Abstract
Delirium is an acute change in cognition and attention, which may include alterations in consciousness and disorganized thinking. While delirium may affect any age group, it is most common in older patients, especially those with preexisting cognitive impairment. Patients with delirium after surgery recover more slowly than those without delirium and, as a result, have increased length of stay and hospital costs. The measured incidence of postoperative delirium varies with the type of surgery, the urgency of surgery, and the type and sensitivity of the delirium assessment. While generally considered a short-term condition, delirium can persist for months and is associated with poor cognitive and functional outcomes beyond the immediate postoperative period. In this article we will provide a guide to assess delirium risk preoperatively, and to prevent, diagnose, and treat this common and morbid condition. Care improvements such as identifying delirium risk preoperatively; training surgeons, anesthesiologists and nurses to screen for delirium; implementing delirium prevention programs; and developing standardized delirium treatment protocols may reduce the risk of delirium and its associated morbidity.
Introduction
Delirium is an acute change in cognition characterized by inattention, fluctuating levels of consciousness, and/or disorganized thinking. Postoperative delirium is common, with significant associated morbidity and cost. Patients with delirium after surgery have high in-hospital mortality (4-17%)1-3 and 1-month4, 6-month5, 12-month6, and long-term mortality remains elevated.7,8 Additionally, delirium is associated with increased postoperative complications9, longer length of stay1, longer intensive care unit stay (ICU)3, and much higher rates of discharge to a nursing home.4,10 As a result, delirium adds significant cost to hospitalization and subsequent medical care.11
The incidence of postoperative delirium depends on the type of surgery. Table 1 highlights the incidence of delirium by surgical type. Hip fracture has the highest incidence of delirium, which is probably due to the urgent nature of the surgery and high comorbidity among these patients. Delirium is also common in patients after surgery for atherosclerosis pathology (cardiac, peripheral vascular, aneurysm repair). Elective and outpatient surgery have a lower, but still significant, incidence of delirium. A major factor in the variation of the reported incidence of delirium is the method for delirium assessment. For example in the cardiac surgery literature the methods used for delirium greatly influence the reported incidence of delirium: chart review only (3%)12, delirium noted during routine clinical care (8%)13, interviews with nurses (9%)7, and daily mental status testing and application of a validated diagnostic algorithm (53%).14
Table 1. Incidence of Postoperative Delirium.
| Surgery | Incidence of Delirium (%) | References |
|---|---|---|
| Abdominal Aortic Aneurysm (infrarenal) | 33-54 | 92-95 |
| Abdominal | 5-51 | 67,92,96,97 |
| Cataract | 4 | 98 |
| Coronary Artery Bypass Graft Surgery | 37 - 52 | 14,99 |
| Elective Orthopedic | 9-15 | 92,100 |
| Head and Neck (major) | 17 | 101 |
| Hip Fracture | 35-65 | 102 |
| Peripheral Vascular | 30-48 | 93,94 |
| Urologic | 4-7 | 99 |
Defining and Diagnosing Delirium
Because of the morbidity associated with delirium, all patients, especially older patients should be screened for delirium, at least, daily and more frequently if they are high risk. An algorithm for the diagnosis of delirium, the Confusion Assessment Method (CAM), which is based on Diagnostic and Statistical Manual of Mental Disorders (DSM)-IIIR criteria15, has been demonstrated to be reliable, sensitive, and specific for diagnosis of delirium compared to expert clinician examination.16,17 The algorithm for the CAM is displayed in Figure 1. Briefly, the criteria are a combination of Feature 1 (acute onset and fluctuating course), Feature 2 (inattention), and either Feature 3 (disorganized thinking) or Feature 4 (abnormal level of consciousness).
Figure 1.
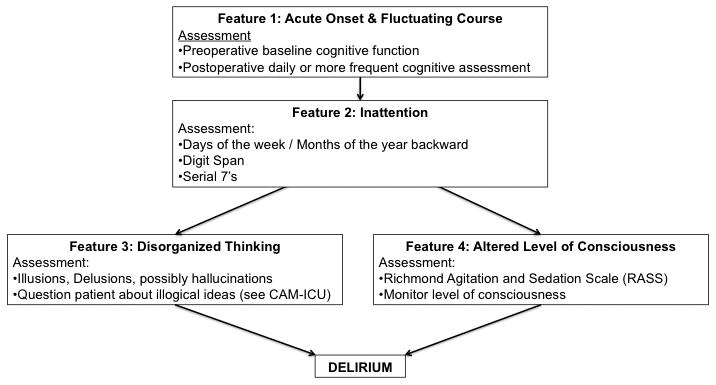
The Algorithm of the Confusion Assessment Method (CAM). The diagnosis of delirium is made with the presence of Feature 1 AND Feature 2 AND either Feature 3 OR Feature 4. Examples of assessments applicable in the postoperative period are included below the features. Adapted from 16,106
There are important elements of the CAM, which need to be clarified. First, attention is best assessed when formal testing (digit span, months of the year backward, serial 7s, etc) is combined with interviewer observations18. Importantly, orientation items have low sensitivity for inattention and delirium and should not be considered the standard assessment for attention.19 Additionally, there are two variants of delirium that are characterized as the hyperactive (agitated) variant and the hypoactive (quiet) variant of delirium. The hyperactive variant, which accounts for only about 25% of cases, is rarely missed, because the patient disrupts the flow of care20. The more common hypoactive variant is often missed because the patient is neither disruptive nor threatening21. For example, a patient with hypoactive delirium would briefly wake when addressed and may comply with some requests, but then quickly falls back to sleep. Several studies have found that the hypoactive variant is detected less frequently and carries a higher mortality, presumably from the delay in diagnosis.21-23 Thus, the CAM is a useful algorithm for diagnosis of delirium, but requires additional assessment of attention and observation.
The CAM-ICU operationalizes the CAM by adding objective assessments for attention, consciousness, and thought.24 The CAM-ICU has been validated in nonverbal ICU patients.24 The advantages of the CAM-ICU are that it can be performed by trained nurses or physicians; can be repeated over time to detect fluctuation and changes; and has been associated with ICU outcomes including mortality25, length of stay26, and cost27. The key elements of the CAM-ICU are the Richmond Agitation and Sedation Scale, a validated measure of consciousness28, the Attention Screening Exam29, and 5 thought questions. This information is used to complete the CAM algorithm for delirium.
Preoperative Assessment for Delirium Risk
Many preoperative risk factors for delirium have been described in the literature, and there are validated prediction rules for noncardiac and cardiac surgery to help identify those patients most at risk for postoperative delirium. The noncardiac surgery prediction rule identified 7 factors: age, impaired cognitive function, impaired physical function, abnormal laboratory values, alcohol abuse, thoracic surgery, and open-aortic surgery.2 The validated prediction rule for delirium after cardiac surgery identified 4 major risk factors: impaired cognitive function, low albumin, preoperative depressive symptoms, and prior stroke or transient ischemic attack.14 Table 2 describes the point scoring system for the prediction rules. In both rules, the incidence of delirium increases with increasing points so that the highest risk group is more likely to develop delirium than the lowest risk group (25× in the noncardiac surgery rule and 4× in the cardiac surgery rule). To complete the prediction rules, a thorough history and physical with screening for cognition, mood, and physical function are required. The paragraphs below describe risk factors for delirium in more detail.
Table 2. Preoperative Prediction Rules for Delirium After Noncardiac and Cardiac Surgery.
| Risk Factor | Criteria | Factor Points | Total Rule Points* | Incidence of Delirium |
|---|---|---|---|---|
| Noncardiac Surgery | ||||
| Cognitive Impairment | TICS <30 | 1 | 0 | 1-2% |
| Age | ≥ 70 years | 1 | 1-2 | 8-19% |
| Physical Function | SAS Class IV | 1 | ≥3 | 45-55% |
| Alcohol Abuse | 1 | |||
| Abnormal sodium (Na), potassium (K), or glucose | Na <130 or >150 mmol/L; K <3.0 or >6.0 mmol/L; Glucose <60 or >300 mg/dL |
1 | ||
| Aortic Aneurysm Surgery | Yes/No | 2 | ||
| Noncardiac Thoracic Surgery | Yes/No | 1 | ||
| Cardiac Surgery | ||||
| Cognitive Impairment | MMSE <24 | 2 | 0 | 18-19% |
| MMSE 24-27 | 1 | 1 | 43-47% | |
| Hypoalbuminemia | <3.5 g/dL | 1 | 2 | 60-63% |
| Depression | GDS >6 | 1 | ≥3 | 86-87% |
| Prior Stroke or TIA | Yes/No | 1 |
Preexisting cognitive impairment
The most common independent risk factor for delirium across studies is preexisting cognitive impairment. Preoperative cognitive screening is beneficial for assessing delirium risk, as well as, documenting baseline performance to detect delirium postoperatively. Cognitive screening should be performed with a standardized screening test. Many brief cognitive tests are available that require less than 10 minutes to complete.30,31 Importantly, orientation items and observation of standard conversation are not sufficient to assess for preoperative cognitive deficits.
Function
Preoperative functional status is an independent risk factor for delirium after noncardiac surgery.2 The Activities of Daily Living (ADLs) and the Instrumental Activities of Daily Living (IADLs) provide an understanding of preoperative function. ADLs measure the ability to perform 7 basic care skills (feeding, bathing, grooming, using the toilet, transferring, and walking).32 The IADLs assesses the ability to perform 7 complex activities (using the telephone, grocery shopping, using transportation, cooking, housekeeping, taking medications, and handling finances).33 In addition to providing risk stratification for delirium, assessing this information preoperatively can inform the patient, the family, and the surgical team about the expected course of recovery postoperatively.
Abnormal Laboratory Values
Abnormal preoperative laboratory values including glucose, sodium, potassium, and albumin are risk factors for delirium (Table 2).2,14 These abnormal laboratory values may represent underlying severe disease or organ system dysfunction, which is a predisposing risk factor for delirium. Hypoalbuminemia may be particularly important, because of its association with malnutrition, drug binding, fluid management34, and perioperative mortality.35 In the delirium prediction rule for medical patients, blood urea nitrogen to creatinine ratio ≥18, a marker of dehydration, was associated with incident delirium.36 Consistent with current practice, preoperative assessment of laboratory values can provide information about patients at high risk for delirium.
Depression
Many studies have identified depression as a risk factor for delirium after surgery.37-40 While the pathophysiology of this relationship remains to be determined, it is known that preoperative depression is associated with postoperative depression and incomplete recovery to independent functioning after surgery.41,42 The assessment of depression in older patients can be easily performed with the 15 question Geriatric Depression Scale, which assesses depressive symptoms using 15 yes / no questions43. The advantage of the Geriatric Depression Scale is that it can be self-completed by the older patient and scored by the clinician in a short time frame (3 minutes).44 In addition to delirium risk, assessment of depressive symptoms may provide insight into a patient's motivation for recovery.
Comorbidities
Patients with multiple comorbidities are at increased risk of delirium. Alcohol abuse and prior stroke or transient ischemic attack deserve particular mention. Withdrawal from alcohol use has long been understood to precipitate delirium tremens, a variant of delirium.45 However, most postoperative delirium is not delirium tremens. Screening for alcohol use preoperatively can allow prevention of alcohol withdrawal with standardized protocols. Additionally, preexisting cerebral damage from prior strokes and transient ischemic attacks14, or a long history of alcohol abuse even in the absence of active drinking has been independently associated with postoperative delirium.2 Collection of this historical information from the patient or proxy before surgery is sufficient and routine cerebral imaging is not required for assessment of delirium risk.
Practical Preoperative Screening Considerations
In many older patients, the five senses decline with age. The combination of decreased sensory input (e.g., no glasses or hearing aids), cognitive impairment, and the perioperative environment may lead to misinterpretation of communication (i.e., talking back to television conversation), alarms (i.e., telephone ringing), and elements of the environment (i.e., window is a picture frame). Additionally, there is evidence in patients that increasing cognitive stimulation through improved sensory input may prevent delirium.46,47 As a result, preoperative assessment should include vision and hearing assessments and patients should be encouraged to bring their glasses and hearing amplifiers for use in the postoperative period to improve sensory input.
Prevention of Delirium
Nonpharmacological
Delirium can be prevented in operative and medical patients46,47 by targeting moderate and high risk patients with clinical modules to improve baseline vulnerabilities and avoid iatrogenic complications. A summary of modules for prevention of postoperative delirium based on successful prevention models is presented in Table 3. For example, the nonpharmacological sleep protocols involved environmental changes conducive to sleep (i.e., lights off, create a relaxing environment, minimizing nighttime interruptions, dedicated time for sleep), which were successful in reducing psychoactive medication use and, ultimately, delirium47. Other key modules include improving sensory input, nutrition, ambulation, and preventing complications.
Table 3. Prevention of Delirium After Surgery.
| Module | Postoperative Interventions |
|---|---|
| Cognitive Stimulation |
|
| Improve Sensory Input |
|
| Mobilization |
|
| Avoidance of Psychoactive Medication |
|
| Fluid and nutrition |
|
| Avoidance of hospital complications |
|
Pharmacological
Haloperidol is a high-potency dopamine antagonist (antipsychotic) medication. In a single-site study, prophylactic administration of haloperidol did not reduce the incidence of delirium after hip fracture, but did reduce the severity and duration of delirium.48 However, severity scales tend to overweight hyperactive symptoms of delirium, so a possible explanation of these findings is the conversion of hyperactive delirium to hypoactive.49 As noted above, such a conversion may be a convenience for the care team, but actually worsens the prognosis of the patient. A follow-up study comparing high potency antipsychotic, atypical antipsychotic, and placebo in ICU patients found no difference in the days alive without delirium.50 As a result, the practice of prophylaxis with antipsychotics should be avoided at present due to increased risk of death, delirium, and complications in older patients attributed to this class of drugs.51
Acetylcholinesterase inhibitors are medications used to stabilize cognitive function in Alzheimer Disease. A randomized controlled trial of rivastigmine for delirium prevention in cardiac surgery found no effect of treatment on delirium incidence or cognitive performance.52 In elective orthopedic surgery, the results have been mixed, with one trial showing no effect53 and another suggesting benefit54. A randomized controlled trial of rivastigmine for delirium treatment was stopped early due to increased mortality in the treatment arm and no effect on delirium.55 Thus, at this time, prevention or treatment of delirium with acetylcholinesterase inhibitors should be avoided.56
Dexmedetomidine is an alpha-2 adrengergic receptor agonist that is used for sedation. Two recent studies have found that the use of dexmedetomidine for sedation in the ICU setting has a reduced rate of delirium compared to midazolam and lorazepam.57,58 Additionally, a randomized trial of intraoperative sedation with dexmedetomidine, propofol, or midazolam found that dexmetometadine was associated with a lower incidence of postoperative delirium.59 Thus, the use of dexmedetomidine in patients at intermediate-risk and high-risk for delirium may have benefits that outweigh the potential adverse events.
Precipitating Factors for Delirium
The delirium risk factors described above are classified as predisposing factors, that is, factors that can be assessed before surgery and increase the patient's risk. Precipitating factors, a distinct class of risk factors, occur intraoperatively and postoperatively and are thought to acutely precipitate the delirium episode. Precipitating factors for delirium have been more difficult to identify than predisposing factors. A primary challenge has been heterogeneity, due to patient factors (age, education, comorbidity), surgery factors (type of surgery, techniques used, hypothermia, bleeding), physiologic factors (inflammation, microembolization, blood-brain barrier function), intraoperative factors (anesthesia, cerebral oxygenation, hypotension), perioperative factors (medication, sleep, complications), and postoperative factors (rehabilitation, depression, social supports). Thus, all of the precipitating factors for postoperative delirium have not been fully elucidated. Table 4 summarizes predisposing (preoperative), and known precipitating (intraoperative, and postoperative) factors that may be associated with delirium.
Table 4. Predisposing and Precipitating Factors for Delirium after Surgery.
| Predisposing Factors | Precipitating Factors | |
|---|---|---|
| Preoperative | Intraoperative | Postoperative |
| Demographics | Type of Operation | Early Complications of Operation |
| Increasing age | Hip fracture | Low hematocrit |
| Male gender | Cardiac surgery | Cardiogenic shock |
| Comorbidities | Vascular surgery | Hypoxemia |
| Impaired Cognition | Complexity of Operation | Prolonged intubation |
| Dementia | Operation time | Sedation Management |
| Mild Cognitive Impairment | Shock / hypotension | Pain |
| Preoperative Memory Complaint | Arrythmia | Later Complications of Operation |
| Atherosclerosis | Decreased cardiac output | Low albumin |
| Intracranial Stenosis | Emergency surgery | Abnormal electrolytes |
| Carotid Stenosis | Operative Factors | Iatrogenic complications |
| Peripheral Vascular Disease | Intraoperative temperature | Pain |
| Prior Stroke / Transient Ischemic Attack | Benzodiazepine administration | Infection |
| Diabetes | Propofol administration | Liver Failure |
| Hypertension | Blood Transfusion | Renal Failure |
| Atrial fibrillation | Anaesthesia Factors | Sleep-wake disturbance |
| Low albumin | Type of anesthesia | Alcohol withdrawal |
| Electrolyte abnormalities | Duration of anesthesia | |
| Psychiatric Disease | Cognitively Active Medications | |
| Anxiety | ||
| Depression | ||
| Benzodiazepine use | ||
| Function | ||
| Impaired functional status | ||
| Sensory impairment | ||
| Lifestyle Factors | ||
| Alcohol use | ||
| Sleep deprivation | ||
| Smoking | ||
Intraoperative Medications
During surgery, numerous medications with cognitive properties are given to patients. Inhaled anesthetics alter electrical activity in the brain60 and have been associated with amyloid deposition and apoptosis61. Induction drugs and benzodiazepines have significant cognitive properties which may precipitate delirium62. While regional anesthesia has the potential to reduce this exposure, studies of general versus regional anesthesia have not demonstrated a reduction in delirium.63 One reason for this may be the concomitant administration of sedatives in addition to the regional anesthetic. In a recent randomized study, lighter depth of sedation, measured with the Bispectral Index, resulted in 50% less postoperative delirium than deep sedation.64
Pain medications may precipitate delirium, particularily meperidine, which increases the odds of delirium over other opioids62. Many of these medications are necessary for the operation; however, the clinically important point is to recognize the risk for delirium associated with cognitively active medications and minimize the exposure to these medications, especially in older patients with cognitive impairment.
Postoperative medications: sedation and analgesia
After surgery, many patients are given medications that can impair cognitive function. For example, in the postoperative tracheally intubated patient, sedatives such as benzodiazepines or propofol are given. In these patients, dexmedetomidine used for sedation may reduce the risk of delirium59. Out of the ICU setting, benzodiazepine and sedative use may be minimized by improving sleep hygiene using nonpharmacological measures such as decreasing environmental noise, creating a relaxing environment, and preserving the circadian rhythm47.
While opioids may precipitate delirium, uncontrolled pain may also precipitate delirium.62 When opioid pain medication is needed after surgery, standardized age-adjusted protocols should be used to treat pain and taper opioid doses. Strong consideration should be given to standing pain medication, especially acetaminophen, which has been shown to reduce total opioid needs and improve patient reports of pain in a postoperative randomized controlled trial65. The advantage of acetaminophen is its limited cognitive properties, compared to opioids. Even in patients who require opioids, administration on a schedule has been shown to reduce total dose needs relative to as needed (PRN) dosing.66 Patient-controlled analgesia improves pain control, however, caution should be used in older patients with cognitive impairments and those who have developed delirium.67 In summary, postoperative sedative use should be minimized, and postoperative analgesia should be administered using rational, carefully designed protocols designed to minimize systemic exposure to opioids with psychoactive properties.
Postoperative Environment
After cardiac surgery, patients are transferred to the ICU environment, which is busy, noisy, and light-filled where patients are approached, assessed, and stimulated constantly. Recent work in the ICU setting found that the environment may contribute to delirium, through sleep deprivation and overstimulation.68 While this environment may be required in the immediate postoperative period, early transfer of medically stable patients to less intense wards should be considered. Additionally, consideration should be given to balance the patient's monitoring needs with the sleep requirements of the patient (i.e., Does the patient need a standing order for vital signs at midnight and 4 a.m.?). Even the non-ICU environment can be disorienting: preservation of the sleep-wake cycle (minimized nighttime interruptions, adequate lighting, sleep hygiene), provision of orienting supplies (clock, calendar, orientation board), and cognitively stimulating activities (glasses, hearing amplifiers, puzzles) may minimize precipitation of delirium.46,47
Iatrogenic Events
Complications of hospitalization and surgery can precipitate delirium. For example, a leading identifiable cause of delirium in older inpatients is urinary tract infection associated with catheter use69. Preventable medical processes such as deep venous thrombosis, pressure ulcer, deconditioning, malnutrition, and dehydration should be assessed using a standardized team-based approach46. Additionally, reduced mobility through formal restraints or informal tethers (i.e., IV lines, oxygen tubing, urinary catheters, etc) can contribute to delirium, loss of function, falls, and increased rehabilitation placement.70 The routine use of care systems to prevent postoperative complications may also prevent delirium.
Evaluation and Treatment of Delirium
Identify and Treat the Etiology
The primary treatment of delirium is to identify and treat its underlying causes. Thus, the clinician is recommended to begin with a broad differential diagnosis and systematically eliminate potential causes. It should be noted that delirium is associated with significant morbidity and mortality and thus, all patients with delirium should be assessed promptly with an interim history, thorough physical examination with a focus on the neurological examination, and targeted laboratory testing based on the history and examination. Most delirious patients require at least basic laboratory including a complete blood count, basic metabolic panel including renal function, and urinalysis. It is also important to carefully review the patient's medications, particularly the nursing administration record where it is clear exactly what medications the patient received and when. It is also important to note that the causes of delirium are often multifactorial, and therefore the search for additional causes and contributors to delirium should not be terminated when a single cause has been identified.
Cerebral Imaging in the Evaluation of Delirium
Prior work has found that in the absence of focal neurological deficits, a head computed tomography has low diagnostic value in the assessment and treatment of the delirious patient.71 MRI scanning in the postoperative patient is difficult, because of the acuity of illness, recently implanted hardware (e.g., staples prostheses, grafts, valves, etc), and the time and cooperation required for scanning. In the patient with delirium, sedation, which can worsen or prolong the delirium, may be needed for imaging72. In the patient after cardiac surgery, there will likely be new foci on imaging related to microemboli during the operation.73 Because the causal link between microemboli and delirium has not been established74, the clinical significance of such findings is unknown. Additionally, stroke thrombolysis is contraindicated in the postoperative surgery patient75, so the potential treatments for stroke are limited in this setting. The treatment of occlusive infarct in postoperative patients is identical to the treatment of cardiac disease (e.g., aspirin, statin, arterial blood pressure control, cardiac risk reduction, rehabilitation). Therefore, cerebral imaging should be restricted to those with new focal neurological findings, or those at very high risk in whom no other cause of delirium can be identified.
Management of Agitation Associated with Delirium
For patients who develop agitation, a through review of the medications and physical examination, including pain assessment, is required. First, the offending precipitant of the delirium (constipation, urinary retention, etc.) should be relieved. Nonpharmacologic treatments should also be initiated, regardless if the cause is identified. For example, elimination of environmental noise, allowing the patient to sleep at night, and reorientation efforts should be implemented. A model of care for the delirious patient found that environmental modifications and staff training could produce reductions in patient agitation, reduction in use of psychoactive medications, with similar length of stay.76 Another useful resource is family members who can serve as a reorienting and reassuring stimulus. Because of the low risk of adverse events, nonpharmacological methods are recommended as a first step.
For patients in whom these nonpharmacological interventions are not sufficient, antipsychotics are considered the first line for the pharmacological management of agitation associated with delirium.51,77 For most patients, haloperidol at a low initial dosage of 0.5 to 1.0mg is a reasonable choice. If there is no response within one hour, a repeat dosage may be considered. If there is no effect after 2-3mg of haloperidol, it is unlikely that the patient is going to respond. Higher doses administered IV are frequently used in ICU patients.50 Antipsychotics administered in the acute setting have not been demonstrated to have increased mortality, but even intermediate-term (6-12 weeks) use of antipsychotics is considered to carry an increased mortality, especially in cognitively impaired patients.78,79 Additionally, electrocardiograms should be performed at baseline and monitored in high-risk patients (i.e., older, atherosclerosis pathology, major surgery) taking high-dose antipsychotics due the risk of QTc prolongation.80 Finally, early evidence suggests that acute administration of antipsychotics may be associated with oropharyngeal dysphagia which may further delay recovery81. At this time, there is no evidence for a incremental benefit of atypical antipsychotics beyond that of haloperidol for the treatment of delirium.51
For patients with contraindications to antipsychotics such as Parkinson's disease, Lewy Body dementia, prior seizures, and prior neuroleptic malignant syndrome; agitation may be better managed with benzodiazepines. In general, benzodiazepines disinhibit patients and patients should be monitored for a paradoxical reaction, where administration of the benzodiazepine results in agitation. Additionally, prior work has shown that benzodiazepines may actually prolong or worsen the course of delirium72. Finally, respiratory depression becomes a risk in older patients with respiratory comorbidities who have just undergone surgery. Thus, other than the specific case of alcohol or chronic sedative withdrawal, use of benzodiazepines should not be considered a first-line therapy and should be reserved for cases in which clinical circumstances limit use of antipsychotics.
Implications of Delirium Beyond the Perioperative Period
Persistent Delirium
While generally thought of as a short-term disorder, delirium can have lasting effects beyond the perioperative period. First, delirium itself can persist for months. In a study of patients with delirium upon admission to a rehabilitation facility after hospitalization, delirium persisted for 6 months in one-third of patients.82 Persistent delirium increased the 1-year mortality and prevented functional recovery. 82,83 There is an increasing body of evidence that persistent delirium can delay both cognitive and functional recovery.10
Post Delirium Mental Health
Delirium may accelerate the cognitive decline in patients with Alzheimer's Disease84. While delirium is felt to be distinct from postoperative cognitive dysfunction, the two syndromes may be highly correlated in the short-term (1 week) 85-87. The impact of delirium on long-term postoperative cognitive dysfunction has been less consistent.85,86,88 In addition to cognitive function, delirium has been associated with postoperative depression,37,38 another factor which may impede recovery. Finally, newer evidence is emerging that (younger) patients with delirium may develop a posttraumatic stress disorder-like syndrome.89,90 As a result, delirium may have long-term mental health complications that are not fully studied and may impact on functional recovery.
Functional Decline
Several studies have demonstrated that postoperative delirium is associated with functional decline and nursing home placement 1-3 months after surgery.4,91 However, these same studies have not demonstrated a consistent association between delirium and functional decline six months or longer after surgery. This difference may be related to reduced statistical power due to decreased functional decline rates in the long term and dropout of the patients with delirium.4,91 Future work in larger epidemiological studies will help determine the relationship between the incidence of delirium and long-term functional change after surgery.
Conclusion
Delirium is an acute change in cognitive function, specifically attention, with associated disorganization of thought and abnormal level of consciousness. Postoperative delirium is very common, especially in older surgical patients, and is associated with substantial morbidity, costs, and mortality. Preoperative delirium risk assessment is critical for identification of those patients who would most benefit from delirium prevention and surveillance protocols. Nonpharmacologic delirium prevention strategies have proven effective at reducing delirium incidence, but pharmacological prevention strategies do not yet have trial-based support. The primary treatment of delirium is to identify and treat the underlying causes. Delirium has substantial long-term consequences, which are currently being better defined through large-scale epidemiological studies. Assessing preoperative delirium risk, using delirium prevention strategies, and implementing standardized treatment protocols are important components of optimal care for older patients undergoing surgery.
Acknowledgments
Funding: Dr. Rudolph is the recipient of a VA Rehabilitation Research Career Development Award and Dr. Marcantonio is a recipient of a Mid-Career Investigator Award in Patient-oriented Research from the National Institute on Aging (K24 AG035075).
Footnotes
The authors declare no conflicts of interest.
Disclosures
Name: James L. Rudolph, MD, SM
Contribution: Study design, conduct of study, data collection, data analysis and manuscript preparation.
Name: Edward R. Marcantonio, MD, SM
Contribution: Study design, conduct of study, data collection, data analysis and manuscript preparation.
Contributor Information
James L. Rudolph, Geriatric Research, Education, and Clinical Center, VA Boston Healthcare System, Boston, MA, USA; Division of Aging, Brigham and Women's Hospital, Boston, MA, USA; Harvard Medical School, Boston, MA.
Edward R. Marcantonio, Divisions of General Medicine and Primary Care and Gerontology, Beth Israel Deaconess Medical Center, Boston, MA, USA; Harvard Medical School, Boston, MA.
References
- 1.Rudolph JL, Jones RN, Rasmussen LS, Silverstein JH, Inouye SK, Marcantonio ER. Independent vascular and cognitive risk factors for postoperative delirium. Am J Med. 2007;120:807–13. doi: 10.1016/j.amjmed.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 2.Marcantonio ER, Goldman L, Mangione CM, Ludwig LE, Muraca B, Haslauer CM, Donaldson MC, Whittemore AD, Sugarbaker DJ, Poss R, Haas S, Cook EF, Orav EJ, Lee TH. A clinical prediction rule for delirium after elective noncardiac surgery. JAMA. 1994;271:134–9. [PubMed] [Google Scholar]
- 3.Norkiene I, Ringaitiene D, Misiuriene I, Samalavicius R, Bubulis R, Baublys A, Uzdavinys G. Incidence and precipitating factors of delirium after coronary artery bypass grafting. Scand Cardiovasc J. 2007;41:180–5. doi: 10.1080/14017430701302490. [DOI] [PubMed] [Google Scholar]
- 4.Marcantonio ER, Flacker JM, Michaels M, Resnick NM. Delirium is independently associated with poor functional recovery after hip fracture. J Am Geriatr Soc. 2000;48:618–24. doi: 10.1111/j.1532-5415.2000.tb04718.x. [DOI] [PubMed] [Google Scholar]
- 5.Robinson TN, Raeburn CD, Tran ZV, Angles EM, Brenner LA, Moss M. Postoperative delirium in the elderly: risk factors and outcomes. Ann Surg. 2009;249:173–8. doi: 10.1097/SLA.0b013e31818e4776. [DOI] [PubMed] [Google Scholar]
- 6.Koster S, Hensens AG, van der Palen J. The long-term cognitive and functional outcomes of postoperative delirium after cardiac surgery. Ann Thorac Surg. 2009;87:1469–74. doi: 10.1016/j.athoracsur.2009.02.080. [DOI] [PubMed] [Google Scholar]
- 7.Gottesman RF, Grega MA, Bailey MM, Pham LD, Zeger SL, Baumgartner WA, Selnes OA, McKhann GM. Delirium after coronary artery bypass graft surgery and late mortality. Ann Neurol. 2010;67:338–44. doi: 10.1002/ana.21899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lundstrom M, Edlund A, Bucht G, Karlsson S, Gustafson Y. Dementia after delirium in patients with femoral neck fractures. J Am Geriatr Soc. 2003;51:1002–6. doi: 10.1046/j.1365-2389.2003.51315.x. [DOI] [PubMed] [Google Scholar]
- 9.Martin BJ, Buth KJ, Arora RC, Baskett RJ. Delirium as a predictor of sepsis in post-coronary artery bypass grafting patients: a retrospective cohort study. Crit Care. 2010;14:R171. doi: 10.1186/cc9273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304:443–51. doi: 10.1001/jama.2010.1013. [DOI] [PubMed] [Google Scholar]
- 11.Franco K, Litaker D, Locala J, Bronson D. The cost of delirium in the surgical patient. Psychosomatics. 2001;42:68–73. doi: 10.1176/appi.psy.42.1.68. [DOI] [PubMed] [Google Scholar]
- 12.Roach GW, Kanchuger M, Mangano CM, Newman M, Nussmeier N, Wolman R, Aggarwal A, Marschall K, Graham SH, Ley C. Adverse cerebral outcomes after coronary bypass surgery. Multicenter Study of Perioperative Ischemia Research Group and the Ischemia Research and Education Foundation Investigators. N Engl J Med. 1996;335:1857–63. doi: 10.1056/NEJM199612193352501. [DOI] [PubMed] [Google Scholar]
- 13.Bucerius J, Gummert JF, Borger MA, Walther T, Doll N, Falk V, Schmitt DV, Mohr FW. Predictors of delirium after cardiac surgery delirium: effect of beating-heart (off-pump) surgery. J Thorac Cardiovasc Surg. 2004;127:57–64. doi: 10.1016/s0022-5223(03)01281-9. [DOI] [PubMed] [Google Scholar]
- 14.Rudolph JL, Jones RN, Levkoff SE, Rockett C, Inouye SK, Sellke FW, Khuri SF, Lipsitz LA, Ramlawi B, Levitsky S, Marcantonio ER. Derivation and validation of a preoperative prediction rule for delirium after cardiac surgery. Circulation. 2009;119:229–36. doi: 10.1161/CIRCULATIONAHA.108.795260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diagnostic and Statistical Manual of Mental Disorders - III revised. Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- 16.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–8. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 17.Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008;56:823–30. doi: 10.1111/j.1532-5415.2008.01674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Keeffe ST, Gosney MA. Assessing attentiveness in older hospital patients: global assessment versus tests of attention. J Am Geriatr Soc. 1997;45:470–3. doi: 10.1111/j.1532-5415.1997.tb05173.x. [DOI] [PubMed] [Google Scholar]
- 19.Stavros KA, Rudolph JL, Jones RN, Marcantonio ER. Delirium and the clinical assessment of attention in older adults. J Am Geriatr Soc. 2008;56:S199–S200. [Google Scholar]
- 20.Liptzin B, Levkoff SE. An empirical study of delirium subtypes. Br J Psychiatry. 1992;161:843–5. doi: 10.1192/bjp.161.6.843. [DOI] [PubMed] [Google Scholar]
- 21.Inouye SK, Foreman MD, Mion LC, Katz KH, Cooney LM., Jr Nurses' recognition of delirium and its symptoms: comparison of nurse and researcher ratings. Arch Intern Med. 2001;161:2467–73. doi: 10.1001/archinte.161.20.2467. [DOI] [PubMed] [Google Scholar]
- 22.Levkoff SE, Evans DA, Liptzin B, Cleary PD, Lipsitz LA, Wetle TT, Reilly CH, Pilgrim DM, Schor J, Rowe J. Delirium - The Occurrence And Persistence Of Symptoms Among Elderly Hospitalized-Patients. Archives Of Internal Medicine. 1992;152:334–40. doi: 10.1001/archinte.152.2.334. [DOI] [PubMed] [Google Scholar]
- 23.Kiely DK, Jones RN, Bergmann MA, Marcantonio ER. Association between psychomotor activity delirium subtypes and mortality among newly admitted postacute facility patients. J Gerontol A Biol Sci Med Sci. 2007;62:174–9. doi: 10.1093/gerona/62.2.174. [DOI] [PubMed] [Google Scholar]
- 24.Ely EW, Margolin R, Francis J, May L, Truman B, Dittus R, Speroff T, Gautam S, Bernard GR, Inouye SK. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Crit Care Med. 2001;29:1370–9. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Pun BT, Ely EW. The importance of diagnosing and managing ICU delirium. Chest. 2007;132:624–36. doi: 10.1378/chest.06-1795. [DOI] [PubMed] [Google Scholar]
- 26.Ely EW, Gautam S, Margolin R, Francis J, May L, Speroff T, Truman B, Dittus R, Bernard R, Inouye SK. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27:1892–900. doi: 10.1007/s00134-001-1132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milbrandt EB, Deppen S, Harrison PL, Shintani AK, Speroff T, Stiles RA, Truman B, Bernard GR, Dittus RS, Ely EW. Costs associated with delirium in mechanically ventilated patients. Crit Care Med. 2004;32:955–62. doi: 10.1097/01.ccm.0000119429.16055.92. [DOI] [PubMed] [Google Scholar]
- 28.Ely EW, Truman B, Shintani A, Thomason JW, Wheeler AP, Gordon S, Francis J, Speroff T, Gautam S, Margolin R, Sessler CN, Dittus RS, Bernard GR. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS) Jama. 2003;289:2983–91. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 29.Hart RP, Levenson JL, Sessler CN, Best AM, Schwartz SM, Rutherford LE. Validation of a cognitive test for delirium in medical ICU patients. Psychosomatics. 1996;37:533–46. doi: 10.1016/S0033-3182(96)71517-7. [DOI] [PubMed] [Google Scholar]
- 30.Fong TG, Jones RN, Rudolph JL, Yang FM, Tommet D, Habtemariam D, Marcantonio ER, Langa KM, Inouye SK. Development and Validation of a Brief Cognitive Assessment Tool: The Sweet 16. Arch Intern Med. 2010 doi: 10.1001/archinternmed.2010.423. [DOI] [PubMed] [Google Scholar]
- 31.Grande LJ, Rudolph JL, Milberg WP, Barber CE, McGlinchey RE. Detecting cognitive impairment in individuals at risk for cardiovascular disease: the “Clock-in-the-Box” screening test. Int J Geriatr Psychiatry. 2010 doi: 10.1002/gps.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. Gerontologist. 1970;10:20–30. doi: 10.1093/geront/10.1_part_1.20. [DOI] [PubMed] [Google Scholar]
- 33.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–86. [PubMed] [Google Scholar]
- 34.Boldt J. Use of albumin: an update. Br J Anaesth. 2010;104:276–84. doi: 10.1093/bja/aep393. [DOI] [PubMed] [Google Scholar]
- 35.Gibbs J, Cull W, Henderson W, Daley J, Hur K, Khuri SF. Preoperative serum albumin level as a predictor of operative mortality and morbidity: results from the National VA Surgical Risk Study. Arch Surg. 1999;134:36–42. doi: 10.1001/archsurg.134.1.36. [DOI] [PubMed] [Google Scholar]
- 36.Inouye SK, Viscoli CM, Horwitz RI, Hurst LD, Tinetti ME. A predictive model for delirium in hospitalized elderly medical patients based on admission characteristics. Ann Intern Med. 1993;119:474–81. doi: 10.7326/0003-4819-119-6-199309150-00005. [DOI] [PubMed] [Google Scholar]
- 37.Greene NH, Attix DK, Weldon BC, Smith PJ, McDonagh DL, Monk TG. Measures of executive function and depression identify patients at risk for postoperative delirium. Anesthesiology. 2009;110:788–95. doi: 10.1097/aln.0b013e31819b5ba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith PJ, Attix DK, Weldon BC, Greene NH, Monk TG. Executive function and depression as independent risk factors for postoperative delirium. Anesthesiology. 2009;110:781–7. doi: 10.1097/aln.0b013e31819b5bc2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kazmierski J, Kowman M, Banach M, Pawelczyk T, Okonski P, Iwaszkiewicz A, Zaslonka J, Sobow T, Kloszewska I. Preoperative predictors of delirium after cardiac surgery: a preliminary study. Gen Hosp Psychiatry. 2006;28:536–8. doi: 10.1016/j.genhosppsych.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 40.Kazmierski J, Kowman M, Banach M, Fendler W, Okonski P, Banys A, Jaszewski R, Rysz J, Mikhailidis DP, Sobow T, Kloszewska I. Incidence and predictors of delirium after cardiac surgery: Results from The IPDACS Study. J Psychosom Res. 2010;69:179–85. doi: 10.1016/j.jpsychores.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 41.Burg MM, Benedetto MC, Rosenberg R, Soufer R. Presurgical depression predicts medical morbidity 6 months after coronary artery bypass graft surgery. Psychosom Med. 2003;65:111–8. doi: 10.1097/01.psy.0000038940.33335.09. [DOI] [PubMed] [Google Scholar]
- 42.Doering LV, Moser DK, Lemankiewicz W, Luper C, Khan S. Depression, healing, and recovery from coronary artery bypass surgery. Am J Crit Care. 2005;14:316–24. [PubMed] [Google Scholar]
- 43.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 44.Bass DS, Attix DK, Phillips-Bute B, Monk TG. An efficient screening tool for preoperative depression: the Geriatric Depression Scale-Short Form. Anesth Analg. 2008;106:805–9. doi: 10.1213/ane.0b013e318163fa75. table of contents. [DOI] [PubMed] [Google Scholar]
- 45.Saitz R. Clinical practice. Unhealthy alcohol use. N Engl J Med. 2005;352:596–607. doi: 10.1056/NEJMcp042262. [DOI] [PubMed] [Google Scholar]
- 46.Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49:516–22. doi: 10.1046/j.1532-5415.2001.49108.x. [DOI] [PubMed] [Google Scholar]
- 47.Inouye SK, Bogardus ST, Jr, Charpentier PA, Leo-Summers L, Acampora D, Holford TR, Cooney LM., Jr A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340:669–76. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 48.Kalisvaart KJ, de Jonghe JF, Bogaards MJ, Vreeswijk R, Egberts TC, Burger BJ, Eikelenboom P, van Gool WA. Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: a randomized placebo-controlled study. J Am Geriatr Soc. 2005;53:1658–66. doi: 10.1111/j.1532-5415.2005.53503.x. [DOI] [PubMed] [Google Scholar]
- 49.Marcantonio ER, Bergmann MA, Kiely DK, Orav EJ, Jones RN. Response letter to dr. Pitkala and colleagues. J Am Geriatr Soc. 2011;59:168–9. [Google Scholar]
- 50.Girard TD, Pandharipande PP, Carson SS, Schmidt GA, Wright PE, Canonico AE, Pun BT, Thompson JL, Shintani AK, Meltzer HY, Bernard GR, Dittus RS, Ely EW. Feasibility, efficacy, and safety of antipsychotics for intensive care unit delirium: the MIND randomized, placebo-controlled trial. Crit Care Med. 2010;38:428–37. doi: 10.1097/ccm.0b013e3181c58715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lonergan E, Britton AM, Luxenberg J, Wyller T. Antipsychotics for delirium. Cochrane Database Syst Rev. 2007:CD005594. doi: 10.1002/14651858.CD005594.pub2. [DOI] [PubMed] [Google Scholar]
- 52.Gamberini M, Bolliger D, Lurati Buse GA, Burkhart CS, Grapow M, Gagneux A, Filipovic M, Seeberger MD, Pargger H, Siegemund M, Carrel T, Seiler WO, Berres M, Strebel SP, Monsch AU, Steiner LA. Rivastigmine for the prevention of postoperative delirium in elderly patients undergoing elective cardiac surgery--a randomized controlled trial. Crit Care Med. 2009;37:1762–8. doi: 10.1097/CCM.0b013e31819da780. [DOI] [PubMed] [Google Scholar]
- 53.Liptzin B, Laki A, Garb JL, Fingeroth R, Krushell R. Donepezil in the prevention and treatment of post-surgical delirium. Am J Geriatr Psychiatry. 2005;13:1100–6. doi: 10.1176/appi.ajgp.13.12.1100. [DOI] [PubMed] [Google Scholar]
- 54.Sampson EL, Raven PR, Ndhlovu PN, Vallance A, Garlick N, Watts J, Blanchard MR, Bruce A, Blizard R, Ritchie CW. A randomized, double-blind, placebo-controlled trial of donepezil hydrochloride (Aricept) for reducing the incidence of postoperative delirium after elective total hip replacement. Int J Geriatr Psychiatry. 2007;22:343–9. doi: 10.1002/gps.1679. [DOI] [PubMed] [Google Scholar]
- 55.van Eijk MM, Roes KC, Honing ML, Kuiper MA, Karakus A, van der Jagt M, Spronk PE, van Gool WA, van der Mast RC, Kesecioglu J, Slooter AJ. Effect of rivastigmine as an adjunct to usual care with haloperidol on duration of delirium and mortality in critically ill patients: a multicentre, double-blind, placebo-controlled randomised trial. Lancet. 2010;376:1829–37. doi: 10.1016/S0140-6736(10)61855-7. [DOI] [PubMed] [Google Scholar]
- 56.Overshott R, Karim S, Burns A. Cholinesterase inhibitors for delirium. Cochrane Database Syst Rev. 2008:CD005317. doi: 10.1002/14651858.CD005317.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pandharipande PP, Pun BT, Herr DL, Maze M, Girard TD, Miller RR, Shintani AK, Thompson JL, Jackson JC, Deppen SA, Stiles RA, Dittus RS, Bernard GR, Ely EW. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. Jama. 2007;298:2644–53. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 58.Riker RR, Shehabi Y, Bokesch PM, Ceraso D, Wisemandle W, Koura F, Whitten P, Margolis BD, Byrne DW, Ely EW, Rocha MG. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. Jama. 2009;301:489–99. doi: 10.1001/jama.2009.56. [DOI] [PubMed] [Google Scholar]
- 59.Maldonado JR, Wysong A, van der Starre PJ, Block T, Miller C, Reitz BA. Dexmedetomidine and the reduction of postoperative delirium after cardiac surgery. Psychosomatics. 2009;50:206–17. doi: 10.1176/appi.psy.50.3.206. [DOI] [PubMed] [Google Scholar]
- 60.Brown EN, Lydic R, Schiff ND. General anesthesia, sleep, and coma. N Engl J Med. 2010;363:2638–50. doi: 10.1056/NEJMra0808281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xie Z, Dong Y, Maeda U, Moir R, Inouye SK, Culley DJ, Crosby G, Tanzi RE. Isoflurane-induced apoptosis: a potential pathogenic link between delirium and dementia. J Gerontol A Biol Sci Med Sci. 2006;61:1300–6. doi: 10.1093/gerona/61.12.1300. [DOI] [PubMed] [Google Scholar]
- 62.Marcantonio ER, Juarez G, Goldman L, Mangione CM, Ludwig LE, Lind L, Katz N, Cook EF, Orav EJ, Lee TH. The relationship of postoperative delirium with psychoactive medications. JAMA. 1994;272:1518–22. [PubMed] [Google Scholar]
- 63.Bryson GL, Wyand A. Evidence-based clinical update: general anesthesia and the risk of delirium and postoperative cognitive dysfunction. Can J Anaesth. 2006;53:669–77. doi: 10.1007/BF03021625. [DOI] [PubMed] [Google Scholar]
- 64.Sieber FE, Zakriya KJ, Gottschalk A, Blute MR, Lee HB, Rosenberg PB, Mears SC. Sedation depth during spinal anesthesia and the development of postoperative delirium in elderly patients undergoing hip fracture repair. Mayo Clin Proc. 2010;85:18–26. doi: 10.4065/mcp.2009.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schug SA, Sidebotham DA, McGuinnety M, Thomas J, Fox L. Acetaminophen as an adjunct to morphine by patient-controlled analgesia in the management of acute postoperative pain. Anesth Analg. 1998;87:368–72. doi: 10.1097/00000539-199808000-00024. [DOI] [PubMed] [Google Scholar]
- 66.Paice JA, Noskin GA, Vanagunas A, Shott S. Efficacy and safety of scheduled dosing of opioid analgesics: a quality improvement study. J Pain. 2005;6:639–43. doi: 10.1016/j.jpain.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 67.Mann C, Pouzeratte Y, Boccara G, Peccoux C, Vergne C, Brunat G, Domergue J, Millat B, Colson P. Comparison of intravenous or epidural patient-controlled analgesia in the elderly after major abdominal surgery. Anesthesiology. 2000;92:433–41. doi: 10.1097/00000542-200002000-00025. [DOI] [PubMed] [Google Scholar]
- 68.Osse RJ, Tulen JH, Bogers AJ, Hengeveld MW. Disturbed circadian motor activity patterns in postcardiotomy delirium. Psychiatry Clin Neurosci. 2009;63:56–64. doi: 10.1111/j.1440-1819.2008.01888.x. [DOI] [PubMed] [Google Scholar]
- 69.Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275:852–7. [PubMed] [Google Scholar]
- 70.Inouye SK. Delirium in older persons. N Engl J Med. 2006;354:1157–65. doi: 10.1056/NEJMra052321. [DOI] [PubMed] [Google Scholar]
- 71.Alsop DC, Fearing MA, Johnson K, Sperling R, Fong TG, Inouye SK. The role of neuroimaging in elucidating delirium pathophysiology. J Gerontol A Biol Sci Med Sci. 2006;61:1287–93. doi: 10.1093/gerona/61.12.1287. [DOI] [PubMed] [Google Scholar]
- 72.Breitbart W, Marotta R, Platt MM, Weisman H, Derevenco M, Grau C, Corbera K, Raymond S, Lund S, Jacobson P. A double-blind trial of haloperidol, chlorpromazine, and lorazepam in the treatment of delirium in hospitalized AIDS patients. Am J Psychiatry. 1996;153:231–7. doi: 10.1176/ajp.153.2.231. [DOI] [PubMed] [Google Scholar]
- 73.Abu-Omar Y, Cader S, Guerrieri Wolf L, Pigott D, Matthews PM, Taggart DP. Short-term changes in cerebral activity in on-pump and off-pump cardiac surgery defined by functional magnetic resonance imaging and their relationship to microembolization. J Thorac Cardiovasc Surg. 2006;132:1119–25. doi: 10.1016/j.jtcvs.2006.04.057. [DOI] [PubMed] [Google Scholar]
- 74.Martin KK, Wigginton JB, Babikian VL, Pochay VE, Crittenden MD, Rudolph JL. Intraoperative cerebral high-intensity transient signals and postoperative cognitive function: a systematic review. Am J Surg. 2009;197:55–63. doi: 10.1016/j.amjsurg.2007.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Adams HP, Jr, Adams RJ, Brott T, del Zoppo GJ, Furlan A, Goldstein LB, Grubb RL, Higashida R, Kidwell C, Kwiatkowski TG, Marler JR, Hademenos GJ. Guidelines for the early management of patients with ischemic stroke: A scientific statement from the Stroke Council of the American Stroke Association. Stroke. 2003;34:1056–83. doi: 10.1161/01.STR.0000064841.47697.22. [DOI] [PubMed] [Google Scholar]
- 76.Flaherty JH, Tariq SH, Raghavan S, Bakshi S, Moinuddin A, Morley JE. A model for managing delirious older inpatients. J Am Geriatr Soc. 2003;51:1031–5. doi: 10.1046/j.1365-2389.2003.51320.x. [DOI] [PubMed] [Google Scholar]
- 77.Campbell N, Boustani MA, Ayub A, Fox GC, Munger SL, Ott C, Guzman O, Farber M, Ademuyiwa A, Singh R. Pharmacological management of delirium in hospitalized adults--a systematic evidence review. J Gen Intern Med. 2009;24:848–53. doi: 10.1007/s11606-009-0996-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294:1934–43. doi: 10.1001/jama.294.15.1934. [DOI] [PubMed] [Google Scholar]
- 79.Wang PS, Schneeweiss S, Avorn J, Fischer MA, Mogun H, Solomon DH, Brookhart MA. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med. 2005;353:2335–41. doi: 10.1056/NEJMoa052827. [DOI] [PubMed] [Google Scholar]
- 80.Consensus statement on high-dose antipsychotic medication. London, UK: Royal College of Psychiatrists; 2006. [Google Scholar]
- 81.Rudolph JL, Gardner KF, Gramigna GD, McGlinchey RE. Antipsychotics and oropharyngeal dysphagia in hospitalized older patients. J Clin Psychopharmacol. 2008;28:532–5. doi: 10.1097/JCP.0b013e318184c905. [DOI] [PubMed] [Google Scholar]
- 82.Kiely DK, Marcantonio ER, Inouye SK, Shaffer ML, Bergmann MA, Yang FM, Fearing MA, Jones RN. Persistent delirium predicts greater mortality. J Am Geriatr Soc. 2009;57:55–61. doi: 10.1111/j.1532-5415.2008.02092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kiely DK, Jones RN, Bergmann MA, Murphy KM, Orav EJ, Marcantonio ER. Association between delirium resolution and functional recovery among newly admitted postacute facility patients. J Gerontol A Biol Sci Med Sci. 2006;61:204–8. doi: 10.1093/gerona/61.2.204. [DOI] [PubMed] [Google Scholar]
- 84.Fong TG, Jones RN, Shi P, Marcantonio ER, Yap L, Rudolph JL, Yang FM, Kiely DK, Inouye SK. Delirium accelerates cognitive decline in Alzheimer disease. Neurology. 2009;72:1570–5. doi: 10.1212/WNL.0b013e3181a4129a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Heilman KM, Gravenstein JS. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18–30. doi: 10.1097/01.anes.0000296071.19434.1e. [DOI] [PubMed] [Google Scholar]
- 86.Rudolph JL, Marcantonio ER, Culley DJ, Silverstein JH, Rasmussen LS, Crosby GJ, Inouye SK. Delirium is associated with early postoperative cognitive dysfunction. Anaesthesia. 2008;63:941–7. doi: 10.1111/j.1365-2044.2008.05523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rudolph JL, Schreiber KA, Culley DJ, McGlinchey RE, Crosby G, Levitsky S, Marcantonio ER. Measurement of post-operative cognitive dysfunction after cardiac surgery: a systematic review. Acta Anaesthesiol Scand. 2010 doi: 10.1111/j.1399-6576.2010.02236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wacker P, Nunes PV, Cabrita H, Forlenza OV. Post-operative delirium is associated with poor cognitive outcome and dementia. Dement Geriatr Cogn Disord. 2006;21:221–7. doi: 10.1159/000091022. [DOI] [PubMed] [Google Scholar]
- 89.Jones C, Griffiths RD, Humphris G, Skirrow PM. Memory, delusions, and the development of acute posttraumatic stress disorder-related symptoms after intensive care. Crit Care Med. 2001;29:573–80. doi: 10.1097/00003246-200103000-00019. [DOI] [PubMed] [Google Scholar]
- 90.Jones C, Backman C, Capuzzo M, Flaatten H, Rylander C, Griffiths RD. Precipitants of post-traumatic stress disorder following intensive care: a hypothesis generating study of diversity in care. Intensive Care Med. 2007;33:978–85. doi: 10.1007/s00134-007-0600-8. [DOI] [PubMed] [Google Scholar]
- 91.Rudolph JL, Inouye SK, Jones RN, Yang FM, Fong TG, Levkoff SE, Marcantonio ER. Delirium: an independent predictor of functional decline after cardiac surgery. J Am Geriatr Soc. 2010;58:643–9. doi: 10.1111/j.1532-5415.2010.02762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Marcantonio ER, Goldman L, Mangione CM, Ludwig LE, Muraca B, Haslauer CM, Donaldson MC, Whittemore AD, Sugarbaker DJ, Poss R, Haas S, Cook EF, Orav EJ, Lee TH. A clinical prediction rule for delirium after elective noncardiac surgery. JAMA. 1994;271:134–9. [PubMed] [Google Scholar]
- 93.Schneider F, Bohner H, Habel U, Salloum JB, Stierstorfer A, Hummel TC, Miller C, Friedrichs R, Muller EE, Sandmann W. Risk factors for postoperative delirium in vascular surgery. Gen Hosp Psychiatry. 2002;24:28–34. doi: 10.1016/s0163-8343(01)00168-2. [DOI] [PubMed] [Google Scholar]
- 94.Bohner H, Hummel TC, Habel U, Miller C, Reinbott S, Yang Q, Gabriel A, Friedrichs R, Muller EE, Ohmann C, Sandmann W, Schneider F. Predicting delirium after vascular surgery: a model based on pre- and intraoperative data. Ann Surg. 2003;238:149–56. doi: 10.1097/01.sla.0000077920.38307.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Benoit AG, Campbell BI, Tanner JR, Staley JD, Wallbridge HR, Biehl DR, Bradley BD, Louridas G, Guzman RP, Fromm RA. Risk factors and prevalence of perioperative cognitive dysfunction in abdominal aneurysm patients. J Vasc Surg. 2005;42:884–90. doi: 10.1016/j.jvs.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 96.Kaneko T, Takahashi S, Naka T, Hirooka Y, Inoue Y, Kaibara N. Postoperative delirium following gastrointestinal surgery in elderly patients. Surg Today. 1997;27:107–11. doi: 10.1007/BF02385897. [DOI] [PubMed] [Google Scholar]
- 97.Olin K, Eriksdotter-Jonhagen M, Jansson A, Herrington MK, Kristiansson M, Permert J. Postoperative delirium in elderly patients after major abdominal surgery. Br J Surg. 2005;92:1559–64. doi: 10.1002/bjs.5053. [DOI] [PubMed] [Google Scholar]
- 98.Milstein A, Pollack A, Kleinman G, Barak Y. Confusion/delirium following cataract surgery: an incidence study of 1-year duration. Int Psychogeriatr. 2002;14:301–6. doi: 10.1017/s1041610202008499. [DOI] [PubMed] [Google Scholar]
- 99.Dyer CB, Ashton CM, Teasdale TA. Postoperative delirium. A review of 80 primary data-collection studies. Arch Intern Med. 1995;155:461–5. doi: 10.1001/archinte.155.5.461. [DOI] [PubMed] [Google Scholar]
- 100.Galanakis P, Bickel H, Gradinger R, Von Gumppenberg S, Forstl H. Acute confusional state in the elderly following hip surgery: incidence, risk factors and complications. Int J Geriatr Psychiatry. 2001;16:349–55. doi: 10.1002/gps.327. [DOI] [PubMed] [Google Scholar]
- 101.Weed HG, Lutman CV, Young DC, Schuller DE. Preoperative identification of patients at risk for delirium after major head and neck cancer surgery. Laryngoscope. 1995;105:1066–8. doi: 10.1288/00005537-199510000-00011. [DOI] [PubMed] [Google Scholar]
- 102.Gustafson Y, Berggren D, Brannstrom B, Bucht G, Norberg A, Hansson LI, Winblad B. Acute confusional states in elderly patients treated for femoral neck fracture. J Am Geriatr Soc. 1988;36:525–30. doi: 10.1111/j.1532-5415.1988.tb04023.x. [DOI] [PubMed] [Google Scholar]
- 103.Fong TG, Fearing MA, Jones RN, Shi P, Marcantonio ER, Rudolph JL, Yang FM, Kiely DK, Inouye SK. Telephone interview for cognitive status: Creating a crosswalk with the Mini-Mental State Examination. Alzheimers Dement. 2009;5:492–7. doi: 10.1016/j.jalz.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 105.Goldman L, Hashimoto B, Cook EF, Loscalzo A. Comparative reproducibility and validity of systems for assessing cardiovascular functional class: advantages of a new specific activity scale. Circulation. 1981;64:1227–34. doi: 10.1161/01.cir.64.6.1227. [DOI] [PubMed] [Google Scholar]
- 106.Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, Truman B, Speroff T, Gautam S, Margolin R, Hart RP, Dittus R. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–10. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]


