Abstract
Background
Although evidence-based guidelines for the treatment of type 2 diabetes mellitus provide clear recommendations for initial therapy, evidence on an optimal treatment strategy after secondary failure is unclear.
Purpose
To compare the efficacy of add-on therapy using basal insulin versus an additional oral antidiabetic agent in patients with type 2 diabetes and secondary failure.
Data sources
We searched the following electronic databases from inception until June 2007: MEDLINE; EMBASE; Cochrane Central Register of Controlled Trials; Web of Science; Scopus; CINAHL; International Pharmaceutical Abstracts; Academic OneFile; PASCAL; Global Health Database; LILACS; HealthSTAR; PubMed. Reference lists of potentially relevant articles and clinical trial databases were searched, pharmaceutical manufacturers were contacted, and grey literature sources were sought.
Study selection
Randomized controlled trials (RCTs) involving subjects with type 2 diabetes with secondary failure who were randomly assigned to receive additional basal insulin therapy (insulin glargine, detemir, or NPH [neutral protamine Hagedorn]) versus another oral antidiabetic agent from any class.
Data extraction
Two reviewers independently screened articles, extracted data and assessed methodological quality. Our primary outcome was glycemic control measured by change in glycosylated hemoglobin (HbA1C) and the proportion of subjects achieving a HbA1C value of ≤ 7%.
Data synthesis
To compare overall efficacy between the 2 treatment strategies, change in HbA1C was pooled across studies using a random-effects model and weighted mean difference (WMD). Eleven RCTs, involving 757 participants with a median age of 56 and a median known duration of diabetes of 11 years, were included in our analysis. Insulin treatment demonstrated a small but statistically significant improvement in HbA1C compared with the use of an additional oral agent as add-on therapy (WMD -0.17; 95% CI [confidence interval] -0.33 to -0.02).
Limitations
The use of surrogate outcomes and the short duration of the trials makes it impossible to gain information on long-term patient-oriented outcomes. The overall quality of the studies was low, primarily in view of inadequate blinding.
Conclusions
Although add-on therapy using injected insulin shows a slight benefit over an additional oral antidiabetic agent, our results indicate that basal insulin therapy and the use of an oral agent as add-on therapy produce comparable results. Non-therapeutic differences must be considered in the choice of treatment strategies. More high-quality studies with adequate safety data using more aggressive insulin titrations are needed.
Lowering blood glucose was shown to decrease the risk of microvascular complications in the United Kingdom Prospective Diabetes Study (UKPDS) trial.1 In this study, patients randomly assigned to the intensive protocol (target fasting plasma glucose (FPG) < 6 mmol/L) showed a significant reduction in microvascular complications and a trend toward reduced macrovascular complications.1 Mainly on the basis of evidence from the UKPDS and other major diabetes clinical trials,2, 3 several organizations have formulated guidelines with clear recommendations for the initial therapy of type 2 diabetes.4-6 However, in view of the progressive nature of type 2 diabetes,7 patients and their clinicians will inevitably need to intensify therapy to maintain glycemic control. The decision to intensify therapy after initial treatment with oral medication has been defined as “secondary failure.”8-10 Although clinical trial evidence conveys the importance of early and sustained blood glucose control,1, 2, 11 the optimal strategy for patients in whom initial oral antidiabetic drug therapy has proven ineffective is not well defined.
Current clinical practice guidelines4-6 for type 2 diabetes recommend the addition of either insulin or another oral agent when monotherapy using an oral agent achieves inadequate control (HbA1C > 7%). However, it unclear which of these options is preferable.
A randomized controlled trial (RCT) assessing the efficacy of intensive glycemic control (HbA1C < 6%) through an extensive protocol involving titration and the addition of various antidiabetic strategies, the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Trial is currently under way; the results, however, are not expected until at least 2010. Previous systematic reviews have not explored whether it is preferable to add insulin therapy or to add an additional oral agent in patients with secondary failure. Goudswaard et al12 focused on switching a patient’s therapeutic regimen to insulin monotherapy versus adding insulin to oral antidiabetic agents. Reviews assessing combination therapy of insulin and oral antidiabetic agents have been limited to a specific class of oral antidiabetic agents, most commonly sulfonylureas,13-15 and assessed whether combination therapy with insulin was beneficial compared with insuln monotherapy. Moreover, these previous reviews predate the launch of the newer long-acting insulins — insulin glargine and detemir.
The objective of this meta-analysis was to evaluate the evidence of the efficacy of adding basal intermediate or long-acting insulin versus the addition of another oral antidiabetic agent in patients with type 2 diabetes whose current oral antidiabetic therapy was failing.
Methods
Search strategy
The search strategy was designed to capture the patient population, consisting of people with type 2 diabetes currently using any class of oral antidiabetic therapy; the population problem, defined as current treatment failure; the intervention of insulin glargine, detemir or NPH (neutral protamine Hagedorn); and the primary outcome measure of change in glycosylated hemoglobin (HbA1C). Our search strategy was developed in consultation with a research librarian well versed in the conduct of systematic reviews and in the use of MeSH (MEDLINE subject headings) and key terms.
The MEDLINE-based search strategy formed the foundation for searching in other databases. We searched the following electronic bibliographic databases from their inception until June 2007: MEDLINE, EMBASE, Cochrane Register of Conrolled Trials, Web of Science, Scopus, CINAHL, International Pharmaceutical Abstracts, Academic OneFile, PASCAL, Global Health Database, LILACS, HealthSTAR, and PubMed. Other literature sources were also searched, including: reference lists of all included studies and relevant narrative reviews; clinical trials databases (ClinicalTrials.gov, CenterWatch Clinical Trials Listing Service, and Current Controlled Trials); OCLC Proceedings First and OCLC Papers First databases to identify studies presented at conferences and proceedings; and Proquest and Index to Theses to identify relevant theses and dissertations. We contacted the pharmaceutical companies producing insulin glargine (Sanofi-Aventis), insulin detemir (Novo Nordisk) and NPH (Novo Nordisk, Lilly) to inquire about other published or unpublished studies.
Selection of studies
Citations identified in the literature search were independently screened by two reviewers (JG, SS) to select potentially relevant articles. The full articles from this list were retrieved and subsequently reviewed by 2 reviewers (JG, LB) for inclusion in the systematic review. Inter-rater agreement at this stage was assessed using Cohen’s kappa statistic. Disagreements between reviewers were reconciled by consensus; a third-party intermediary was not required. Reviewers were not blinded to the authors, journal, or publisher of the studies. Non-English abstracts and articles were assessed by one reviewer (SK).
Studies were included if they had the following characteristics: RCTs, whether parallel or crossover design; participants inadequately controlled on their current oral antidiabetic regimen, defined as an HbA1C > 7% or a fasting plasma glucose (FPG) > 7 mmol/L; participants insulin naïve at baseline; subjects randomly assigned to receive the addition of either basal insulin therapy (insulin glargine, detemir, or NPH) or another oral antidiabetic agent from any class (biguanide, sulfonylurea, thiazolidinedione, non-sulfonylurea secretagogue, or glucosidase inhibitor). We use the term “basal” to mean administration of an intermediate or long-acting insulin as 100% of daily insulin dose; specifically, these would be regimens using NPH, glargine, or detemir.4 We felt that crossover trials were suitable for our clinical question, as diabetes management is a chronic condition of which we do not expect a carry-over effect of treatment in respect to blood glucose levels. Data from crossover trials were entered as a parallel study.
In addition to the above criteria, studies must have reported (or given the information to calculate) change in HbA1C (%) from baseline. Glycemic control was our primary outcome, measured by change in HbA1C and the proportion of individuals achieving an HbA1C ≤ 7%. Secondary outcomes included change in FPG (mmol/L), change in weight (kg) and the proportion of participants who experienced ≥ 1 hypoglycemic event as defined by the study investigators.
Data extraction and management
Two reviewers (JG, LB) independently extracted the data from all articles that met predefined eligibility criteria. Data were recorded on a standardized form, and all discrepancies were resolved by consensus. Both reviewers independently extracted data from 2 studies using a preliminary data extraction form. Minor revisions to the extraction form were made after this trial period to provide the content found in Textbox 1. We attempted to contact authors to verify, interpret and obtain missing data. In addition to extracting data, the reviewers assessed the overall methodological quality of studies using the Jadad scale.16 Methodological quality was assessed on the basis of information reported in the published article only. In addition, the scale devised by Schulz and colleagues17 was used to assess allocation of concealment. Funding sources for included studies were also considered.
Textbox 1.
Data extraction
If the mean change and its respective standard deviation were missing, we calculated the mean change from baseline by subtracting the mean baseline HbA1C from the mean HbA1C at the last follow-up date. Standard deviation (SD) was calculated using standard formulas,18 using a correlation coefficient of 0.5 to allow estimation of the combined SDs. In one study19 we had to estimate the values of HbA1C and fasting plasma glucose from inspection of graphs, as the exact values were not included in the publication. We substituted the mean SD from the other studies that used an identical comparison agent.
Data synthesis
We chose a random-effects model for our meta-analysis, as this is more conservative than a fixed-effects model and therefore less likely to overestimate treatment effects.20 Statistical, clinical, and methodological heterogeneity were assessed to determine the appropriateness of pooling data across studies. We evaluated statistical heterogeneity using the I2 statistic. A value of I2 greater than 50% was considered indicative of significant heterogeneity.18 We recognized the potential for variability in key clinical characteristics such as duration of diabetes, baseline HbA1C, and age; however, we used the method described by Tobias21 to explore the impact of each study on the overall summary effect.
We further explored sources of potential heterogeneity through subgroup and sensitivity analyses. Subgroups defined a priori included stratification by the type of insulin (NPH, glargine, detemir) and the comparative oral agent (metformin, thiazolidinedione, acarbose). Sensitivity analyses were performed on the following factors, defined a priori: fixed-effects versus random-effects model; parallel versus crossover design; and duration of follow-up.
All continuous variables (changes in HbA1C, FPG, and weight) were expressed using a weighted mean difference (WMD) and 95% confidence interval (CI). All dichotomous outcomes (proportion of subjects achieving HbA1C ≤ 7%, and proportion of subjects experiencing ≥ 1 hypoglycemic event) were expressed using relative risk (RR) and 95% CI. We chose RR as a measure of effect, given considerations of consistency and interpretability. Publication bias was assessed by examining the symmetry of a funnel plot, where sample size is plotted against the treatment effect. A funnel plot was inspected for our primary outcome only, in view of the small number of studies that addressed our secondary outcomes.
Results
Search strategy
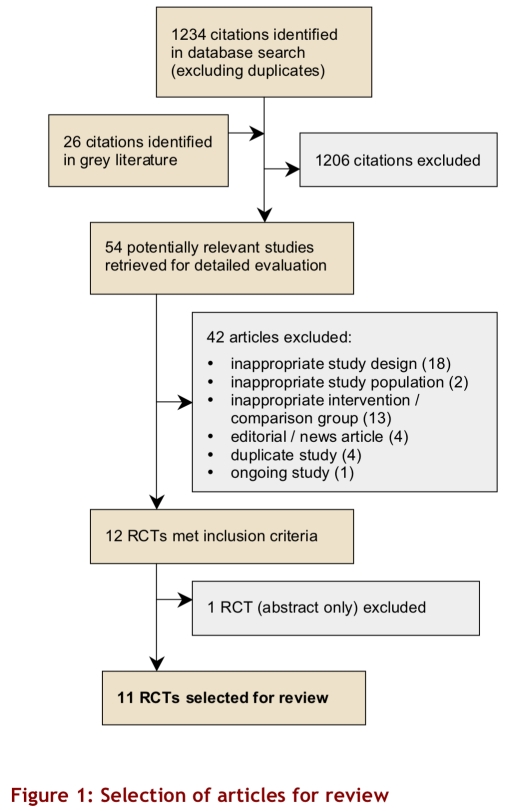
Our search strategy identified 1234 unique citations, and an additional 26 citations were identified from grey literature sources (Fig. 1). Screening of title, abstracts, and keywords identified 54 citations potentially relevant to the review question, and the full text for these studies was retrieved. Seven non-English articles were assessed by 1 reviewer (SK), who found that none met the eligibility criteria. Two reviewers assessed the remaining 47 potentially relevant articles and found that 12 studies met the eligibility criteria independently (kappa = 0.74). The reviewers arrived at a consensus that 11 studies met all of the eligibility criteria.
Figure 1.
Selection of articles for review
Included studies
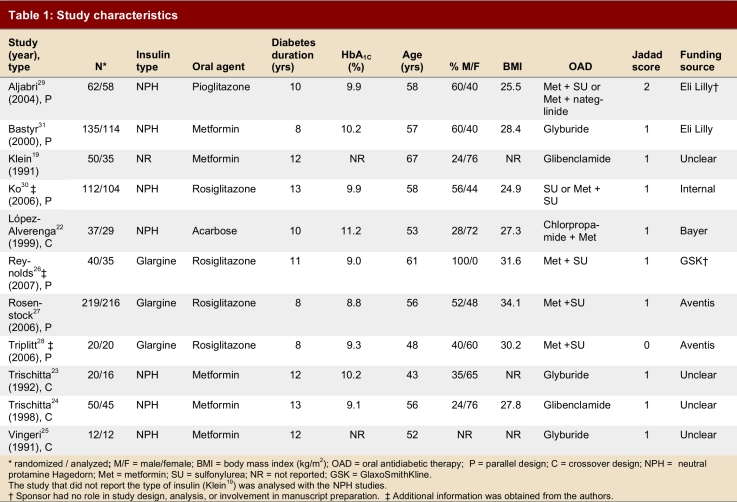
Seven studies used a parallel design; 4 studies22-25 used a crossover design. Crossover studies tended to have smaller sample sizes, contributing 119 to a total of 757 participants. Trial duration ranged from 12 weeks to 1 year of follow-up. Sample sizes ranged from 12 to 219 participants. Three studies used insulin glargine,26-28 7 studies used NPH insulin,22-25, 29, 30 and 1 study did not specify the type of insulin.19 Five studies used a thiazolidinedione (n = 1 for pioglitazone and n = 4 for rosiglitazone),26-30 5 studies used metformin,19, 23-25, 31 and 1 study used acarbose22 as comparison agents. Baseline HbA1C ranged from 8.8% to 11.2 %.
The overall quality of the studies was low (Jadad range 0–2), and only 1 study adequately described the allocation concealment method.30 One study22 was described as double-blinded; this was misleading, as the insulin arm was not blinded, and only the acarbose arm was masked with a placebo. Three studies27, 29, 31 explicitly stated that they were “open label” studies. The average percentage of dropouts per study was 13% of the number of subjects randomly assigned to a study arm. Reasons for dropouts were given in all studies, except the 2 that had no dropouts.25, 28 Although 2 studies described an intention-to-treat analysis,27, 30 in fact no study performed an intention-to-treat analysis.
Six studies were sponsored by a pharmaceutical company.26-29, 31 Baseline clinical and demographic data for each study are listed in Table 1. Most studies did not explicitly state their primary outcome. In the study by Rosenstock and colleagues27 the primary outcome was identical to that of our systematic review: glycemic control measured using HbA1C.
Table 1.
Study characteristics
Outcomes
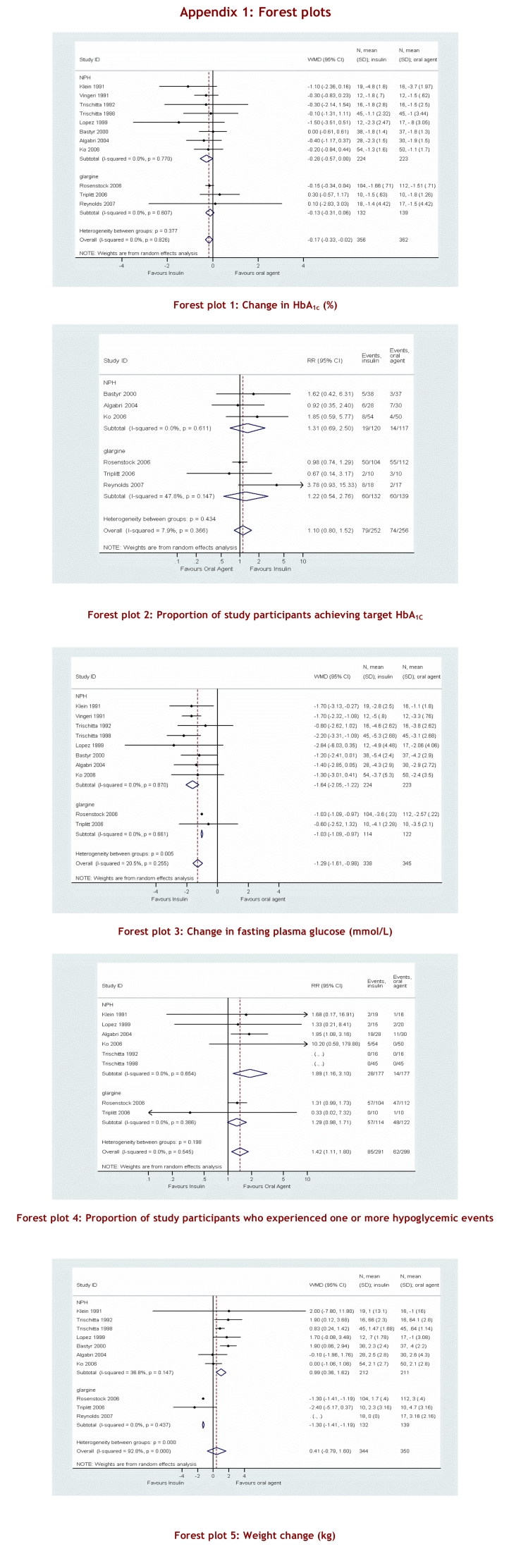
To compare the overall efficacy of the two treatment options — addition of basal insulin versus another oral antidiabetic agent — outcome results from each study were pooled and an overall summary measure of effect was calculated. When all studies were pooled, the addition of basal insulin demonstrated a statistically significant improvement in HbA1C in comparison with the use of an oral agent as add-on therapy, but this difference was not clinically significant (WMD 0.17; 95% CI -0.33 to -0.02)(see Appendix 1 for Forest plots of meta-analyses). The pooled analyses of patients achieving HbA1C ≤ 7% favoured the addition of insulin; however, this finding did not reach statistical significance (RR 1.10; 95% CI 0.80–1.52). A third measure of glycemic control was change in FPG from baseline, where an improvement in the insulin arm versus the oral agent arm was found (WMD -1.29; 95% CI -1.61 to -0.98). With respect to adverse events, more patients experienced at least one hypoglycemic event in the insulin group than in the oral agent group (RR 1.42; 95% CI 1.11–1.80). Weight gain was not pooled into an overall meta-analysis in view of the significant heterogeneity among studies.
Results were categorized into clinically meaningful subgroups according to the type of insulin used. Eight studies compared a once-daily injection of NPH versus an oral antidiabetic as add-on therapy.19, 22-25, 29-31 Two of these studies used a thiazolidinedione,29, 30 5 studies used metformin19, 23-25, 31 and 1 study used acarbose22 as a comparator. No differences between groups were demonstrated for overall glycemic control as measured by change in HbA1C or proportion achieving an HbA1C ≤ 7%.
A greater change in FPG was observed in the NPH group than in the oral therapy group (WMD -1.64; 95% CI 2.05 to -1.22). The proportion of participants who experienced a hypoglycemic event was higher in the NPH treatment group (RR 1.89; 95% CI 1.16–3.10), as was the change in weight in kilograms from baseline (WMD 1.19; 95% CI 0.61–1.76). As expected, when NPH was compared with metformin only, even more weight gain was seen in the NPH group (WMD 1.29; 95% CI 0.62–1.96).
Three studies compared the addition of insulin glargine to an oral agent.26-28 Rosiglitazone was the only oral agent used in all 3 studies. Glycemic control did not differ significantly between groups, although the point estimates favour the addition of insulin glargine for both change in HbA1C (WMD -0.13; 95% CI -0.31 to 0.06) and the proportion of subjects achieving a target HbA1C ≤ 7% (RR 1.22; 95% CI 0.76–2.76). A significant difference was seen in favour of insulin for change in FPG (WMD 1.03; 95% CI -1.09 to -0.97) as well as weight gain (WMD -1.30; 95% CI -1.41 to -1.19). No difference was demonstrated between groups with respect to hypoglycemia (RR 1.29; 95% CI 0.98–1.71).
Sensitivity analyses, using a fixed-effects model, stratification by study design, or stratification by study duration, did not result in a substantial change in the magnitude or direction of the summary effect. To test the robustness of our summary measure of effect for change in HbA1C, we used the method developed by Tobias,21 by which each study is omitted and the summary effect measure is compared with the original result. The WMD did not change by more than 10%, with the exception that when the study by Rosenstock and colleagues27 was omitted the WMD changed by 28% in favour of insulin treatment. The possibility of publication bias was suggested by asymmetry in the funnel plot.
Discussion
Management of type 2 diabetes mellitus is multifaceted, incorporating blood glucose, blood pressure, lipid, and weight control. Although guidelines recommend tight glucose control to reduce the risk of microvascular complications,4-6 many patients remain above recommended glycemic targets.32 The progressive nature of type 2 diabetes further exacerbates the difficulty in achieving and maintaining glycemic control.33 The objective of this review was to evaluate the efficacy of 2 different treatment strategies in people with type 2 diabetes in whom initial oral antidiabetic therapy had failed. We compared the addition of a basal insulin injection with the addition of another oral antidiabetic agent.
The results of this systematic review indicate that, when used as add-on therapy, basal insulin therapy and an oral agent achieve comparable glycemic control. Although insulin showed a statistically significant benefit, the difference was small and of limited clinical importance. The clinical impact of a 0.17% reduction in HbA1C associated with insulin therapy versus the addition of oral therapy must be viewed in light of the absence of large-scale quality trials. The 95% CI showed potential benefit ranging from a 0.02% to a 0.33% reduction in HbA1C. We reported pooled estimates of the WMD in change in HbA1C from baseline, comparing insulin and oral agent treatment according to the type of insulin agent used. Although the overall pooled estimate favoured the addition of basal insulin, analysis stratified by insulin type to obtain an indirect comparison34 showed no apparent difference between NPH or glargine in comparison with the addition of an oral antidiabetic agent. Another outcome of interest with respect to glycemic control was the number of patients in each treatment group who achieved the target HbA1C ≤ 7%.4, 5 The small number of patients who achieved an optimal HbA1C was likely related to the conservative dosing of insulin. A much larger magnitude of effect was observed with respect to change in FPG, but this might be expected insofar as insulin dosing was titrated on the basis of FPG levels in all of the studies. In view of the significant heterogeneity between NPH and glargine groups, the magnitude of effect must be considered in context. Insulin glargine was generally used as a third-line agent, whereas NPH was added as a second-line agent. Therefore, the magnitude of effect may have been influenced by other factors, such as differences in postprandial blood glucose control, which could account for the diminished effect observed in the change in HbA1C.27
The relative safety of the 2 treatment strategies was evaluated using 2 secondary outcomes: proportion of subjects experiencing ≥ 1 hypoglycemic event, and change in weight. As expected, hypoglycemic events were more frequent in the insulin group than in the oral agent group. This appears to have been driven mostly by the large number of studies that used metformin as the comparison agent. The magnitude of effect is diminished and is statistically non-significant when only studies using a thiazolidinedione are considered. Overall, there was no difference in weight gain when insulin versus an oral agent was used as add-on therapy. The significant heterogeneity observed (I2 92.8%; p < 0.001) is explained in part by subgroup analysis. Of the 7 studies that used NPH and reported weight as an outcome measure, 4 used metformin as the comparison oral agent and showed a non-significant increase in weight gain among the the NPH users (WMD 1.29; 95% CI 0.62–1.96). This is consistent with metformin use in general, which is advocated for overweight patients.4 In the insulin glargine subgroup, insulin users experienced significantly less weight gain than those who used rosiglitazone as an add-on agent (WMD -1.30; 95% CI -1.41 to -1.19).
Limitations
Several limitations should be considered in the interpretation of our results. First, the overall quality of the studies included in the meta-analysis was poor, as indicated by their average Jadad score. We identified several recurring problems of methodology. For example, although all studies used random allocation, the process of randomization and concealment was not adequately described. Moreover, the lack of blinding was an important limitation across all studies. Proper blinding would require a double-dummy design whereby participants would administer an injection and an oral tablet concurrently. Second, follow-up times were relatively short, considering that people with type 2 diabetes receive treatment for the rest of their lives. Two studies had a follow-up of 1 year.19, 30 However, the 2 treatment groups might not show comparable efficacy after 2, 5, or 10 years. Longer follow-up times would increase the external validity of the results. A third limitation is that our primary outcomes are surrogate markers and lack information on long-term outcomes, such as microvascular or cardiovascular events. A fourth consideration concerns the limit to which a triple oral therapy can lower HbA1C. The addition of a third oral agent is unlikely to decrease HbA1C levels by greater than 1.5% to 2.0%; therefore, insulin may be a more appropriate option for those whose diabetes is very poorly controlled (> 9.5%) with secondary oral antidiabetic therapy. Evidence for this exists in the findings of Rosenstock and colleagues,27 which show that the glucose-lowering benefit of insulin glargine, as measured by FPG, was greater when baseline HbA1C was ≥ 9.5%. A fifth limitation is the absence of data for secondary outcomes. Hypoglycemic event reporting was inconsistent, and definitions of hypoglycemia were rare (n = 3).27-29 Similarly, reporting on weight change was inconsistent between studies. Consistent reporting of other side-effects such as edema or pain at the injection site would aid in the applicability of the results.
Although every effort was made to minimize biases in the review process, potential biases still exist. These biases were limited the involvement of 2 independent reviewers involved at each major stage in the review process. Publication bias was suggested by asymmetry observed on the funnel plot, although other sources of bias, including selection bias, true heterogeneity, data irregularities, artefact, or chance may explain this asymmetry.35
The results of this systematic review are relevant for clinicians working with patients with poorly controlled type 2 diabetes who are using either a sulfonylurea as monotherapy or in combination with metformin. The choice of treatment regimens for add-on therapy should be evaluated in light of current HbA1C levels and the risk of hypoglycemia. Non-therapeutic reasons such as cost and patient preference or adverse effects should be given adequate weight in view of the small magnitude of benefit observed for insulin use as add-on therapy. The optimal strategy for adding basal insulin therapy to an oral antidiabetic regimen remains to be demonstrated. More rigorous studies are required to establish the ideal treatment strategy for people with type 2 diabetes experiencing secondary failure on oral antidiabetic therapy.
Acknowledgments
The authors thank Tamara Durec for assistance designing and implementing the search strategy; Stefan Kuhle for evaluating all non-English articles; Donna Dryden for providing comments on an early draft; and Drs Raymond Reynolds, Curtis Triplitt, and Gary Ko for providing additional data from their respective studies.
Biographies
J.M. Gamble, BSc, BSc(Pharm), is a master’s student in clinical epidemiology at the School of Public Health, University of Alberta, and a research associate with the Institute of Health Economics, Edmonton, Alberta.
Scot H. Simpson, PharmD, MSc, is an assistant professor in the Faculty of Pharmacy and Pharmaceutical Sciences at the University of Alberta and is a research fellow at the Institute of Health Economics in Edmonton, Alberta.
Lauren C. Brown, BSc(Pharm), MSc, is a PhD candidate in the School of Public Health at the University of Alberta and a research associate with the Institute of Health Economics in Edmonton, Alberta.
Jeffrey A. Johnson, PhD, is a professor in the School of Public Health at the University of Alberta and is a research fellow at the Institute of Health Economics in Edmonton, Alberta.
Appendix 1.
Forest plots.
Appendix 2.
MEDLINE final search strategy.
Footnotes
Competing interests: None declared.
Funding source: The Alliance for Canadian Health Outcomes Research in Diabetes (ACHORD) New Emerging Team (NET) grant is sponsored by the Canadian Diabetes Association, the Heart and Stroke Foundation of Canada, the Kidney Foundation of Canada and CIHR (Institute of Nutrition, Metabolism and Diabetes and the Institute of Circulatory and Respiratory Health). Mr. Gamble is supported by a full-time Health Research Studentship through the Alberta Heritage Foundation for Medical Research (AHFMR) and a Canada Graduate Scholarship from the Canadian Institutes of Health Research (CIHR). Dr. Simpson is a New Investigator supported by the CIHR. Ms. Brown is supported by a full-time Health Studentship through the AHFMR. Dr. Johnson holds a Canada Research Chair in Diabetes Health Outcomes, is a Health Scholar through the AHFMR and is the Chair of a NET grant to ACHORD.
References
- 1.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–53. [PubMed] [Google Scholar]
- 2.Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, Kojima Y, Furuyoshi N, Shichiri M. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28(2):103–17. doi: 10.1016/0168-8227(95)01064-k. [DOI] [PubMed] [Google Scholar]
- 3.Abraira C, Colwell J A, Nuttall F Q, Sawin C T, Nagel N J, Comstock J P, Emanuele N V, Levin S R, Henderson W, Lee H S. Veterans Affairs Cooperative Study on glycemic control and complications in type II diabetes (VA CSDM). Results of the feasibility trial. Veterans Affairs Cooperative Study in Type II Diabetes. Diabetes Care. 1995;18(8):1113–23. doi: 10.2337/diacare.18.8.1113. [DOI] [PubMed] [Google Scholar]
- 4.Canadian Diabetes Association. 2003 Clinical Practice Guidelines. Can J Diabetes. 2003:S1–S52. doi: 10.1016/j.jcjd.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 5.American Diabetes Association. Standards of medical care in diabetes--2007. Diabetes Care. 2007 Jan;30(Suppl 1):S4–S41. doi: 10.2337/dc07-S004. http://care.diabetesjournals.org/cgi/pmidlookup?view=long&pmid=17192377. [DOI] [PubMed] [Google Scholar]
- 6.Rydén Lars, Standl Eberhard, Bartnik Małgorzata, Van den Berghe Greet, Betteridge John, de Boer Menko-Jan, Cosentino Francesco, Jönsson Bengt, Laakso Markku, Malmberg Klas, Priori Silvia, Ostergren Jan, Tuomilehto Jaakko, Thrainsdottir Inga, Vanhorebeek Ilse, Stramba-Badiale Marco, Lindgren Peter, Qiao Qing, Priori Silvia G, Blanc Jean-Jacques, Budaj Andrzej, Camm John, Dean Veronica, Deckers Jaap, Dickstein Kenneth, Lekakis John, McGregor Keith, Metra Marco, Morais João, Osterspey Ady, Tamargo Juan, Zamorano José Luis, Deckers Jaap W, Bertrand Michel, Charbonnel Bernard, Erdmann Erland, Ferrannini Ele, Flyvbjerg Allan, Gohlke Helmut, Juanatey Jose Ramon Gonzalez, Graham Ian, Monteiro Pedro Filipe, Parhofer Klaus, Pyörälä Kalevi, Raz Itamar, Schernthaner Guntram, Volpe Massimo, Wood David Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC); European Association for the Study of Diabetes (EASD) Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary. The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD) Eur Heart J. 2007;28(1):88–136. doi: 10.1093/eurheartj/ehl260. http://eurheartj.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=17220161. [DOI] [PubMed] [Google Scholar]
- 7.U.K. prospective diabetes study 16. Overview of 6 years' therapy of type II diabetes: a progressive disease. U.K. Prospective Diabetes Study Group. Diabetes. 1995;44(11):1249–58. [PubMed] [Google Scholar]
- 8.Eurich Dean T, Simpson Scot H, Majumdar Sumit R, Johnson Jeffrey A. Secondary failure rates associated with metformin and sulfonylurea therapy for type 2 diabetes mellitus. Pharmacotherapy. 2005;25(6):810–6. doi: 10.1592/phco.2005.25.6.810. [DOI] [PubMed] [Google Scholar]
- 9.Nichols Gregory A, Alexander Charles M, Girman Cynthia J, Kamal-Bahl Sachin J, Brown Jonathan B. Contemporary analysis of secondary failure of successful sulfonylurea therapy. Endocr Pract. 2007;13(1):37–44. doi: 10.4158/EP.13.1.37. [DOI] [PubMed] [Google Scholar]
- 10.Groop L C, Pelkonen R, Koskimies S, Bottazzo G F, Doniach D. Secondary failure to treatment with oral antidiabetic agents in non-insulin-dependent diabetes. Diabetes Care. 1986;9(2):129–33. doi: 10.2337/diacare.9.2.129. [DOI] [PubMed] [Google Scholar]
- 11.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329(14):977–86. doi: 10.1056/NEJM199309303291401. http://content.nejm.org/cgi/pmidlookup?view=short&pmid=8366922&promo=ONFLNS19. [DOI] [PubMed] [Google Scholar]
- 12.Goudswaard A N, Furlong N J, Rutten G E H M, Stolk R P, Valk G D. Insulin monotherapy versus combinations of insulin with oral hypoglycaemic agents in patients with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2004 Oct 18;4(4):CD003418. doi: 10.1002/14651858.CD003418.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson J L, Wolf S L, Kabadi U M. Efficacy of insulin and sulfonylurea combination therapy in type II diabetes. A meta-analysis of the randomized placebo-controlled trials. Arch Intern Med. 1996;156(3):259–64. [PubMed] [Google Scholar]
- 14.Peters A L, Davidson M B. Insulin plus a sulfonylurea agent for treating type 2 diabetes. Ann Intern Med. 1991;115(1):45–53. doi: 10.7326/0003-4819-115-1-45. [DOI] [PubMed] [Google Scholar]
- 15.Pugh J A, Wagner M L, Sawyer J, Ramirez G, Tuley M, Friedberg S J. Is combination sulfonylurea and insulin therapy useful in NIDDM patients? A metaanalysis. Diabetes Care. 1992;15(8):953–9. doi: 10.2337/diacare.15.8.953. [DOI] [PubMed] [Google Scholar]
- 16.Jadad A R, Moore R A, Carroll D, Jenkinson C, Reynolds D J, Gavaghan D J, McQuay H J. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 17.Schulz K F, Chalmers I, Hayes R J, Altman D G. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273(5):408–12. doi: 10.1001/jama.273.5.408. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. 4.2.6. Chichester (UK): John Wiley & Sons; 2006. [Google Scholar]
- 19.Klein W. Sulfonylurea-metformin-combination versus sulfonylurea-insulin-combination in secondary failures of sulfonylurea monotherapy. Results of a prospective randomized study in 50 patients. Diabete Metab. 1991;17(1 Pt 2):235–40. [PubMed] [Google Scholar]
- 20.Scott I, Greenberg P, Poole P, Campbell D. Cautionary tales in the interpretation of systematic reviews of therapy trials. Intern Med J. 2006;36(9):587–99. doi: 10.1111/j.1445-5994.2006.01140.x. [DOI] [PubMed] [Google Scholar]
- 21.Tobias A. Assessing the influence of a single study in the meta-analysis estimate. Stata Technical Bulletin. 1999:15–17. [Google Scholar]
- 22.López-Alvarenga J C, Aguilar-Salinas C A, Velasco-Perez M L, Arita-Melzer O, Guillen L E, Wong B, Brito G, Mercado V, Gómez-Pérez F J, Rull-Rodrigo J A. Acarbose vs. bedtime NPH insulin in the treatment of secondary failures to sulphonylurea-metformin therapy in type 2 diabetes mellitus. Diabetes Obes Metab. 1999;1(1):29–35. doi: 10.1046/j.1463-1326.1999.00007.x. [DOI] [PubMed] [Google Scholar]
- 23.Trischitta V, Italia S, Mazzarino S, Buscema M, Rabuazzo A M, Sangiorgio L, Squatrito S, Vigneri R. Comparison of combined therapies in treatment of secondary failure to glyburide. Diabetes Care. 1992;15(4):539–42. doi: 10.2337/diacare.15.4.539. [DOI] [PubMed] [Google Scholar]
- 24.Trischitta V, Italia S, Raimondo M, Guardabasso V, Licciardello C, Runello F, Mazzarino S, Sangiorgi L, Anello M, Vigneri R. Efficacy of combined treatments in NIDDM patients with secondary failure to sulphonylureas. Is it predictable? J Endocrinol Invest. 1998;21(11):744–7. doi: 10.1007/BF03348039. [DOI] [PubMed] [Google Scholar]
- 25.Vigneri R, Trischitta V, Italia S, Mazzarino S, Rabuazzo M A, Squatrito S. Treatment of NIDDM patients with secondary failure to glyburide: comparison of the addition of either metformin or bed-time NPH insulin to glyburide. Diabete Metab. 1991;17(1 Pt 2):232–4. [PubMed] [Google Scholar]
- 26.Reynolds L Raymond, Kingsley Felicia J, Karounos Dennis G, Tannock Lisa R. Differential effects of rosiglitazone and insulin glargine on inflammatory markers, glycemic control, and lipids in type 2 diabetes. Diabetes Res Clin Pract. 2007 Jan 18;77(2):180–7. doi: 10.1016/j.diabres.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 27.Rosenstock Julio, Sugimoto Danny, Strange Poul, Stewart John A, Soltes-Rak Erika, Dailey George. Triple therapy in type 2 diabetes: insulin glargine or rosiglitazone added to combination therapy of sulfonylurea plus metformin in insulin-naive patients. Diabetes Care. 2006;29(3):554–9. doi: 10.2337/diacare.29.03.06.dc05-0695. http://care.diabetesjournals.org/cgi/pmidlookup?view=long&pmid=16505505. [DOI] [PubMed] [Google Scholar]
- 28.Triplitt Curtis, Glass Leonard, Miyazaki Yoshiniro, Wajcberg Estela, Gastaldelli Amalia, De Filippis Elena, Cersosimo Eugenio, DeFronzo Ralph A. Comparison of glargine insulin versus rosiglitazone addition in poorly controlled type 2 diabetic patients on metformin plus sulfonylurea. Diabetes Care. 2006;29(11):2371–7. doi: 10.2337/dc06-0564. http://care.diabetesjournals.org/cgi/pmidlookup?view=long&pmid=17065670. [DOI] [PubMed] [Google Scholar]
- 29.Aljabri Khaled, Kozak Sharon E, Thompson David M. Addition of pioglitazone or bedtime insulin to maximal doses of sulfonylurea and metformin in type 2 diabetes patients with poor glucose control: a prospective, randomized trial. Am J Med. 2004;116(4):230–5. doi: 10.1016/j.amjmed.2003.07.023. [DOI] [PubMed] [Google Scholar]
- 30.Ko Gary T C, Tsang Patrick C C, Wai Hendena P S, Kan Eva C Y, Chan Hamish C K. Rosiglitazone versus bedtime insulin in the treatment of patients with conventional oral antidiabetic drug failure: a 1-year randomized clinical trial. Adv Ther. 2006;23(5):799–808. doi: 10.1007/BF02850321. [DOI] [PubMed] [Google Scholar]
- 31.Bastyr E J, Stuart C A, Brodows R G, Schwartz S, Graf C J, Zagar A, Robertson K E. Therapy focused on lowering postprandial glucose, not fasting glucose, may be superior for lowering HbA1C. IOEZ Study Group. Diabetes Care. 2000;23(9):1236–41. doi: 10.2337/diacare.23.9.1236. http://care.diabetesjournals.org/cgi/pmidlookup?view=long&pmid=10977012. [DOI] [PubMed] [Google Scholar]
- 32.Harris Stewart B, Ekoé Jean-Marie, Zdanowicz Yola, Webster-Bogaert Susan. Glycemic control and morbidity in the Canadian primary care setting (results of the diabetes in Canada evaluation study) Diabetes Res Clin Pract. 2005;70(1):90–7. doi: 10.1016/j.diabres.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 33.Turner R C, Cull C A, Frighi V, Holman R R. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA. 1999;281(21):2005–12. doi: 10.1001/jama.281.21.2005. http://jama.ama-assn.org/cgi/pmidlookup?view=long&pmid=10359389. [DOI] [PubMed] [Google Scholar]
- 34.Song Fujian, Altman Douglas G, Glenny Anne-Marie, Deeks Jonathan J. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analyses. BMJ. 2003;326(7387):472. doi: 10.1136/bmj.326.7387.472. http://bmj.com/cgi/pmidlookup?view=long&pmid=12609941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sterne J A, Egger M, Smith G D. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323(7304):101–5. doi: 10.1136/bmj.323.7304.101. http://bmj.com/cgi/pmidlookup?view=long&pmid=11451790. [DOI] [PMC free article] [PubMed] [Google Scholar]