Abstract
Solid and liquid media supplemented only with a cyanobacterial extract (CE) and free of fetal calf serum (FCS), blood, and its derivatives support the growth of Helicobacter pylori. A total of 11 strains of H. pylori isolated from gastric biopsy samples were successfully subcultured in Mueller-Hinton agar supplemented with 0.4% CE. When this medium was used for primary isolation of H. pylori, a low isolation rate (30%) was observed because of the abundant growth of contaminants. The growth kinetics of eight isolates and H. pylori reference strain NCTC 11638 in Mueller-Hinton broth (MHB) supplemented with 0.7% CE were estimated by use of growth parameters, and the results were compared with those obtained with MHB-5% FCS. For four strains the cellular concentrations obtained with CE were statistically higher (P < 0.05) than those obtained with FCS, and in some cases these values were similar to the highest values reported in the literature. Depending on the strain, the specific growth rates obtained with CE were similar to or increased compared with those obtained with FCS. The replacement of FCS by CE in H. pylori cultures would facilitate the retrieval of cultures with high cellular densities as a source of cellular and extracellular proteins free of serum. Also, CE has advantages over conventional supplements, such as easier conservation and compliance with the pressing tendency at present to avoid the use of products derived from animals.
Helicobacter pylori is a gram-negative microaerobic bacterium whose presence in the gastric mucosa is associated with the development of gastroduodenal pathologies ranging from gastritis to peptic ulcers and neoplasias.
This microorganism is difficult to culture in vitro, particularly in liquid media. However, its growth has been demonstrated in several nutrient-rich media, such as brucella broth (23), brain heart infusion broth (21, 23), tryptic soy broth (21, 23), and Mueller-Hinton broth (MHB) (21, 23). Fetal calf serum (FCS) is one of the most commonly used supplements (4, 5, 13, 14, 20, 21, 25). Solid media are usually supplemented with blood and blood derivatives (23).
Other typical supplements added to growth media include horse serum (23), lysed erythrocytes or hemin (21), yeast extract (YE) (23), peptone (23), IsoVitaleX (11), Vitox (13), starch (5), cyclodextrins (23), ferrous sulfate plus sodium pyruvate (FP), and/or mucin (10). Although some of these enhance the growth of H. pylori, they do not replace the serum or blood derivatives in the culture media (10).
Most of the supplements used to grow H. pylori are difficult to obtain and conserve, and they are usually costly. Besides, their incorporation into liquid culture media may interfere with cellular growth evaluation (10, 15). It has also been observed that proteins derived from serum may associate with bacterial cells, thus making the purification of cellular and extracellular proteins more difficult (15). Also, FCS can be contaminated with several viruses (19). Among these, the bovine diarrhea virus cannot be eliminated by filtration, so its presence in the culture media affects the quality of the products derived from microorganisms, such as antigens, enzymes, and DNA (3, 5, 24).
When the drawbacks mentioned above are considered, the search for new alternative supplements that may replace FCS, blood, or other blood derivatives becomes relevant. Besides, the development of serum-free media that allows a flourishing growth of H. pylori would facilitate the retrieval of cultures with high cellular densities, which could be used as a source of cellular and extracellular proteins and for the isolation of RNA and DNA.
It has recently been found (12) that cyanobacterial and algal extracts can successfully replace nutrients in culture media not only in cultures of microorganisms but also in tissue cultures, thus increasing their applications in the biotechnological field. Filamentous cyanobacteria are particularly suitable for the generation of a variety of valuable chemical products, some of which have nutritional value, such as vitamins, enzymes, fatty acids, and polypeptides, in addition to proteins and carbohydrates (6, 12, 18).
The aim of the study described here was to analyze the effect of incorporation of a cyanobacterial extract (CE) into H. pylori cultures as a substitute for serum in liquid media and blood in solid media. The culture of H. pylori in MHB supplemented only with CE was characterized by measurement of growth parameters. The results obtained were compared with those obtained with media supplemented with FCS.
MATERIALS AND METHODS
Strains and isolation procedure.
A total of 13 H. pylori strains were used in this study. Eleven were clinical isolates of H. pylori identified as VHP124, VHP180, VHP181, VHP324, VHP346, VHP349, VHP474, VHP529, VHP530, VHP571, and VHP590. These strains were isolated from gastric biopsy specimens collected at the Gastroenterology Unit of the San Luis Public Hospital, San Luis, Argentina. H. pylori NCTC 11638, which was used as a reference strain, and strain AHP2 were obtained from the Microbiology Service of the Hospital Universitario de la Princesa, Madrid, Spain. Primary isolation was carried out on plates containing Mueller-Hinton agar (MHA) medium supplemented with 5% sheep blood (SB), vancomycin (10 mg/liter), trimethoprim (5 mg/liter), and polymyxin B solution (2,500 U/liter) (Merck). Cultures were incubated at 37°C for 7 to 10 days in a GasPak jar (Oxoid, Basingstoke, United Kingdom), whose atmosphere was evacuated three times and replaced with a microaerobic gas mixture composed of 5% oxygen, 10% carbon dioxide, and 85% nitrogen.
For primary isolation in MHA-0.4% CE, gastric biopsy specimens were collected from 20 dyspeptic patients (8 females, 12 males) undergoing endoscopy. The medium was supplemented with the same antibiotic mixture included with MHA-5% SB, and the other culture conditions were similar to those described above.
The identification of local strains was done on the basis of typical colonial morphology (small and translucent colonies); the presence of curved gram-negative cells; and urease, catalase, and oxidase production.
Confirmation of the strain identities at the molecular level was carried out by PCR with primers (5′ to 3′) specific for a species-specific antigen (TGGCGTGTCTATTGACAGCGAGC and CCTGCTGGGCATACTTCACCATG; Promega) previously described by Hammar et al. (7) and visualization of a 298-bp fragment on a 1.8% agarose gel stained with ethidium bromide.
CE.
CE was obtained from the biomass of a Nostoc sp., a filamentous nitrogen-fixing heterocystic cyanobacterium (22). The biomass was collected by centrifugation, washed twice with distilled water, and freeze-dried. The freeze-dried biomass was mixed with distilled water to give a 10% (wt/vol) suspension, and the mixture was heated at 95°C for 30 min. The extract was purified by centrifugation at 8,800 × g for 15 min, lyophilized, and stored until use.
Growth media and culture conditions.
Three-day-old cultures on MHA-5% SB were harvested by scraping the bacterial growth with a sterile swab. The cells were washed once with MHB and resuspended to a final concentration of 1 × 108 to 5 × 108 CFU/ml in the same medium. Then, 0.1 ml of the bacterial suspension was subcultured onto MHA plates with 0.4 or 0.6% CE as the only supplement (MHA-CE) and incubated under the same conditions used for primary isolation. Nine H. pylori strains (strains VHP181, VHP324, VHP346, VHP529, VHP530, VHP571 VHP590, and AHP2) were tested for growth in liquid media, with H. pylori NCTC 11638 used as a reference strain. The cells were washed with MHB and then resuspended in MHB to provide the inocula, as described above. The cultures were initiated with concentrations of 1 × 104 to 3 × 104 CFU/ml and carried out in 125-ml Erlenmeyer flasks containing 20 ml of MHB supplemented with 0.3, 0.7, or 1% CE or with MHB supplemented with 5% FCS as a reference medium. The pH was initially adjusted to 6.8 to 7.2 and was allowed to evolve freely during the remainder of the experiment. The cultures were incubated at 37°C for 120 h and agitated at 120 rpm under the same conditions described above. Growth studies were carried out without any special adaptation of the H. pylori strains to the media assayed.
Enumeration of H. pylori.
At daily intervals, cultures were serially diluted (1:10) in MHB and the H. pylori isolates were enumerated in duplicate by plating onto the surfaces of plates with MHA-5% SB. The plates were incubated at 37°C for 5 to 7 days under microaerobic conditions. The results presented are the means of three separate experiences.
Growth parameters.
Growth kinetics were characterized by determination of the following parameters: lag period (in hours), generation time (in hours), and final biomass concentration (in numbers of CFU per milliliter) (17).
Statistical analyses.
In tests for the differences between the mean values for two populations, P values of <0.05 (t statistic) were considered statistically significant and P values between 0.05 and <0.1 were defined as marginally significant.
RESULTS
Growth in solid media.
All H. pylori strains isolated from gastric biopsy samples in MHA-5% SB were successfully subcultured in MHA-CE. It was observed that the growth of H. pylori strains was similar in medium containing CE at a concentration of either 0.4 or 0.6%. When H. pylori strains were previously cultured in MHB-0.4% CE, marked confluent growth was observed.
Primary isolation.
A lower rate of isolation was observed with MHA-0.4% CE than with MHA-5% SB: 30% (6 of 20 samples) and 43.3% (9 of 20 samples), respectively. Even though both media were supplemented with the same antimicrobial agents, abundant growth of contaminants, especially Candida spp., was observed in MHA-0.4% CE.
Growth in liquid media.
No lag phases were detected in the strains studied by the Lodge and Hinshelwood method (17). We previously observed that a second passage in MHB of the inoculum obtained in MHA-5% SB increased the lag period of all strains in about 8 to 12 h (data not shown).
The effect of the CE concentration on H. pylori growth was determined by culturing strains VHP571, VHP590, and AHP2 in the presence of 0.3, 0.7, or 1% CE. The final cellular concentrations of strains VHP571 and VHP590 obtained with CE at 0.3 and 0.7% were compared, and marginally higher values were obtained with 0.7% CE on day 4 of culture (P < 0.07 and P < 0.065, respectively), with no significant differences between the two concentrations for strain AHP2. The increase in the CE concentration in the culture medium to 1% had no effect on the cellular growth of the three strains, with the values obtained being similar to those obtained with CE at 0.7%. Because of these results, a CE concentration of 0.7% was used in the liquid media in further experiments.
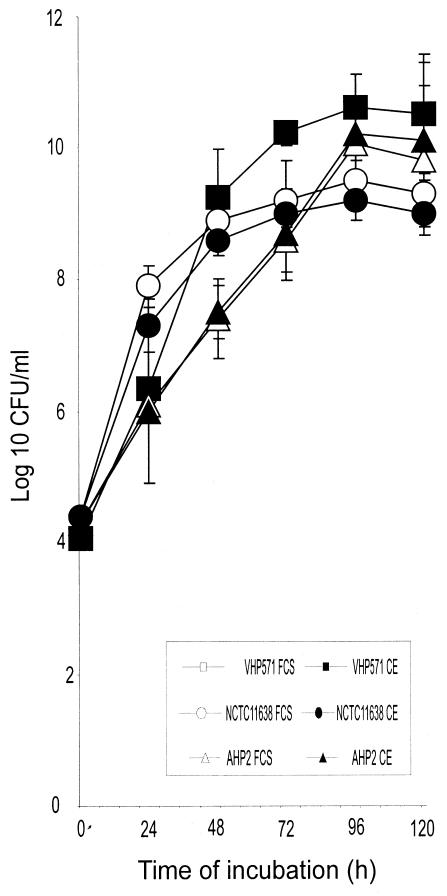
Table 1 summarizes the growth parameters for eight clinical isolates of H. pylori and reference strain NCTC 11638 obtained with MHB supplemented with 0.7% CE or 5% FCS. The evolution of the growth of H. pylori strains VHP571 and AHP2 and reference strain NCTC 11638 in medium supplemented with 0.7% CE or 5% FCS are shown in Fig. 1.
TABLE 1.
Growth parameters obtained for H. pylori cultures in liquid medium supplemented with FCS or CE
| H. pylori strain | Supplement | Growth parameters
|
P value at the following sampling times (h)b
|
|||||
|---|---|---|---|---|---|---|---|---|
| Generation time (h) | Final biomass (CFU/ml)a | 24 h | 48 h | 72 h | 96 h | 120 h | ||
| NCTC 11638 | FCS | 3.3 | 9.3 ± 0.2c | NS | NS | NS | NS | NS |
| CE | 3.5 | 9.0 ± 0.2 | ||||||
| AHP2 | FCS | 4.62 | 9.8 ± 1.13 | NS | NS | NS | NS | NS |
| CE | 4.62 | 10.1 ± 1.16 | ||||||
| VHP181 | FCS | 3.6 | 8.56 ± 0.1 | 0.01 | 0.03 | 0.002 | 0.019 | 0.005 |
| CE | 3.46 | 9.14 ± 0.14 | ||||||
| VHP324 | FCS | 3.15 | 9.16 ± 0.6 | NS | NS | NS | NS | NS |
| CE | 3.01 | 9.43 ± 0.15 | ||||||
| VHP346 | FCS | 4.3 | 8.26 ± 0.15 | 0.03 | 0.01 | 0.07 | 0.005 | 0.0009 |
| CE | 2.77 | 9.83 ± 0.24 | ||||||
| VHP529 | FCS | 3.3 | 9.21 ± 0.2 | NS | NS | NS | NS | NS |
| CE | 3.46 | 9.06 ± 0.14 | ||||||
| VHP530 | FCS | 4.3 | 9.7 ± 0.2 | NS | 0.09 | 0.02 | 0.06 | NS |
| CE | 2.8 | 10.5 ± 0.81 | ||||||
| VHP571 | FCS | 4.3 | 9.65 ± 0.25 | NS | NS | NS | 0.054 | NS |
| CE | 2.39 | 10.5 ± 0.9 | ||||||
| VHP590 | FCS | 4.7 | 9.3 ± 0.3 | NS | 0.01 | NS | NS | NS |
| CE | 3.1 | 9.7 ± 0.25 | ||||||
Obtained on day 5.
P values of <0.05 indicate significant differences. P values between 0.05 and <0.1 were defined as marginally significant. NS, not significant.
Standard error.
FIG. 1.
Growth of H. pylori strains NCTC 11638, VHP571, and AHP2 with agitation under microaerobic conditions in MHB with 5% FCS or 0.7% CE. Growth was estimated as the number of CFU per milliliter.
The generation times of the nine H. pylori strains in MHB-0.7% CE ranged from 2.39 h for VHP571 to 4.62 h for AHP2, while in MHB-5% FCS they ranged from 3.15 h for VHP324 to 4.7 h for VHP590. In general, a 35% decrease in the generation time was observed with MHB-0.7% CE for all strains except AHP2, VHP529, and NCTC 11638, for which the generation times were similar with both supplements.
For seven H. pylori strains the final cellular concentrations obtained with MHB-0.7% CE were higher than those obtained with MHB-5% FCS, but the increases were significant (P < 0.05) for only four strains. For strain VHP181 significant increases were observed at all sampling times; for strain VHP346 significant increases were detected at 24, 48, 96, and 120 h of incubation; for strain VHP590 a significant increase was detected at 48 h of incubation; and for strain VHP571 a marginally significant increase (P = 0.054) was observed at 96 h of incubation.
Coccoid forms were observed by optical microscopy in cultures with CE or FCS; however, their numbers in cultures with CE were smaller on day 4 of culture. For three strains (VHP571, VHP590, and AHP2) the proportion of coccal forms was 7.4% in FCS, whereas it decreased to 2.63% in CE.
DISCUSSION
The use of CE at low concentrations (0.3 to 1%) proved to be a satisfactory substitute for YE for the culture of some rhizobia (22). The results of this study showed that when CE is added at concentrations similar to those used for culture of rhizobia to media commonly used for H. pylori, it can be used as a replacement for FCS and SB, which are usually used at much higher concentrations (5 to 10%) (13, 23).
Solid media supplemented with CE supported the growth of all the H. pylori strains assayed, which showed that it could be a substitute for blood. For primary isolation the low selectivity of MHA-0.4% CE yielded a lower rate of recovery (30%) than MHA-SB (43.3%), with abundant growth of contaminants that obscured the growth of H. pylori and interfered with its isolation, as has been described for other nonselective media (16). Even though both media possessed identical antibiotic supplements, the different isolation rates can be ascribed to the fact that CE is a more suitable supplement for the growth of contaminants. Further studies with additional antimicrobial agents and other concentrations of CE are planned in order to make MHA-CE more suitable for primary isolation.
Three strains of H. pylori had different responses to CE when the CE concentration in liquid media was varied. CE at 0.7% was found to be the more suitable concentration for supporting growth and was chosen for further studies.
Among the strains studied, the lag periods of the cultures developed in media supplemented with CE or FCS could not be estimated by extrapolation by the Lodge and Hinshelwood method (17) because the first sample used to determine viable counts was taken at 24 h of incubation, when the cultures had already reached the exponential growth phase. It can be assumed that the use of an adequate and active inoculum obtained directly from young, 3-day cultures in rich solid media such as MHA-SB will reduce or even eliminate the lag periods. This behavior was observed when different H. pylori strains were cultured in richer nonselective media such as brucella broth with 1% IsoVitaleX (11), brain heart infusion (BHI) broth-horse serum with 0.075% FP, BHI broth supplemented with 0.025% FP and 0.15% mucin (10), and BHI broth-5% horse serum-0.25% YE (25).
The generation times of four of the H. pylori strains analyzed ranged from 2.39 to 3.1 h and were 35% shorter with CE than with FCS. These values are similar to those reported for different H. pylori strains (3.2 to 4.3 h for strain NB2-1, 2.19 h for strain 26695, and 3.72 h for strain WV99) in cultures of supplemented rich media such as BHI-7% horse serum containing different concentrations of FP and mucin (10).
In this study the final biomass values obtained with MHB-0.7% CE ranged between 1.1 × 109 CFU/ml for strain NCTC 11638 and 3.16 × 1010 CFU/ml for strain VHP590. These values were similar to the highest that have been obtained until now for strains of H. pylori; a final concentration above 1 × 1010 CFU/ml was obtained with BHI-1% IsoVitaleX broth after 48 h of incubation, although a sharp decrease in viability (1 × 104 CFU/ml) was observed in this medium after 96 h of culture (11); in another study a final biomass value of 2 × 1010 CFU/ml was obtained on day 5 in BHI medium containing 10% FCS and 0.1% YE (21). Other published studies have reported values that range from 108 to 1010 CFU/ml (1, 11, 15, 25). The final biomass values obtained with MHB-0.7% CE were higher than those obtained in media supplemented with 5% FCS for four of the nine strains studied, but the differences were statistically significant for only two of them.
It has been found that the growth response of H. pylori in supplemented liquid media varies depending on the strain (8). For well-characterized strain H. pylori NCTC 11638, the final cellular concentration obtained in MHB supplemented with FCS doubled (1.99 × 109 CFU/ml) compared with that obtained in the same medium with CE (1 × 109 CFU/ml); however, this difference was not statistically significant. In other studies the final cellular concentrations for this strain were markedly lower: 3.9 × 107 CFU/ml in MHB-5% FCS and 7.9 × 107 CFU/ml in MHB-7% horse blood-1% YE (23).
It has been suggested that clinical isolates are more fastidious than laboratory-adapted or culture collection strains (8); however, for the majority of clinical isolates, MHB-0.7% CE yielded final cellular concentrations higher than those found for the reference strain. Similar results have been reported for another reference strain, H. pylori NCTC 11637, which performed less satisfactorily in terms of the maximum growth achieved (6.2 × 107 CFU/ml) than 15 clinical isolates, all of which reached 1 × 108 CFU/ml (25). The results obtained in this study show that CE can satisfactorily replace FCS and can even improve the growth of both clinical isolates and laboratory-adapted strains of H. pylori.
The presence in the cultures of nondividing coccal forms, which are extremely difficult to subculture, is a drawback of H. pylori cultivation that must be avoided. By using CE as a supplement, a very low percentage of coccal forms was observed among the strains studied. These coccal forms have been considered senescent (9) or dormant (2) cells that appear under certain conditions, especially in old cultures, and have decreased urease activity and a decreased ability to be subcultured (9). It is not clear if nutritional factors are involved. Coccal forms have predominantly been found after 24 to 36 h of culture even in a very rich and balanced medium containing tryptone, yeast extract, newborn calf serum, erythrocyte lysate, and trace minerals (2).
The promising results obtained with media supplemented with CE for H. pylori culture may be attributed to the contribution of nutrients by this extract, such as 16 different amino acids, soluble (35%) and crude (45.6%) proteins, carbohydrates (1.9%), reducing sugars (2.8%), and different minerals (22). It has also been demonstrated that special components in media used to culture H. pylori, i.e., bovine serum albumin, reduce the toxic effects of fatty acids by adsorbing them and that cyclodextrins reduce the effective free concentrations of substrates and/or products to levels below those which are inhibitory or toxic (15). It can be assumed that some substances present in the CE may perform similar functions.
The use of media that do not require the addition of blood or blood derivatives and that allow adequate growth to high cellular concentrations is important in the culture of H. pylori for the purpose of obtaining antigens, vaccines, and/or enzymes. Finally, the use of the CE has another advantage with respect to conventional supplements, such as its low cost. A cost comparison of CE and FCS shows a remarkable difference between the costs of the two supplements. While the cost of 100 ml of FCS, which can produce 2,000 ml of culture medium, ranges from $100 to $239, 14 g of CE, which generates the same culture volume, costs $0.60, based on an estimated cost of $20/kg of cyanobacterial biomass. Additionally, CE is easy to prepare and store (22) and complies with the pressing tendency at present to avoid the use of products derived from animals.
Acknowledgments
We thank Patricia Gómez and Rubén Majul for providing the gastric biopsy specimens and Gabriela Favier for statistical analyses.
This work was supported by funds of C y T projects 8802 and 9303 from the Universidad Nacional de San Luis, San Luis, Argentina.
REFERENCES
- 1.Albertson, N., I. Wenngren, and J. E. Sjostrom. 1998. Growth and survival of Helicobacter pylori in defined medium and susceptibility to Brij 78. J. Clin. Microbiol. 36:1232-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen, A. P., D. A. Elliot, M. Lawson, P. Barland, V. B. Hatcher, and E. G. Puszkin. 1997. Growth and morphological transformation of Helicobacter pylori in broth media. J. Clin. Microbiol. 35:2918-2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolin, S. R., J. F. Ridpath, J. Black, M. Macy, and R. Roblin. 1994. Survey of cell lines in the American Type Culture Collection for bovine viral diarrhea virus. J. Virol. Methods 48:212-221. [DOI] [PubMed] [Google Scholar]
- 4.Buck, G. E., and J. S. Smith. 1987. Medium supplement for growth of Campylobacter pyloridis. J. Clin. Microbiol. 25:597-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coudron, P. E., and C. W. Stratton. 1995. Factors affecting growth and susceptibility testing of Helicobacter pylori in liquid media. J. Clin. Microbiol. 33:1028-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fingerhut, U., L. E. Webb, and C. J. Soeder. 1984. Increased yields of Rhizobium japonicum by an extract of the green alga, Scenedesmus obliquus (276-3a). Appl. Microbiol. Biotechnol. 19:358-360. [Google Scholar]
- 7.Hammar, M., T. Yszkiewicz, T. Wadstrom, and P. O'Toole. 1992. Rapid detection of Helicobacter pylori in gastric biopsy material by polymerase chain reaction. J. Clin. Microbiol. 30:54-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hazzel, S. L., D. C. Markesich, D. J. Evans, D. G. Evans, and D. Y. Graham. 1989. Influence of media supplements on growth and survival of Campylobacter pylori. Eur. J. Clin. Microbiol. Infect. Dis. 8:597-602. [DOI] [PubMed] [Google Scholar]
- 9.Jerris, R C. 1995. Helicobacter, p. 492-498. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 6th ed. ASM Press, Washington, D.C.
- 10.Jiang, X., and M. P. Doyle. 2000. Growth supplements for Helicobacter pylori. J. Clin. Microbiol. 38:1984-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitsos, C. M., T. K. Christian, and H. Stadtlander. 1998. Helicobacter pylori in liquid culture: evaluation of growth rates and ultrastructure. Curr. Microbiol. 37:88-93. [DOI] [PubMed] [Google Scholar]
- 12.Maeda, T., K. Tanaka, H. Katsuda, T. Takaoka, and M. Okuda. 1981. Cell culture method. Publication no. EP 0 049 632 A2. European Patent Office, London, United Kingdom.
- 13.Morgan, D. R., R. Fredman, C. E. Depew, and W. G. Kraft. 1987. Growth of Campylobacter pylori in liquid media. J. Clin. Microbiol. 25:2123-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morshed, M. G., M. Karita, H. Konishi, K. Okita, and T. Nakazawa. 1994. Growth medium containing cyclodextrin and low concentration of horse serum for cultivation of Helicobacter pylori. Microbiol. Immunol. 38:897-900. [DOI] [PubMed] [Google Scholar]
- 15.Olivieri, R., M. Bugnoli, D. Armenllini, S. Bianciardi, R. Rappuoli, P. F. Bayeli, L. Abate, E. Esposito, L. De Gregorio, J. Aziz, C. Basagni, and N. Figura. 1993. Growth of Helicobacter pylori in media containing cyclodextrins. J. Clin. Microbiol. 31:160-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piccolomini, R., G. Di Bonaventura, D. Festi, G. Catamo, F. Laterza, and M. Neri. 1997. Optimal combination of media for primary isolation of Helicobacter pylori from gastric biopsy specimens J. Clin. Microbiol. 35:1541-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pirt, S. J. 1975. Principles of microbe and cell cultivation. Blackwell Scientific Publications, Oxford, United Kingdom.
- 18.Pushparaj, B., E. Pelosi, P. Carlozzi, and G. Torzillo. 1995. Yield and biochemical composition of a marine cyanobacterium (Nodularia sp.) in outdoor culture. Aquat. Microb. Ecol. 9:13-16. [Google Scholar]
- 19.Ridpath, S. R., and J. F. Ridpath. 1998. Prevalence of bovine viral diarrhea virus genotypes and antibody against those viral genotypes in fetal bovine serum. J. Vet. Diagn. Investig. 10:135-139. [DOI] [PubMed] [Google Scholar]
- 20.Seeker, D. A., D. S. Tompkins, and G. Alderson. 1991. Gas-permeable Lifecell tissue culture flasks give improved growth of Helicobacter pylori in a liquid medium. J. Clin. Microbiol. 29:1060-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shahamat, M., U. E. H. Mai, C. Paszko-Kolva, H. Yamamoto, and R. R. Colwell. 1991. Evaluation of liquid media for growth of Helicobacter pylori. J. Clin. Microbiol. 29:2835-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silva, P. G., D. M. González, E. Aguilar, and H. J. Silva. 1998. Nutritional evaluation of Cyanobacterium (Nostoc sp.) extract in Rhizobium cultures. World J. Microbiol. Biotechnol. 14:223-228. [Google Scholar]
- 23.Walsh, E. J., and A. P. Moran. 1997. Influence of medium composition on the growth and antigen expression of Helicobacter pylori. J. Appl. Microbiol. 83:67-75. [DOI] [PubMed] [Google Scholar]
- 24.Weber, E. L. 2000. Suero fetal bovino: contaminación con virus de la diarrea viral bovina. Banco Argentino de Células. [Online.] http://www.abac.org.ar/suerofetalbovino.html. [PubMed]
- 25.Xia, R. X., L. English, C. T. Keane, and C. A. O'Morain. 1993. Enhanced cultivation of Helicobacter pylori in liquid media. J. Clin. Pathol. 46:750-753. [DOI] [PMC free article] [PubMed] [Google Scholar]