Abstract
Cerebral cavernous malformations (CCM) are characterized by abnormal dilated intracranial capillaries that predispose to hemorrhage. The development of some CCMs in humans has been attributed to mutations in the CCM1 genes. Currently, contradictory results have been generated regarding the vascular endothelial cell population changes in Ccm1 deficiency in zebrafish. We hypothesize that the inconsistent results simply reflect the spatial and temporal difference for the observed vascular endothelial cells during zebrafish embryonic development. Using high resolution images in vivo, we demonstrated that the loss of Ccm1 in zebrafish embryos leads to marked increases in apoptosis in vascular endothelium at the end stage of microvascular angiogenesis. In vivo zebrafish studies were further substantiated by in vitro findings in human endothelial cells that elucidated the biochemical pathways of CCM1 deficiency. We found that that loss of CCM1 in vitro promotes apoptosis through decreased activation of the integrin-linked kinase survival signaling pathway. In summary, Ccm1 has been identified as a key modulator in maintaining microvascular integrity during zebrafish embryonic angiogenesis.
Keywords: Cerebral cavernous malformation, Angiogenesis, Intersegmental vessels, Apoptosis, Vascular endothelial cell
Introduction
Cerebral cavernous malformations (CCMs) are vascular lesions characterized by distended intracranial capillaries with a thin layer of vascular endothelial cells. This vasculature lacks supportive parenchyma, compromising vascular integrity and thus potentially causing hemorrhage and stroke. Loss-of-function mutation in one of three CCM genes: KRIT1 (CCM1), MGC4607 (CCM2), and PDCD10 (CCM3) results in the pathogenesis of CCM. On the molecular level, CCM1 interacts with integrin cytoplasmic domain associated protein-1-alpha (ICAP1α), suggesting a role in β1 integrin-mediated signaling [1–3].
Our prior in vitro observation documented a decrease in the VEC population upon depletion of CCM1 [3]. The phenomenon could be explained by decreased cell proliferation, increased apoptosis, or a combination of both [3]; we already reported the negative effects on cell proliferation upon depletion of CCM1 [3], in this experiment we also found an increased apoptosis in the endothelial cells upon depletion of CCM1 in vitro. However, whether the Ccm1 deficiency in vivo would result in the number changes of vascular endothelial cell in animal model still remains controversial. In the first Ccm1 zebrafish study, no cell number change in both cardiomyocyte and endothelial cells was found in the enlarged heart, a major phenotype in Ccm1 zebrafish, which lead to the conclusion that Ccm1 regulates the concentric growth of myocardium without changing the fate of cardiomyocyte or endothelial cells. Two recent studies in zebrafish embryos found that Ccm1 mutations cause the dilation of major vessels (especially primitive veins) and the progressive thinning of endothelial cells lining these primitive vessels [4, 5]. While one of these studies reported significantly increased endothelial cells in the primitive vessels of Ccm1 mutants [5], the other emphasized that the endothelial cell number or contacts in these primitive vessels were unchanged [4]. Nevertheless, both studies agreed that Ccm1 proteins regulate the genesis of vascular endothelial cells, particularly vascular tubular formation during vasculogenesis, further supporting the perturbed vasculogenesis hypothesis found in mouse models [4–6].
To understand the apparent discrepancy among these in vitro and in vivo observations, we posit that the difference simply reflects the fact that these in vivo observations were performed at different anatomical regions during different embryonic developmental stages (such as cardiogenesis, vasculogenesis, and angiogenesis). By studying the vasculature during angiogenesis in Ccm1 zebrafish in vivo and cultured endothelial cells in vitro, we examined angiogenic property and vascular integrity during angiogenesis in Ccm1 zebrafish embryonic vasculature and cellular signaling in human endothelium upon depletion of CCM1. Our results demonstrate the concordant angiogenic effect by Ccm1 in both in vitro and in vivo models and the molecular mechanisms by which CCM1 deficiency leads to increased apoptosis during microvascular development in endothelium.
Materials and Methods
Zebrafish Care, Manipulation, and Imaging
Acquisition and maintenance of zebrafish lines used in this experiment and generation of a double transgenic line by crossing a Ccm1 mutant strain (KRIT1ty219c/+(AB)) with a Tg (fli1:EGFP)y1/+(AB) strain have been carried out in our lab. Antisense oligonucleotide morpholinos (MOs) to Ccm1 gene (MO1 and MO2, GeneTools) and injection procedure were previously described [7]. Fluorescent images were acquired with a Nikon 90i microscopy with a Coolsnap ES CCD camera (Roper Scientific) and processed with Metamorph 6.3 (MDS, Inc.).
Endogenous Alkaline Phosphatase Staining
Embryos were fixed with 4% paraformaldehyde in PBST for 30 min at room temperature and permeabilized by incubation with acetone for 30 min at −20°C. After 2 × 5 min wash with PBST, embryos were washed with NTMT buffer (50 mM MgCl2, 100 mM NaCl, 0.1% Tween-20, 100 mM Tris pH9.5), 3 × 5 min at room temperature. NBT (2.25 uL of 75 mg/ml stock solution in 70% dimethylformamide) and BCIP (1.75 uL of 50 mg/ml stock solution in dimethylformamide) were then added to each milliliter of NTMT to initiate staining. After 15–30 min, embryos were washed three times in PBST and stored in 87% glycerol for microscopy examination.
Whole-Mount Immunofluorescent Staining of Zebrafish and TUNEL Assay
In immunostaining, fixed zebrafish embryos were permeabilized, blocked, and incubated with primary antibodies overnight at 4°C. Embryos were then washed and incubated with Alexa fluor 488 or 563 conjugated secondary antibodies for 1 h, followed by washing, mounting, and imaging. In TUNEL assay, fixed and permeabilized zebrafish embryos were labeled using the In Situ Cell Death Detection Kit, POD (Roche, Penzberg, Germany).
Morpholino Analysis
Antisense oligonucleotide morpholinos (MOs) to Ccm1 gene (MO1 and MO2, GeneTools) were diluted in 1× Danieau’s buffer to 0.5 mM concentration as previously described [7]. The injection of MO solution (0.1, 0.2, 0.5, 1, and 2 nL volume) or control buffer was performed on fertilized eggs at one-cell stage from Tg (fli:EGFP) zebrafish under AB wild-type background. After injection, the embryos were allowed to develop to designated stage at 28.5°C.
Endothelial Cell Culture, RNAi, and Apoptosis Assay
Human CCM1 (KRIT1) and ICAP1α-specific siRNA oligos and control siRNA duplex SCRAMBLE II and Luciferase GL2 duplex were synthesized at DHARMACON as previously described [3]. HUVEC (105) cells cultured in EGM2 medium (Clonetics) were seeded in each well for siRNA treatment with Oligofectamine (Invitrogen), and the cell apoptosis assay was carried out using the colorimetric kit (Roche).
Antibodies and Immunocytochemistry and Western Blot
β-actin (C-2) and PARP (H250), pBad (ser136), Bad (C-7), pAKT1 (ser473, Thr308), AKT1 (B-1), pPDK1 (Ser241), PDK1 (C-20 or V-17) and ILK1 (C-19, Santa Cruz), pSer-specific antibodies (Poly-Z-PS1, Invitrogen), Caspase 3 (8G10), Caspase 7, and pBad (ser112, ser155, Cell Signaling Technology) primary antibodies for HUVEC cells, Tie-2 (176222, R&D System), and active caspase-3 primary antibodies (C92-605, Pharmingen) for zebrafish were purchased. Protein concentrations of the RNAi treatment lysates were measured with RC DC protein assay (Bio-Rad). Equal amounts of protein for each treatment were then subsequently loaded on SDS-PAGE. Proteins were detected with designated antibodies followed by chemiluminescence (GE).
Statistical Testing
For multiple comparisons, one-way analysis of variance (ANOVA) was used to detect the differences in the mean values among the treatment groups. Pairwise comparison (t test) was used to test the difference between each treatment.
Results
1. Decrease in VEC population due to Ccm1 knockout
Overt signs of decreased VEC GFP fluorescence in Ccm1/Tg (fli1: EGFP) zebrafish were found during our observation of zebrafish embryonic development. In examination of Ccm1 mutant (san) zebrafish with additional two VEC specific stainings, the significant decreases in VEC signal were still present, indicating that the VEC population indeed declines at the end of angiogenesis (Fig. 1). The decrease in the VEC population within angiogenic vessels could be explained by the established fact that cord-like primary and secondary angiogenic vessels, which have not accomplished angiogenic events, will regress after circulation is initiated [8].
Fig. 1.
Loss of endogenous alkaline phosphatase staining (EAP) and tie-2 immunostaining (Tie2) of vascular endothelial cells in the trunk of Ccm1 mutant zebrafish. At developmental stages 48 or 72 hpf, wild-type and Ccm1 mutant (KRIT1ty219c/+(AB)) embryos were subjected to staining assay. There is diminished alkaline phosphatase staining (left) and tie-2 immunostaining (right) in the trunks of Ccm1 mutants (lower panels) compared with wild type embryos (upper panels)
To determine whether a loss of Ccm1 function can impact microvascular integrity by promoting apoptosis, we next examined the apoptotic events of VECs in san and wild-type embryos with TUNEL assays under a Tg (fli1: EGFP) background. From developmental stage 48–72 hpf, a period during which angiogenesis fails in san embryos, TUNEL assays show a larger increase in VEC apoptosis in san embryos than in wild-type. The fluorescent signals in these assays colocalize with the microvasculatures of ISV, CV, DLAV, small vessels in the brain, and lens of the eyes (Fig. 2). No differences in TUNEL assays of wild-type and san zebrafish were found at 24 hpf prior to angiogenesis (data not shown). In agreement with the increased apoptosis seen in the TUNEL assays, there was a substantial increase in VEC apoptotic fragments observed in the subsequent immunostaining of cleaved active caspase 3 from san zebrafish at developmental stage 48–72 hpf (Fig. 3).
Fig. 2.
TUNEL assay of apoptosis in the vascular endothelial cells. At developmental stage 48 hpf, wild-type (left panels) and Ccm1 mutant (right panels) embryos with Tg (Fli1:EGFP) background were subjected to TUNEL assay for apoptosis. Vasculature (EGFP, upper panels) and apoptosis signals (TUNEL, middle panels) were then examined under multi-wavelength fluorescence microscopy. The lower panels show the alignment of EGFP and TUNEL signals. Note that in Ccm1 mutants, there is enhanced apoptotic signal along the vasculature compared with wild-type embryos (indicated with white arrows; calibration bars, 50μm)
Fig. 3.
Caspase-3 active fragment immunostaining for apoptosis in vascular endothelial cells. At developmental stage 48 hpf, wild-type (left panels) and san mutant (right panels) zebrafish with Tg (Fli1:EGFP) background were subjected to caspase-3 active fragment immunostaining for apoptosis. Vasculature (EGFP, upper panels) and apoptosis signals (Caspase-3, middle panels) were then examined under multi-wavelength fluorescence microscopy. The lower panels show the alignment of EGFP and apoptosis signals. There is a clearly enhanced apoptosis signal in the Ccm1 mutant’s vasculature compared with the wild-type (marked with white arrows; calibration bars, 50 μm)
2. Decrease in VEC population due to Ccm1 knockdown
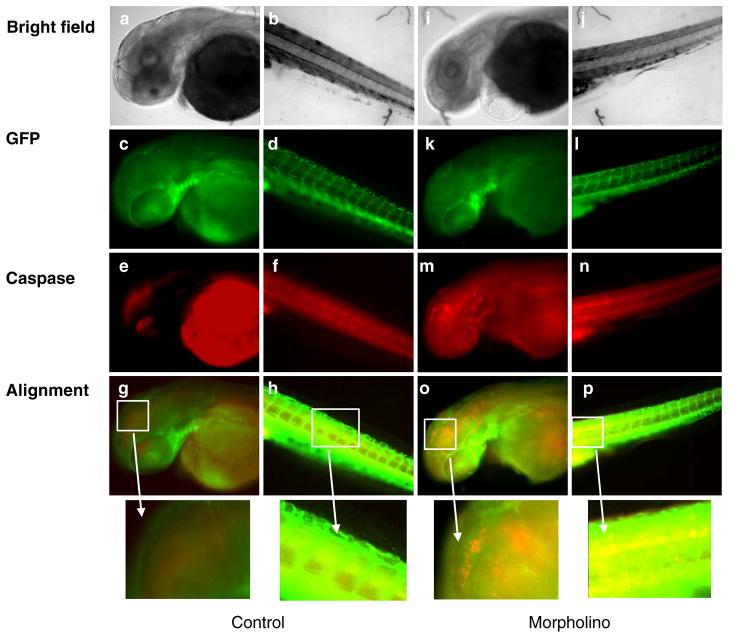
To validate the above observations in san zebrafish, we next performed TUNEL assay on Ccm1 MO zebrafish. As before, no difference in the quantity of apoptotic events in primitive and primary angiogenic vessels was observed at 24 hpf (data not shown). In subsequent TUNEL assays and Caspase-3 active fragment stainings at developmental stage 48–72 hpf, Ccm1 MO treatment showed enhanced apoptosis in the VECs compared with control MO treatment (Figs. 4 and 5). These experiments confirm a significant increase in VEC apoptosis with MO knockdown of Ccm1 protein during the embryonic stages when secondary angiogenesis are occurring.
Fig. 4.
Ccm1 knockdown enhances vascular endothelial TUNEL signals and perturbs microvasculature. Enhanced TUNEL signals in Ccm1 knockdown zebrafish are coincident with vasculature in Tg (fli1:EGFP) background embryos at 48 hpf. Left panel: a 48 hpf wild-type embryo that was injected with 1× Danieau’s buffer only (a–h). Right panel: a 48 hpf wild-type embryo that was injected with 1 nl of 0.5 mM Ccm1 antisense morpholino (i–p). Panels a, e, i, and m show the fluorescence image of EGFP-labeled vascular endothelial cells. Panels b, f, j, and n are TUNEL fluorescence images. Panels c, g, k, and o are merged views for GFP and TUNEL signals. Panels d, h, l, and p are partially magnified views of c, g, k, and o. There is enhanced TUNEL signal in the vasculature of Ccm1 MO embryos (l and p, marked by white arrows) compared with control (d and h)
Fig. 5.
Ccm1 knockdown enhances vascular endothelial cell apoptosis. Ccm1 knockdown enhances vascular endothelial cell apoptosis in Tg (fli1:egfp) zebrafish embryos. Left panel: Control, a 48 hpf embryo that had been injected with 1× Danieau’s buffer only at one-cell stage (a–h). Right panel: a 48 hpf embryos that had been injected with 1 nl of 0.5 mM Ccm1 antisense morpholino (i–p). Panels a, b, i, and j are bright field images; panels c, d, k, and l show the fluorescence image of GFP-labeled vascular endothelial cells. Panels e, f, m, and n are caspase-3 active fragment staining fluorescence images. Panels g, h, o, and p are merged views for GFP and caspase-3 signals; partial magnified views are also shown in the figure. Note that there is enhanced caspase-3 signal in the vasculature of Ccm1 MO embryos (o and p) compared with control (g and h)
As reported previously, sufficient doses of Ccm1 MO treatment induce changes in vasculature similar to those observed in san zebrafish [7]. These vascular changes were present at stage 48 hpf, which is temporally concordant with the increase in apoptosis. This temporal concordance supports a relationship between VEC apoptosis, failed angiogenesis, and loss of Ccm1 function.
3. Silencing of CCM1 enhances VEC apoptosis in vitro
The in vivo data presented above provides evidence that failure of angiogenesis due to Ccm1 deficiency leads to increased apoptosis. To corroborate this evidence, the effect of CCM1 RNAi on VEC programmed cell death was further explored in vitro. These experiments showed significant increases of apoptosis among CCM1 siRNA-treated HUVEC cells compared with controls (Fig. 6a). This demonstrates that silencing of CCM1 significantly enhances human vascular endothelial cell apoptosis.
Fig. 6.
Silencing of CCM1 and ICAP1α leads to increased apoptosis via an ILK-mediated pathway. a Histogram indicating increased apoptotic cell death in HUVECs treated with CCM1 siRNA compared to mock control and to treatment with irrelevant siRNA. One-way ANOVA shows significantly increased absorbance values in CCM1 depleted cells compared to scramble-treated and mock controls (F= 7.678, P<0.005, n=3). Pairwise comparison shows that there are significant differences between the CCM1 siRNA-treated cells and each of the controls (P≤0.001 for each comparison). b The Western blot demonstrates that depletion of CCM1 and ICAP1α leads to decreased serine phosphorylation of ILK compared to a luciferase siRNA-treated control. Depletion of CCM1 and ICAP1α also leads to decreased ILK-mediated phosphorylation of AKT1 at Ser473. Additionally, the downstream effects include decreased phosphorylation of BAD on Ser136, decreases in procaspase 7 and 3 (indicating cleavage and increased caspase activity), and increases in cleaved PARP (cPARP). These results are consistent with increased apoptosis and decreased cell survival
4. Silencing of CCM1 and ICAP1α perturbs the ILK-AKT1-BAD cell survival signaling pathway
After documenting the presence of increased apoptosis in VEC, the focus of our research shifted to understanding the molecular mechanisms underlying the VEC regression. In this investigation, another role for CCM1 in the microvascular development was uncovered: the promotion of VEC survival in blood vessels during angiogenesis.
Integrin-linked kinase (ILK) is not only an effector of β1 integrin, but also a major regulator of cell survival signaling via the AKT1-BAD pathway. The phosphorylation status of ILK was examined in order to ascertain any effect of CCM1 deficiency on this apoptosis-suppressing pathway. We found that the serine phosphorylation level of ILK was diminished upon the depletion of CCM1 and its cellular effector, ICAP1α, compared to Luciferase siRNA controls in HUVECs (Fig. 6b). This diminished phosphorylation implies that the depletion of CCM1 causes a sharp decrease in ILK kinase activity.
We next explored whether depletion of CCM1 and ICAP1α has significant negative impact on the AKT1-BAD cascade in HUVECs (Fig. 6b). In our experiments, significant drops in phosphorylation of AKT1 at Ser473 (the ILK-specific site) were detected upon silencing of CCM1 and ICAP1α compared to Luciferase siRNA and Mock controls. In contrast, no differences in phosphorylation at Thr308 existed among any of the treatments (data not shown). Next, we examined an AKT1 downstream target and found significantly decreased phosphorylation of pBAD at Ser136 (the AKT1 specific site) upon the depletion of CCM1 and ICAP1α compared to controls. At the same time, the phosphorylation status of Ser155 (phosphorylated by PKA and RSK) and Ser112 (phosphorylated by PKA and RSK) of pBAD did not change (data not shown). Finally, by screening the end products of the survival pathway, we found a larger number of PARP degradation fragments upon depletion of CCM1 and ICAP1α compared to Mock and Luciferase siRNA controls. This result indicates that caspase 3 might be involved in this apoptotic process [9]. However, when we screened procaspase 3 and its parallel partner procaspase 7, we found surprisingly that, in addition to a noticeable decreased procaspase 3, procaspase 7 decreased dramatically upon depletion of CCM1 and ICAP1α (Fig. 6b). These findings indicate that caspase 7 mediated apoptotic cascade plays a major role in loss of CCM1 and its cellular effector, ICAP1α, induced VEC apoptosis.
Given the results of these in vitro studies, we conclude that CCM1 plays a pivotal role in ILK-mediated cell survival signal pathways via its effect on integrin-mediated ILK activation. Silencing of CCM1 and ICAP1α inhibits ILK activity and consequently inactivates downstream signaling kinases AKT1 and BAD. The resulting dephosphorylation of pBAD initiates activation of caspases (mainly via caspases 7 cleavages) and cleavage of PARP, which leads to increased VEC apoptosis.
Discussion
Using both in vivo animal models and in vitro human VEC cell lines, we have demonstrated that defective embryonic angiogenesis helps to precipitate the regression of ISVs due to disruption of a CCM1-mediated ILK survival signal pathway in the VECs. This study has established a paradigm that links CCM1 molecular function to VEC morphogenesis and integrity during microvascular angiogenesis.
Our studies have further elucidated the role of Ccm1 in zebrafish embryonic development. Prior research reported an unchanged or increased VEC population in Ccm deficiency [4, 5, 7]. In contrast, our studies found a decrease in VECs, specifically in microvasculature during late stage of angiogenesis, resulted from increased apoptosis as part of the process of microvascular failure in Ccm1 deficiency in zebrafish embryos. With in vitro human VEC studies, we showed that CCM1 deficiency induces VEC apoptosis through β1 integrin-mediated signaling mechanism. CCM1 mediates an AKT1-BAD survival pathway through ILK-mediated signaling, and disruption of this pathway by CCM1 deficiency increases VEC apoptosis, thereby reducing the VEC population.
Blood vessel integrity is essential for vascular homeostasis during embryogenesis. Most of the components that determine vascular integrity have been conserved among vertebrates. For this reason, understanding the molecular mechanism controlling angiogenesis and vascular regression in zebrafish could be invaluable in treating human diseases. A key component of this molecular mechanism in zebrafish is apoptosis [10, 11]. Apoptosis is essential for lumen formation, as apoptosis serves to remove redundant VECs. Vascular regression is a programmed process; endothelial cell apoptosis is required for this capillary regression [12]. The inhibition of apoptosis has been shown to block capillary lumen formation. Therefore, apoptosis of capillary VECs serves to open capillary lumens in microvascular morphogenesis [13]. The observation that zebrafish blood vessels carrying a high circulatory volume enlarge, whereas those with low or no flow regress [14], further accentuates the importance of apoptosis during vascular lumen formation.
While apoptosis is required for embryonic vascular development, excessive apoptosis among VECs would lead to the destruction of microvessels. This phenomenon has been proven true in the disruption of several genes controlling endothelial cell survival and vascular integrity. For example, Birc2 (also known as cIap1) is a critical regulator of vascular integrity and endothelial cell survival. Birc2 inhibits a caspase-8-dependent apoptotic program that leads to vessel regression [5]. Neuropilin-1 (NRP-1) mediates VEC survival through the inactivation of p53. An in vitro study using HUVEC cells demonstrated that NRP-1 inhibits an Akt1-mediated caspase-3-dependent apoptotic program that leads to vessel regression [15]. Defective ISVs due to increased VEC apoptosis were observed in both loss of Birc2 and loss of NRP-1 mutant zebrafish embryos [15, 16]. Furthermore, the phenotype of defective ISVs was reported in several zebrafish lines that were knockouts of several upstream regulators of Akt1 [17–19]. Similar to NRP-1, our research found that krit1 inhibits an Akt1-BAD mediated, caspase 7-dependent apoptotic program.
The intricate balance between vascular morphogenesis and regression is controlled by a dynamic gene regulation. For example, β1 integrin usually supports vascular morphogenesis; however, at least one in vitro experiment showed that activated β1 integrin inhibits capillary vacuoles and tube formation [20]. In san zebrafish, we found that β1 integrin signaling mediates a caspase-7-dependent apoptotic program that leads to vessel regression. Through this mechanism, an absence of CCM1 dramatically increases apoptosis in the angiogenic cord network after failed angiogenesis, leading to vessel regression. Thus, not only does CCM1 deficiency contribute to defective angiogenesis of ISVs, CCM1 deficiency also increases apoptosis in the ISVs.
All three CCM genes have been proven to interact with each other and form a CCM complex [21–24] that interacts with other genes [25, 26]. CCM3 gene (PDCD10) has been indicated to associate with apoptotic events [27]. We have shown the anti-apoptotic property of CCM1 in this report, however, whether CCM genes involve in apoptotic events independently or they act coordinately within the CCM complex need to be further addressed.
Acknowledgments
We thank Chris Carr and Betty Chen at UMMC Neurosurgery, Hal Dietz, Richard Clatterbuck, and Sharmila Basu at Johns Hopkins University for their invaluable technical help and discussion during the experiments and in the preparation of this manuscript.
Contributor Information
Huiling Liu, Department of Neurosurgery, University of Mississippi Medical Center, Jackson, MS 39216, USA.
Daniele Rigamonti, Department of Neurological Surgery, The Johns Hopkins University School of Medicine, Baltimore, MD 21205, USA.
Ahmed Badr, COE for Neurosciences, Departments of Anesthesiology, Biomedical Sciences, Neurosurgery, Texas Tech University Health Science Center, 4800 Alberta Avenue, El Paso, TX 79905, USA.
Jun Zhang, Email: jun.zhang2000@gmail.com, COE for Neurosciences, Departments of Anesthesiology, Biomedical Sciences, Neurosurgery, Texas Tech University Health Science Center, 4800 Alberta Avenue, El Paso, TX 79905, USA.
References
- 1.Zhang J, Clatterbuck RE, Rigamonti D, Chang DD, Dietz HC. Interaction between krit1 and icap1alpha infers perturbation of integrin beta1-mediated angiogenesis in the pathogenesis of cerebral cavernous malformation. Hum Mol Genet. 2001;10:2953–2960. doi: 10.1093/hmg/10.25.2953. [DOI] [PubMed] [Google Scholar]
- 2.Zawistowski JS, Serebriiskii IG, Lee MF, Golemis EA, Marchuk DA. Krit1 association with the integrin-binding protein icap-1: a new direction in the elucidation of cerebral cavernous malformations (ccm1) pathogenesis. Hum Mol Genet. 2002;11:389–396. doi: 10.1093/hmg/11.4.389. [DOI] [PubMed] [Google Scholar]
- 3.Zhang J, Basu S, Rigamonti D, Dietz HC, Clatterbuck RE. Krit1 modulates beta1-integrin-mediated endothelial cell proliferation. Neurosurgery. 2008;63:571–578. doi: 10.1227/01.NEU.0000325255.30268.B0. discussion 578. [DOI] [PubMed] [Google Scholar]
- 4.Hogan BM, Bussmann J, Wolburg H, Schulte-Merker S. Ccm1 cell autonomously regulates endothelial cellular morphogenesis and vascular tubulogenesis in zebrafish. Hum Mol Genet. 2008;17:2424–2432. doi: 10.1093/hmg/ddn142. [DOI] [PubMed] [Google Scholar]
- 5.Jin S-W, Herzog W, Santoro MM, Mitchell TS, Frantsve J, Jungblut B, Beis D, Scott IC, D’Amico LA, Ober EA, Verkade H, Field HA, Chi NC, Wehman AM, Baier H, Stainier DYR. A transgene-assisted genetic screen identifies essential regulators of vascular development in vertebrate embryos. Dev Biol. 2007;307:29–42. doi: 10.1016/j.ydbio.2007.03.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitehead KJ, Plummer NW, Adams JA, Marchuk DA, Li DY. Ccm1 is required for arterial morphogenesis: implications for the etiology of human cavernous malformations. Development. 2004;131:1437–1448. doi: 10.1242/dev.01036. [DOI] [PubMed] [Google Scholar]
- 7.Mably JD, Chuang LP, Serluca FC, Mohideen M-APK, Chen J-N, Fishman MC. Santa and valentine pattern concentric growth of cardiac myocardium in the zebrafish. Development. 2006;133:3139–3146. doi: 10.1242/dev.02469. [DOI] [PubMed] [Google Scholar]
- 8.Isogai S, Lawson ND, Torrealday S, Horiguchi M, Weinstein BM. Angiogenic network formation in the developing vertebrate trunk. Development. 2003;130:5281–5290. doi: 10.1242/dev.00733. [DOI] [PubMed] [Google Scholar]
- 9.Takagi Y, Hattori I, Nozaki K, Ishikawa M, Hashimoto N. DNA fragmentation in central nervous system vascular malformations. Acta Neurochir (Wien) 2000;142:987–994. doi: 10.1007/s007010070053. [DOI] [PubMed] [Google Scholar]
- 10.Blum Y, Belting HG, Ellertsdottir E, Herwig L, Luders F, Affolter M. Complex cell rearrangements during intersegmental vessel sprouting and vessel fusion in the zebrafish embryo. Dev Biol. 2008;316:312–322. doi: 10.1016/j.ydbio.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 11.Kamei M, Saunders WB, Bayless KJ, Dye L, Davis GE, Weinstein BM. Endothelial tubes assemble from intracellular vacuoles in vivo. Nature. 2006;442:453–456. doi: 10.1038/nature04923. [DOI] [PubMed] [Google Scholar]
- 12.Lang RA. Apoptosis in mammalian eye development: lens morphogenesis, vascular regression and immune privilege. Cell Death Differ. 1997;4:12–20. doi: 10.1038/sj.cdd.4400211. [DOI] [PubMed] [Google Scholar]
- 13.Fierlbeck W, Liu A, Coyle R, Ballermann BJ. Endothelial cell apoptosis during glomerular capillary lumen formation in vivo. J Am Soc Nephrol. 2003;14:1349–1354. doi: 10.1097/01.asn.0000061779.70530.06. [DOI] [PubMed] [Google Scholar]
- 14.Snider P, Conway SJ. Developmental biology: the power of blood. Nature. 2007;450:180–181. doi: 10.1038/450180a. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Dutta SK, Kojima T, Xu X, Khosravi-Far R, Ekker SC, Mukhopadhyay D. Neuropilin-1 modulates p53/caspases axis to promote endothelial cell survival. PLoS ONE. 2007;2:e1161. doi: 10.1371/journal.pone.0001161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santoro MM, Samuel T, Mitchell T, Reed JC, Stainier DY. Birc2 (ciap1) regulates endothelial cell integrity and blood vessel homeostasis. Nat Genet. 2007;39:1397–1402. doi: 10.1038/ng.2007.8. [DOI] [PubMed] [Google Scholar]
- 17.Croushore JA, Blasiole B, Riddle RC, Thisse C, Thisse B, Canfield VA, Robertson GP, Cheng KC, Levenson R. Ptena and ptenb genes play distinct roles in zebrafish embryogenesis. Dev Dyn. 2005;234:911–921. doi: 10.1002/dvdy.20576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez F, Vacaru A, Overvoorde J, den Hertog J. The receptor protein-tyrosine phosphatase, dep1, acts in arterial/venous cell fate decisions in zebrafish development. Dev Biol. 2008;324:122–130. doi: 10.1016/j.ydbio.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 19.Liu L, Zhu S, Gong Z, Low BC. K-ras/pi3k-akt signaling is essential for zebrafish hematopoiesis and angiogenesis. PLoS ONE. 2008;3:e2850. doi: 10.1371/journal.pone.0002850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gamble J, Meyer G, Noack L, Furze J, Matthias L, Kovach N, Harlant J, Vadas M. B1 integrin activation inhibits in vitro tube formation: effects on cell migration, vacuole coalescence and lumen formation. Endothelium. 1999;7:23–34. doi: 10.3109/10623329909165309. [DOI] [PubMed] [Google Scholar]
- 21.Hilder TL, Malone MH, Bencharit S, Colicelli J, Haystead TA, Johnson GL, Wu CC. Proteomic identification of the cerebral cavernous malformation signaling complex. J Proteome Res. 2007;6:4343–4355. doi: 10.1021/pr0704276. [DOI] [PubMed] [Google Scholar]
- 22.Voss K, Stahl S, Schleider E, Ullrich S, Nickel J, Mueller TD, Felbor U. Ccm3 interacts with ccm2 indicating common pathogenesis for cerebral cavernous malformations. Neurogenetics. 2007;8:249–256. doi: 10.1007/s10048-007-0098-9. [DOI] [PubMed] [Google Scholar]
- 23.Zawistowski JS, Stalheim L, Uhlik MT, Abell AN, Ancrile BB, Johnson GL, Marchuk DA. Ccm1 and ccm2 protein interactions in cell signaling: implications for cerebral cavernous malformations pathogenesis. Hum Mol Genet. 2005;14:2521–2531. doi: 10.1093/hmg/ddi256. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J, Rigamonti D, Dietz HC, Clatterbuck RE. Interaction between krit1 and malcavernin: implications for the pathogenesis of cerebral cavernous malformations. Neurosurgery. 2007;60:353–359. doi: 10.1227/01.NEU.0000249268.11074.83. discussion 359. [DOI] [PubMed] [Google Scholar]
- 25.Ma X, Zhao H, Shan J, Long F, Chen Y, Zhang Y, Han X, Ma D. Pdcd10 interacts with ste20-related kinase mst4 to promote cell growth and transformation via modulation of the erk pathway. Mol Biol Cell. 2007;18:1965–1978. doi: 10.1091/mbc.E06-07-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uhlik MT, Abell AN, Johnson NL, Sun W, Cuevas BD, Lobel-Rice KE, Horne EA, Dell’Acqua ML, Johnson GL. Rac-mekk3-mkk3 scaffolding for p38 mapk activation during hyperosmotic shock. Nat Cell Biol. 2003;5:1104–1110. doi: 10.1038/ncb1071. [DOI] [PubMed] [Google Scholar]
- 27.Wang YG, Liu HT, Zhang YM, Ma DL. Cdna cloning and expression of an apoptosis-related gene, human tfar-15 gene. Science in China C Life Sci. 1999;29:331–336. doi: 10.1007/BF03183610. [DOI] [PubMed] [Google Scholar]