Abstract
The use of stable isotopically labeled substrates and analysis by mass spectrometry have provided substantial insight into rates of synthesis, disposition, and utilization of lipids in vivo. The information to be gained from such studies is of particular benefit to therapeutic research where the underlying causes of disease may be related to the production and utilization of lipids. When studying biology through the use of isotope tracers, care must be exercised in interpreting the data to ensure that any response observed can truly be interpreted as biological and not as an artifact of the experimental design or a dilutional effect on the isotope. We studied the effects of dosing route and tracer concentration on the mass isotopomer distribution profile as well as the action of selective inhibitors of microsomal triglyceride transfer protein (MTP) in mice and diacylglycerol acyltransferase 1 (DGAT1) in nonhuman primates, using a stable-isotopically labeled approach. Subjects were treated with inhibitor and subsequently given a dose of uniformly 13C-labeled oleic acid. Samples were analyzed using a rapid LC-MS technique, allowing the effects of the intervention on the assembly and disposition of triglycerides, cholesteryl esters, and phospholipids to be determined in a single 3 min run from just 10 μl of plasma.
Keywords: cholesteryl ester, diacylglycerol acyltransferase, fatty acid, liquid chromatography-mass spectrometry, mass isotopomer distribution analysis, microsomal triglyceride transfer protein, nonhuman primate, phosphatidylcholine, stable isotope tracer, triglycerides
Understanding the synthesis and disposition of triglycerides, cholesteryl esters, and phospholipids is an important component in developing effective therapies to treat cardiovascular and metabolic disease. The kinetics of synthesis and disposition can be ascertained by introducing a labeled substrate and measuring its incorporation into and decay from bulk lipid pools, which provides useful information on the flux of lipid through the relevant synthetic and metabolizing pathways. Such techniques have been used to study the kinetics of synthesis of VLDL triglycerides (1–3), cholesterol (4, 5), and phospholipids (6), as well as many other aspects of lipid disposition. When introducing a labeled substrate into a biological system, the pattern of labeling in downstream metabolites conveys information about the synthesis of the product. By experimentally measuring this labeling pattern and comparing the results to those predicted by combinatorial probability, it is possible to determine kinetic parameters. This technique has been termed mass isotopomer distribution analysis (MIDA); discussions on consideration for use and limits of applicability of the method have been presented previously (7, 8). A central tenet of MIDA is that differences in the observed concentration of a particular isotopomeric product can be due solely to the relative abundances of the labeled precursor and the endogenous form of the substrate, i.e., the labeling of the precursor pool. In effect, introduction of the label represents an intervention on the system, and this carries important ramifications for interpreting observed effects when a second intervention, e.g., dosing of a pharmacological agent, is carried out in tandem with dosing of the labeled substrate. Observed differences in the synthesis of a particular mass isotopomer could be due to isotope dilution, effects of the pharmacological agent, or both. Thus, when using a stable isotopically labeled tracer approach to evaluate the effects of drugs in vivo, it behooves the researcher to assess the labeling of the precursor pool (to evaluate comparability among subjects and between treated and control groups) in order to determine whether an observed effect can truly be attributed to action of the drug (9, 10).
When stable-isotopically labeled substrates are utilized to interrogate metabolite synthesis in biological systems, analysis of the samples is often accomplished using gas-chromatography mass-spectroscopy (GC-MS) analysis (11). This technique generally requires that complex molecules with high boiling points, such as triglycerides, phospholipids, and cholesteryl esters, must first be separated from one another using off-line methods such as thin layer chromatography and second be saponified and derivatized so that analysis can be carried out on an appropriate molecular component (e.g., labeled fatty acid or glycerol). In contrast to GC-MS-based approaches, LC-MS requires less sample preprocessing and enables the analysis of intact molecular lipids. The ability of LC-MS to separate different classes of lipids, such as triglycerides, phosphatidylcholines, and cholesteryl esters, in a single run is another significant advantage and allows the disposition of the tracer between different lipid pools to be easily explored. A recent publication by Qi et al. (12) describes the utility of an LC-MS-based approach to monitoring the effects of pharmacological inhibitors of DGAT1 on di- and triglyceride assembly from stable-isotopically labeled oleic acid in vitro and in vivo. Although the authors demonstrated the capability of the LC-MS method to measure multiple labeled forms of di- and triglyceride simultaneously, little detail on the mass isotopomer distribution profile was presented, and no analysis of precursor pool labeling was described.
In this report, we present data from a series of 5 experiments in which administration of uniformly [13C]-labeled oleic acid has been used to elucidate lipid assembly in vivo, using a rapid LC-MS method to measure the labeling pattern of triglycerides, phospholipids, and cholesteryl esters. Initial experiments explored the effects of the route of administration and dose of the isotope on the labeling pattern of various triglycerides. By administering high doses of the labeled oleate, we demonstrate that it is possible to steer lipid synthesis in vivo toward defined mass isotopomers and also show that a single isotopomer can be representative of the total pool, allowing bulk behavior to be inferred from the analysis of a single biomarker. Subsequent experiments using selective inhibitors of microsomal triglyceride transfer protein (MTP) in mice and DGAT1 in nonhuman primates highlight the utility of this approach to assess perturbations in lipid assembly resulting from pharmacological intervention. Comparability between treated and control groups in labeling of the precursor pool is demonstrated for each experiment, allowing the true pharmacological effects to be clearly interpreted. Similarity in the mass isotopomer distribution profile between two preclinical species, mouse and nonhuman primate, has been explored for oral dosing and highlights the utility of the stable isotope method for translational studies in pharmaceutical research.
MATERIALS AND METHODS
Animal studies
All animals were maintained in Association for Assessment and Accreditation of Laboratory Animal Care-accredited facilities. All experimental procedures were approved by the Institutional Animal Care and Use Committee and were in conformance with the National Research Council's Guide for the Care and Use of Laboratory Animals. [13C18] oleic acid was obtained from Sigma-Isotec (St. Louis, MO) as either the free acid or potassium salt form. Oleic acid was used as the isotope rather than other possible forms (e.g., triolein) so that the same tracer could be administered in experiments designed to investigate intestinal lipid assembly or hepatic synthesis of complex lipids from a nonesterified fatty acid (NEFA) precursor. All formulations of the isotope were prepared on the day of study or, at most, the day prior. To determine tracer incorporation into different lipid pools, blood was collected into tubes containing EDTA and processed to plasma by centrifugation (1,500 g). All samples were stored at −80°C prior to LC-MS analysis.
Experiment 1: oral administration of isotope: effect of different vehicles
Male C57BL/6 mice (Taconic Farms, Inc., Germantown, NY) ranging in body weight from 18–22 g were group housed and maintained on a 12 h light/12 h dark cycle in a temperature-controlled environment (22°C). Animals had free access to food and water and were fed a standard rodent chow (product no. 7012, 5% dietary fat; 3.75 kcal/g; Teklad, Madison, WI). Mice were fasted overnight (16 h) and then dosed orally at 10 ml/kg body weight with the isotope prepared in 20% vitamin E d-α-tocopheryl polyethylene glycol succinate (vitamin E TPGS), olive oil, or heavy cream. The total dose of isotope administered was 480 mg/kg body weight. [13C18] oleic acid (free form) was mixed directly with the vehicle under study; formulations of the tracer in aqueous vehicles (TPGS and heavy cream) appeared as emulsions, whereas formulation of the fatty acid in olive oil appeared to form a solution. The free acid was used to interrogate intestinal lipid assembly rather than a labeled triglyceride in order to be able to use the same form of the isotope for oral and intravenous dosing. Blood was obtained via tail nick at 0, 1, 2, 3, and 4 h after tracer for LC-MS analysis.
Experiment 2, intravenous administration of isotope: effect of different doses
Dosing solutions were prepared by dissolving an appropriate weight of the potassium salt of [13C18] oleic acid in Intralipid 20 (Sigma). In general, the fatty acid was found to go into solution with brief vortexing followed by 10 min of sonication. Male C57BL/6 mice were pseudofasted for 4 h (food was removed from cages during lights on) and subsequently dosed with 10, 50, or 150 mg/kg of the isotope. Doses were administered via tail vein injection using a volume of 10 ml/kg of body weight. Blood was collected via tail nick at 30 min following administration of the tracer.
Experiment 3, effect of a DGAT1 inhibitor on distribution of isotope given as an oral bolus
Studies were performed in female African green monkeys (Chlorocebus aethiops; weight range, 6–10 kg). On the morning of study, overnight-fasted animals were treated with vehicle (20% TPGS) or one of two doses of DGAT1 inhibitor (0.3 and 3.0 mg/kg) (13), with the solution mixed into a yogurt treat for conscious dosing 1 h prior to tracer administration. [13C18] oleic acid (free form) was administered in 20% TPGS to sedated animals via p.o. gavage at a dose of 50 mg/kg body weight. Blood was collected under light sedation (10 mg/kg ketamine) via femoral vein injection at the time of dosing (0 h) and at 1, 2, 4, 6, and 24 h.
Experiment 4, effect of an MTP inhibitor on distribution of isotope given as an IV Bolus
Dosing solutions of the isotope were prepared by dissolving the potassium salt of [13C18] oleic acid in Intralipid 20. Male C57BL/6 mice were studied, and food was removed from lights on (animals were pseudofasted for a total of 4 h prior to administration of the isotope). At 1 h prior to isotope administration, animals were given an oral dose of vehicle (0.25% methylcellulose in water) or 50 mg/kg of a selective inhibitor of MTP (14, 15). A 50 mg/kg dose of the [13C18] oleic acid tracer was subsequently administered via tail vein, using a dosing volume of 10 ml/kg body weight. Blood was collected via tail nick at 15, 30, 60, and 120 min after tracer.
Experiment 5, effect of an MTP inhibitor on distribution of isotope given as an oral bolus
Male C57BL/6 mice were fasted overnight (16 h) and then treated orally with vehicle (0.25% methylcellulose in water) or 50 mg/kg of the MTP inhibitor. One hour later, animals were given an oral dose of corn oil (10 ml/kg body weight) containing 15 mg/ml of [13C18] oleic acid. Dosing solutions were prepared by mixing the free form of the acid directly with corn oil; the total dose of isotope administered was 150 mg/kg. Blood was collected at 0, 0.5, 1, 2, and 4 h after tracer.
Ultra performance LC-MS/MS analysis
All analyses were carried out using 10 μl of plasma. Proteins were first precipitated by addition of 190 μl of methanol containing class-specific internal standards, followed by brief vortexing. Samples were then diluted with a further 600 μl of pentanol and vortexed again. Insoluble material was pelleted by centrifugation, and an aliquot of the supernatant was transferred to a 96 well plate for analysis. Triglycerides (TG), phosphatidylcholines (PC), and cholesteryl esters (CE) were separated using an HSS-T3, 2.1 × 50 mm, 1.7 μm column (Waters, Milford, MA) on an Acquity Ultra Performance LC system (Waters). The sample injection volume was 2 μl. Lipid classes were resolved using a binary solvent system and a linear gradient. The column was initially conditioned with 90% solvent A. Immediately following injection, the solvent composition was ramped to 95% solvent B over 2.5 min. At 2.6 min, the solvent composition was returned to 90% solvent A and held for 0.4 min before the next injection. The flow rate was held constant throughout the run at 400 μl /min. Solvent A was 10 mM ammonium formate in 40% H2O:60% acetonitrile, and solvent B was 10% acetonitrile: 90% isopropanol (16). All solvents used were of HPLC grade or better. Triglycerides and cholesteryl esters were analyzed as the ammoniated adducts [M+NH4]+ and phosphatidylcholines as [M+H]+ by using ESI+ ionization. All analytes were quantified using multiple reaction monitoring with a Xevo triple quadrupole mass spectrometer (Waters). For triglycerides in which at least one equivalent of the [13C]-labeled fatty acid tracer was incorporated, improved specificity was obtained when the fragment ion selected for quantitation preserved the 13C label (enhanced discrimination against isobaric chemical interferences).
Individual molecular lipids were quantified by internal, single-point calibration using isotopically labeled internal standards. Because variations in the ionization efficiency of molecular lipids with different acyl chain compositions are known (17–19), we believe that the use of labeled internal standards with acyl chain composition identical to the analyte is required for accurate quantitation. The internal standards used in the investigations reported here were as follows: for triglycerides, 100 nM 13C21-triolein (TG 54:3; catalog number 646253; Sigma-Isotec); 125 nM 2H5-rac-glycerol-1-palmitate-2,3-di(oleate) (TG 52:2; catalog number 730076; Sigma-Isotec) and 125 nM 2H5-rac-glycerol-1-palmitate-2-linoleate-3-oleate (TG 52:3; catalog number 730068; Sigma-Isotec); for phosphatidylcholines, 125nM 2H9-1-palmitoyl-2-oleoyl-rac-glycero-3-phosphocholine (PC 34:1; catalog number 730041; Sigma-Isotec); and for cholesteryl esters, 500 nM 2H6-cholesteryl-13C7-oleate (catalog number 729671; Sigma-Isotec). The concentrations stated refer to the final amount of each internal standard in the plasma samples after dilution with methanol and pentanol. Early studies on oral formulation development or intravenous dose ranging (identified as experiments 1 and 2 in Materials and Methods) made use of only the 13C21-triolein internal standard for triglycerides, a diheptadecanoyl internal standard for phosphatidylcholines, and a heptadecanoyl internal standard for cholesteryl esters. As a result, accurate quantitation could not be achieved for lipids with acyl chain compositions differing from these standards. For these investigations, the abundance of each molecular lipid is reported as corrected response (equal to the peak area for the analyte divided by the peak area for the class-specific internal standard), rather than absolute concentration. In cases where data are presented as corrected response, values for different isotopomers of a single triglyceride can be compared directly to one another and accurately represent differences in relative abundance in the sample (i.e., concentration). However, corrected responses for the isotopomers of different triglycerides should not be compared to each other (e.g., 52:2 to 54:3) as potential differences in ionization efficiency cannot be properly accounted for.
For tissue analyses, a 50 mg sample (wet weight) was homogenized in 1 ml of dichloromethane-methanol (2:1) containing 25 μM of the isotopically labeled, class-specific internal standards noted above. Homogenization was carried out in 2 ml polypropylene tubes containing 14 mm ceramic beads, using a Precellys 24 homogenizer (Bertin Technologies, Montigny-le-Bretonneux, France). Samples were homogenized at 5,500 rpm in 2 × 30 s bursts with a 15 s pause between cycles. Following homogenization, 200 μl of water was added to each sample, and the tubes were vigorously vortexed. The aqueous and organic phases were separated by centrifugation, and then an aliquot of the lower (lipid-containing) layer was transferred to a 96 well plate and diluted 50-fold with pentanol for analysis.
GC-MS analysis
Plasma (100 μl) was extracted by following a modification of the method of Folch et al. (20). Briefly, 50 μl of 0.9% saline was added to each sample, followed by 200 μl of methanol. Samples were vigorously vortexed to precipitate proteins. Four hundred microliters of chloroform containing the internal standards heptadecanoyl-TG, -PC, and -CE were then added, and the samples were vortexed again. Phases were separated by centrifugation, and the lower layer was withdrawn to a new vessel. The samples were then extracted with two additional aliquots of chloroform (400 μl each); the chloroform extracts from each round were combined and evaporated to dryness under N2. The dried extracts were reconstituted in 100 μl of chloroform for lipid separation by TLC. Silica TLC plates (20 × 20 cm; Whatman) were scored into 1.2 cm lanes, and samples were spotted at the origin alongside a lane containing a mixture of authentic lipid standards. Plates were developed in a solvent system composed of 80 parts hexane-20 parts diethyl ether-1 part acetic acid. Lipid bands were visualized by spraying with a 0.1% solution of dichlorofluorescein in ethanol and illuminated with ultraviolet light. The bands corresponding to triglycerides were scraped into glass tubes for further processing. Triglyceride-bound fatty acids were converted to the free methyl esters following the procedure of Morrison and Smith (21). Following derivatization, the methyl esters were extracted into hexane, and an aliquot was removed for GC-MS analysis. Samples were analyzed using an Agilent 6890 gas chromatography unit linked to an Agilent 5973 mass selective detector (Agilent, Palo alto, CA) using electron impact ionization at 70 eV. GC was performed using an Agilent HP-5MS capillary column, 30.0 m × 250 μm × 0.25 μm. Samples (4 μl) were injected at a 5:1 split. The inlet temperature was set at 270°C, and the helium gas carrier flow was set at 1 ml/min. The oven temperature was initially set at 150°C, raised at 20°C/min to 280°C and then ramped to 310°C at 40°C/min; this final temperature was held for 5 min. The mass selective detector was set for selected ion monitoring of m/z 296 and 297 for the methyl ester of endogenous 12C oleate and m/z 314 and 315 for the methyl ester of the 13C18 oleate tracer, using a 10 ms dwell time per ion.
Selective inhibitors of MTP and DGAT1
To explore the effects of pharmacological perturbation on the disposition of the tracer fatty acid, selective inhibitors of MTP and DGAT1 were synthesized. The specific inhibitors used in these investigations were selected from molecules disclosed in the scientific or patent literature (13–15).
Determination of precursor labeling
Labeling of the precursor (oleate) pool was calculated based on the ratio of the M2/M1 isotopomers of plasma triglycerides following exposure to the [13C18] oleic acid tracer. The precursor labeling (p) was calculated using the equation p = 2(M2/M1)/[1 + 2 (M2/M1)] (22).
Statistical analysis
Statistical analyses of data were performed by unpaired, two-tailed Student's t-test or ANOVA, using Prism software (GraphPad Software, La Jolla, CA). Data are presented as means ± standard errors of the mean (SEM) and P values <0.05 were considered significant. GraphPad software was also used to determine areas under the plasma timecourse curve (AUC).
RESULTS
LC-MS method development and identification of lipid markers
The predominant TG and PC molecular species in mouse plasma were initially identified by neutral loss scanning for oleate (m/z 299.2) or precursor scanning for the choline head group (m/z 184.3), respectively. Figure 1 shows an example of the chromatographic separation obtained as well as the extracted mass spectra. The most intense species were identified initially by m/z. Authentic standards were injected and matched to peaks in biological samples on the basis of retention time; further characterization by accurate mass quadrupole-time-of-flight (Q-TOF) MS/MS was carried out to confirm the fatty acid compositions reported in Table 1 (Synapt G2; Waters; data not shown) (16). Selected triglycerides, phosphatidylcholine 34:1, and cholesteryl oleate were all measured in subsequent experiments. The appropriate stable-isotopically labeled internal standards for these lipid markers were custom synthesized by Sigma-Isotec as described above in Materials and Methods.
Fig. 1.
(a) LC separation of selected PC and TG lipid markers analyzed using multiple reaction monitoring is shown (retention times for diglycerides and cholesteryl esters are also indicated). Phosphatidylcholines were initially identified by scanning for precursors of m/z 184.3 (b); oleate containing triglycerides were identified by scanning for a neutral loss of m/z 299.2 (c). Fatty acid compositions for the major markers are reported in Table 1 and were confirmed by accurate mass MS/MS with a Waters Q-TOF (Synapt G2).
TABLE 1.
Abundant molecular species of PC and oleate-containing TG in mouse plasma identified by precursor and neutral loss scanning
| Lipid marker | Nominal m/za | Fatty acid compositionb |
|---|---|---|
| PC 34:1 | 760.6 | 16:0/18:1 |
| PC 34:2 | 758.6 | 16:0/18:2 |
| PC 36:1 | 788.6 | 18:0/18:1 |
| PC 36:2 | 786.6 | 18:0/18:2 |
| TG 52:3 | 874.8 | 16:0/18:1/18:2 |
| TG 52:2 | 876.8 | 16:0/18:1/18:1 |
| TG 54:3 | 902.8 | 18:1/18:1/18:1 |
| TG 54:4 | 900.8 | 18:1/18:1/18:2 |
Triglycerides were detected as the NH4+ adducts.
Order of notation does not connote regiochemistry.
Experiment 1: oral administration of isotope: effect of different vehicles
Figure 2 shows the relationship between the precursor and product labeling. Panel (a) demonstrates the different molecular species of triolein that can arise from the combination of endogenous and [13C18]-labeled oleic acid. Panel (b) contains the theoretical mass isotopomer distribution profile for triolein; increased labeling of the precursor pool promotes the formation of multiply labeled triglyceride. Here, the designation M0 refers to a molecule of triglyceride that contains only the natural 12C form of the fatty acid. M1, M2, and M3 refer to molecules where 1, 2, or 3 of the side chains are composed of the uniformly [13C18]-labeled tracer fatty acid. The nature of the precursor pool from which lipids are assembled is dependent upon the synthesizing organ. For example, triglycerides ingested from dietary sources are broken down to free fatty acids and monoglycerides through the action of lipases. These substrates are then taken up by the enterocyte and reassembled into triglycerides, cholesteryl esters, and phospholipids for secretion to the systemic circulation via chylomicron particles in the lymph (23). As such, the fatty acid composition of the diet makes a substantial contribution to the precursor pool, although fatty acids may also be derived from lipids stored intracellularly in the enterocyte or taken up from the systemic circulation (24, 25). The labeling of the precursor pool and mass isotopomer distribution profile for an orally ingested tracer will thus be determined in part by the size and natural fatty acid composition of the meal ingested. Table 2 reports data for the isotopomer profile for two triglycerides, 54:3 and 54:4, as well as the labeling of their respective precursor pools following oral administration of [13C18] oleic acid in various formulations to mice that had been fasted for 16 h prior to testing. When the tracer fatty acid was mixed in a vehicle containing endogenous 12C lipid (olive oil or heavy cream), the labeled substrate was largely incorporated as a single equivalent into newly synthesized triglyceride. Conversely, when the tracer was administered in aqueous TPGS, the predominant triglyceride detected in plasma was the fully labeled (M3) triolein; the response for the M3 isotopomer exceeded the response for the endogenous 12C form of TG 54:3 under these conditions. In this case, the precursor pool effectively consists of the [13C18] oleic acid tracer and whatever lipid can be derived from intracellular stores or the systemic circulation.
Fig. 2.
(a) The different molecular species of triolein than can arise from the combination of endogenous and [13C18]-labeled oleic acid are shown. (b) The theoretical mass isotopomer distribution profile for triolein; increased labeling of the precursor pool promotes the formation of multiply labeled triglyceride.
TABLE 2.
Precursor pool labeling and relative amounts of isotopomers formed for TG 54:3 and 54:4
| Oral Formulation | 1h | 2h | 3h | 4h | |
|---|---|---|---|---|---|
| Olive oil | |||||
| TG 54:3 | p (%) | 41 ± 2 | 39 ± 2 | 34 ± 1 | 38 ± 3 |
| M0 | 3.10 ± 0.78 | 2.88 ± 0.48 | 3.62 ± 0.94 | 2.31 ± 0.66 | |
| M1 | 0.38 ± 0.10 | 0.34 ± 0.05 | 0.40 ± 0.10 | 0.23 ± 0.07 | |
| M2 | 0.13 ± 0.03 | 0.11 ± 0.01 | 0.11 ± 0.03 | 0.07 ± 0.02 | |
| M3 | 0.02 ± 0.01 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | |
| TG 54:4 | p (%) | 29 ± 2 | 24 ± 1 | 21 ± 1 | 21 ± 1 |
| M0 | 3.29 ± 0.63 | 2.87 ± 0.39 | 3.10 ± 0.50 | 2.09 ± 0.42 | |
| M1 | 0.17 ± 0.04 | 0.15 ± 0.02 | 0.19 ± 0.04 | 0.11 ± 0.03 | |
| M2 | 0.03 ± 0.01 | 0.02 ± 0.00 | 0.02 ± 0.01 | 0.01 ± 0.00 | |
| Heavy cream | |||||
| TG 54:3 | p (%) | 36 ± 2 | 32 ± 2 | 38 ± 5 | 37 ± 3 |
| M0 | 3.11 ± 0.27 | 2.52 ± 0.32 | 1.37 ± 0.19 | 1.57 ± 0.24 | |
| M1 | 0.70 ± 0.08 | 0.55 ± 0.13 | 0.17 ± 0.03 | 0.16 ± 0.04 | |
| M2 | 0.19 ± 0.02 | 0.13 ± 0.04 | 0.05 ± 0.01 | 0.04 ± 0.01 | |
| M3 | 0.19 ± 0.04 | 0.07 ± 0.01 | 0.02 ± 0.01 | 0.01 ± 0.01 | |
| TG 54:4 | p (%) | 42 ± 2 | 38 ± 3 | 30 ± 1 | 29 ± 3 |
| M0 | 4.40 ± 0.38 | 3.85 ± 0.42 | 2.50 ± 0.32 | 2.87 ± 0.39 | |
| M1 | 0.40 ± 0.09 | 0.41 ± 0.13 | 0.17 ± 0.04 | 0.16 ± 0.05 | |
| M2 | 0.15 ± 0.04 | 0.14 ± 0.06 | 0.04 ± 0.01 | 0.04 ± 0.02 | |
| 0.25% TPGS | |||||
| TG 54:3 | p (%) | 94 ± 1 | 85 ± 2 | 79 ± 2 | 74 ± 3 |
| M0 | 0.40 ± 0.02 | 0.31 ± 0.02 | 0.33 ± 0.02 | 0.30 ± 0.04 | |
| M1 | 0.10 ± 0.01 | 0.11 ± 0.01 | 0.08 ± 0.01 | 0.04 ± 0.01 | |
| M2 | 0.85 ± 0.07 | 0.39 ± 0.10 | 0.17 ± 0.04 | 0.06 ± 0.01 | |
| M3 | 21.06 ± 2.57 | 2.02 ± 0.62 | 0.44 ± 0.12 | 0.10 ± 0.03 | |
| TG 54:4 | p (%) | 95 ± 1 | 83 ± 3 | 75 ± 3 | 62 ± 4 |
| M0 | 2.31 ± 0.12 | 1.62 ± 0.09 | 1.76 ± 0.12 | 1.60 ± 0.21 | |
| M1 | 0.29 ± 0.02 | 0.46 ± 0.02 | 0.34 ± 0.04 | 0.17 ± 0.03 | |
| M2 | 2.49 ± 0.15 | 1.38 ± 0.36 | 0.59 ± 0.14 | 0.15 ± 0.03 |
Summary of precursor pool labeling (p, %) and relative amounts of isotopomers (M0, M1, M2, M3) formed for TG 54:3 and 54:4 at different time points following po administration of 13C18-oleic acid (480 mg/kg) in formulations containing different amounts of lipid. Data are presented as means ± SEM; values listed for the isotopomers are in units of corrected response (see Materials and Methods for details).
Experiment 2: intravenous administration of isotope: effect of different vehicles
Hepatic lipid synthesis draws from precursor pools that are different from those drawn upon by intestinal lipid synthesis. Fatty acid substrates can be derived from chylomicron remnants, synthesized de novo in the hepatocyte, or taken up from the systemic circulation after lipolysis of adipose triglyceride. Depending on nutritional state, systemically derived NEFA can be the dominant contributor (1). Hepatic assembly of lipids can thus be probed by administering a fatty acid tracer intravenously to form part of the plasma NEFA pool. Table 3 reports data for the precursor labeling and isotopomer profile for two triglycerides, 52:2 and 54:3, measured 30 min after iv administration of increasing doses of [13C18] oleic acid (formulated in Intralipid) to mice that had been fasted for 4 h prior to testing. Labeling of the oleate precursor pool(s) increases with increasing dose of the isotope for both triglycerides, although the magnitude of precursor labeling is different. The isotopomer distribution shifts toward the multiply labeled forms of each triglyceride as the dose is increased, as predicted by MIDA theory.
TABLE 3.
Precursor pool labeling (p) and relative amounts of isotopomers formed for TG 52:2 and 54:3
| Mean ± SEM amounts of isotopomer formed | |||||
|---|---|---|---|---|---|
| Dose | p (%) | M0 | M1 | M2 | M3 |
| 10 mg/kg | |||||
| TG 52:2 | 22 ± 2 | 63.04 ± 7.03 | 0.56 ± 0.11 | 0.08 ± 0.02 | — |
| TG 54:3 | 38 ± 1 | 74.95 ± 10.97 | 0.23 ± 0.05 | 0.07 ± 0.01 | 0.02 ± 0.01 |
| 50 mg/kg | |||||
| TG 52:2 | 53 ± 2 | 66.44 ± 5.42 | 2.06 ± 0.37 | 1.11 ± 0.15 | — |
| TG 54:3 | 80 ± 1 | 91.50 ± 17.48 | 1.02 ± 0.08 | 2.14 ± 0.22 | 2.72 ± 0.41 |
| 150 mg/kg | |||||
| TG 52:2 | 73 ± 1 | 58.11 ± 6.94 | 2.10 ± 0.38 | 2.80 ± 0.53 | — |
| TG 54:3 | 87 ± 1 | 84.41 ± 19.02 | 1.25 ± 0.21 | 4.11 ± 0.83 | 11.64 ± 2.57 |
Summary of precursor pool labeling (p, %) and relative amounts of isotopomers (M0, M1, M2, M3) formed for TG 52:2 and 54:3 measured 30 min after iv administration of the [13C18] oleic acid label to 4 h-fasted mice. Data are presented as means ± SEM; values listed for the isotopomers are in units of corrected response (see Materials and Methods for details).
Experiment 3: effect of a DGAT1 inhibitor on distribution of isotope given as an oral bolus
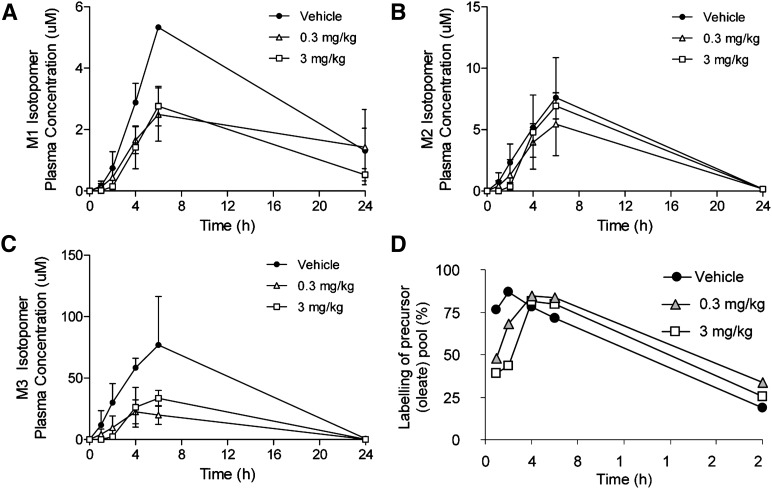
To investigate the effects of pharmacological perturbation on lipid synthesis in the intestine, African green monkeys were treated with ascending doses of a selective DGAT1 inhibitor. At 1 h after compound administration, animals were given an oral dose (50 mg/kg body weight) of [13C18] oleic acid formulated in 20% TPGS, and blood was collected at serial time points to measure tracer incorporation into circulating triglycerides. Figure 3 shows the effects of the DGAT1 inhibitor on incorporation of the labeled oleic acid into the different mass isotopomers of triolein (TG 54:3) as well as the precursor pool labeling over the time course for each dosed group, as determined by LC-MS. As observed in the formulation experiments conducted in mice, the M3 isotopomer was found to predominate over lesser labeled forms. This led us to investigate whether the effect of the DGAT1 compound on assembly of this single isotopomer could be shown to be representative of oleate incorporation into the total pool of circulating triglyceride. To achieve this, the triglyceride fractions from aliquots of the same plasma samples were isolated by TLC and saponified. Total triglyceride-bound [13C18] oleic acid was determined by GC-MS. Figure 4 shows that the AUC values obtained by the two different analytical methodologies are quite comparable, demonstrating under these conditions that incorporation of isotope into a single molecular species of triglyceride can be representative of the behavior of the bulk pool.
Fig. 3.
Effect of a DGAT1 inhibitor on the distribution of isotope given as an oral bolus is shown. African green monkeys were treated with a single dose of vehicle, 0.3, or 3 mg/kg of a DGAT1 inhibitor. At 1 h later, a 50 mg/kg oral dose of [13C18]-oleic acid was administered in 0.25% TPGS (aqueous). The various isotopomers of triolein were measured at subsequent time points in the plasma by LC-MS. (a) M1 isotopomer; (b) M2 isotopomer; (c) M3 isotopomer; (d) percent labeling of the oleate precursor pool with the [13C18] tracer.
Fig. 4.
Comparison of LC-MS and GC-MS for measuring incorporation of isotope given as an oral bolus into plasma triglycerides of African green monkeys treated with a DGAT1 inhibitor is shown. (a) AUC for the M3 isotopomer of triolein determined by LC-MS; (b) AUC for total triglyceride containing the 13C18 oleic acid tracer determined by GC-MS. mpk, mg/kg.
Experiment 4: effect of an MTP inhibitor on distribution of isotope given as an IV bolus
To investigate the effects of pharmacological perturbation on lipid synthesis in the liver, C57Bl/6 mice were pseudofasted and treated with a single dose (50 mg/kg body weight) of an MTP inhibitor. At 1 h after compound administration, mice were injected with 50 mg/kg body weight of the [13C18] oleic acid via tail vein, and blood was collected at serial time points afterward. Previous data in mice administered a 50 mg/kg intravenous dose of the isotope showed substantial incorporation into the M3 isotopomer of triolein (Table 3). Figure 5 shows the effects of the MTP inhibitor on appearance of the M3 isotopomer of triolein in plasma, as well as on the M1 isotopomer of PC 34:1. The enrichment of the oleate precursor pool measured over the time course is reported in Table 4. The data collected 30 min after administration of the isotope agree very well with data from experiment 2. More importantly, the enrichment of the oleate precursor pool was found to be highly comparable between that of the vehicle and the compound-treated groups, giving confidence that the inhibition of isotope incorporation into triglyceride shown in Fig. 5 can be unambiguously attributed to the action of the MTP inhibitor.
Fig. 5.
Effects of a single dose of an MTP inhibitor on the disposition of [13C18] oleic acid administered intravenously to mice are shown. (a) The role of MTP in assembling lipids synthesized in the liver into lipoprotein particles for secretion into the systemic circulation is shown. (b and c) Time course of incorporation for [13C18] oleic acid into selected triglyceride and phosphatidylcholine lipid markers, respectively, is shown. (d) Effects of the MTP inhibitor on the AUC values for the triolein containing exclusively 12C- versus 13C-oleate are shown. The inhibitory effect of the compound is clearly seen for TG labeled with the tracer fatty acid, whereas no statistically significant differences between treated versus untreated animals could be discerned based on the 12C form of the triglyceride. **, P < 0.01.
TABLE 4.
Precursor pool labeling determined from the M2/M1 ratio for TG 54:3
| Mean ± SEM % of oleate precursor pool labeling (p) | |||||
|---|---|---|---|---|---|
| Time (min) | 5 | 15 | 30 | 60 | 120 |
| Vehicle | 49 ± 7 | 89 ± 1 | 88 ± 1 | 85 ± 2 | 80 ± 2 |
| MTPi | 49 ± 5 | 89 ± 1 | 82 ± 3 | 81 ± 3 | 56 ± 6 |
The [13C18] oleic acid label (50 mg/kg) was administered to mice in an Intralipids formulation 1 h after an oral dose of the MTP inhibitor to investigate the effects on distribution of isotope given as an intravenous bolus.
Experiment 5: effect of an MTP inhibitor on distribution of isotope given as an oral bolus
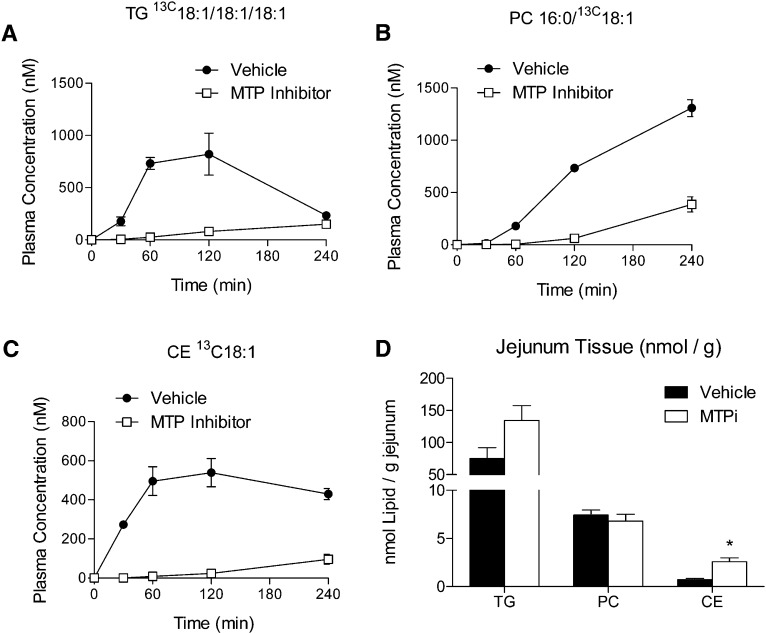
Figure 6 shows the appearance of the [13C18] oleic acid tracer in various TG, PC, and CE lipids following oral administration (150 mg/kg in corn oil) to overnight-fasted mice that had been treated 1 h prior with vehicle or MTP inhibitor. Data are shown for lipids appearing in the systemic circulation (plasma) as well as for those retained in the intestinal (jejunal) tissue. Table 5 reports data for the enrichment of the oleate precursor pool measured over the plasma time course and, as in the previous experiment, shows that equivalent labeling was achieved in both the vehicle- and the compound-treated animals. Also, as in the previous experiment, treatment with the MTP inhibitor profoundly reduced the amount of labeled lipid appearing in plasma. The data in Fig. 6 also show that these same labeled lipids accumulate in the intestinal tissue, a finding consistent with the known mechanism of MTP, which does not alter lipid synthesis but rather modulates secretion of lipid via lipoprotein particles.
Fig. 6.
Effects of a single dose of an MTP inhibitor on the disposition of [13C18] oleic acid administered orally to mice are shown. A 150 mg/kg dose of the isotope was mixed with corn oil and given to mice 1 h after treatment with the MTP inhibitor. The effects of the inhibitor on the disposition of the tracer in various plasma lipid pools is shown for (a) triglyceride, M1 isotopomer of triolein; (b) PC 34:1; and (c) CE 18:1. The tissue concentration of the same molecular lipids in a sample from the jejunum is shown in panel d. *, P < 0.05.
TABLE 5.
Precursor pool labeling as determined from the M2/M1 ratio for TG 54:3
| Mean ± SEM % of oleate precursor pool labeling (p) | ||||
|---|---|---|---|---|
| Time (min) | 30 | 60 | 120 | 240 |
| Vehicle | 32 ± 3 | 18 ± 1 | 12 ± 2 | 9 ± 1 |
| MTPi | 30 ± 8 | 15 ± 4 | 10 ± 2 | 8 ± 2 |
The [13C18] oleic acid label was administered (150 mg/kg) in corn oil to investigate the effects of an MTP inhibitor on distribution of isotope given as an oral bolus.
DISCUSSION
Experiments 1 and 2 outlined in Materials and Methods were designed to explore the effects of route of administration and relative doses of the stable [13C18]-labeled oleic acid substrate on the isotopomeric profile of complex lipids isolated from the plasma of mice. The data generated from these experiments, presented in Tables 2 and 3, show experimental evidence for the profiles predicted by MIDA theory (7). As expected, in the case of oral dosing of the tracer in the presence of larger amounts of dietary lipid (olive oil and heavy cream), the distribution profile favors incorporation of a single equivalent of tracer (i.e., the unlabeled dietary lipid dilutes the tracer labeling minimizing the generation of doubly and triply labeled triglycerides [ ). Conversely, administration of the tracer fatty acid in aqueous TPGS, a vehicle containing no lipid, results in assembly of fully labeled triglyceride. Most notably, the predominant labeled triglyceride is the M3 isotopomer of triolein (TG 54:3), and the response for this molecular species far surpasses the response for circulating levels of 12C-labeled TG 54:3. The data generated via intravenous administration of the tracer in Intralipid (a vehicle containing 20% endogenous triglyceride) shows complementary data: as the dose of the tracer fatty acid is increased, the distribution profile shifts toward greater incorporation of the label, and formation of the M2 or M3 isotopomer is favored. These general trends are consistent for the 3 different triglycerides investigated, TGs 52:2, 54:3, and 54:4, but it is interesting to note that these different species also show different levels of precursor pool enrichment. It has been documented that intestinal assembly of triglyceride can draw from multiple pools of fatty acid (e.g., those derived from the diet and those taken up from the systemic circulation (26, 27). In agreement with this, our data show that the synthesis of discrete molecular triglycerides can also draw from different pools of oleate during assembly. Use of the MIDA approach enabled by LC-MS analysis of the intact triglycerides provides a convenient means to assess precursor enrichment regardless of which or how many different pools of oleate are accessed during assembly. The result is a snapshot of precursor labeling taken at the precise moment of synthesis without interference from bound oleic acid that was not available for assembly of new lipid.
These findings, which are in strong agreement with the MIDA theory, have practical implications for experimental design when investigating lipid biology and, particularly, the effects of pharmacological intervention. The utility of the LC-MS approach to analyze intact molecular lipids allows the relative ratios of isotopomers to be empirically determined. From this information, quantitative information on the labeling of the precursor pool can be derived through simple calculations (22). To confidently determine that differences in the assembly/synthesis of newly made lipid are due to a pharmacological perturbation, it is important to document whether the labeling of the precursor pool is equivalent between treated and control subjects. Provided this requirement is met, it can be safely concluded that differences in the appearance of labeled lipids are truly due to the effects of the treatment and not to differences in isotope (precursor) dilution. The data contained in Figs. 3 and 4 illustrate the utility of LC-MS in evaluating the impact of pharmacological intervention while providing a means for assessing labeling of the precursor pool. These figures also illustrate the additional information that can be derived from evaluating the mass isotopomer distribution profile and how this can help to further understanding of the underlying biology.
Consider the triglyceride excursion curves shown in Fig. 3 for African green monkeys treated with a DGAT1 inhibitor and orally exposed to the tracer fatty acid dosed in TPGS. The classical model of lipid assembly in the gut involves lipolysis of intact triglyceride to 2-monoglyceride and free fatty acids via the action of pancreatic lipase, which are then taken up by the enterocyte. Once inside the cell, the substrates are reassembled into intact lipid by the action of enzymes such as MGAT and DGAT (in the case of triglycerides), ACAT (cholesteryl esters), and AGPAT (phospholipids) (28). This is commonly called the MGAT pathway, and in this paradigm, the expectation is that the majority of triglyceride would preserve the fatty acid in the sn2 position of the monoglyceride taken up by the enterocyte (29). It then follows that when introducing a labeled fatty acid substrate, the greatest degree of incorporation one would typically expect would be 2 equivalents of the tracer. The data in Fig. 3, however, illustrate a different case based on the design of the experiment. In this case, the free fatty acid tracer was administered orally in aqueous TPGS, i.e., in the absence of endogenous triglyceride that could be cleaved via lipase action to generate 2-monoglyceride. The free fatty acid tracer, able to be taken up by the enterocyte, is then resynthesized into triglycerides. The other substrates required for TG synthesis would either be lipid stored intracellularly in the enterocyte (that could provide a source of mono- or diglyceride) or glycerol-3-phosphate (G3P) that could be synthesized de novo. In the latter case, classical biochemistry would dictate that 1 equivalent of the oleate tracer could be conjugated to G3P via the action of GPAT to form lysophosphatidic acid, which can then be converted via phosphatidic acid to di- and triglyceride containing additional equivalents of the tracer (30). This route, referred to as the GPAT pathway, could be seen to result in the formation of the M3 isotopomer of triolein, expected to be a rare if not completely improbable occurrence. Indeed, triglyceride assembly via the GPAT pathway in the enterocyte is largely considered to be a minor contributor to overall synthesis under normal conditions (31). The data in Fig. 3 show evidence that assembly of both the singly labeled (M1) and fully labeled (M3) isotopomers of triolein are inhibitable by DGAT1 selective compounds. The data shown in Fig. 3d demonstrate that the precursor pool labeling was equivalent between the control- and DGAT1 compound-treated groups over the course of the experiment, providing confidence that the decreased incorporation of the tracer into newly synthesized TG is truly due to inhibition of the enzyme. The finding that the M3 isotopomer is the dominant version of triolein formed is comparable to the data presented in Table 2 for the mouse study, illustrating an important concept for pharmaceutical development. The fact that similar isotopomer distribution profiles were observed for both mice and nonhuman primates for labeled oleate dosed orally in TPGS illustrates that methods developed in one preclinical model have the potential to be translatable to higher species. This is a substantial benefit of the stable-isotopically labeled tracer approach but does rely on a complete analysis of the isotopomer profile to understand similarities and potential differences between species.
The data shown in Fig. 3 illustrate the effects of DGAT1 inhibition on the assembly of a single triglyceride, TG 54:3 (triolein). The question as to whether the behavior of this single molecular species is representative of the behavior of the bulk pool of total triglyceride must be considered. To address this question in the context of this particular experiment, aliquots of the same plasma samples analyzed by LC-MS and reported in Fig. 5 were analyzed by GC-MS. Plasma TG was isolated by TLC and saponified, and the liberated fatty acids were derivatized to methyl esters for analysis by GC-MS as described above. The ratio of [13C18]-labeled oleate to endogenous 12C oleate was determined over the time course, and AUC values were calculated. Figure 4 shows a comparison of the AUC values for [13C18]-oleate incorporation into the M3 isotopomer of triolein as determined by LC-MS and the AUC values for isotope incorporation into total TG as determined by GC-MS. The data are quite comparable, suggesting that triolein can serve as a single biomarker for bulk triglyceride assembly. It must be noted that this conclusion can only be drawn for the specific experimental design employed, i.e., the administration of high doses of oleic acid in the absence of competing, endogenous lipid. This approach allows us to steer TG synthesis toward a single molecular marker (M3 triolein) which can then be taken to be representative of bulk behavior.
Figures 5 and 6 highlight the utility of an LC-MS approach to measuring effects on disposition of isotope administered orally and intravenously as a consequence of pharmacological intervention, as well as trafficking of the fatty acid tracer between different lipid pools. Figure 5a shows the role of MTP in assembling lipid in the liver into VLDL for secretion to the systemic circulation. Figures 5b and c show the dramatic decrease in secretion of newly synthesized lipid appearing in the plasma following iv administration of the tracer when MTP is inhibited. Data for tracer incorporation into TG and PC markers are both obtained from the same sample in a single injection, allowing disposition of the tracer between these pools to be examined. It must be noted that the appearance of labeled lipid in the plasma will not be solely determined by hepatic lipogenesis as the iv tracer will be exposed to other tissues. However, the liver is known to be a major site for removal of plasma NEFA (32), a significant portion of which is resynthesized into triglyceride, particularly under conditions of fasting (1, 33). Figure 5d illustrates a universal advantage of employing stable-isotopically labeled tracers to measure lipid synthesis. The secretion of newly synthesized triglyceride (i.e., those containing at least 1 equivalent of the tracer fatty acid) in mice treated with the MTP inhibitor is markedly reduced over the time course of the experiment, whereas changes in the steady-state levels of endogenous triglyceride were not found to be statistically significant.
Figure 6 shows an illustration of these same concepts applied to oral administration of the tracer fatty acid. In this case, the [13C18]-labeled oleate was mixed with corn oil, and trafficking of the fatty acid between secreted (plasma) and storage (intestinal tissue) TG, PC, and CE lipid pools was measured. The effects of the MTP inhibitor on secretion of these newly formed lipids is similar to the data shown in Fig. 5, with substantial decreases observed compared with that of control. The time course of appearance of labeled PC in plasma is markedly different from that of TG or CE, with both of the neutral lipids appearing much earlier in the profile. This suggests that the label may initially be incorporated into TG and CE in the enterocyte for secretion via chylomicrons and that accrual of the isotope by PC may be the result of synthesis/exchange mechanisms taking place in the periphery. These data support the conclusions of others that the enterocyte may draw from a pool of preformed phospholipid for chylomicron secretion (34). Analysis of the lipids retained in the intestinal tissue yields additional insight into disposition of the tracer. Increases in tissue levels of triglyceride and cholesteryl ester (the main neutral lipid components supplied via MTP to chylomicron particles for secretion [28]) are observed. The appearance of increased triglyceride in the intestinal tissue of mice following treatment with this MTP inhibitor has previously been reported (14). Here, we show that tissue cholesteryl ester is also increased, providing additional information on the ultimate fate of the fatty acid tracer flowing through this pathway when perturbed by the MTP inhibitor. In both experiments, determination of the enrichment of the precursor pool via the ratio of the M2:M1 isotopomers of TG 54:3 demonstrated comparability between the vehicle- and compound-treated animals (Tables 4 and 5). This confirms that the data presented in Figs. 5 and 6 are truly representative of the effects of the inhibitor and ensure that the pharmacological action can be cleanly interpreted.
CONCLUSIONS
The use of LC-MS to follow the disposition of stable-isotopically labeled oleic acid in vivo provides all the tools required to interrogate biology and confidently assess pharmacological effects. Analysis of the mass isotopomer distribution profile at the same time as the efficacy of the drug is being assessed provides an empirical means to document labeling of the precursor pool, an important consideration when using these techniques for therapeutic research. By using an appropriate experimental design, we have also shown that it is possible to infer bulk behavior from the analysis of a single biomarker. This allows analysts to develop targeted methods requiring small sample volumes and short run times, enabling rapid assessment of pharmacological effects and facilitating quick decision making. Although these are all significant advantages, it is important to draw attention to the limitations of the experiment. For the investigations presented here, we selected oleate as the tracer fatty acid based on findings that it serves as a reliable probe for lipid synthesis (35) and on our own evaluations of the suitability of this substrate for the systems under study. To date, we have conducted no independent studies to compare data obtained using different substrates such as palmitate, stearate, or linoleate under these experimental conditions, each of which could be envisioned to provide different (perhaps complementary) information. As an illustration, it has been reported that the choice of fatty acid substrate is a large determinant of the data that are ultimately obtained when investigating the synthesis and disposition of lipids (35, 36), i.e., not all fatty acid tracers are equivalent. A further consideration here is that we cannot derive data for the synthesis of lipids that do not draw from the tracer-containing (i.e., oleate) pool. An additional limitation placed on experimental design using this approach is the requirement to administer a sufficient amount of the isotope to generate measurable amounts of multiply labeled triglyceride. In the experiments reported here, the lowest level of precursor enrichment was 22% (for TG 52:2) when the isotope was administered intravenously in Intralipids at a dose of 10 mg/kg, although we have been able to measure oleate enrichments as low as 10% for triolein in other experiments (data not shown). Our general experience has been that a 10 mg/kg dose of the [13C18] oleic acid tracer administered to mice p.o. in nonlipid containing vehicle or i.v. in Intralipid has been sufficient to generate M2 isotopomers for each of the triglycerides studied in this report and to result in labeling of PC 34:1 and CE 18:1. The need to administer the isotope at doses high enough to form multiply labeled species should be weighed against the goals of the experiment to ensure that the amount of isotope given, or the means of delivery, does not perturb the system under study. Provided that these limitations are properly understood, the use of stably-labeled tracers for exploring lipid synthesis and assembly can provide a rapid and reliable evaluation of pharmacological effects in vivo.
Footnotes
Abbreviations:
- AUC
- areas under the plasma timecourse curve
- CE
- cholesteryl ester
- MIDA
- mass isotopomer distribution analysis
- MTP
- microsomal triglyceride transfer protein
- NEFA
- nonesterified fatty acid
- PC
- phosphatidylcholine
- Q-TOF
- quadrupole-time-of-flight
- TG
- triglyceride
- TPGS
- D-α-tocopheryl polyethylene glycol succinate
REFERENCES
- 1.Barrows B., Parks E. 2006. Contributions of different fatty acid sources to very low-density lipoprotein-triacylglycerol in the fasted and fed states. J. Clin. Endocrinol. Metab. 91: 1446–1452. [DOI] [PubMed] [Google Scholar]
- 2.Lemieux S., Patterson B., Carpentier A., Lewis G., Steiner G. 1999. A stable isotope method using a [2H5] glycerol bolus to measure very low density lipoprotein triglyceride kinetics in humans. J. Lipid Res. 40: 2111–2117. [PubMed] [Google Scholar]
- 3.Magkos F., Patterson B., Mittendorfer B. 2007. Reproducibility of stable isotope-labeled tracer measures of VLDL-triglyceride and VLDL-apolipoprotein B-100 kinetics. J. Lipid Res. 48: 1204–1211. [DOI] [PubMed] [Google Scholar]
- 4.Di Buono M., Jones P., Beaumier L., Wykes L. 2000. Comparison of deuterium incorporation and mass isotopomer distribution analysis for measurement of human cholesterol biosynthesis. J. Lipid Res. 41: 1516–1523. [PubMed] [Google Scholar]
- 5.Jones P. J., Leitch C. A., Li Z. C., Connor W. E. 1993. Human cholesterol synthesis measurement using deuterated water. Theoretical and procedural considerations. Arterioscler. Thromb. 13: 247–253. [DOI] [PubMed] [Google Scholar]
- 6.DeLong C. J., Shen Y. J., Thomas M. J., Cui Z. 1999. Molecular distinction of phosphatidylcholine synthesis between the CDP-choline pathway and phosphatidylethanolamine methylation pathway. J. Biol. Chem. 274: 29683–29688. [DOI] [PubMed] [Google Scholar]
- 7.Hellerstein M. K., Neese R. A. 1999. Mass isotopomer distribution analysis at eight years: theoretical, analytic, and experimental considerations. Am. J. Physiol. 276: E1146–E1170. [DOI] [PubMed] [Google Scholar]
- 8.Previs S. F., Cline G. W., Shulman G. I. 1999. A critical evaluation of mass isotopomer distribution analysis of gluconeogenesis in vivo. Am. J. Physiol. 277: E154–E160.. [DOI] [PubMed] [Google Scholar]
- 9.Parks E. J., Hellerstein M. K. 2006. Recent advances in liver triacylglycerol and fatty acid metabolism using stable isotope labeling techniques. J. Lipid Res. 47: 1651–1660. [DOI] [PubMed] [Google Scholar]
- 10.Murphy E. J. 2006. Stable isotope methods for the in vivo measurement of lipogenesis and triglyceride metabolism. J. Anim. Sci. 84: E94–E104. [DOI] [PubMed] [Google Scholar]
- 11.Wittmann C. 2007. Fluxome analysis using GC-MS. Microb. Cell Fact. 6: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qi J., Lang W., Giardino E., Caldwell G. W., Smith C., Minor L. K., Darrow A. L., Willemsens G., Dewaepenaert K., Roevens P., et al. 2010. High-content assays for evaluating cellular and hepatic diacylglycerol acyltransferase activity. J. Lipid Res. 51: 3559–3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Birch A. M., Birtles S., Buckett L., Kemmitt P., Smith G., Smith T., Turnbull A., Wang S. 2009. Discovery of a potent, selective, and orally efficacious pyrimidinooxazinyl bicyclooctaneacetic acid diacylglycerol acyltransferase-1 inhibitor. J. Med. Chem. 52: 1558–1568. [DOI] [PubMed] [Google Scholar]
- 14.Chandler C. E., Wilder D., Pettini J., Savoy Y., Petras S., Chang G., Vincent J., Harwood H., Jr 2003. CP-346086: an MTP inhibitor that lowers plasma cholesterol and triglycerides in experimental animals and in humans. J. Lipid Res. 44: 1887–1901. [DOI] [PubMed] [Google Scholar]
- 15.Fox B., Furukawa N., Hao X., Iio K., Inaba T., Jackson S., Kayser F., Labelle M., Li K., Matsui T. 2004. Fused bicyclic nitrogen-containing heterocycles. Patent No. WO2004047755. [Google Scholar]
- 16.Castro-Perez J. M., Kamphorst J., DeGroot J., Lafeber F., Goshawk J., Yu K., Shockcor J., Vreeken R., Hankemeier T. 2010. Comprehensive LC-MS E lipidomic analysis using a shotgun approach and its application to biomarker detection and identification in osteoarthritis patients. J. Proteome Res. 9: 2377–2389. [DOI] [PubMed] [Google Scholar]
- 17.Gakwaya R., Li X., Wong Y., Chivukula S., Collins E., Evans J. 2007. Examining the collision-induced decomposition spectra of ammoniated triglycerides. III. The linoleate and arachidonate series. Rapid Commun. Mass Spectrom. 21: 3262–3268. [DOI] [PubMed] [Google Scholar]
- 18.Li X., Evans J. 2005. Examining the collision-induced decomposition spectra of ammoniated triglycerides as a function of fatty acid chain length and degree of unsaturation. I. The OXO/YOY series. Rapid Commun. Mass Spectrom. 19: 2528–2538. [DOI] [PubMed] [Google Scholar]
- 19.Han X., Gross R. 2001. Quantitative analysis and molecular species fingerprinting of triacylglyceride molecular species directly from lipid extracts of biological samples by electrospray ionization tandem mass spectrometry. Anal. Biochem. 295: 88–100. [DOI] [PubMed] [Google Scholar]
- 20.Folch J., Lees M., Sloane-Stanley G. H. 1957. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226: 497–509. [PubMed] [Google Scholar]
- 21.Morrison W. R., Smith L. M. 1964. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride–methanol. J. Lipid Res. 5: 600–608. [PubMed] [Google Scholar]
- 22.Diraison F., Pachiaudi C., Beylot M. 1996. In vivo measurement of plasma cholesterol and fatty acid synthesis with deuterated water: determination of the average number of deuterium atoms incorporated* 1. Metabolism . 45: 817–821. [DOI] [PubMed] [Google Scholar]
- 23.Mansbach C. M., Siddiqi S. A. 2010. The biogenesis of chylomicrons. Annu. Rev. Physiol. 72: 315–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iqbal J., Hussain M. 2009. Intestinal lipid absorption. Am. J. Physiol. Endocrinol. Metab. 296: E1183–E1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mansbach C. M., Dowell R. 1992. Uptake and metabolism of circulating fatty acids by rat intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 263: G927–G933. [DOI] [PubMed] [Google Scholar]
- 26.Storch J., Zhou Y., Lagakos W. 2008. Metabolism of apical versus basolateral sn-2-monoacylglycerol and fatty acids in rodent small intestine. J. Lipid Res. 49: 1762–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gangl A., Ockner R. 1975. Intestinal metabolism of plasma free fatty acids. Intracellular compartmentation and mechanisms of control. J. Clin. Invest. 55: 803–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi Y., Burn P. 2004. Lipid metabolic enzymes: emerging drug targets for the treatment of obesity. Nat. Rev. Drug Discov. 3: 695–710. [DOI] [PubMed] [Google Scholar]
- 29.Emken E., Adlof R., Duval S., Shane J., Walker P., Becker C. 2004. Effect of triacylglycerol structure on absorption and metabolism of isotope-labeled Palmitic and Linoleic acids by humans. Lipids . 39: 1–9. [DOI] [PubMed] [Google Scholar]
- 30.Johnston J. M., Rao G. A., Lowe P. A. 1967. The separation of the alpha-glycerophosphate and monoglyceride pathways in the intestinal biosynthesis of triglycerides. Biochim. Biophys. Acta . 137: 578–580. [DOI] [PubMed] [Google Scholar]
- 31.Kayden H. J., Senior J. R., Mattson F. H. 1967. The monoglyceride pathway of fat absorption in man. J. Clin. Invest. 46: 1695–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Havel R. J., Kane J. P., Balasse E., Segel N., Basso L. 1970. Splanchnic metabolism of free fatty acids and production of triglycerides of very low density lipoproteins in normotriglyceridemic and hypertriglyceridemic humans. J. Clin. Invest. 49: 2017–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aarsland A., Chinkes D., Wolfe R. 1996. Contributions of de novo synthesis of fatty acids to total VLDL-triglyceride secretion during prolonged hyperglycemia/hyperinsulinemia in normal man. J. Clin. Invest. 98: 2008–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luchoomun J., Hussain M. M. 1999. Assembly and secretion of chylomicrons by differentiated Caco-2 cells. J. Biol. Chem. 274: 19565–19572. [DOI] [PubMed] [Google Scholar]
- 35.Mittendorfer B., Liem O., Patterson B., Miles J., Klein S. 2003. What does the measurement of whole-body fatty acid rate of appearance in plasma by using a fatty acid tracer really mean? Diabetes . 52: 1641–1648. [DOI] [PubMed] [Google Scholar]
- 36.Hagenfeldt L., Wahren J., Pernow B., Raf L. 1972. Uptake of individual free fatty acids by skeletal muscle and liver in man. J. Clin. Invest. 51: 2324–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]