Kinlin LM, Fisman DN. Let the sun shine in: effects of ultraviolet radiation on invasive pneumococcal disease risk in Philadelphia, Pennsylvania. BMC Infect Dis. 2009;9:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Grant WB, Giovannucci E. The possible roles of solar ultraviolet-B radiation and vitamin D in reducing case-fatality rates from the 1918–1919 influenza pandemic in the United States. Dermatoendocrinol. 2009;1:215–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cannell JJ, Vieth R, Umhau JC, Holick MF, Grant WB, Madronich S, Garland CF, Giovannucci E. Epidemic influenza and vitamin D. Epidemiol Infect. 2006;134:1129–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Roth DE, Jones AB, Prosser C, Robinson JL, Vohra S. Vitamin D status is not associated with the risk of hospitalization for acute bronchiolitis in early childhood. Eur J Clin Nutr. 2009;63:297–9 [DOI] [PubMed] [Google Scholar]
- 97.McNally JD, Leis K, Matheson LA, Karuananyake C, Sankaran K, Rosenberg AM. Vitamin D deficiency in young children with severe acute lower respiratory infection. Pediatr Pulmonol. 2009;44:981–8 [DOI] [PubMed] [Google Scholar]
- 98.Rehman PK. Sub-clinical rickets and recurrent infection. J Trop Pediatr. 1994;40:58. [DOI] [PubMed] [Google Scholar]
- 99.Aloia JF, Li-Ng M. Re: epidemic influenza and vitamin D. Epidemiol Infect. 2007;135:1095–6, author reply 1097–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr. 2010;91:1255–60 [DOI] [PubMed] [Google Scholar]
- 101.Manaseki-Holland S, Qader G, Isaq Masher M, Bruce J, Zulf Mughal M, Chandramohan D, Walraven G. Effects of vitamin D supplementation to children diagnosed with pneumonia in Kabul: a randomised controlled trial. Trop Med Int Health. 2010;15:1148–55 [DOI] [PubMed] [Google Scholar]
- 102.Avenell A, Cook JA, Maclennan GS, Macpherson GC. Vitamin D supplementation to prevent infections: a sub-study of a randomised placebo-controlled trial in older people (RECORD trial, ISRCTN 51647438). Age Ageing. 2007;36:574–7 [DOI] [PubMed] [Google Scholar]
- 103.Li-Ng M, Aloia JF, Pollack S, Cunha BA, Mikhail M, Yeh J, Berbari N. A randomized controlled trial of vitamin D3 supplementation for the prevention of symptomatic upper respiratory tract infections. Epidemiol Infect. 2009;137:1396–404 [DOI] [PubMed] [Google Scholar]
- 104.de Batlle J, Romieu I, Anto JM, Mendez M, Rodriguez E, Balcells E, Ferrer A, Gea J, Rodriguez-Roisin R, et al. Dietary habits of firstly admitted Spanish COPD patients. Respir Med. 2009;103:1904–10 [DOI] [PubMed] [Google Scholar]
- 105.Fielding J, Maloney JJ. Calciferol, streptomycin, and para-aminosalicylic acid in pulmonary tuberculosis. Lancet. 1951;2:614–7 [DOI] [PubMed] [Google Scholar]
- 106.Aloia JF, Talwar SA, Pollack S, Yeh J. A randomized controlled trial of vitamin D3 supplementation in African American women. Arch Intern Med. 2005;165:1618–23 [DOI] [PMC free article] [PubMed] [Google Scholar]