Abstract
Dietary SFA and cholesterol are major targets for reducing plasma total and LDL cholesterol as a strategy to decrease cardiovascular disease risk. However, many studies show that excess adiposity attenuates the expected lipid and lipoprotein response to a plasma cholesterol–lowering diet. Diets low in SFA and cholesterol are less effective in improving the lipid profile in obese individuals and in patients with metabolic syndrome. In contrast, lean persons are more responsive to reductions in dietary SFA and cholesterol. Multiple mechanisms likely contribute to the altered plasma lipid responses to dietary changes in individuals with excess adiposity. The greater rate of hepatic cholesterol synthesis in obese individuals suppresses the expression of hepatic LDL receptors (LDLR), thereby reducing hepatic LDL uptake. Insulin resistance develops as a result of adipose-tissue induced inflammation, causing significant changes in enzymes necessary for normal lipid metabolism. In addition, the LDLR-mediated uptake in obesity is attenuated by alterations in neuroendocrine regulation of hormonal secretions (e.g. growth hormone, thyroid hormone, and cortisol) as well as the unique gut microbiota, the latter of which appears to affect lipid absorption. Reducing adipose tissue mass, especially from the abdominal region, is an effective strategy to improve the lipid response to dietary interventions by reducing inflammation, enhancing insulin sensitivity, and improving LDLR binding. Thus, normalizing adipose tissue mass is an important goal for maximizing the diet response to a plasma cholesterol–lowering diet.
Introduction
Cardiovascular disease (CVD2) remains a major global health problem. Dietary modifications that lower atherogenic lipids and lipoproteins are effective in the prevention and treatment of CVD. Because of individual variability in the plasma lipid response to changes in dietary SFA and cholesterol, there is keen interest in unraveling the underlying biological mechanisms that account for these differences. Obesity, type II diabetes, and metabolic syndrome (MetS) increase CVD risk. In fact, MetS increases the risk of developing CVD by ∼2-fold (1). MetS typically is characterized by any 3 of the following 5 risk factors: increased waist circumference (>102 cm for men, >88 cm for women), elevated TG (≥1.69 mmol/L), decreased HDL cholesterol (HDL-C) (<1.03 mmol/L for men, < 1.29 mmol/L for women), elevated blood pressure (≥130/≥85 mm Hg), and glucose (≥6.10 mmol/L) (1). Obesity (BMI > 30 kg/m2), which presents with dyslipidemia and elevated cholesterol levels (2, 3), plays a major role in the development of MetS, which increases the risk of type II diabetes (4). In most developed countries, the prevalence of MetS is between 20 and 30% of the adult population and likely will follow a parallel increase with the projected future rise in obesity (5). Because of the exploding obesity epidemic, research efforts have escalated to better understand all aspects of the pathophysiology, including how obesity affects lipid and lipoprotein metabolism.
Numerous factors affect variability in lipid response to diet. Genetics plays a role in the lipid response to dietary fat and cholesterol (6, 7). A strong environmental factor that affects diet response is obesity. Excess adiposity appreciably affects lipid metabolism and inflammation. Release of proinflammatory markers by adipocytes (adipokines) leads to insulin resistance (8, 9). There is a significant link between obesity and insulin resistance, a predominant characteristic of MetS. Greater cholesterol synthesis and lower cholesterol absorption can arise as a consequence of insulin resistance, often causing a diminished plasma lipid response to diet (10). Dietary interventions are effective for improving the lipid and lipoprotein profile. Replacing SFA with MUFA, PUFA, and/or carbohydrate (CHO) reduces LDL cholesterol (LDL-C) (11, 12). A mechanism by which replacing SFA with PUFA lowers LDL-C is via an increase in LDL receptor (LDLR)-mediated uptake of LDL-C from circulation (13, 14). LDLR-mediated uptake, however, is impaired by obesity. Consequently, obese individuals are less responsive to dietary interventions aimed at improving the lipid/lipoprotein profile. A greater understanding of the factors that diminish lipid uptake in obese individuals likely will increase our understanding of why they have a blunted lipid response to dietary interventions. The purpose of this review is to summarize the evidence and discuss the possible mechanisms that contribute to the blunted lipid response to dietary change that is associated with obesity. This review will discuss the effects of dietary SFA and cholesterol on changes in plasma lipids and lipoproteins and the effect of adiposity on these responses.
Current state of knowledge
Obesity causes a blunted lipid response to dietary SFA and cholesterol
A number of clinical studies have reported an inverse relationship between BMI and the lipid response to dietary SFA and cholesterol (Table 1) (9, 15–25). The lipid and lipoprotein response is greater in lean individuals compared to obese. Early intervention studies assessed lipid response to SFA and cholesterol by the removal or addition of eggs in the diet (18, 22, 23). Bronsgeest-Schoute et al. (18) recruited participants who typically consumed at least 1 egg/d; for 3 wk they were not allowed to consume any egg products. The experimental diet contained ∼264 mg/d cholesterol, which was less than the 742 mg/d cholesterol prior to egg removal. A small but significant decrease in total cholesterol (TC) was reported (−0.16 ± 0.40 mmol/L; P < 0.05) with an inverse correlation (r = −0.321; P < 0.05) between BMI and the reduction in TC. When participants were classified on the basis of BMI, only those who were not obese had a reduction in TC (−0.23 ± 0.43 mmol/L; P < 0.01). Katan and Beynen (23) reported a similar response when participants consumed egg yolks after a low-cholesterol diet. The TC response was inversely related to BMI (r = −0.50; P < 0.01), habitual cholesterol consumption (r = −0.62; P < 0.01), and rate of cholesterol synthesis (r = −0.40; P < 0.05). Therefore, participants with a lower body weight, lower cholesterol consumption, and low absolute cholesterol synthesis rate demonstrated the greatest response to dietary cholesterol.
Table 1.
Effect of obesity on the lipid response to dietary SFA and cholesterol in intervention studies1
| Participant characteristics |
|||||
| Reference | n (M/F) | Status | Dietary intervention | Length | Lipid/lipoprotein response |
| Bronsgeest-Schoute et al., 1979 (18) | 44 (25/19) | Habitual egg consumers | Removal of eggs from normal diet | 3 wk | BMI inversely correlated with cholesterol response (r = −0.321) |
| Clifton et al.,1995 (25) | 120 (53/67) | Healthy adults (BMI 26.5 kg/m2)2 | Low-fat diet with: 1. Full cream milk and egg yolk 2. Skim milk and long-chain glucose polymers | 3 wk (each) | BMI inversely correlated with HDL-C response in men (r = −0.45); WHR and plasma insulin inversely correlated with HDL-C response (r = −0.30, r = −0.45 for men, and r = −0.38, r = −0.60 for women) |
| Cole et al., 1992 (15) | 19 (0/19) | Moderately hypercholesterolemic (BMI 28.2 kg/m2)2 | AAD (15.7% SFA) AHA Phase 3 diet (4.7% SFA) | 5 mo | BMI < 24 kg/m2 greatest response in TC, LDL-C, and HDL-C; BMI correlated with ↑ in VLDL-C (r = 0.596) and TG (r = 0.535) |
| Cox et al., 1995 (24) | 67 (28/39) | Hypercholesterolemic (BMI 22–30 kg/m2) | High-SFA diet (26% SFA,10% MUFA, 2% PUFA) High-PUFA diet (9% SFA, 6% MUFA, 23% PUFA) | 6 wk (each) | Hypo-responders had greater BMI (26.0 kg/m2) compared to hyper-responders (25.0 kg/m2), although not significant |
| Denke et al., 2000 (19) | 92 (46/46) | Adults (BMI 28.8 kg/m2)2 | Butter-added diet (16% SFA) Margarine-added diet (9% SFA) | 5 wk (each) | BMI > 30 kg/m2 less LDL-C lowering response compared to BMI < 21 kg/m2 (−0.23 ± 0.44 mmol/L vs. −0.34 ± 0.44 mmol/L) |
| 134 (71/63) | Children (BMI 19.3 kg/m2)2 | ||||
| Erlinger et al., 2003 (31) | 100 (48, 52) | Hypertensive (BMI 29.6 kg/m2)2 | Control diet (37% fat: 16% SFA; 300 mg/d cholesterol) DASH diet (27% fat, 6% SFA, 151 mg/d cholesterol) at 3 different sodium levels (150, 100, 50 mmol/d) | 30 d (each) | High CRP associated with significant ↓ LDL-C reduction compared to low CRP (−11.8 vs. −3%) |
| Hannah et al., 1997 (16) | 63 (30/33) | Moderately hypercholesterolemic (BMI 20–35 kg/m2) | Four diets (all 30% fat; 10% SFA): 17% MUFA, 3% PUFA 14% MUFA, 6% PUFA 10% MUFA, 10% PUFA 6% MUFA, 14% PUFA | 6 wk (each) | BMI inversely correlated with cholesterol response in women (r = −0.33), but not significant in men |
| Hilpert et al., 2005 (32) | 32 (14/18) | Hypercholesterolemic (BMI 26.16 kg/m2)2 | Step I diet with 25g/d soy protein isolate + 90 mg/d isoflavones Step I with 25 g/d milk protein isolate | 6 wk (each) | High CRP significantly ↑ LDL-C (4.8%), whereas low CRP ↓ LDL-C (−3.5%) |
| Jansen et al., 1998 (17) | 41 (41/0) | 1. BMI < 25 kg/m2 2. BMI 25–30 kg/m2 | SFA-rich diet (20% SFA) Step I diet (10% SFA) MUFA-rich diet (10% SFA) | 28 d (each) | BMI 25–30 kg/m2 ↓ LDL-C response compared to BMI < 25 kg/m2 (−9 vs. −21%) |
| Katan and Beynen, 1987 (23) | 32 (21/11) | Healthy adults | Three controlled trials of low-cholesterol followed by high-cholesterol diet | 28, 24, 54 d | BMI inversely correlated with cholesterol response (r = −0.50), consumption (r = −0.62), and synthesis (r = 0.40) |
| Knopp et al., 2004 (20) | 194 (75/119) | Insulin sensitive (BMI 23.2 kg/m2)2 | 0 eggs/d Step I diet | 1 mo (each) | BMI > 27.5 kg/m2 significantly ↓ LDL-C response than BMI < 27.5 kg/m2. ↓ LDL-C response associated with insulin resistance |
| Insulin resistant (BMI 24.5 kg/m2)2 | 2 eggs/d Step I diet | ||||
| Obese insulin resistant (BMI 31.5 kg/m2)2 | 4 eggs/d Step I diet | ||||
| Lefevre et al., 2005 (9) | 86 (86/0) | Healthy adults (BMI 19.6–33.2 kg/m2) | AAD (14.1% SFA) Step I diet (8.8% SFA) Step II diet (6.2% SFA) | 6 wk (each) | BMI and fasting insulin levels correlated with change in LDL-C (r = 0.22, r = 0.26) |
| Mukuddem-Peterson et al., 2007 (29) | 64 (29/35) | MetS (BMI ≥ 30 kg/m2) | Control diet: no nuts Walnut diet: 63–108 g/d (20% kcal) Cashew diet: 63–108 g/d (20% kcal) | 8 wk | No significant difference in lipid/lipoproteins between groups |
| Oh and Miller, 1985 (22) | 21 (21/0) | Normolipidemic | Hyper-responders: 3 eggs/d Hypo-responders: 6 eggs/d | 42 d | Hypo-responders had greater BMI (26.1 kg/m2) compared to hyper-responders (24.7 kg/m2) |
WHR, waist-to-hip ratio.
BMI reported as mean.
Dietary fat modifications designed to improve lipid and lipoprotein levels also appear to be less effective in obese individuals (15, 16, 19, 24, 26). Many clinical studies have reported a TC- and LDL-C–lowering effect of diets low in SFA and cholesterol and high in unsaturated fat provided by a variety of nuts. Based on these studies, an intake of 1–2 oz/d (∼28–57 g/d) of nuts was reported to reduce LDL-C by 2–19% (27, 28). However, a recent pooled analysis of intervention studies found that the lipid-lowering effects of nut consumption were greatest among participants with low BMI (26). In agreement with this analysis, Mukuddem-Petersen et al. (29) reported no lipid/lipoprotein effects of a weight maintenance diet that contained either walnuts or cashews in obese individuals. This indicates that the benefits of nut consumption are less when body weight is elevated.
Cole et al. (15) evaluated the effects of a low-fat, low-cholesterol diet in accordance with recommendations of the AHA Phase 3 diet (15% protein, 65% CHO, <20% fat, <200 mg cholesterol, and PUFA:SFA ratio > 1) on the lipid profile of moderately hypercholesterolemic premenopausal women. After 5 mo, the low-fat diet reduced TC and LDL-C from baseline by ∼7 and 11%, respectively (P < 0.05). TG levels increased by 20–30% over the first month and remained increased for the entire 5 mo period, whereas HDL-C levels decreased by 12% after 2 mo and 5% after 5 mo (P < 0.05). When individuals were classified according to BMI, the TC, LDL-C, and HDL-C levels decreased in the lean group (BMI < 24 kg/m2), whereas in the obese group (BMI > 30 kg/m2) the decrease in LDL-C was slightly less (∼10 vs. 12% in the lean group). Although obese women had an attenuated cholesterol-lowering response, BMI was correlated with change in VLDL-C (r = 0.596; P = 0.007) and TG levels (r = 0.535; P = 0.018) following the dietary intervention, demonstrating that obese women had a greater TG-raising response to a low-fat, low-cholesterol diet compared to lean women. Thus, not all individuals respond similarly to particular diet interventions; rather, it may be more appropriate to make diet recommendations on a person-to-person basis to account for individual variability and obesity-related complications. A crossover study by Hannah et al. (16) reported similar results using a low-fat diet with different proportions of SFA, MUFA, and PUFA. Baseline BMI and cholesterol levels were similar among men and women with moderately elevated cholesterol levels (LDL-C, 3.37–4.91 mmol/L); however, in women there was a relationship between BMI and the LDL-C–lowering effect (r = −0.35; P < 0.01) but not for men (r = −0.03; P = 0.50). A plausible explanation may be the greater amount of body fat (30.7 ± 8.1% vs. 21.5 ± 7.7%, respectively) and the distribution of central fat in women compared to men (16). Because a larger percentage of women are obese compared to men (35.5 vs. 32.2%, respectively), they may be more predisposed for altered lipid metabolism (30).
Denke et al. (19) conducted a 2-period crossover trial to determine if individual differences in lipid response were a familial trait. Families followed two 5-wk dietary periods, one with margarine (cholesterol-lowering diet) and the other with butter (cholesterol-raising diet), and received an individualized diet prescription for portion sizes and frequency of consumption of study foods. LDL-C was lower in the margarine group in adults (−0.41 mmol/L; P < 0.001) and children (−0.29 mmol/L; P < 0.001) compared to butter. Dietary responsiveness was determined by the LDL-C levels on the margarine diet minus the LDL-C levels on the butter diet. Although obese participants had higher baseline LDL-C levels, they had less of a LDL-C–lowering response compared to those with a BMI < 21 kg/m2 (−0.23 ± 0.44 vs. −0.34 ± 0.44 mmol/L, respectively). Predictive models demonstrated that for every 1-kg/m2 increase in BMI, LDL-C increased by 0.02 ± 0.006 mmol/L in children (P = 0.008) and in adults (P = 0.01). Therefore, the body weight of both children and adults predicted their lipid response to the test diets. Mixed linear models used to estimate the variance explained by family membership (shared genes and environment) indicated that family membership accounted for 19% of the variance in percent LDL-C change (P = 0.007) when adults and children were included in the same model. When considered separately, family membership explained 40% of the variability in percent LDL-C change (P = 0.002). This demonstrates that responsiveness to a cholesterol-lowering diet is a shared trait among families; whether these traits are habitual or heritable requires further study (19). However, it is clear that body weight may be a predictor of dietary responsiveness.
Inflammation decreases benefits of diet interventions
Inflammation, a common complication of obesity, affects the lipid response to dietary modifications (20, 31, 32). Elevated acute-phase C-reactive protein (CRP), a sensitive marker of systemic inflammation, is associated with higher BMI (33). Erlinger et al. (31) conducted an ancillary study to the Dietary Approaches to Stop Hypertension (DASH) trial to test whether CRP levels influence the cholesterol-lowering effects of a low-fat/low-cholesterol diet. Participants (the majority were overweight or obese African American women) with elevated blood pressure (systolic, 120–159 mm Hg and diastolic, 80–95 mm Hg) and relatively normal lipid levels (TC < 6.73 mmol/L) were randomly assigned to either the control (37% fat: 16% SFA, 13% MUFA, 8% PUFA) or DASH diet (27% fat: 6% SFA, 13% MUFA, 8% PUFA) for 12 wk. Individuals were divided into 2 groups based on CRP levels. Participants with baseline CRP levels below the median (<2.37 mg/L) significantly reduced TC by 9.8% (P < 0.0001) and LDL-C by 11.8% (P < 0.0001) following the DASH diet; however, participants with elevated CRP levels (>2.37 mg/L) experienced small, nonsignificant reductions in TC and LDL-C (3%; P ≥ 0.10). The DASH diet had no significant effect on TG in participants with low baseline CRP, whereas participants with high baseline CRP levels reported a significant increase in TG following the DASH diet (19.8%; P < 0.0001). This study demonstrates that higher CRP levels independent of weight status increased TG, with no significant improvements in TC and LDL-C, suggesting that inflammation attenuates the benefits of a healthy dietary intervention.
Similarly, Hilpert et al. (32) reported that individuals with high CRP levels were less responsive to a low-SFA, high-fiber diet with or without soy. Moderately hypercholesterolemic participants (TC > 5.27 mmol/L, LDL-C > 50th percentile, and TG < 90th percentile) were randomly assigned to a Step I diet with 25 g/d of soy protein or 25 g/d of milk protein for 6 wk in a crossover design. Only participants with low CRP (<3.5 mg/L) had decreases LDL-C (−3.5%; P < 0.01) and the LDL-C:HDL-C ratio (−4.8%; P < 0.01) compared to the run-in diet, independent of soy or milk consumption (P < 0.01). In contrast, participants with high CRP (>3.5 mg/L) had an increase in LDL-C (4.8%; P < 0.01) and the LDL-C:HDL-C ratio (5.2%; P < 0.01). The authors concluded that whereas consumption of a Step I diet would be beneficial for individuals with CRP levels < 3.5 mg/L, it also may not be effective for individuals with elevated CRP levels (32). Therefore, increased inflammation as evident by elevated CRP appears to blunt and quite possibly aggravate the lipid response to dietary modifications.
Insulin resistance diminishes lipid response independent of body weight
Adiposity-associated inflammation is closely linked to the development of insulin resistance (34). Clinical studies have demonstrated an association between insulin resistance and decreased lipid response to dietary SFA and cholesterol (Table 1) (9, 20, 35). Knopp et al. (20) evaluated the lipid response, particularly LDL-C change, to various amounts of egg yolks in a double-blind crossover study. Participants were randomized to insulin-sensitive, insulin-resistant, or obese insulin-resistant (BMI ≥ 27.5 kg/m2) groups and instructed to consume 0, 2, or 4 eggs/d or an equivalent egg substitute during three 1-mo intervention periods. The consumption of 4 eggs/d increased LDL-C from baseline in insulin-sensitive (7.8%; P < 0.001) and insulin-resistant participants (3.3%; P < 0.05) but not in obese insulin-resistant participants (2.4%; P > 0.05). A similar trend was reported for HDL-C changes; 4 eggs/d increased HDL-C from baseline in insulin-sensitive (8.8%; P < 0.001), insulin-resistant (5.2%; P < 0.001), and obese insulin-resistant participants (3.6%; P < 0.01). This study demonstrates that obese individuals are less responsive to dietary SFA and cholesterol but also that insulin resistance decreases the lipid response, independent of body weight.
Lefevre et al. (9) conducted a double-blind, crossover study to examine the effect of adiposity and insulin resistance on the lipid response to traditional Step I and Step II diets. In a randomized, crossover study, healthy male participants were fed 3 diets that varied in fat content for 6 wk each: an average American diet (AAD) consisting of 38% of energy as fat (14% SFA), Step I Diet (30% fat, 9% SFA), and the Step II diet (25% fat, 6% SFA). The Step I and Step II diets lowered LDL-C by 6.8 and 11.7% (P < 0.05) compared to the AAD; however, they also lowered HDL-C by 7.5 and 11.2% (P < 0.05) and increased TG by 14.3 and 16.2%, respectively (P < 0.05). BMI was correlated with the change in LDL-C when comparing the changes from the AAD to the Step II diet (r = 0.22; P = 0.04). Similarly, fasting insulin levels and the change in LDL-C were correlated (r = 0.26; P = 0.01). This demonstrates that elevated BMI and insulin levels decrease the lipid response to a lower fat diet that is low in SFA. The authors concluded that insulin resistance and elevated insulin levels, along with increased adiposity, diminish LDL-C reduction, increase TG levels, and ultimately increase the TC:HDL-C ratio in response to reductions in fat, SFA, and cholesterol (9).
Weight reduction improves insulin sensitivity and LDL uptake
Weight reduction is a standard intervention for obese and/or insulin-resistant individuals. By decreasing adiposity, obese individuals can lower inflammation and improve insulin sensitivity. Losing weight would be expected to contribute to a normalization of lipid metabolism. Numerous studies have reported beneficial effects of weight reduction on the postprandial lipid response in overweight individuals (36–39). However, obese and insulin-resistant participants may require more aggressive dietary interventions to alter the lipid response, specifically, a greater weight reduction. An improvement in lipid absorption would be expected following weight loss and/or an improvement in insulin sensitivity. Simonen et al. (38) evaluated cholesterol and lipoprotein metabolism in obese type II diabetics following weight reduction and whether changes could be maintained during a prolonged follow-up period. Participants were randomized to either a very low-energy diet or a low-energy diet for 3 mo followed by a 21-mo weight maintenance period (energy balance was zero) individually tailored by a registered dietitian. After 2 y, body weight decreased by 6.0 ± 1.0 kg (P < 0.01) with a 6% reduction in BMI (P < 0.05). Cholesterol absorption efficiency increased by 28% (P < 0.05) and the proportion of plant sterols in serum increased by 20–31% (P < 0.05), although cholesterol synthesis (the difference between fecal steroids of cholesterol origin and dietary cholesterol) did not significantly change. Also, the TG content of serum, VLDL, LDL, and HDL was reduced by 13–24% (P < 0.05) and blood glucose was reduced by 14% (P < 0.05), with a nonsignificant decrease in serum insulin. Therefore, the authors concluded that weight reduction tended to normalize cholesterol metabolism in addition to improving glucose metabolism, demonstrating that insulin resistance and cholesterol absorption efficiency are interrelated (38). These results suggest that weight reduction improves insulin sensitivity and LDLR binding, which would be expected to enhance the lipid response to dietary changes by increasing clearance of remnant-like particles (RLP).
James et al. (37) demonstrated that a weight loss of 10 kg in obese, insulin-resistant males improved insulin sensitivity as assessed by a lower homeostatic model assessment (HOMA) score. The HOMA score is the product of fasting insulin and fasting glucose divided by 22.50 (40). It typically is used to estimate an individual’s state of insulin resistance and β-cell function. BMI and HOMA score were positively correlated (r = 0.567; P < 0.01), signifying the association between obesity and insulin resistance. The 10-kg weight loss also increased LDLR binding by 27.5% (P < 0.05). Although no significant change in cholesterol synthesis was reported, weight loss was correlated with decreases in TC and LDL-C (r = 0.635, P < 0.05; r = 0.738, P < 0.01). In addition, no change in postprandial TG response was observed following weight loss. The authors concluded that a greater change in LDLR binding would be required to alter chylomicron metabolism and in effect improve clearance of hepatic lipoproteins (37). Similarly, a greater improvement in insulin sensitivity may be necessary to significantly reduce cholesterol synthesis (37). Volek et al. (39) found that obese women who lost a modest amount of weight on either a low-CHO diet (−2.96 ± 1.45 kg) or a low-fat diet (−1.06 ± 2.07 kg) significantly improved the AUC for TG following a fat-rich meal (−29 and −25%, respectively). Insulin sensitivity only improved after the low-CHO diet; however, fasting insulin levels in both diets were lower than the post-weight loss amount reported by James et al. (37.4 ± 16.1 pmol/L for low CHO and 50.5 ± 33.9 pmol/L for low fat vs. 56.25 ± 6.94 pmol/L). This may explain why Volek et al. (39) observed an improved postprandial lipid response following small weight reductions regardless of whether the participant was following a low-CHO or low-fat diet.
Dallongeville et al. (36) conducted a similar intervention study to assess the lipid response to a high-fat or high-CHO meal before and after weight loss. Obese women followed an energy-restricted diet (800 kcal/d) for 7 wk and reduced their body weight by ∼10% (P < 0.0001) with a concomitant decrease in TG (P = 0.0102), TC (P < 0.0001), LDL-C (P = 0.0003), and HDL-C (P = 0.0009). Postprandial reduction in the AUC for insulin was correlated with BMI (r = 0.50; P = 0.045). Weight loss was associated with lower postprandial TG (P < 0.0003), TC (P < 0.0001), and HDL-C (P < 0.002) following both high-fat and high-CHO meals. Even after adjusting for baseline values, postprandial TG remained significantly lower after both meals, suggesting that weight loss can improve the postprandial TG response beyond simply decreasing baseline lipid levels (36). It can be assumed that weight loss enhances insulin sensitivity in part by decreasing adipose tissue-induced inflammation. RLP-cholesterol concentrations were lower after the test meals, but the response did not differ from the pre-weight loss values. Therefore, a 10% reduction in body weight was insufficient to improve the RLP-cholesterol clearance following a high-fat or high-CHO meal (36). Considering the participants were still obese following weight reduction (BMI, 33.5 ± 4.6 kg/m2), additional weight loss would be expected to further improve insulin sensitivity and potentially augment RLP-cholesterol and chylomicron clearance.
Considering that adipocyte function seems to differ depending on the anatomical location of the tissue depot, measuring the distribution of adipose tissue may help predict how an obese individual will respond to weight loss and/or dietary interventions Abdominal adiposity is strongly associated with insulin resistance, hence the use of waist circumference to help identify MetS, because abdominal obesity is highly correlated with metabolic risk factors (41). Collectively, abdominal adiposity appears to be positively associated with an abnormal postprandial lipid response (35, 42). Thus, a reduction in adipose tissue from the abdominal region would be expected to result in greater lipid responsiveness to dietary modifications. Any decrease in body fat, especially adipose tissue from the abdominal region, likely would enhance insulin sensitivity and lead to considerable improvements in the postprandial lipid response to dietary SFA and cholesterol.
Potential mechanisms altering lipid metabolism
Cholesterol synthesis impairs LDL uptake.
Dietary cholesterol enters the pool of cholesterol that is transported to the liver, where it can suppress synthesis of LDLR, causing an increase in the conversion of VLDL remnants to LDL and a decrease in cholesterol clearance from the plasma, thereby increasing LDL-C levels (43). Regulation of this by the sterol regulatory element binding protein (SREBP) was first introduced by Brown and Goldstein (44). SREBP regulate transcription of several genes involved in the metabolism and absorption of cholesterol and lipids, determining LDLR activity based on the cholesterol needs of the membranes. When cellular cholesterol levels are low, SREBP are cleaved and translocated to the nucleus to activate transcription of the LDLR gene as well as genes encoding for HMG-CoA reductase, the rate-limiting enzyme in cholesterol synthesis. This causes an increase in cholesterol uptake and synthesis to meet membrane cholesterol requirements. In contrast, influx of dietary cholesterol suppresses synthesis of LDLR by preventing SREBP cleavage, reducing cholesterol uptake by the cells, in addition to suppressing cholesterol synthesis by inhibiting enzymes responsible for cholesterol production, specifically HMG-CoA reductase. However, higher rates of cholesterol synthesis in obese individuals generate increased amounts of circulating cholesterol, suppressing synthesis of LDLR and increasing LDL-C levels as a result of decreased clearance (23, 45). Thus, due to the large amount of cholesterol already present from greater cholesterol synthesis, the increased dietary cholesterol would have little to no effect on the already suppressed LDLR. Increased hepatic cholesterol also generates oxysterols, the oxygenated derivatives of cholesterol. Oxysterols are important intermediates or end products in cholesterol excretion as well as modulators of other biological processes. The addition of oxygen to cholesterol decreases the half-life and promotes the degradation and excretion of oxysterols, which traverse lipophilic membranes and the blood-brain barrier much faster than cholesterol (46). Furthermore, oxysterols act as ligands for liver X receptors (LXR) to stimulate reverse cholesterol transport (RCT) and bile acid synthesis in order to prevent cholesterol overload in the cell (47). Bile acids are synthesized from cholesterol in the liver and secreted into the intestine to facilitate lipid absorption where they are reabsorbed and transported back to the liver. Activating cholesterol 7α hydroxylase (CYP7A1), the rate-limiting enzyme of bile acid synthesis, increases the conversion of cholesterol to bile acids and stimulates biliary cholesterol excretion. However, regulation of CYP7A1 is species specific. In rodents, LXR activation induces CYP7A1, yet in humans LXR activation has little effect and may actually repress CYP7A1 (48). This could explain in part human susceptibility to diet-induced hypercholesterolemia. However, LXR inhibition of CYP7A1 may actually be a physiologic adaptation to limit bile acid formation and emulsification of dietary lipids, thereby reducing intestinal cholesterol absorption (48). Rudel et al. (49) demonstrated that monkeys fed a high-fat, high-cholesterol diet lowered CYP7A1 mRNA levels, decreased bile acid production, and reduced intestinal cholesterol absorption. Therefore, increased dietary and endogenous cholesterol associated with obesity may lower intestinal cholesterol absorption by inhibiting bile acid synthesis and excretion via LXR activation. Clinical studies are needed though to determine the effects of high-fat and high-cholesterol diets on cholesterol absorption and regulation of bile acid pool size. In addition, LXR activation also stimulates lipogenesis by inducing SREBP-1c transcription, which can lead to hypertriglyceridemia (50). Therefore, further evidence is necessary to understand the various effects of LXR activation in obese individuals.
Adipose tissue#x2013induced inflammation.
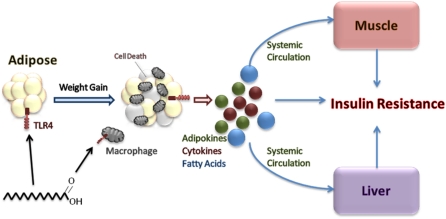
Excess adipose tissue results in inflammation that leads to insulin resistance (Fig. 1). The increase in adipocyte size and ensuing expansion of adipose tissue mass increases FFA release into the circulation and decreases oxygen delivery to the cells (8). This leads to an increase in cellular stress, adipocyte death, and expression of inflammatory genes, enhancing the activation of the proinflammatory c-Jun N-terminal kinase 1 (JNK1) and inhibitor of kappaB kinase (IKK)/NF-κB pathways (51, 52). Macrophages accumulate in the adipose tissue and remodel the tissue (8). Additional proinflammatory cytokines (TNFα and IL-6) and chemokines are released, which can initiate the JNK1 and IKK/NF-κB pathways in nearby adipocytes, causing further macrophage recruitment to local sites of injury, or circulate to the liver and initiate a similar process. Adipocytes also secrete a variety of adipokines, many of which affect insulin sensitivity. For instance, leptin and adiponectin have been shown to promote insulin sensitivity, whereas resistin and retinol-binding protein 4 interfere with insulin action and diminish insulin sensitivity (8, 53, 54). Shi et al. (55) observed that adipose tissue and macrophages exposed to fatty acids also can trigger the release of proinflammatory cytokines through Toll-like receptors (TLR), specifically TLR4. Specific nod-like receptors also are important for upregulating transcription of proinflammatory genes via NF-κB and MAPK by sensing intracellular microbial components (56). However, Vandanmagsar et al. (57) recently found that the nod-like receptor, pyrin domain-containing-3 (Nlrp3) inflammasome are involved in the recognition of nonmicrobial “danger signals” that cause caspase-1 activation and the secretion of proinflammatory cytokines, particularly IL-1β and IL-18. Expression of Nlrp3 mRNA in abdominal subcutaneous adipose tissue of 10 obese men with type II diabetes was examined to determine the effects of a 1-y weight loss intervention (≥7% of initial body weight) through decreased caloric intake and increased physical activity. Following weight loss, relative Nlrp3 mRNA expression in adipose tissue decreased from 5.9 ± 2.4 to 3.0 ± 0.8 (P < 0.05), coupled with lower glycemia and improved insulin sensitivity (57). The authors concluded that the expression of Nlrp3 inflammasome senses obesity-associated danger signals, thereby inducing inflammation and the subsequent downstream effects on insulin signaling (57).
Figure 1.
Excess adipose tissue leads to insulin resistance. Weight gain and excess nutrition increase adipose tissue and adipocyte size. Decreased oxygen delivery and elevated stress occur within the adipocytes, resulting in cell death, initiation of the inflammatory response, and recruitment of macrophages to the site of injury. Exposure to fatty acids can initiate the inflammatory process as well via TLR4 on adipocytes and macrophages. Release of proinflammatory cytokines further activates the inflammatory process in nearby adipocytes, resulting in localized insulin resistance. Proinflammatory cytokines, adipokines, and fatty acids also enter systemic circulation, causing insulin resistance in both liver and muscle.
Eventually, an inflammatory environment in insulin target cells, specifically adipocytes and hepatocytes, causes localized insulin resistance due to stimulation of adipocyte lipolysis and complications associated with the inflammatory response, including reduced adiponectin, increased resistin, and increased hepatic glucose production (8). Activation of JNK1 and IKKB reduces the effects of insulin on glucose uptake by phosphorylation of insulin receptor substrate, thereby diminishing downstream insulin signaling (58). The proinflammatory cytokine TNFα can contribute independently to insulin resistance by reducing insulin receptor expression, insulin receptor substrate and GLUT4 gene expression, adiponectin, hormone sensitive lipase, and insulin-mediated glucose uptake (59, 60). CRP is synthesized and secreted by the liver in response to proinflammatory cytokines, specifically IL-6 (33). Not surprisingly, TNFα, IL-6, and CRP typically are elevated in insulin-resistant states (33, 61–63). Food intake can stimulate the production of TNFα and IL-6, leading to an increase in CRP levels, but food ingestion also can stimulate production of satiety factors (CCK and leptin) and incretins responsible for augmenting insulin sensitivity. PUFA are capable of binding to PPARγ, a ligand-activated transcription factor highly expressed in adipose tissue, which when activated can squelch the NF-κB pathway and inhibit cytokine production (64). In addition, important resolution metabolites (resolvins and protectins) are synthesized from EPA+DHA and act as potent antiinflammatory and immunoregulatory agents. Therefore, the reduction in CVD risk reported when SFA is replaced with PUFA may be due in part to inhibition of proinflammatory cytokine production. Insulin secretion can stimulate the synthesis of long-chain PUFA (LCPUFA), which would be expected to enhance insulin action and reduce oxidative stress by suppressing the synthesis of TNFα and IL-6. However, SFA, trans-fatty acids, and hyperglycemia interfere with LCPUFA synthesis [both (n-6) and (n-3) fatty acid pathways], thereby inhibiting the control of LCPUFA on TNFα and IL-6 as well as impairing the nutrient-sensing system responsible for enhancing insulin sensitivity (65). LCPUFA-CoA activates neural pathways that regulate plasma glucose levels by producing counter-regulatory responses, including brain-derived neurotrophic factor, in the hypothalamus and the intestine to reduce food intake and increase insulin sensitivity (65). The increase in lipid availability is sensed by the hypothalamus, thereby regulating glucose production in response (66). Prolonged fat consumption can diminish this regulatory system by increasing the amount of circulating FFA (66, 67). Sustained elevation of FFA hinders the feedback regulation from the hypothalamus to the liver, pancreas, and gut (65). Skeletal muscle often develops insulin resistance as a result of enhanced fatty acid flux from cytokine-stimulated adipocyte lipolysis (8). In an obese individual, lipid also can accumulate in the muscle and liver independent of adipocyte lipolysis, initiating a proinflammatory state and the development of insulin resistance. Inflammation has been demonstrated to impair RCT at various steps in the pathway, conserving cholesterol stores in the body and preventing cholesterol flux through liver to bile and feces (68). The inhibition of RCT likely contributes to insulin resistance and MetS, negatively altering the lipid profile and potentially accelerating the development of CVD. The assortment of connections between inflammation and insulin resistance continues to expand; however, a detailed description is beyond the focus of this review. The key point is that excess adipose tissue and nutrient intake causes an increase in inflammation, which leads to the development of insulin resistance and the ensuing decrease in lipid response to changes in dietary SFA and cholesterol.
Insulin resistance promotes lipid synthesis and excretion.
Cholesterol synthesis typically is greater than cholesterol absorption in an insulin-resistant state. Postprandial hyperinsulinemia results in defective clearance of endogenous and exogenous TG-rich lipoproteins (35). Insulin normally enhances LDLR activity, but in the presence of insulin resistance, LDLR activity is blunted and LDL binding declines, resulting in impaired receptor-mediated LDL-C removal and decreased chylomicron remnant clearance (69). The decrease in LDL-C uptake leads to an increase in endogenous cholesterol production, perhaps by the stimulation of LXRα. Hyperinsulinemia has been shown to stimulate LXRα (70), which is known to regulate lipogenesis and cholesterol excretion (70). However, it is possible that decreased cholesterol absorption is secondary to increased cholesterol synthesis. Both states are related to insulin resistance; therefore, concurrent changes in both cholesterol absorption and synthesis make it difficult to determine which state is affected primarily by insulin resistance. Greater cholesterol production likely would lead to a further decline in LDLR activity and, consequently, a resistance to reductions in LDL-C that are associated with dietary fat and cholesterol modifications (9).
Disruption of insulin signaling and stimulation of the LXRα pathway increases the expression of intestinal ATP-binding cassette (ABC) transporters, specifically ABCG5 and ABCG8 (71, 72). ABCG5 and ABCG8 regulate the secretion of cholesterol and sterols from intestinal enterocytes into the intestinal lumen and from hepatocytes into the biliary space (73). Therefore, upregulation of ABCG5 and ABCG8 promotes biliary cholesterol secretion and decreased cholesterol absorption, which leads to the increase in hepatic cholesterol synthesis (74). High-cholesterol and high-fat diets also have been shown to increase the mRNA of ABCG5 and ABCG8 (75, 76). In addition, single nucleotide polymorphisms in the ABCG5 and ABCG8 genes can alter cholesterol metabolism and various lipid responses (77, 78). Thus, ABCG5 and ABCG8 are important factors to consider in regulating endogenous cholesterol homeostasis. Hyperinsulinemia enhances expression of the ABCG5 and ABCG8 genes, stimulating cholesterol excretion and decreasing cholesterol absorption. However, proinflammatory cytokines, IL-6 and TNFα, as well as insulin, have been shown to inhibit CYP7A1 gene transcription, thereby decreasing bile acid synthesis as an adaptive response to protect hepatocytes from injury (79, 80). Evidence suggests that these cell-signaling pathways crosstalk to regulate bile acid synthesis to maintain hepatic bile acid homeostasis (80). Bile acid sequestrants, common drugs used to treat hypercholesterolemia, work by binding to bile acids in the intestine and preventing them from being reabsorbed into the circulation. This decrease in bile acids stimulates CYP7A1 to synthesize more bile acids from cholesterol, resulting in lower plasma cholesterol levels.
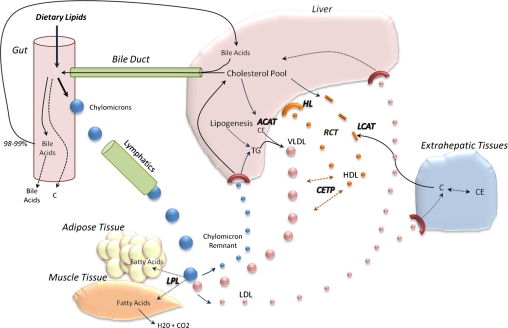
In contrast, Niemann-Pick C1 Like 1 (NPC1L1) protein regulates cholesterol influx into intestinal enterocytes (81). NPC1L1 is expressed in the liver as well, although its functions there are less well understood. Variations in the NPC1L1 gene can affect lipid responses to dietary modifications (77, 82). Diets high in cholesterol downregulate the expression of NPC1L1 and reduce cholesterol absorption (83). This is consistent with the SREBP pathway. Recent evidence suggests that NPC1L1 may affect transcription factors responsible for regulating insulin sensitivity and lipid metabolism (84). Several studies have shown that inhibition of NPC1L1 in rats significantly reduces cholesterol uptake, lowers weight gain, and decreases insulin resistance (85–87). Although lower lipid levels have been associated with NPC1L1 inhibition in humans, the effects on insulin sensitivity have yet to be elucidated. It certainly is possible though that increased adiposity and insulin resistance alters lipid metabolism by decreasing NPC1L1 gene expression. The cholesterol-lowering drug Zetia (Merck and Schering-Plough) (ezetimibe) works by inhibiting NPC1L1, thereby decreasing intestinal cholesterol absorption and possibly dietary fat as well (85). Adipose tissue lipoprotein lipase (LPL) also can become resistant to the effects of insulin, thereby amplifying postprandial hypertriglyceridemia through decreased TG clearance (88). Elevated VLDL due to diminished LPL activity would decrease HDL-C through the action of cholesterol ester transfer protein (CETP). CETP regulates the transport of cholesterol esters from HDL and LDL to VLDL in exchange for TG; when VLDL concentrations are high, as often is the case with insulin resistance, HDL and LDL molecules become TG rich (Fig. 2). Hepatic lipase hydrolyzes the TG and phospholipids of HDL and LDL, resulting in smaller lipid-depleted particles. Due to the very small size, surface apoA-1 of the HDL particle can be filtered and degraded in the kidney, leading to a reduction in HDL-C. Small LDL particles, too large for renal clearance, have a lower affinity for LDLR and longer half-life compared to large LDL particles; thus, they remain in circulation for a longer duration (89, 90). Evidence suggests that small LDL particles are more susceptible to oxidative stress, can more effectively penetrate the arterial wall, and have increased binding to arterial proteoglycans (89, 90). Therefore, decreased HDL-C in combination with increased small LDL particles associated with the decline in LPL activity could potentially alter normal lipid metabolism and significantly augment CVD risk. These lipoprotein changes in addition to increased cholesterol synthesis and decreased cholesterol absorption all relate to insulin resistance initiated by adipose tissue-induced inflammation.
Figure 2.
Lipid metabolism and transport. Dietary fat and cholesterol are transported as chylomicrons through the lymphatic system. LPL hydrolyze TG in chylomicrons, releasing glycerol and FFA. Cholesterol and TG in the liver get packaged and transported as VLDL. Lecithin-cholesterol acyltransferase (LCAT) esterifies free cholesterol (C), forming the core of newly synthesized HDL molecules. CETP transfers TG to HDL in exchange for cholesterol ester (CE), whereas LPL hydrolyzes TG in VLDL, resulting in dense LDL molecules taken up by extrahepatic tissues and/or liver. Lipid-rich HDL gets taken back up by the liver in a process known as RCT. Cholesterol can also be used to synthesize bile acids and/or get excreted. ACAT, acyl-CoA:cholesterol acyltransferase; HL, hepatic lipase.
Neuroendocrine regulation of lipid uptake
Lipid metabolism also is regulated by the neuroendocrine system. The brain influences the metabolic response to food intake by altering hormonal regulation. In addition, the vagus nerve allows for crosstalk between adipose tissue and the liver. Multiple studies have shown that obese individuals exhibit different ingestive behaviors than lean individuals (91, 92); therefore, it is possible that unique neural connections also influence lipid metabolism. For example, neuron-specific disruption of the insulin receptor gene has been shown to cause insulin resistance as well as increase body fat and plasma leptin levels in mice, with no effect on brain development (93). This section highlights a few of the many hormones that likely have a role in the impaired LDL-C uptake associated with obesity.
Growth hormone.
Growth hormone (GH) stimulates LDLR expression and increases clearance of plasma LDL-C (94, 95). Rudling et al. (96) demonstrated that GH was responsible for resistance to hypercholesterolemia in cholesterol-fed rats. The presence of GH maintained hepatic LDLR resistance to the suppressive action of dietary cholesterol in rats, suggesting that decreased GH secretion would increase sensitivity to dietary cholesterol, thereby causing increased plasma LDL-C levels. In addition, administration of GH to aging rats restored bile acid synthesis to levels observed in younger rats (97). It is possible that the age-dependent decline in GH contributes to the increase in lipid levels associated with aging as well as obesity. Compared to normal weight individuals, those who are obese have lower GH production, decreased GH secretion, and a shorter GH half-life (98). Therefore, it would be expected that obese individuals also have decreased LDLR expression and less lipid clearance.
GH also is important for the expression of hepatic estrogen receptors. Estrogen has a stimulatory effect on the expression of LDLR; thus, higher lipid levels associated with postmenopausal women may be attributed in part to the reduction in estrogen (99). Elevated estrogen levels upregulate expression of NPC1L1, in addition to ABCG5 and ABCG8, via the intestinal estrogen receptor-α pathway (100). Although expression of ABCG5 and ABCG8 increases, the net effect appears to favor the influx of cholesterol into the enterocyte. This supports the theory that cholesterol absorption efficiency is determined by the net effect between influx and efflux. There is some evidence that estrogen stimulates HMG-CoA reductase, the rate-limiting step in cholesterol synthesis (100, 101). As a result, cholesterol production increases with a concomitant increase in the excretion rate of biliary cholesterol, thereby augmenting endogenous cholesterol from bile into the intestine for absorption by the enterocyte (100). Collectively, the biological effects of estrogen on lipid metabolism suggest that women would respond differently than men. If estrogen stimulates LDLR expression, it would be hypothesized that women respond greater to dietary modifications based on the enhanced lipid absorption. Men with gallstone disease reported a greater plasma clearance of LDL-C when treated with estrogen, indicating that estrogen stimulated LDLR expression (101). However, Hannah et al. (16) observed that BMI was inversely correlated with lipid response in women, but not men. Therefore, additional mechanisms may be altering the neuroendocrine regulation of lipid metabolism.
Thyroid hormones.
The relationship between obesity and thyroid hormones (TH) is controversial. Hypothyroidism often is associated with weight gain, whereas hyperthyroidism is connected with weight loss. Although it is logical to conclude that a decrease in TH is associated with obesity, based on greater body weight and elevated lipid levels, this may not be the case. Thyroid-stimulating hormone (TSH), also known as thyrotropin, regulates the thyroid gland secretion of thyroxine (T4), and triiodothyronine (T3). T3 is the primary TH acting on tissues. In the liver and peripheral tissues, 5-deiodinase converts T4 to T3, but during lower energy intake, 5-deiodinase is inhibited. This leads to a decrease in metabolic rate and a greater opportunity for weight gain. A positive correlation between TSH and BMI has been demonstrated in several studies (102–104). The increase in TSH is likely an adaptation to avoid storing energy as fat. Elevated TH levels result in a greater metabolic rate, but in a fasting state, TH levels decrease with a parallel reduction in metabolic rate. Thus, reducing caloric intake may not be sufficient to treat obesity. Weight loss has been shown to normalize TH levels, indicating that elevated TSH and T3 may be a consequence rather than a cause of obesity (105). TSH also has been related to insulin resistance, yet considering both are affected by adiposity, it is difficult to establish any casual relationship (105). Therefore, the association between TH and obesity is not entirely understood.
Early clinical evidence reported an inverse correlation between plasma cholesterol levels and TH (106). TH has been shown to upregulate the gene expression of LDLR via a TH responsive element on the LDLR gene and stimulates enzymes involved in lipid metabolism in part by activating SREBP (107–109). Shin and Osborne (109) hypothesized that as TH levels fall, SREBP-2 levels decrease and there is a resulting decrease in LDLR mRNA. Therefore, the decline in SREBP-2 resulting from less TH can cause a reduction in LDL-C uptake by the liver, leading to higher plasma lipid levels. TH also stimulates cholesterol synthesis by inducing HMG-CoA reductase activity (108), although overall LDL-C levels tend to decrease due to the increase in LDLR activity and cholesterol excretion in bile. In addition, CETP, LPL, and hepatic lipase are all stimulated by TH, resulting in a decrease in HDL-C. Therefore, lower TH levels associated with hypothyroidism would be expected to cause an increase in plasma lipid levels, specifically LDL-C, and decrease the lipid response to changes in dietary SFA and cholesterol. Clearly, more research is needed to understand how hormones regulate lipid metabolism and how variations in hormone secretion induced by an increase in adiposity affect the response of plasma lipids to dietary SFA and cholesterol.
Glucocorticoids.
Abnormalities in glucocorticoid signaling may contribute to the altered lipid metabolism observed in MetS and obesity. Glucocorticoids are produced in response to stress, providing potent antiinflammatory actions that can suppress TNFα synthesis; however, glucocorticoids also have been shown to impair glucose utilization and clearance, thereby inducing hyperglycemia and eventual insulin resistance (110). Glucocorticoids bind the glucocorticoid receptor (GR), translocating the GR complex to the nucleus and activating transcription of antiinflammatory genes via glucocorticoid response elements. GR can then bind to several other different transcription factors, such as NF-κB, and decrease gene transcription (110). A number of studies have shown that obese individuals have lower central GR response to circulating glucocorticoids, indicating less sensitivity to glucocorticoid negative feedback regulation (111, 112). This suggests that excess adipose tissue can cause opposing downstream events that closely interact during the inflammatory process and formation of insulin resistance.
Cortisol is the principal glucocorticoid in the body. Cortisol acts as an antagonist to insulin. The resulting increase in plasma glucose can induce insulin resistance (113), hence the reported association of glucose intolerance with abnormal cortisol actions, independent of obesity (114). Elevated cortisol levels also depress TSH secretion, which would inhibit the conversion of T4 to T3, leading to a reduction in LDL-C uptake by the liver (115). Obese individuals may have greater cortisol secretion due to adipose tissue-induced inflammatory stress; however, it is unclear whether increased cortisol secretion is a cause or consequence of obesity. Collectively, abnormal cortisol secretion associated with excess body fat would be expected to decrease lipid uptake by impairing insulin action, thereby leading to increased plasma lipid levels.
Gut microbiota
Recent evidence suggests that gut microbiota (microorganisms living in the gastrointestinal tract), which differ between obese and lean individuals, play a pivotal role in metabolism and may explain in part why certain individuals are more prone to obesity (116). Firmicutes is the predominant bacteria in the gut of obese humans. Firmicutes break down hard-to-digest polysaccharides, allowing for enhanced digestion and absorption, thereby increasing energy absorption (65). However, following weight loss, the amount of Firmicutes becomes similar to that of lean individuals (117). These changes in gut microbiota may influence the alterations in lipid metabolism associated with obesity.
Gut microbiota have been shown to regulate plasma lipids by contributing to bile acid metabolism. Primary bile acids synthesized in the liver can be metabolized into secondary bile acids by intestinal bacteria through the process of deconjugation and dehydroxylation. Excessive bile acid production associated with obesity likely would lead to a greater amount of secondary bile acids. Some evidence suggests that elevated secondary bile acids can cause DNA damage and contribute to a wide range of disease states from MetS to cancer (118). Further studies are needed though to better understand the effects of primary and secondary bile acids on lipid metabolism and how changes in gut microbiota may affect the composition of the bile acid pool.
Although less understood, certain components of the outer membrane of Gram-negative bacteria in the intestine, such as LPS, could trigger inflammatory responses, causing the development of insulin resistance and the ensuing decrease in lipid response. Creely et al. (119) discovered that individuals with type II diabetes had 76% more circulating LPS than healthy lean participants. LPS activated the innate immune pathway and stimulated the secretion of proinflammatory cytokines, likely explaining the presence of insulin resistance in the type II diabetics. High-fat and/or high-energy diets also may increase plasma LPS in humans. Cani et al. (120) found that mice fed high-energy diets for 4 wk significantly increased the amount of LPS-containing microbiota in the gut. In addition, higher fat intake led to greater amounts of LPS in plasma. This suggests that fat may be more efficient than CHO in transporting LPS from the gut into circulation (121). Enhanced LPS absorption by high-fat intake can stimulate the secretion of TNFα and IL-6, resulting in low-grade systemic inflammation (121). Therefore, gut microbiota could have an active role in the promotion and/or inhibition of inflammation and thus a profound effect on lipid metabolism. Research in this area continues to gain more attention with the discovery of new symbiotic functions for a variety of intestinal microbes. Clinical studies are needed to determine the activity of intestinal bacteria and the effects of diet and obesity on altering gut microbiota and the immune system.
Conclusions
A cholesterol-lowering diet favorably modifies plasma lipids and lipoproteins; however, the responses typically are blunted by excess adipose tissue and complications associated with obesity, including insulin resistance. Substitution of MUFA and PUFA for SFA and/or CHO decreases LDL-C, a response that is blunted in overweight/obese individuals and in response to insulin resistance and inflammation. There is a pressing need to better understand the mechanisms by which adiposity alters normal lipid metabolism. Although several explanations have been proposed, it is unclear what the mechanisms are that explain the decreased response to diet observed in obesity. Several complications of obesity regulate lipid metabolism either by increasing synthesis or decreasing absorption of lipids. This clearly is the case with insulin resistance resulting from adipose tissue-induced inflammation, leading to changes in enzyme activity, especially HMG-CoA reductase and LPL, needed for normal lipid metabolism. Additional factors, including specific hormone changes and gut microbiota, also hinder the lipid response to changes in dietary SFA and cholesterol. In contrast, a reduction in adipose tissue mass enhances LDLR binding by decreasing inflammation and augmenting insulin sensitivity. Consequently, weight loss is recommended for overweight/obese individuals to realize the maximal benefits of dietary interventions low in SFA and cholesterol.
Acknowledgments
All authors wrote the paper. M.R.F. had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Author disclosures: M. R. Flock, M. H. Green, and P. M. Kris-Etherton, no conflicts of interest.
Abbreviations used: ABC, ATP-binding cassette; AAD, average American diet; CETP, cholesterol ester transfer protein; CHO, carbohydrate; CRP, C-reactive protein; CVD, cardiovascular disease; CYP7A1, cholesterol 7α hydroxylase; DASH, Dietary Approaches to Stop Hypertension; GH, growth hormone; GR, glucocorticoid receptor; HDL-C, HDL cholesterol; HOMA, homeostatic model assessment; IKK, inhibitor of kappaB kinase; JNK1, c-Jun N-terminal kinase 1; LCPUFA, long-chain PUFA; LDL-C, LDL cholesterol; LDLR, LDL receptor; LPL, lipoprotein lipase; LXR, liver X receptor; MetS, metabolic syndrome; Nlrp3, nod-like receptor, pyrin domain-containing-3; NPC1L1, Niemann-Pick C1 Like 1; RCT, reverse cholesterol transport; RLP, remnant-like particle; SREBP, sterol regulatory element binding protein; T3, triiodothyronine; T4, thyroxine; TC, total cholesterol; TH, thyroid hormone; TLR, Toll-like receptor; TSH, thyroid-stimulating hormone.
Literature Cited
- 1.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52 [DOI] [PubMed] [Google Scholar]
- 2.Garrison RJ, Wilson PW, Castelli WP, Feinleib M, Kannel WB, McNamara PM. Obesity and lipoprotein cholesterol in the Framingham offspring study. Metabolism. 1980;29:1053–60 [DOI] [PubMed] [Google Scholar]
- 3.Keys A, Aravanis C, Blackburn H, Van Buchem FS, Buzina R, Djordjevic BS, Fidanza F, Karvonen MJ, Menotti A, et al. Coronary heart disease: overweight and obesity as risk factors. Ann Intern Med. 1972;77:15–27 [DOI] [PubMed] [Google Scholar]
- 4.Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2003;163:427–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol. 2008;28:629–36 [DOI] [PubMed] [Google Scholar]
- 6.Boerwinkle E, Brown SA, Rohrbach K, Gotto AM, Jr, Patsch W. Role of apolipoprotein E and B gene variation in determining response of lipid, lipoprotein, and apolipoprotein levels to increased dietary cholesterol. Am J Hum Genet. 1991;49:1145–54 [PMC free article] [PubMed] [Google Scholar]
- 7.Tikkanen MJ, Xu CF, Hamalainen T, Talmud P, Sarna S, Huttunen JK, Pietinen P, Humphries S. XbaI polymorphism of the apolipoprotein B gene influences plasma lipid response to diet intervention. Clin Genet. 1990;37:327–34 [DOI] [PubMed] [Google Scholar]
- 8.Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest. 2008;118:2992–3002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lefevre M, Champagne CM, Tulley RT, Rood JC, Most MM. Individual variability in cardiovascular disease risk factor responses to low-fat and low-saturated-fat diets in men: body mass index, adiposity, and insulin resistance predict changes in LDL cholesterol. Am J Clin Nutr. 2005;82:957–63 [DOI] [PubMed] [Google Scholar]
- 10.Gylling H, Miettinen TA. Cholesterol absorption, synthesis, and LDL metabolism in NIDDM. Diabetes Care. 1997;20:90–5 [DOI] [PubMed] [Google Scholar]
- 11.Berglund L, Lefevre M, Ginsberg HN, Kris-Etherton PM, Elmer PJ, Stewart PW, Ershow A, Pearson TA, Dennis BH, et al. Comparison of monounsaturated fat with carbohydrates as a replacement for saturated fat in subjects with a high metabolic risk profile: studies in the fasting and postprandial states. Am J Clin Nutr. 2007;86:1611–20 [DOI] [PubMed] [Google Scholar]
- 12.Lichtenstein AH, Matthan NR, Jalbert SM, Resteghini NA, Schaefer EJ, Ausman LM. Novel soybean oils with different fatty acid profiles alter cardiovascular disease risk factors in moderately hyperlipidemic subjects. Am J Clin Nutr. 2006;84:497–504 [DOI] [PubMed] [Google Scholar]
- 13.USDA Dietary Guidelines for Americans, 2010 [cited 2011 Feb 2]. Available from: http://www.health.gov/dietaryguidelines/dga2010/DietaryGuidelines2010.pdf
- 14.Mustad VA, Etherton TD, Cooper AD, Mastro AM, Pearson TA, Jonnalagadda SS, Kris-Etherton PM. Reducing saturated fat intake is associated with increased levels of LDL receptors on mononuclear cells in healthy men and women. J Lipid Res. 1997;38:459–68 [PubMed] [Google Scholar]
- 15.Cole TG, Bowen PE, Schmeisser D, Prewitt TE, Aye P, Langenberg P, Dolecek TA, Brace LD, Kamath S. Differential reduction of plasma cholesterol by the American Heart Association Phase 3 Diet in moderately hypercholesterolemic, premenopausal women with different body mass indexes. Am J Clin Nutr. 1992;55:385–94 [DOI] [PubMed] [Google Scholar]
- 16.Hannah JS, Jablonski KA, Howard BV. The relationship between weight and response to cholesterol-lowering diets in women. Int J Obes Relat Metab Disord. 1997;21:445–50 [DOI] [PubMed] [Google Scholar]
- 17.Jansen S, Lopez-Miranda J, Salas J, Castro P, Paniagua JA, Lopez-Segura F, Ordovas JM, Jimenez-Pereperez JA, Blanco A, et al. Plasma lipid response to hypolipidemic diets in young healthy non-obese men varies with body mass index. J Nutr. 1998;128:1144–9 [DOI] [PubMed] [Google Scholar]
- 18.Bronsgeest-Schoute DC, Hermus RJ, Dallinga-Thie GM, Hautvast JG. Dependence of the effects of dietary cholesterol and experimental conditions on serum lipids in man. III. The effect on serum cholesterol of removal of eggs from the diet of free-living habitually egg-eating people. Am J Clin Nutr. 1979;32:2193–7 [DOI] [PubMed] [Google Scholar]
- 19.Denke MA, Adams-Huet B, Nguyen AT. Individual cholesterol variation in response to a margarine- or butter-based diet: A study in families. JAMA. 2000;284:2740–7 [DOI] [PubMed] [Google Scholar]
- 20.Knopp RH, Retzlaff B, Fish B, Walden C, Wallick S, Anderson M, Aikawa K, Kahn SE. Effects of insulin resistance and obesity on lipoproteins and sensitivity to egg feeding. Arterioscler Thromb Vasc Biol. 2003;23:1437–43 [DOI] [PubMed] [Google Scholar]
- 21.Nabeno-Kaeriyama Y, Fukuchi Y, Hayashi S, Kimura T, Tanaka A, Naito M. Delayed postprandial metabolism of triglyceride-rich lipoproteins in obese young men compared to lean young men. Clin Chim Acta. 2010;411:1694–9 [DOI] [PubMed] [Google Scholar]
- 22.Oh SY, Miller LT. Effect of dietary egg on variability of plasma cholesterol levels and lipoprotein cholesterol. Am J Clin Nutr. 1985;42:421–31 [DOI] [PubMed] [Google Scholar]
- 23.Katan MB, Beynen AC. Characteristics of human hypo- and hyperresponders to dietary cholesterol. Am J Epidemiol. 1987;125:387–99 [DOI] [PubMed] [Google Scholar]
- 24.Cox C, Mann J, Sutherland W, Ball M. Individual variation in plasma cholesterol response to dietary saturated fat. BMJ. 1995;311:1260–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clifton PM, Abbey M, Noakes M, Beltrame S, Rumbelow N, Nestel PJ. Body fat distribution is a determinant of the high-density lipoprotein response to dietary fat and cholesterol in women. Arterioscler Thromb Vasc Biol. 1995;15:1070–8 [DOI] [PubMed] [Google Scholar]
- 26.Sabate J, Oda K, Ros E. Nut consumption and blood lipid levels: a pooled analysis of 25 intervention trials. Arch Intern Med. 2010;170:821–7 [DOI] [PubMed] [Google Scholar]
- 27.Griel AE, Kris-Etherton PM. Tree nuts and the lipid profile: a review of clinical studies. Br J Nutr. 2006;96 Suppl 2:S68–78 [DOI] [PubMed] [Google Scholar]
- 28.Mukuddem-Petersen J, Oosthuizen W, Jerling JC. A systematic review of the effects of nuts on blood lipid profiles in humans. J Nutr. 2005;135:2082–9 [DOI] [PubMed] [Google Scholar]
- 29.Mukuddem-Petersen J, Stonehouse Oosthuizen W, Jerling JC, Hanekom SM, White Z. Effects of a high walnut and high cashew nut diet on selected markers of the metabolic syndrome: a controlled feeding trial. Br J Nutr. 2007;97:1144–53 [DOI] [PubMed] [Google Scholar]
- 30.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–41 [DOI] [PubMed] [Google Scholar]
- 31.Erlinger TP, Miller ER III, Charleston J, Appel LJ. Inflammation modifies the effects of a reduced-fat low-cholesterol diet on lipids: results from the DASH-sodium trial. Circulation. 2003;108:150–4 [DOI] [PubMed] [Google Scholar]
- 32.Hilpert KF, Kris-Etherton PM, West SG. Lipid response to a low-fat diet with or without soy is modified by C-reactive protein status in moderately hypercholesterolemic adults. J Nutr. 2005;135:1075–9 [DOI] [PubMed] [Google Scholar]
- 33.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–5 [DOI] [PubMed] [Google Scholar]
- 34.Shah A, Mehta N, Reilly MP. Adipose inflammation, insulin resistance, and cardiovascular disease. JPEN J Parenter Enteral Nutr. 2008;32:638–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mekki N, Christofilis MA, Charbonnier M, Atlan-Gepner C, Defoort C, Juhel C, Borel P, Portugal H, Pauli AM, et al. Influence of obesity and body fat distribution on postprandial lipemia and triglyceride-rich lipoproteins in adult women. J Clin Endocrinol Metab. 1999;84:184–91 [DOI] [PubMed] [Google Scholar]
- 36.Dallongeville J, Gruson E, Dallinga-Thie G, Pigeyre M, Gomila S, Romon M. Effect of weight loss on the postprandial response to high-fat and high-carbohydrate meals in obese women. Eur J Clin Nutr. 2007;61:711–8 [DOI] [PubMed] [Google Scholar]
- 37.James AP, Watts GF, Barrett PH, Smith D, Pal S, Chan DC, Mamo JC. Effect of weight loss on postprandial lipemia and low-density lipoprotein receptor binding in overweight men. Metabolism. 2003;52:136–41 [DOI] [PubMed] [Google Scholar]
- 38.Simonen P, Gylling H, Howard AN, Miettinen TA. Introducing a new component of the metabolic syndrome: low cholesterol absorption. Am J Clin Nutr. 2000;72:82–8 [DOI] [PubMed] [Google Scholar]
- 39.Volek JS, Sharman MJ, Gomez AL, DiPasquale C, Roti M, Pumerantz A, Kraemer WJ. Comparison of a very low-carbohydrate and low-fat diet on fasting lipids, LDL subclasses, insulin resistance, and postprandial lipemic responses in overweight women. J Am Coll Nutr. 2004;23:177–84 [DOI] [PubMed] [Google Scholar]
- 40.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9 [DOI] [PubMed] [Google Scholar]
- 41.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Final report. Circulation. 2002;106:3143–421 [PubMed] [Google Scholar]
- 42.Wideman L, Kaminsky LA, Whaley MH. Postprandial lipemia in obese men with abdominal fat patterning. J Sports Med Phys Fitness. 1996;36:204–10 [PubMed] [Google Scholar]
- 43.Katan MB. The response of lipoproteins to dietary fat and cholesterol in lean and obese persons. Curr Cardiol Rep. 2006;8:446–51 [DOI] [PubMed] [Google Scholar]
- 44.Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–40 [DOI] [PubMed] [Google Scholar]
- 45.Keys A, Anderson JT, Grande F. Serum cholesterol response to changes in the diet. IV. Particular saturated fatty acids in the diet. Metabolism. 1965;14:776–87 [DOI] [PubMed] [Google Scholar]
- 46.Björkhem I. Do oxysterols control cholesterol homeostasis? J Clin Invest. 2002;110:725–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wojcicka G, Jamroz-Wisniewska A, Horoszewicz K, Beltowski J. Liver X receptors (LXRs). Part I: structure, function, regulation of activity, and role in lipid metabolism. Postepy Hig Med Dosw (Online). 2007;61:736–59 [PubMed] [Google Scholar]
- 48.Goodwin B, Watson MA, Kim H, Miao J, Kemper JK, Kliewer SA. Differential regulation of rat and human CYP7A1 by the nuclear oxysterol receptor liver x receptor-alpha. Mol Endocrinol. 2003;17:386–94 [DOI] [PubMed] [Google Scholar]
- 49.Rudel L, Deckelman C, Wilson M, Scobey M, Anderson R. Dietary cholesterol and downregulation of cholesterol 7 alpha-hydroxylase and cholesterol absorption in African green monkeys. J Clin Invest. 1994;93:2463–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56:901–11 [DOI] [PubMed] [Google Scholar]
- 52.Wang B, Wood IS, Trayhurn P. Dysregulation of the expression and secretion of inflammation-related adipokines by hypoxia in human adipocytes. Pflugers Arch. 2007;455:479–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang P, Mariman E, Renes J, Keijer J. The secretory function of adipocytes in the physiology of white adipose tissue. J Cell Physiol. 2008;216:3–13 [DOI] [PubMed] [Google Scholar]
- 54.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169–80 [DOI] [PubMed] [Google Scholar]
- 55.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kanneganti T-D, Lamkanfi M, Núñez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007;27:549–59 [DOI] [PubMed] [Google Scholar]
- 57.Vandanmagsar B, Youm Y-H, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med 2011;17:179–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Luca C, Olefsky JM. Inflammation and insulin resistance. FEBS Lett. 2008;582:97–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stephens JM, Lee J, Pilch PF. Tumor necrosis factor-alpha-induced insulin resistance in 3T3–L1 adipocytes is accompanied by a loss of insulin receptor substrate-1 and GLUT4 expression without a loss of insulin receptor-mediated signal transduction. J Biol Chem. 1997;272:971–6 [DOI] [PubMed] [Google Scholar]
- 60.Ruan H, Hacohen N, Golub TR, Van Parijs L, Lodish HF. Tumor necrosis factor-alpha suppresses adipocyte-specific genes and activates expression of preadipocyte genes in 3T3–L1 adipocytes: nuclear factor-kappaB activation by TNF-alpha is obligatory. Diabetes. 2002;51:1319–36 [DOI] [PubMed] [Google Scholar]
- 61.Roytblat L, Rachinsky M, Fisher A, Greemberg L, Shapira Y, Douvdevani A, Gelman S. Raised interleukin-6 levels in obese patients. Obes Res. 2000;8:673–5 [DOI] [PubMed] [Google Scholar]
- 62.Hotamisligil GS, Spiegelman BM. Tumor necrosis factor alpha: a key component of the obesity-diabetes link. Diabetes. 1994;43:1271–8 [DOI] [PubMed] [Google Scholar]
- 63.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chinetti G, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors (PPARs): nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflamm Res. 2000;49:497–505 [DOI] [PubMed] [Google Scholar]
- 65.Das UN. Metabolic syndrome pathophysiology: the role of essential fatty acids. Singapore: Wiley-Blackwell; 2010 [Google Scholar]
- 66.Lam TK, Pocai A, Gutierrez-Juarez R, Obici S, Bryan J, Aguilar-Bryan L, Schwartz GJ, Rossetti L. Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat Med. 2005;11:320–7 [DOI] [PubMed] [Google Scholar]
- 67.Obici S, Feng Z, Morgan K, Stein D, Karkanias G, Rossetti L. Central administration of oleic acid inhibits glucose production and food intake. Diabetes. 2002;51:271–5 [DOI] [PubMed] [Google Scholar]
- 68.McGillicuddy FC, de la Llera Moya M, Hinkle CC, Joshi MR, Chiquoine EH, Billheimer JT, Rothblat GH, Reilly MP. Inflammation impairs reverse cholesterol transport in vivo. Circulation. 2009;119:1135–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lewis GF, Carpentier A, Adeli K, Giacca A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev. 2002;23:201–29 [DOI] [PubMed] [Google Scholar]
- 70.Tobin KA, Ulven SM, Schuster GU, Steineger HH, Andresen SM, Gustafsson JA, Nebb HI. Liver X receptors as insulin-mediating factors in fatty acid and cholesterol biosynthesis. J Biol Chem. 2002;277:10691–7 [DOI] [PubMed] [Google Scholar]
- 71.Yu L, York J, von Bergmann K, Lutjohann D, Cohen JC, Hobbs HH. Stimulation of cholesterol excretion by the liver X receptor agonist requires ATP-binding cassette transporters G5 and G8. J Biol Chem. 2003;278:15565–70 [DOI] [PubMed] [Google Scholar]
- 72.Pihlajamaki J, Gylling H, Miettinen TA, Laakso M. Insulin resistance is associated with increased cholesterol synthesis and decreased cholesterol absorption in normoglycemic men. J Lipid Res. 2004;45:507–12 [DOI] [PubMed] [Google Scholar]
- 73.Tsubakio-Yamamoto K, Nishida M, Nakagawa-Toyama Y, Masuda D, Ohama T, Yamashita S. Current therapy for patients with sitosterolemia–effect of ezetimibe on plant sterol metabolism. J Atheroscler Thromb. 2010;17:891–900 [DOI] [PubMed] [Google Scholar]
- 74.Klett EL, Lee MH, Adams DB, Chavin KD, Patel SB. Localization of ABCG5 and ABCG8 proteins in human liver, gall bladder and intestine. BMC Gastroenterol. 2004;4:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Berge KE, Tian H, Graf GA, Yu L, Grishin NV, Schultz J, Kwiterovich P, Shan B, Barnes R, et al. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science. 2000;290:1771–5 [DOI] [PubMed] [Google Scholar]
- 76.van der Velde AE, Vrins CL, van den Oever K, Seemann I, Oude Elferink RP, van Eck M, Kuipers F, Groen AK. Regulation of direct transintestinal cholesterol excretion in mice. Am J Physiol Gastrointest Liver Physiol. 2008;295:G203–8 [DOI] [PubMed] [Google Scholar]
- 77.Zhao HL, Houweling AH, Vanstone CA, Jew S, Trautwein EA, Duchateau GS, Jones PJ. Genetic variation in ABC G5/G8 and NPC1L1 impact cholesterol response to plant sterols in hypercholesterolemic men. Lipids. 2008;43:1155–64 [DOI] [PubMed] [Google Scholar]
- 78.Santosa S, Demonty I, Lichtenstein AH, Ordovas JM, Jones PJ. Single nucleotide polymorphisms in ABCG5 and ABCG8 are associated with changes in cholesterol metabolism during weight loss. J Lipid Res. 2007;48:2607–13 [DOI] [PubMed] [Google Scholar]
- 79.Li T, Jahan A, Chiang JY. Bile acids and cytokines inhibit the human cholesterol 7 alpha-hydroxylase gene via the JNK/c-jun pathway in human liver cells. Hepatology. 2006;43:1202–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li T, Ma H, Chiang JYL. TGFβ1, TNFα, and insulin signaling crosstalk in regulation of the rat cholesterol 7α-hydroxylase gene expression. J Lipid Res. 2008;49:1981–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Altmann SW, Davis HR, Jr, Zhu LJ, Yao X, Hoos LM, Tetzloff G, Iyer SP, Maguire M, Golovko A, et al. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303:1201–4 [DOI] [PubMed] [Google Scholar]
- 82.Cohen JC, Pertsemlidis A, Fahmi S, Esmail S, Vega GL, Grundy SM, Hobbs HH. Multiple rare variants in NPC1L1 associated with reduced sterol absorption and plasma low-density lipoprotein levels. Proc Natl Acad Sci USA. 2006;103:1810–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Davis HR, Jr, Altmann SW. Niemann-Pick C1 Like 1 (NPC1L1) an intestinal sterol transporter. Biochim Biophys Acta 2009;1791:679–83 [DOI] [PubMed] [Google Scholar]
- 84.Ahmed MH, Byrne CD. Potential therapeutic uses for ezetimibe beyond lowering LDL-c to decrease cardiovascular events. Diabetes Obes Metab. 2010;12:958–66 [DOI] [PubMed] [Google Scholar]
- 85.Labonte ED, Camarota LM, Rojas JC, Jandacek RJ, Gilham DE, Davies JP, Ioannou YA, Tso P, Hui DY, et al. Reduced absorption of saturated fatty acids and resistance to diet-induced obesity and diabetes by ezetimibe-treated and Npc1l1−/− mice. Am J Physiol Gastrointest Liver Physiol. 2008;295:G776–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Deushi M, Nomura M, Kawakami A, Haraguchi M, Ito M, Okazaki M, Ishii H, Yoshida M. Ezetimibe improves liver steatosis and insulin resistance in obese rat model of metabolic syndrome. FEBS Lett. 2007;581:5664–70 [DOI] [PubMed] [Google Scholar]
- 87.Nomura M, Ishii H, Kawakami A, Yoshida M. Inhibition of hepatic Neiman-Pick C1-Like 1 improves hepatic insulin resistance. Am J Physiol Endocrinol Metab. Epub 2009 Aug 4. [DOI] [PubMed] [Google Scholar]
- 88.Picard F, Boivin A, Lalonde J, Deshaies Y. Resistance of adipose tissue lipoprotein lipase to insulin action in rats fed an obesity-promoting diet. Am J Physiol Endocrinol Metab. 2002;282:E412–8 [DOI] [PubMed] [Google Scholar]
- 89.Berneis KK, Krauss RM. Metabolic origins and clinical significance of LDL heterogeneity. J Lipid Res. 2002;43:1363–79 [DOI] [PubMed] [Google Scholar]
- 90.Griffin BA. Lipoprotein atherogenicity: an overview of current mechanisms. Proc Nutr Soc. 1999;58:163–9 [DOI] [PubMed] [Google Scholar]
- 91.Stoeckel LE, Weller RE, Cook EW III, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage. 2008;41:636–47 [DOI] [PubMed] [Google Scholar]
- 92.Rothemund Y, Preuschhof C, Bohner G, Bauknecht HC, Klingebiel R, Flor H, Klapp BF. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage. 2007;37:410–21 [DOI] [PubMed] [Google Scholar]
- 93.Brüning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Müller-Wieland D, et al. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–5 [DOI] [PubMed] [Google Scholar]
- 94.Lind S, Rudling M, Ericsson S, Olivecrona H, Eriksson M, Borgstrom B, Eggertsen G, Berglund L, Angelin B. Growth hormone induces low-density lipoprotein clearance but not bile acid synthesis in humans. Arterioscler Thromb Vasc Biol. 2004;24:349–56 [DOI] [PubMed] [Google Scholar]
- 95.Rudling M, Norstedt G, Olivecrona H, Reihner E, Gustafsson JA, Angelin B. Importance of growth hormone for the induction of hepatic low density lipoprotein receptors. Proc Natl Acad Sci USA. 1992;89:6983–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rudling M, Angelin B. Loss of resistance to dietary cholesterol in the rat after hypophysectomy: importance of the presence of growth hormone for hepatic low density lipoprotein-receptor expression. Proc Natl Acad Sci USA. 1993;90:8851–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Galman C, Matasconi M, Persson L, Parini P, Angelin B, Rudling M. Age-induced hypercholesterolemia in the rat relates to reduced elimination but not increased intestinal absorption of cholesterol. Am J Physiol Endocrinol Metab. 2007;293:E737–42 [DOI] [PubMed] [Google Scholar]
- 98.Scacchi M, Pincelli AI, Cavagnini F. Growth hormone in obesity. Int J Obes Relat Metab Disord. 1999;23:260–71 [DOI] [PubMed] [Google Scholar]
- 99.Parini P, Angelin B, Rudling M. Importance of estrogen receptors in hepatic LDL receptor regulation. Arterioscler Thromb Vasc Biol. 1997;17:1800–5 [DOI] [PubMed] [Google Scholar]
- 100.Duan LP, Wang HH, Ohashi A, Wang DQ. Role of intestinal sterol transporters Abcg5, Abcg8, and Npc1l1 in cholesterol absorption in mice: gender and age effects. Am J Physiol Gastrointest Liver Physiol. 2006;290:G269–76 [DOI] [PubMed] [Google Scholar]
- 101.Angelin B, Olivecrona H, Reihner E, Rudling M, Stahlberg D, Eriksson M, Ewerth S, Henriksson P, Einarsson K. Hepatic cholesterol metabolism in estrogen-treated men. Gastroenterology. 1992;103:1657–63 [DOI] [PubMed] [Google Scholar]
- 102.Nyrnes A, Jorde R, Sundsfjord J. Serum TSH is positively associated with BMI. Int J Obes (Lond). 2005;30:100–5 [DOI] [PubMed] [Google Scholar]
- 103.Knudsen N, Laurberg P, Rasmussen LB, Bulow I, Perrild H, Ovesen L, Jorgensen T. Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. J Clin Endocrinol Metab. 2005;90:4019–24 [DOI] [PubMed] [Google Scholar]
- 104.Reinehr T, Isa A, de Sousa G, Dieffenbach R, Andler W. Thyroid hormones and their relation to weight status. Horm Res. 2008;70:51–7 [DOI] [PubMed] [Google Scholar]
- 105.Reinehr T. Obesity and thyroid function. Mol Cell Endocrinol. 2010;316:165–71 [DOI] [PubMed] [Google Scholar]
- 106.Thompson GR, Soutar AK, Spengel FA, Jadhav A, Gavigan SJ, Myant NB. Defects of receptor-mediated low density lipoprotein catabolism in homozygous familial hypercholesterolemia and hypothyroidism in vivo. Proc Natl Acad Sci USA. 1981;78:2591–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bakker O, Hudig F, Meijssen S, Wiersinga WM. Effects of triiodothyronine and amiodarone on the promoter of the human LDL receptor gene. Biochem Biophys Res Commun. 1998;249:517–21 [DOI] [PubMed] [Google Scholar]
- 108.Liberopoulos EN, Elisaf MS. Dyslipidemia in patients with thyroid disorders. Hormones (Athens). 2002;1:218–23 [DOI] [PubMed] [Google Scholar]
- 109.Shin D-J, Osborne TF. Thyroid hormone regulation and cholesterol metabolism are connected through sterol regulatory element-binding protein-2 (SREBP-2). J Biol Chem. 2003;278:34114–8 [DOI] [PubMed] [Google Scholar]
- 110.Qi D, Rodrigues B. Glucocorticoids produce whole body insulin resistance with changes in cardiac metabolism. Am J Physiol Endocrinol Metab. 2007;292:E654–67 [DOI] [PubMed] [Google Scholar]
- 111.Paulmyer-Lacroix O, Boullu S, Oliver C, Alessi M-C, Grino M. Expression of the mRNA coding for 11{beta}-hydroxysteroid dehydrogenase type 1 in adipose tissue from obese patients: an in situ hybridization study. J Clin Endocrinol Metab. 2002;87:2701–5 [DOI] [PubMed] [Google Scholar]
- 112.Rask E, Walker BR, Soderberg S, Livingstone DEW, Eliasson M, Johnson O, Andrew R, Olsson T. Tissue-specific changes in peripheral cortisol metabolism in obese women: increased adipose 11{beta}-hydroxysteroid dehydrogenase type 1 activity. J Clin Endocrinol Metab. 2002;87:3330–6 [DOI] [PubMed] [Google Scholar]
- 113.Rizza RA, Mandarino LJ, Gerich JE. Cortisol-induced insulin resistance in man: impaired suppression of glucose production and stimulation of glucose utilization due to a postreceptor defect of insulin action. J Clin Endocrinol Metab. 1982;54:131–8 [DOI] [PubMed] [Google Scholar]
- 114.Andrews RC, Herlihy O, Livingstone DEW, Andrew R, Walker BR. Abnormal cortisol metabolism and tissue sensitivity to cortisol in patients with glucose intolerance. J Clin Endocrinol Metab. 2002;87:5587–93 [DOI] [PubMed] [Google Scholar]
- 115.Samuels MH. Effects of variations in physiological cortisol levels on thyrotropin secretion in subjects with adrenal insufficiency: a clinical research center study. J Clin Endocrinol Metab. 2000;85:1388–93 [DOI] [PubMed] [Google Scholar]
- 116.DiBaise JK, Zhang H, Crowell MD, Krajmalnik-Brown R, Decker GA, Rittmann BE. Gut microbiota and its possible relationship with obesity. Mayo Clin Proc. 2008;83:460–9 [DOI] [PubMed] [Google Scholar]
- 117.Bajzer M, Seeley RJ. Physiology: obesity and gut flora. Nature. 2006;444:1009–10 [DOI] [PubMed] [Google Scholar]
- 118.Cronin J, Williams L, McAdam E, Eltahir Z, Griffiths P, Baxter J, Jenkins G. The role of secondary bile acids in neoplastic development in the oesophagus. Biochem Soc Trans. 2010;38:337–42 [DOI] [PubMed] [Google Scholar]
- 119.Creely SJ, McTernan PG, Kusminski CM, Fisher M, Da Silva NF, Khanolkar M, Evans M, Harte AL, Kumar S. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am J Physiol Endocrinol Metab. 2007;292:E740–7 [DOI] [PubMed] [Google Scholar]
- 120.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–72 [DOI] [PubMed] [Google Scholar]
- 121.Amar J, Burcelin R, Ruidavets JB, Cani PD, Fauvel J, Alessi MC, Chamontin B, Ferrieres J. Energy intake is associated with endotoxemia in apparently healthy men. Am J Clin Nutr. 2008;87:1219–23 [DOI] [PubMed] [Google Scholar]