Abstract
A case of life-threatening Salmonella enterica serotype Enteritidis pneumonia in a febrile patient with lung cancer is described. The organism was isolated from the sputum, the protected specimen brush material of bronchial secretions, and the stool. Despite the early administration of appropriate and adequate treatment, the patient died 7 days after the onset of the infection.
CASE REPORT
The patient was a 72-year-old white male with small cell lung cancer, diagnosed 10 months prior to the present admission. He was initially treated for his cancer with three cycles of chemotherapy with cisplatin and etoposide, with partial response. The therapeutic effort was continued with radiation treatment of the primary tumor followed by second-line chemotherapy with cisplatin and paclitaxel. Five weeks after he completed radiation treatment, radiation pneumonitis was suspected and was treated with corticosteroids (prednisolone, 50 mg/day for 15 days). He continued on corticosteroids in tapered doses, and 20 days prior to the present admission he received the first cycle of his second-line chemotherapy.
His past medical history was significant for a myocardial infarction in 1979. He had undergone surgery for coronary arterial bypass grafting in 1998. He was on proton pump inhibitors (omeprazole) for gastric protection during the time he was receiving steroids for radiation pneumonitis. He was also on nitrites, calcium channel antagonists, and low-dose acetylsalicylic acid.
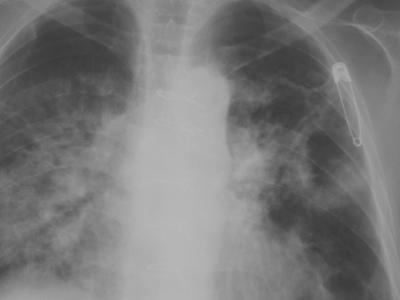
He was admitted with a low-grade fever of 37.5°C, dyspnea, tachypnea, chest discomfort, and a productive cough with purulent sputum. His vital signs on admission were the following: pulse rate of 126 beats/min, blood pressure of 150/75 mm Hg, and respiration rate of 20 breaths/min. Examination of the chest revealed decreased breath sounds and rales over the left middle and lower lung fields. A chest X-ray showed diffuse consolidation of the lower lobe of the right lung (Fig. 1). The white blood cell count was 8,500/mm3 with an absolute granulocyte count of 7,700/mm3 and with marked lymphocytopenia (absolute lymphocyte count, 100/mm3). Serology for human immunodeficiency virus serotypes 1 and 2 was negative.
FIG. 1.
Chest radiograph taken on admission showed diffuse consolidation of the lower lobe of the right lung.
Sputum, protected specimen brush (PSB) material of bronchial secretions, and three sets of blood specimens were taken for cultures on admission. He was also started on empirical antibiotic treatment with trimethoprim-sulfamethoxazole (240/1,200 mg intravenously [i.v.], four times a day), ceftazidime (2 g i.v., three times a day), and clindamycin (600 mg i.v., three times a day). He also received bronchodilators.
Twenty-four hours later, sputum and PSB sample cultures yielded a gram-negative aerobic rod identified as Salmonella enterica serotype Enteritidis, at a concentration of 2 × 108 CFU/ml in the sputum and 3 × 103 CFU/ml in the PSB sample, while all blood cultures were negative. Stool specimens cultured for enteric pathogens also yielded the same organism. Antimicrobial susceptibility testing was performed by the disk-diffusion method following the recommendations of the National Committee for Clinical Laboratory Standards (12), and MICs of the antibiotics were determined by the E-test. Susceptibilities and MICs were identical for all three isolates. The isolates were sensitive to commonly used antibiotics, such as ampicillin (MIC = 0.75 μg/ml), ceftazidime (MIC = 0.125 μg/ml), ceftriaxone (MIC = 0.094 μg/ml), cefotaxime (MIC = 0.094 μg/ml), ciprofloxacin (MIC = 0.016 μg/ml), and trimethoprim-sulfamethoxazole (MIC = 0.064 μg/ml).
Two days after admission, although the patient was receiving two antibiotics to which Salmonella was sensitive, he developed a fever of 39°C, his respiratory function worsened (severe dyspnea, tachypnea, and oxygen saturation of 70%), and his general condition deteriorated. He was transferred to the intensive care unit (ICU). The patient was initially treated with noninvasive positive-pressure ventilation. Three additional sets of blood cultures taken while the patient was febrile were negative. Three days after his admission to the hospital and 24 h after his admission in the ICU, the patient developed respiratory failure due to the extensive pulmonary involvement and was put on mechanical ventilation via an endotracheal tube. The clinical course was consistent with acute respiratory distress syndrome (ARDS), although the tracheal secretion cultures were negative. His condition continued to deteriorate, and finally he expired 5 days after his transfer to the ICU.
Discussion.
Infections due to nontyphoid salmonellae are increasing worldwide (9, 14). Salmonella enterica has been the enteropathogen most frequently isolated in Greece, with serotype Enteritidis being the most common (10).
Clinical forms of salmonellosis are gastroenteritis, bacteremia, focal infections such as septic arthritis, osteomyelitis, cholecystitis, endocarditis, meningitis, and a carrier state (13). An increased incidence of salmonellosis has been described in patients with impaired cell-mediated immunity because Salmonella is an intracellular pathogen, and an intact cellular immunity is required for its eradication. Hence, conditions predisposing to salmonellosis include infection with human immunodeficiency virus, diabetes mellitus, prolonged corticosteroid therapy, alcohol abuse, some types of chemotherapy, and some types of malignancies, mainly leukemias and lymphomas (2-4, 8, 15, 17). Wolfe et al. evaluated salmonella infections in patients with neoplastic diseases. Among the 86 patients described, more than half had hematologic malignancies (17).
Salmonella pneumonia due to nontyphoid salmonellae is rare (1, 5). Prior lung disease is associated with a higher risk for lung involvement. In a review of 36 patients with Salmonella pneumonia, empyema, or lung abscess, Cohen et al. noted that 13 of them (36%) had prior abnormalities of the lung or pleura. Among them seven had lung malignancies (5).
The most common serotypes isolated from salmonella pulmonary infections are S. enterica serotype Typhimurium and S. enterica serotype Choleraesuis (1, 5, 7). Serotype Enteritidis has been considered the causative agent in only four cases of respiratory infection according to the available literature (1).
In the present case the isolation of the bacterium from the sputum and the PSB samples in high concentrations make the diagnosis certain. Additionally, the organism was isolated from stools. Many factors may have contributed to the development of the infection, such as cancer of the lung, chemotherapy with paclitaxel, already known to cause lymphopenia and lymphocyte dysfunction (16), irradiation of the lung, and prolonged administration of corticosteroids. The patient had no symptoms from the gastrointestinal tract, and the fact that Salmonella was isolated from his stools does not necessarily mean that he was a carrier. The patient was most likely swallowing infected respiratory secretions, and this could account for the positive cultures. Since he was on antacids for a long period, we hypothesize that the source of the infection could be aspiration of colonized or infected gastric secretions; due to the decreased gastric acidity, Salmonella from the gallbladder and the upper intestinal tract could have colonized the stomach and the esophagus. Alternatively, it could be a self-limited bacteremia with seeding of the lung. Other mechanisms known from the literature to be implicated in the pathogenesis of pulmonary Salmonella infections include extension of the infection from a nearby site (5).
Salmonella pneumonia requires at least 2 weeks of parenteral or oral antibiotic therapy (11). Mortality is high in patients over the age of 60, with underlying malignancies and immunosuppression due to antineoplastic treatments (1, 5). Aguado et al. reported a mortality rate of 63% among immunosuppressed patients with pleuropulmonary diseases caused by nontyphoid salmonellae (1). In the present case, although appropriate and adequate antibiotic therapy was instituted early on, the patient succumbed a week later due to respiratory failure from extensive pulmonary involvement and development of ARDS. ARDS is a very dangerous complication of severe infection associated with high mortality (6). Additionally, the present patient was debilitated, suffering from progressive neoplasia and severe lymphopenia due to previous anticancer treatments. All these adverse factors may account for the lack of response to two effective antibiotic treatments.
Nontyphoid Salmonella organisms, although uncommon, should be considered among the pathogens responsible for gram-negative pneumonia in immunocompromised patients with lung cancer undergoing immunosuppressive treatment. The disease is severe and associated with high mortality. However, early institution of empirical antibiotic treatment for severe pneumonia that includes a third- or fourth generation cephalosporin, an antipseudomonal penicillin-beta lactamase inhibitor combination, or a quinolone would be appropriate in treating nontyphoidal Salmonella pneumonia.
REFERENCES
- 1.Aguado, J. M., G. Obeso, J. J. Cabanillas, M. Fernandez-Guerrero, and J. Ales. 1990. Pleuropulmonary infections due to nontyphoid strains of Salmonella. Arch. Intern. Med. 150:54-56. [PubMed] [Google Scholar]
- 2.Berkeley, D., and J. Mangels. 1980. Salmonella pneumonia in a patient with lung cancer. Am. J. Clin. Pathol. 74:476-478. [DOI] [PubMed] [Google Scholar]
- 3.Canney, P. A., S. N. Larsson, J. H. Hay, and M. A. Yussuf. 1985. Case report: Salmonella pneumonia associated with chemotherapy for non-Hodgkin's lymphoma. Clin. Radiol. 36:459-460. [DOI] [PubMed] [Google Scholar]
- 4.Casado, J. L., E. Navas, B. Frutos, A. Moreno, P. Martin, J. Hermida, and A. Guerrero. 1997. Salmonella lung involvement in patients with HIV infection. Chest 112:1197-1201. [DOI] [PubMed] [Google Scholar]
- 5.Cohen, J. I., J. A. Bartlett, and G. R. Corey. 1987. Extra-intestinal manifestations of Salmonella infections. Medicine 66:349-388. [DOI] [PubMed] [Google Scholar]
- 6.Gikas, A., G. Samonis, A. Christidou, J. Papadakis, D. Kofteridis, Y. Tselentis, and N. Tsaparas. 1998. Gram-negative bacteremia in non-neutropenic patients: a 3-year review. Infection 26:155-159. [DOI] [PubMed] [Google Scholar]
- 7.Gratten, J., J. Barker, and A. Rongar. 1983. Pneumonia due to Salmonella choleraesuis in infants in Papua, New Guinea. Lancet ii:580-581. [DOI] [PubMed] [Google Scholar]
- 8.Han, T., J. E. Sokal, and E. Neter. 1967. Salmonellosis in disseminated malignant diseases. N. Engl. J. Med. 276:1045-1052. [DOI] [PubMed] [Google Scholar]
- 9.Hohmann, E. L. 2001. Nontyphoidal salmonellosis. Clin. Infect. Dis. 32:263-269. [DOI] [PubMed] [Google Scholar]
- 10.Maraki, S., A. Georgiladakis, Y. Tselentis, and G. Samonis. 2003. A 5-year study of bacterial pathogens associated with acute diarrhoea on the island of Crete, Greece, and their resistance to antibiotics. Eur. J. Epidemiol. 18:85-90. [DOI] [PubMed] [Google Scholar]
- 11.Miller, S., and D. A. Pegues. 2000. Salmonella species including Salmonella typhi, p. 2344-2363. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases, 5th ed. Churchill Livingstone, Philadelphia, Pa.
- 12.National Committee for Clinical Laboratory Standards. 2001. Performance standards for antimicrobial susceptibility testing; 11th informational supplement. M100-S11. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 13.Saphra, I., and J. W. Winter. 1957. Clinical manifestations of salmonellosis in man: an evaluation of 7,779 human infections identified in the New York Salmonella Center. N. Engl. J. Med. 256:1128-1134. [DOI] [PubMed] [Google Scholar]
- 14.Shimoni, Z., S. Pitlik, L. Leibovici, Z. Samra, H. Konigsberger, M. Drucker, V. Agmon, S. Ashkenazi, and M. Weinberger. 1999. Nontyphoid Salmonella bacteremia: age-related differences in clinical presentation, bacteriology and outcome. Clin. Infect. Dis. 28:822-827. [DOI] [PubMed] [Google Scholar]
- 15.Sinkovics, J. G., and J. P. Smith. 1969. Salmonellosis complicating neoplastic diseases. Cancer 24:631-636. [DOI] [PubMed] [Google Scholar]
- 16.Souglakos, J., A. Kotsakis, C. Kouroussis, S. Kakolyris, D. Mavroudis, K. Kalbakis, S. Agelaki, J. Vlachonikolis, V. Georgoulias, and G. Samonis. 2002. Nonneutropenic febrile episodes associated with docetaxel-based chemotherapy in patients with solid tumors. Cancer 95:1326-1333. [DOI] [PubMed] [Google Scholar]
- 17.Wolfe, M. S., D. Armstrong, D. B. Louria, and A. Blevins. 1971. Salmonellosis in patients with neoplastic disease: a review of 100 episodes at Memorial Cancer Center over a 13 year period. Arch. Intern. Med. 128:546-554. [DOI] [PubMed] [Google Scholar]