Apolipoprotein C-III (apoC-III) is a protein of 79 amino acids that is synthesized in the liver and to a lesser degree in the intestine (1). In the circulation, apoC-III is a constituent of both apoB- and apoA-I-containing lipoproteins. It is not evenly distributed between these lipoproteins however, with the majority of apoC-III found in the HDL fraction in normolipidemic individuals and on triglyceride-rich lipoproteins in patients with elevated levels of plasma triglyceride (2, 3). Furthermore, some VLDLs, intermediate density lipoproteins (IDLs) and LDLs contain many molecules of apoC-III, whereas others contain none (4). Semi-quantitative analysis suggests that less than half of apoA-I-containing lipoproteins in plasma (i.e., HDL) contain apoC-III (5).
ApoC-III plays a pivotal role in regulating the plasma metabolism of VLDL, IDL, and LDL, primarily by inhibiting receptor-mediated uptake of these lipoproteins by the liver (6–8). VLDL containing apoC-III are channeled down the lipolytic cascade to LDL, particularly to denser LDL that have a slower clearance rate from plasma (9). ApoC-III also enhances the hepatic assembly and secretion of VLDL (10), and overproduction of apoC-III and of apoB lipoproteins that contain apoC-III is a common feature of patients with hypertriglyceridemia (8, 9, 11). Thus, apoC-III is intricately involved in establishing hypertriglyceridemia and its associated dense LDL phenotype. Much less is known, however, about the function of apoC-III when it is a component of HDL. ApoC-III has been shown to inhibit hepatic lipase (12) and interact with receptors such as scavenger receptor class B type IQ1: Please confrim or amend spellouts for SR-BI and TNF. (13) and ABCA1 (14), thus having the potential to affect the function or metabolism of HDL.
ApoC-III can also stimulate several processes involved in atherogenesis and vascular inflammation. ApoC-III stimulates blood-born monocytes and endothelial cells to produce cytokines such as tumor necrosis factor-α and adhesion molecules, and it activates insulin-resistance pathways in endothelial cells causing endothelial dysfunction (15, 16). Interaction of apoC-III with TLR-2 at the cell surface acts as an initiating event in this function. ApoC-III in LDL binds to vascular proteoglycans that may lead to LDL retention in the arterial wall (17). ApoC-III also stimulates adipocytes to produce cytokines and suppresses their production of adiponectin (18). These actions, converting adipocytes into a proinflammatory phenotype, may act indirectly to promote the development of atherosclerosis. More direct evidence for the atherogenicity associated with apoC-III comes from transgenic animal studies that have found that ldlr−/− mice overexpressing human apoC-III develop enhanced atherosclerotic lesions on a Western diet (19). Furthermore, apoC-III and the VLDL and LDL that contain it are strong independent predictors of cardiovascular events and of progression of coronary atherosclerosis (20, 21). In summary, evidence from several sources link apoC-III with atherosclerosis, providing a strong rationale to better understand factors affecting the plasma metabolism of apoC-III.
In the April issue of the Journal of Lipid Research, Ooi and colleagues (22) evaluated the metabolism of apoC-III in patients with chronic renal failure, a condition associated with a high incidence of cardiovascular disease. The principal finding was a low fractional catabolic rate (FCR) for apoC-III in VLDL of renal patients compared with controls. The FCR of VLDL apoC-III was strongly correlated with the FCR of VLDL apoB. These results provide additional evidence for an effect of apoC-III on clearance of triglyceride-rich lipoproteins and for the involvement of apoC-III in the dyslipidemia of patients with chronic renal impairment.
Ooi and colleagues, in this and previous research articles (22–24), have used their apoC-III kinetic data to make general conclusions about the plasma metabolism of apoC-III. They found that their tracer enrichment-time curves for apoC-III in VLDL and in HDL were similar. Consequently, the FCR for VLDL and HDL apoC-III were the same. From this observation, they concluded that apoC-III equilibrates rapidly and completely between these lipoproteins (24). Because VLDL is cleared much more rapidly from plasma than HDL, these results and conclusions suggest that apoC-III transfers or exchanges between apoB- and apoA-I-containing lipoproteins (and perhaps also between different apoB-containing subspecies), but avoids being catabolized when these lipoproteins are cleared from the circulation. This scenario would suggest that apoC-III continually “jumps off” lipoproteins before they are taken up by cells. In addition, for a one-pool model of apoC-III kinetics to adequately explain plasma apoC-III metabolism, apoC-III must be cleared as free apoC-III or as a single lipoprotein type (i.e., “terminally catabolized triglyceride-rich lipoproteins”). Because free apoC-III is not found in human plasma (5, 25), and we find it hard to conceptualize how apoC-III escapes tissue uptake during the normal course of VLDL and HDL clearance, we have to question this oversimplified view of apoC-III metabolism.
We view the one-pool model for plasma apoC-III metabolism as only one of several possible interpretations of apoC-III tracer enrichment-time curves. Ooi and colleagues have not measured the movement of apoC-III between lipoproteins, so their interpretation that apoC-III rapidly and completely exchanges between lipoproteins is by inference only and not by direct measurement. One possibility is that enrichment curves could reflect an average of several distinctly different apoC-III pools within VLDL or HDL (which is incidentally the case for VLDL apoB and for HDL apoA-I when it is isolated and analyzed as one fraction). The pattern of apoC-III distribution within triglyceride-rich lipoproteins certainly does not suggest a random equilibration of apoC-III. ApoC-III-immunoaffinity chromatography separation of VLDL has demonstrated that VLDL containing apoC-III have as many as 100 apoC-III molecules per particle, yet a significant portion of VLDL do not contain apoC-III at all (4, 26).
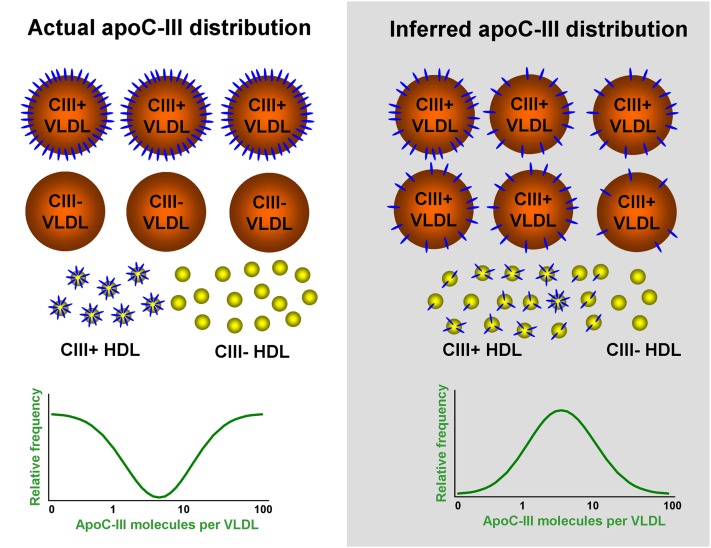
Another problem with the one-pool concept for plasma apoC-III metabolism is that it fails to explain the asymmetrical presence of apoC-III among triglyceride-rich lipoproteins and HDL. ApoC-III is present across the entire spectrum of differently-sized plasma lipoproteins from large VLDL to small HDL. However, apoC-III is present on only 30–70% of VLDL, a smaller percentage of IDL, 5–15% of LDL, and a minority of HDL particles. The actual apoC-III distribution pattern, as demonstrated in Fig. 1, suggests that apoC-III transfer between various lipoproteins is a regulated process and requires other factors. If apoC-III is freely and rapidly exchangeable in a single pool, then exchange would be restricted to apoC-III-containing lipoprotein subtypes. This would require an unknown property of apoC-III-containing lipoproteins that permitted apoC-III to transfer freely among them and not to nonapoC-III containing lipoproteins.
Fig. 1.
Comparison between the actual lipoprotein distribution of apoC-III and the distribution inferred from the one-pool concept of plasma apoC-III metabolism. There are on average ∼20–50 apoC-III molecules on each VLDL particle. About 50% of VLDL contain apoC-III (CIII+), and the other half of VLDL do not contain apoC-III at all (CIII−). The apoC-III distribution pattern within VLDL has an inverse bell shape (left panel). On the other hand, the one-pool concept of plasma apoC-III metabolism suggests that apoC-III exchanges freely and randomly within VLDL and HDL and also between VLDL and HDL. In this scenario, the vast majority of VLDL and HDL would contain some apoC-III molecules and would have a normal distribution pattern (right panel). There would be few VLDL or HDL containing large numbers of apoC-III molecules and one would be unlikely to find lipoproteins without apoC-III.
Therefore, an unanswered question that needs to be resolved is the extent to which apoC-III exchanges between VLDL and HDL. Although some studies have demonstrated exchange and equilibration of apoC-III between VLDL and HDL (24, 27), others have found nonexchangeable pools of apoC-III that do not completely equilibrate between VLDL and HDL (11, 28). Based on in vitro experiments, Boyle et al. (29) found two kinetic pools of apoC-III that transferred between VLDL and HDL at different speeds. ApoC-III in the fast pool rapidly transferred from donor to recipient lipoproteins in a matter of minutes, whereas apoC-III in the slow pool followed a monoexponential rate of exchange with a t1/2 of 3 h. The distribution of apoC-III between fast and slow pools was variable but apparently depended on the size of the donor particles. These results suggest that the two kinetically distinct pools may be related to conformational changes in individual apoC-III molecules on the lipoprotein surface. In a separate study, labeled apoC-III disappearance from plasma was found to follow tri-exponential kinetics, and differences in plasma and urine radioactivity curves suggested the presence of kinetically distinct pools of apoC-III (30). These findings for apoC-III are analogous to the properties of apoE, which is a fixed and exchangeable component within VLDL of various sizes (31).
Our tracer studies of apoB metabolism have shown that apoC-III-containing VLDL and IDL are converted to VLDL, IDL, and LDL without apoC-III (8, 9). More than half of apoC-III-containing VLDL and IDL follow this pathway, losing their triglyceride content together with apoC-III. They are thus transformed into slowly turning over lipoproteins that do not contain apoC-III. Partial loss of apoC-III also happens to triglyceride-rich lipoproteins with apoC-III as evidenced by their diminishing apoC-III content as they become smaller (26). It remains to be studied whether apoC-III molecules released from VLDL and IDL are relocated to other apoB lipoproteins or HDL or are cleared from circulation directly. Similarly, it is not entirely clear what processes regulate the transfer of apoC-III from HDL to VLDL. Although apoC-III can readily transfer from HDL to VLDL or chylomicron-sized lipid emulsions (24, 30), in our tracer studies, we have not found evidence in vivo for acquisition of apoC-III by circulating VLDL that do not yet have apoC-III. It is possible that if indeed apoC-III does move from HDL to VLDL in significant amounts, the preferred recipient VLDL are the large particles that already possess considerable amounts of apoC-III. One additional reason to question the simplicity of a one-pool model of plasma apoC-III metabolism is that apoC-III exists as three isoforms, i.e., apoC-III0, apoC-III1 and apoC-III2, corresponding to 0-2 sialic acid molecules on the protein. The isoform with most sialic acid, apoC-III2, tends to have a higher FCR compared with monosialylated apoC-III1, and apoC-III0, the less-predominant isoform, has a significantly slower FCR and lower production rates compared with the other two isoforms (32). These data suggest heterogeneity among apoC-III isoforms, regarding both secretion and clearance, which should be taken into consideration when dealing with disease conditions that directly affect apoC-III sialyation, including chronic renal failure (33).
Current modeling of apoC-III kinetic data has assumed that apoC-III is secreted on both VLDL and HDL, and that the distribution of secretion is proportional to their respective pool sizes. However, it is not clear if this assumption is correct. Recent experiments with hepatocytes expressing apoC-III show that the vast majority of apoC-III is found on HDL instead of VLDL in the media of cultured cells (10). It is unclear whether apoC-III is secreted together with HDL or if HDL acquires apoC-III after secretion. On the other hand, the same group reported that apoC-III seemed to play an important role in packaging lipids onto VLDL precursors (10, 34). Indeed, the majority of apoC-III in the microsomal lumen was found associated with IDL-sized lipid droplets, which later fused with VLDL precursors to form VLDL. Therefore, it is possible that apoC-III is secreted as an integral component of lipoproteins and is not a result of random acquisition by acceptor particles. Once again, apoC-III appears to have a specific and not simply a random role in regulating VLDL production by the liver.
In conclusion, it is our opinion that: 1) hepatic apoC-III production (i.e., synthesis and secretion) plays an important role in determining the size or number of triglyceride-rich VLDL secreted by the liver; 2) in the blood, different lipoproteins (whether apoB- or apoA-I-containing) have different numbers of apoC-III molecules, which may be determined by the structure or composition of the lipoproteins themselves; and 3) irrespective of whether all apoC-III is exchangeable or not, it significantly affects the metabolism of the particle on which it resides, and in so doing, plays a central role in determining the concentration in the circulation of potentially atherogenic VLDL, IDL, and small dense LDL.
Footnotes
This article is available online at http://www.jlr.org
REFERENCES
- 1.Jong M. C., Hofker M. H., Havekes L. M. 1999. Role of apoCs in lipoprotein metabolism. Functional differences between apoC1, apoC2, and apoC3. Arterioscler. Thromb. Vasc. Biol. 19: 472–484. [DOI] [PubMed] [Google Scholar]
- 2.Fredenrich A., Giroux L. M., Tremblay M., Krimbou L., Davignon J., Cohn J. S. 1997. Plasma lipoprotein distribution of apoC-III in normolipidemic and hypertriglyceridemic subjects: comparison of the apoC-III to apoE ratio in different lipoprotein fractions. J. Lipid Res. 38: 1421–1432. [PubMed] [Google Scholar]
- 3.Schonfeld G., George P. K., Miller J., Reilly P., Witztum J. 1979. Apolipoprotein C-II and C-III levels in hyperlipoproteinemia. Metabolism. 28: 1001–1010. [DOI] [PubMed] [Google Scholar]
- 4.Khoo C., Campos H., Judge H., Sacks F. M. 1999. Effects of estrogenic oral contraceptives on the lipoprotein B particle system defined by apolipoproteins E and C-III content. J. Lipid Res. 40: 202–212. [PubMed] [Google Scholar]
- 5.Asztalos B. F., Schaefer E. J., Horvath K. V., Yamashita S., Miller M., Franceschini G., Calabresi L. 2007. Role of LCAT in HDL remodeling: investigation of LCAT deficiency states. J. Lipid Res. 48: 592–599. [DOI] [PubMed] [Google Scholar]
- 6.Sehayek E., Eisenberg S. 1991. Mechanisms of inhibition by apolipoprotein C of apolipoprotein E-dependent cellular metabolism of human triglyceride-rich lipoproteins through the low density lipoprotein receptor pathway. J. Biol. Chem. 266: 18259–18267. [PubMed] [Google Scholar]
- 7.Aalto-Setala K., Fisher E. A., Chen X., Chajek-Shaul T., Hayek T., Zechner R., Walsh A., Ramakrishnan R., Ginsberg H. N., Breslow J. L. 1992. Mechanism of hypertriglyceridemia in human apolipoprotein (apo) CIII transgenic mice. Diminished very low density lipoprotein fractional catabolic rate associated with increased apo CIII and reduced apo E on the particles. J. Clin. Invest. 90: 1889–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng C., Khoo C., Ikewaki K., Sacks F. M. 2007. Rapid turnover of apolipoprotein C-III-containing triglyceride-rich lipoproteins contributing to the formation of LDL subfractions. J. Lipid Res. 48: 1190–1203. [DOI] [PubMed] [Google Scholar]
- 9.Zheng C., Khoo C., Furtado J., Sacks F. M. 2010. Apolipoprotein C-III and the metabolic basis for hypertriglyceridemia and the dense low-density lipoprotein phenotype. Circulation. 121: 1722–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sundaram M., Zhong S., Khalil M. B., Links P. H., Zhao Y., Iqbal J., Hussain M. M., Parks R. J., Wang Y., Yao Z. 2010. Expression of apolipoprotein C-III in McA-RH7777 cells enhances VLDL assembly and secretion under lipid-rich conditions. J. Lipid Res. 51: 150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Batal R., Tremblay M., Barrett P. H., Jacques H., Fredenrich A., Mamer O., Davignon J., Cohn J. S. 2000. Plasma kinetics of apoC-III and apoE in normolipidemic and hypertriglyceridemic subjects. J. Lipid Res. 41: 706–718. [PubMed] [Google Scholar]
- 12.Kinnunen P. K., Ehnolm C. 1976. Effect of serum and C-apoproteins from very low density lipoproteins on human postheparin plasma hepatic lipase. FEBS Lett. 65: 354–357. [DOI] [PubMed] [Google Scholar]
- 13.Xu S., Laccotripe M., Huang X., Rigotti A., Zannis V. I., Krieger M. 1997. Apolipoproteins of HDL can directly mediate binding to the scavenger receptor SR-BI, an HDL receptor that mediates selective lipid uptake. J. Lipid Res. 38: 1289–1298. [PubMed] [Google Scholar]
- 14.Remaley A. T., Stonik J. A., Demosky S. J., Neufeld E. B., Bocharov A. V., Vishnyakova T. G., Eggerman T. L., Patterson A. P., Duverger N. J., Santamarina-Fojo S., et al. 2001. Apolipoprotein specificity for lipid efflux by the human ABCAI transporter. Biochem. Biophys. Res. Commun. 280: 818–823. [DOI] [PubMed] [Google Scholar]
- 15.Kawakami A., Aikawa M., Alcaide P., Luscinskas F. W., Libby P., Sacks F. M. 2006. Apolipoprotein CIII induces expression of vascular cell adhesion molecule-1 in vascular endothelial cells and increases adhesion of monocytic cells. Circulation. 114: 681–687. [DOI] [PubMed] [Google Scholar]
- 16.Kawakami A., Osaka M., Tani M., Azuma H., Sacks F. M., Shimokado K., Yoshida M. 2008. Apolipoprotein C-III links hyperlipidemia with vascular endothelial cell dysfunction. Circulation. 118: 731–742. [DOI] [PubMed] [Google Scholar]
- 17.Hiukka A., Stahlman M., Pettersson C., Levin M., Adiels M., Teneberg S., Leinonen E. S., Hulten L. M., Wiklund O., Oresic M., et al. 2009. ApoCIII-enriched LDL in type 2 diabetes displays altered lipid composition, increased susceptibility for sphingomyelinase, and increased binding to biglycan. Diabetes. 58: 2018–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abe Y., Kawakami A., Osaka M., Uematsu S., Akira S., Shimokado K., Sacks F. M., Yoshida M. 2010. Apolipoprotein CIII induces monocyte chemoattractant protein-1 and interleukin 6 expression via Toll-like receptor 2 pathway in mouse adipocytes. Arterioscler. Thromb. Vasc. Biol. 30: 2242–2248. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Masucci-Magoulas L., Goldberg I. J., Bisgaier C. L., Serajuddin H., Francone O. L., Breslow J. L., Tall A. R. 1997. A mouse model with features of familial combined hyperlipidemia. Science. 275: 391–394. [DOI] [PubMed] [Google Scholar]
- 20.Blankenhorn D. H., Alaupovic P., Wickham E., Chin H. P., Azen S. P. 1990. Prediction of angiographic change in native human coronary arteries and aortocoronary bypass grafts. Lipid and nonlipid factors. Circulation. 81: 470–476. [DOI] [PubMed] [Google Scholar]
- 21.Sacks F. M., Alaupovic P., Moye L. A., Cole T. G., Sussex B., Stampfer M. J., Pfeffer M. A., Braunwald E. 2000. VLDL, apolipoproteins B, CIII, and E, and risk of recurrent coronary events in the Cholesterol and Recurrent Events (CARE) trial. Circulation. 102: 1886–1892. [DOI] [PubMed] [Google Scholar]
- 22.Ooi E. M., Chan D. T., Watts G. F., Chan D. C., Ng T. W., Dogra G. K., Irish A. B., Barrett P. H. 2011. Plasma apolipoprotein C-III metabolism in patients with chronic kidney disease. J. Lipid Res. 52: 794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan D. C., Nguyen M. N., Watts G. F., Barrett P. H. 2008. Plasma apolipoprotein C-III transport in centrally obese men: associations with very low-density lipoprotein apolipoprotein B and high-density lipoprotein apolipoprotein A-I metabolism. J. Clin. Endocrinol. Metab. 93: 557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen M. N., Chan D. C., Dwyer K. P., Bolitho P., Watts G. F., Barrett P. H. 2006. Use of Intralipid for kinetic analysis of HDL apoC-III: evidence for a homogeneous kinetic pool of apoC-III in plasma. J. Lipid Res. 47: 1274–1280. [DOI] [PubMed] [Google Scholar]
- 25.Krimbou L., Tremblay M., Davignon J., Cohn J. S. 1997. Characterization of human plasma apolipoprotein E-containing lipoproteins in the high density lipoprotein size range: focus on pre-beta1-LpE, pre-beta2-LpE, and alpha-LpE. J. Lipid Res. 38: 35–48. [PubMed] [Google Scholar]
- 26.Zheng C., Khoo C., Furtado J., Ikewaki K., Sacks F. M. 2008. Dietary monounsaturated fat activates metabolic pathways for triglyceride-rich lipoproteins that involve apolipoproteins E and C-III. Am. J. Clin. Nutr. 88: 272–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huff M. W., Fidge N. H., Nestel P. J., Billington T., Watson B. 1981. Metabolism of C-apolipoproteins: kinetics of C–II, C–III1 and C–III2, and VLDL-apolipoprotein B in normal and hyperlipoproteinemic subjects. J. Lipid Res. 22: 1235–1246. [PubMed] [Google Scholar]
- 28.Bukberg P. R., Le N. A., Ginsberg H. N., Gibson J. C., Rubinstein A., Brown W. V. 1985. Evidence for non-equilibrating pools of apolipoprotein C-III in plasma lipoproteins. J. Lipid Res. 26: 1047–1057. [PubMed] [Google Scholar]
- 29.Boyle K. E., Phillips M. C., Lund-Katz S. 1999. Kinetics and mechanism of exchange of apolipoprotein C-III molecules from very low density lipoprotein particles. Biochim. Biophys. Acta. 1430: 302–312. [DOI] [PubMed] [Google Scholar]
- 30.Malmendier C. L., Lontie J. F., Grutman G. A., Delcroix C. 1988. Metabolism of apolipoprotein C-III in normolipemic human subjects. Atherosclerosis. 69: 51–59. [DOI] [PubMed] [Google Scholar]
- 31.Gianturco S. H., Gotto A. M., Jr, Bradley W. A. 1985. Hypertriglyceridemia: lipoprotein receptors and atherosclerosis. Adv. Exp. Med. Biol. 183: 47–71. [DOI] [PubMed] [Google Scholar]
- 32.Mauger J. F., Couture P., Bergeron N., Lamarche B. 2006. Apolipoprotein C-III isoforms: kinetics and relative implication in lipid metabolism. J. Lipid Res. 47: 1212–1218. [DOI] [PubMed] [Google Scholar]
- 33.Holdsworth G., Stocks J., Dodson P., Galton D. J. 1982. An abnormal triglyceride-rich lipoprotein containing excess sialylated apolipoprotein C-III. J. Clin. Invest. 69: 932–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sundaram M., Zhong S., Bou Khalil M., Zhou H., Jiang Z. G., Zhao Y., Iqbal J., Hussain M. M., Figeys D., Wang Y., et al. 2010. Functional analysis of the missense APOC3 mutation Ala23Thr associated with human hypotriglyceridemia. J. Lipid Res. 51: 1524–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]