Abstract
The aim of this study was to assess the independent contributions of plasma levels of lipoprotein(a) (Lp(a)), Lp(a) cholesterol, and of apo(a) isoform size to prospective coronary heart disease (CHD) risk. Plasma Lp(a) and Lp(a) cholesterol levels, and apo(a) isoform size were measured at examination cycle 5 in subjects participating in the Framingham Offspring Study who were free of CHD. After a mean follow-up of 12.3 years, 98 men and 47 women developed new CHD events. In multivariate analysis, the hazard ratio of CHD was approximately two-fold greater in men in the upper tertile of plasma Lp(a) levels, relative to those in the bottom tertile (P < 0.002). The apo(a) isoform size contributed only modestly to the association between Lp(a) and CHD and was not an independent predictor of CHD. In multivariate analysis, Lp(a) cholesterol was not significantly associated with CHD risk in men. In women, no association between Lp(a) and CHD risk was observed. Elevated plasma Lp(a) levels are a significant and independent predictor of CHD risk in men. The assessment of apo(a) isoform size in this cohort does not add significant information about CHD risk. In addition, the cholesterol content in Lp(a) is not a significant predictor of CHD risk.
Keywords: apolipoprotein, cholesterol, epidemiology, triglycerides
Lipoprotein(a) (Lp(a)) was first described by Berg in 1963 (1). Lp(a) is a lipoprotein similar in structure to LDL, but differs from LDL in having apo(a), a large glycoprotein, attached to apoB-100 by a disulfide bond (2). Apo(a) shares strong homology with several regions of plasminogen (3), including the protease domain, the kringle 5 domain, and 10 types of the kringle 4 domain. It has been shown that apo(a) is highly polymorphic in size due to different numbers of the kringle 4 type 2 (kringle-42) domain, ranging from a minimum of 3 to greater than 40 (4). Plasma levels of Lp(a) are highly heritable (2, 5) and are inversely correlated with the apo(a) isoform size, with subjects carrying small isoforms having high plasma Lp(a) levels (5). Metabolic studies have shown that the inverse association between plasma Lp(a) levels and apo(a) isoform size is due to differences in the hepatic secretion of apo(a), with subjects carrying small apo(a) isoforms having increased apo(a) secretion (6, 7). Boerwinkle et al. (8) indicated that 69% of the variability in plasma Lp(a) levels is accounted for by the number of apo(a) kringle-42 repeats, and an additional 21% of the variation is explained by other sequences within the gene coding for apo(a). Recently, the rs3798220 single-nucleotide polymorphism (SNP) in the apo(a) locus was found to be a strong predictor of Lp(a) levels and coronary heart disease (CHD) risk (9).
Plasma Lp(a) levels are associated with CHD risk (10, 11). The mechanisms by which Lp(a) increases CHD risk are not well defined, but may include both a prothrombotic effect due to the similarity of apo(a) to the fibrinolytic pro-enzyme plasminogen (12, 13), and an atherogenic effect mediated by the preferential binding of oxidized phospholipids by Lp(a) as well as Lp(a) deposition in the arterial wall (14).
The goal of this study was to assess prospectively the risk of CHD associated with elevated plasma Lp(a) levels and the contribution of apo(a) isoform size to this association in the Framingham Offspring Study (FOS). In addition, we have compared the CHD risk prediction associated with elevated Lp(a) levels as assessed by two different immunoassays and by the measurement of Lp(a) cholesterol levels.
METHODS
Subjects
The FOS is a longitudinal population-based study which started in 1975 and enrolled the children of the participants in the original Framingham Heart Study cohort, and their spouses (15). All participants are Caucasian. During the 5th examination cycle (1991–1995), subjects underwent a medical history, physical examination, electrocardiogram (ECG) evaluation, and a blood draw for the assessment of plasma lipid and lipoprotein levels. At cycle 5, there were 1,328 men and 1,562 women who were free of CHD and had their plasma Lp(a) levels measured. These subjects were followed until completion of cycle 8, with a mean follow-up of 12.3 years, for the occurrence of new CHD events. All suspected CHD events were assessed by a panel of three physicians who evaluated the available evidence. CHD was defined as: myocardial infarction diagnosed by ECG, myocardial infarction diagnosed by enzymatic elevations and history, sudden CHD death, and nonsudden CHD death.
Lp(a) assessment
Blood was drawn after a 12 h overnight fast into 0.1% EDTA tubes. Plasma was separated by centrifugation at 2,500 rpm for 30 min at 4°C and immediately frozen and stored at −80°C. Plasma levels of total cholesterol (TC), triglycerides (TGs), and HDL-cholesterol (HDL-C) were measured by automated enzymatic methods in an Abbott Diagnostics ABA-200 analyzer using Abbott A-Gent enzymatic reagents. LDL-cholesterol (LDL-C) levels were calculated using the Friedewald formula when TG levels were <400 mg/dl (16).
Lp(a) protein levels were measured using an ELISA. This assay utilizes as capturing antibody a monoclonal antibody against apo(a) that does not recognize the kringle-42 domain and is independent of apo(a) isoform size (17). Lp(a) protein levels are expressed in nmol/l. The Lp(a) standard used for this assay was a lyophilized serum having kringles 18 and 25 and an assigned value of 123 nmol/l using World Health Organization/International Federation of Clinical Chemistry and Laboratory Medicine reference material (18). Inter- and intra-assay coefficients of variation were 3.2% and 3.5%, respectively. In some individuals, only one apo(a) isoform is expressed. However, in most individuals, apo(a) is expressed by both alleles, with one isoform more prevalent than the other. Apo(a) isoforms were measured with methodology previously described (4), in which the size of apo(a) isoforms are visualized on agarose gel electrophoresis. The apo(a) isoform size thus visualized is directly proportional to the number of kringle-42 repeats (4). The predominant apo(a) isoform size was used in statistical analysis. These measurements were carried out at the Northwest Lipid Metabolism and Diabetes Research Laboratories (NWRL), University of Washington, Seattle, WA.
Lp(a) levels were also measured with two other assays in plasma obtained at examination cycle 5: an immunoturbidimetric assay from Wako Chemicals USA (Richmond, VA), which utilizes an antibody that does not cross-react with plasminogen or apoB (inter- and intra-assay coefficients of variation (CVs) <3%), and a lectin-based assay (Genzyme Diagnostics; Framingham, MA), which utilizes lectin to capture Lp(a) and then measures the cholesterol content in the particle (inter-and intra-assay CV: 3.8% and 3.2%, respectively) (19). Commercially available standards were used (Wako Chemicals USA). Measurements with these two assays were carried out at the Lipid Metabolism Laboratory, Tufts University, Boston, MA.
The genotyping for the rs3798220 SNP, located in the apo(a) locus, was performed according to established methodology (20).
Statistical analysis
Variables were assessed for normal distribution, and a log-transformation was applied to skewed variables before analysis. Means and standard deviations are reported for normally distributed variables, and median and inter-quartile ranges are reported for skewed variables. Analyses were stratified by sex. Differences in baseline characteristics for subjects who developed new CHD events during follow-up (CHD cases) versus subjects who did not develop CHD (controls) were compared using Student's t-tests for continuous variables and χ2 tests for dichotomous variables. Cox proportional hazards models were used to estimate the hazard ratio (HR) of CHD associated with high levels of Lp(a). In these models, subjects in the upper tertile of Lp(a) levels (or lower tertile of apo(a) isoform size) were compared with subjects in the lower tertile of Lp(a) (or upper tertile of apo(a) isoform size). Models were adjusted for possible confounding risk factors for CHD, including age, body mass index (BMI), smoking, hypertension, diabetes, TC, TG, HDL-C, and use of lipid-lowering medications.
RESULTS
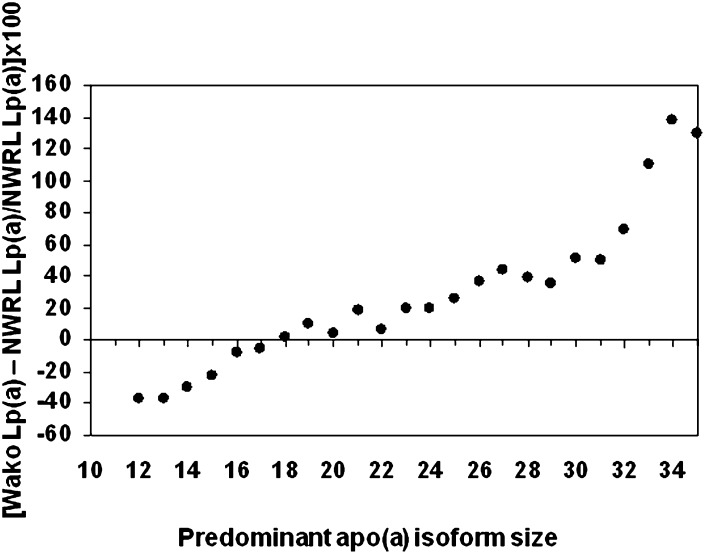
Plasma Lp(a) concentrations were measured with three different assays in FOS participants at examination cycle 5. The percentile distribution of Lp(a) concentrations measured with the different methods is illustrated in Table 1. The Lp(a) protein values obtained by the NWRL ELISA were highly correlated to the Lp(a) concentrations obtained by the Wako immunoturbidimetric assay (R = 0.930, P < 0.0001). However, after conversion of the Lp(a) Wako measurements from mg/dl to nmol/l using a factor based on the predominant allele size, this assay showed a strong apo(a) isoform size-dependent bias (Fig. 1), consistent with the concept that the Wako assay overestimates the Lp(a) values in samples with large apo(a) isoforms and underestimates the Lp(a) values in samples with small apo(a) isoforms. A bias greater than 10% was detected in 73% of the population. The cholesterol content of Lp(a), measured using the lectin-affinity chromatography method, was correlated with both the NWLR Lp(a) protein and the Wako Lp(a) concentrations (R = 0.765, P < 0.0001 and R = 0.789, P < 0.0001, respectively). However, a comparison of the Lp(a) cholesterol values with the other Lp(a) measurements, based on percentiles shown in Table 1, suggested that the Lp(a) cholesterol method may overestimate the amount of Lp(a) cholesterol in samples with low Lp(a) values. The predominant apo(a) isoform size was significantly and inversely related to plasma levels of Lp(a) as assessed with the different assays (NWRL method, R = −0.573, P < 0.0001; Wako method, R = −0.514, P < 0.0001; and Lp(a)-C, R = −0.441, P < 0.0001).
TABLE 1.
Percentile distribution of plasma Lp(a) concentrations in the Framingham Offspring Study population
| 20th | 30th | 40th | 50th | 60th | 70th | 80th | |
|---|---|---|---|---|---|---|---|
| Men | |||||||
| Lp(a) NWRL (nmol/l) | 3.1 | 6.9 | 11.9 | 18.8 | 28.8 | 48.8 | 99.4 |
| Lp(a) Wako (mg/dl) | 4.3 | 6.1 | 8.2 | 10.9 | 15.1 | 22.6 | 36.3 |
| Lp(a)-C Genzyme (mg/dl) | 2.2 | 2.9 | 3.8 | 4.8 | 6.1 | 7.9 | 11.1 |
| Women | |||||||
| Lp(a) NWRL (nmol/l) | 5.0 | 10.0 | 15.6 | 23.1 | 35.0 | 59.4 | 108.1 |
| Lp(a) Wako (mg/dl) | 5.1 | 7.1 | 9.5 | 13.1 | 18.3 | 26.5 | 43.2 |
| Lp(a)-C Genzyme (mg/dl) | 2.2 | 2.8 | 3.7 | 4.7 | 6.3 | 8.4 | 11.1 |
Only participants in whom plasma Lp(a) levels were measured with all three assays were included in analyses; men n = 1,050, and women n = 1,233.
Fig. 1.
Average percent bias of the Lp(a) Wako assay according to the predominant apo(a) isoform size in FOS subjects.
Men who developed new CHD events during the 12.3 years follow-up were significantly older and had a higher prevalence of CHD risk factors than men who did not develop CHD (Table 2). Median plasma Lp(a) levels were approximately two-fold higher in male CHD cases than in male control subjects for both immuno-based Lp(a) methods (NWRL, P = 0.003; and Wako, P = 0.01) (Table 2). The difference in median plasma levels of Lp(a) cholesterol between men with incident CHD and controls was marginally significant (P = 0.045). The average predominant apo(a) isoform size was significantly smaller in male CHD cases than in controls (P = 0.025) (Table 2). During follow-up, only 3.1% of women developed CHD, as opposed to 7.9% of men. Women with CHD, similar to men, were significantly older and had a higher prevalence of CHD risk factors than women without CHD (Table 2). The difference in plasma lipid levels and in CHD risk factors between cases and controls was more marked in women than in men. In contrast to men, median plasma Lp(a) protein, Lp(a), or Lp(a) cholesterol levels were not significantly different between women CHD cases and controls, and the average predominant apo(a) isoform was marginally larger in cases than in controls (P = 0.044).
TABLE 2.
Baseline characteristics, plasma lipid and Lp(a) levels, and predominant apo(a) isoform size in subjects who developed CHD during a mean follow-up of 12.3 years, and in subjects free of CHD
| Men |
Women |
|||||
|---|---|---|---|---|---|---|
| Variable | Controls n = 1,230 | CHD cases n = 98 | P | Controls n = 1,505 | CHD cases n = 47 | P |
| Age, y | 55 ± 10 | 59 ± 10 | <0.0001 | 54 ± 10 | 61 ± 9 | <0.0001 |
| BMI, kg/m2 | 28.17 ± 4.25 | 28.93 ± 3.49 | 0.046 | 26.74 ± 5.41 | 28.27 ± 5.66 | 0.057 |
| TC, mg/dl | 201 ± 33 | 207 ± 39 | 0.117 | 208 ± 37 | 232 ± 37 | <0.0001 |
| TG, mg/dl | 128 [88, 192] | 149 [104, 207] | 0.011a | 112 [81, 162] | 172 [106, 223] | <0.0001a |
| LDL-C, mg/dlb | 121 ± 30 | 124 ± 34 | 0.457 | 118 ± 33 | 141 ± 31 | <0.0001 |
| HDL-C, mg/dl | 43 ± 11 | 41 ± 10 | 0.064 | 56 ± 16 | 47 ± 12 | <0.0001 |
| Lp(a) protein, nmol/L [NWRL] | 18.1 [4.4, 65] | 36.3 [10.6, 124.4] | 0.003a | 20.6 [5.6, 71.3] | 18.8 [4.4, 53.1] | 0.353a |
| Lp(a), mg/dl [Wako]c | 10.8 [4.9, 30] | 20.6 [7.6, 38] | 0.010a | 13 [5.9, 34] | 12 [5.5, 56] | 0.71a |
| Lp(a)-C, mg/dl [Genzyme]d | 4.9 [2.6, 9.5] | 5.9 [3.2, 11.6] | 0.045a | 4.7 [2.5, 9.3] | 5.5 [2.6, 12.2] | 0.49a |
| Predominant isoform | 24.0 ± 5.5 | 22.7 ± 5.5 | 0.025 | 23.9 ± 5.6 | 25.6 ± 6.2 | 0.044 |
| Systolic blood pressure, mm Hg | 129 ± 17 | 134 ± 18 | 0.006 | 124 ± 20 | 138 ± 21 | <0.0001 |
| Diastolic blood pressure, mm Hg | 77 ± 10 | 77 ± 10 | 0.689 | 73 ± 10 | 77 ± 9 | 0.003 |
| Hypertension, % | 34 | 55 | <0.0001 | 29 | 62 | <.0001 |
| Smoking, % | 19 | 24 | 0.307 | 19 | 37 | 0.003 |
| Diabetes, % | 8 | 18 | 0.001 | 5 | 17 | 0.0002 |
| Lipid-lowering medications, % | 7 | 19 | <0.0001 | 5 | 13 | 0.030 |
| Postmenopausal, % | 65 | 89 | 0.0005 | |||
| Hormone therapy, % | 17 | 13 | 0.436 | |||
Data shown as mean ± SD, with the exception of TG and Lp(a), where values are shown as median [IQR]. HDL-C, HDL-cholesterol; LDL-C, LDL cholesterol.
P value after variable was log-transformed.
LDL-C concentration obtained after the subtraction of Lp(a) cholesterol.
Wako method: men, 1,045 controls and 86 cases; women, 1,300 controls and 41 cases.
Genzyme method: men, 1,131 controls and 94 cases; women, 1,376 controls and 44 cases.
When subjects were divided according to tertiles of plasma Lp(a) levels, as assessed by either the NWRL or Wake method, a greater number of CHD cases, relative to controls, were observed in men in the upper tertile of plasma Lp(a) levels as compared with men in the lower tertile (Table 3). This was not observed in women (Table 3).
TABLE 3.
Number of new CHD cases by tertiles of baseline plasma Lp(a) values
| Men |
Women |
|||||
|---|---|---|---|---|---|---|
| 10.6–8.1 nmol/l | 28.8–38.8 nmol/l | 339.4–469.4 nmol/l | 10.2–6.8 mg/dl | 26.9–20.3 mg/dl | 320.7–145.8 mg/dl | |
| Men | ||||||
| Controls, n | 420 | 414 | 396 | 357 | 355 | 333 |
| CHD cases, n | 21 | 32 | 45 | 18 | 25 | 43 |
| CHD cases, % | 4.8 | 7.2 | 10.2 | 4.8 | 6.6 | 11.4 |
| Women | ||||||
| 10.6–10.0 nmol/L | 210.6–43.8 nmol/l | 344.4–483.1 nmol/l | 10.1–7.8 mg/dl | 27.9–23.1 mg/dl | 323.2–150.2 mg/dl | |
| Controls, n | 503 | 500 | 502 | 435 | 431 | 434 |
| CHD cases, n | 17 | 17 | 13 | 14 | 13 | 14 |
| CHD cases, % | 3.3 | 3.3 | 2.5 | 3.1 | 2.9 | 3.1 |
In multivariate Cox proportional hazards models, the HR of incident CHD was greater than 2-fold in men in the upper tertile of Lp(a) levels than in men in the lower tertile (Table 4), both with the NWRL and the Wako Lp(a) measurements. The risk of CHD associated with high Lp(a) levels was not modified after adjusting for several risk factors for CHD, including age, BMI, smoking, hypertension, diabetes, and plasma levels of TC, log-TG, and HDL-C (Table 4, model 3), or further adjustment for use of cholesterol-lowering medications and niacin [a medication known to affect plasma Lp(a) levels] (Table 4, model 4). Apo(a) isoform size is known to be a significant determinant of plasma Lp(a) levels, and after further adjustment for the predominant isoform size, the association between high Lp(a) levels and CHD was still significant (Table 4, model 5). In separate multivariate models, the HR for incident CHD was significantly higher in men in the lower tertile of apo(a) isoform size than in those in the higher tertile (Table 4). However, the strength of this association was lower than that observed for Lp(a) levels, and the significance was abolished after adjustment for plasma Lp(a) levels measured with either the NWRL or the Wako methods (Table 4, model 6). In similar multivariate Cox proportional hazards models, there was no significantly increased HR in men in the upper tertile of Lp(a) cholesterol levels than in men in the lower tertile (HR = 1.39, CI = 0.79–2.46, P = 0.25). In women, the risk of CHD associated with high Lp(a) concentrations was not significant after adjustment for age, BMI, smoking, hypertension, diabetes, plasma lipids, and use of lipid-lowering medications or hormone therapy (NWRL: HR = 0.53, CI = 0.22–1.25, P = 0.15; Wako: HR = 0.85, CI = 0.37–1.96, P = 0.71; Lp(a) cholesterol: HR = 0.79, CI = 0.35–1.78, P = 0.57). A power calculation analysis, fixing the event rate to 2.8%, as observed in women in this study, and assuming an HR of 2.49, as observed in men in this population, determined that our power to detect differences in CHD risk between the lower and the upper tertile of Lp(a) in women was less than 70%.
TABLE 4.
Hazard ratios for incident CHD in FOS men by baseline Lp(a) levels (upper tertile vs. lower tertile) or apo(a) isoform size (lower tertile vs. upper tertile)
| HR | CI | P | HR | CI | P | |
|---|---|---|---|---|---|---|
| Model | Lp(a) (NWRL) |
Lp(a) (Wako) |
||||
| 1 | 2.23 | 1.33–1.74 | 0.003 | 2.36 | 1.36–4.09 | 0.003 |
| 2 | 2.48 | 1.46–4.21 | 0.0008 | 2.69 | 1.51–4.78 | 0.0008 |
| 3 | 2.57 | 1.50–4.40 | 0.0006 | 2.69 | 1.51–4.81 | 0.0008 |
| 4 | 2.36 | 1.37–4.07 | 0.002 | 2.52 | 1.40–4.53 | 0.003 |
| 5 | 2.45 | 1.20–4.99 | 0.013 | 2.74 | 1.35–5.58 | 0.004 |
| Predominant apo(a) isoform | ||||||
| 1 | 1.70 | 1.04–2.77 | 0.03 | 1.70 | 1.04–2.77 | 0.03 |
| 2 | 1.76 | 1.07–2.88 | 0.03 | 1.76 | 1.07–2.88 | 0.03 |
| 3 | 1.78 | 1.09–2.93 | 0.03 | 1.78 | 1.09–2.93 | 0.03 |
| 4 | 1.68 | 1.02–2.77 | 0.04 | 1.68 | 1.02–2.77 | 0.04 |
| 6 | 1.15 | 0.59–2.25 | 0.67 | 1.08 | 0.54–2.12 | 0.83 |
Model 1 covariate: age; Model 2 covariates: age, BMI, smoking, hypertension, and diabetes; Model 3 covariates: age, BMI, smoking, hypertension, diabetes, TC, log-TG, and HDL-C; Model 4 covariates: age, BMI, smoking, hypertension, diabetes, TC, log-TG, HDL-C, and use of cholesterol-lowering medications and/or niacin; Model 5 covariates as in Model 4, plus predominant apo(a) isoform size; Model 6 covariates as in Model 4, plus Lp(a) levels.
The allele frequency of the rs3798220 SNP was 1.56 in men and 1.55 in women. This SNP was associated with plasma Lp(a) levels both in men and women (P < 0.0001). However, in the fully adjusted multivariate Cox proportional hazards models, this SNP was not significantly associated with CHD risk in men (HR = 1.29, CI = 0.39–4.21, P = 0.66) or women (HR = 1.53, CI = 0.36–6.54, P = 0.56).
DISCUSSION
Elevated levels of Lp(a) have long been known to be associated with premature CHD. In cross-sectional analysis of the Lipid Research Clinics Coronary Primary Prevention Trial and the Framingham Heart Study, we had previously shown that elevated levels of Lp(a), whether assessed by immunoassay or electrophoresis, were significantly associated with CHD risk (21–23). Recently, there has been a resurgence of interest in Lp(a), with studies conducted in large numbers of subjects indicating an independent association of Lp(a) levels with CHD risk. The Emerging Risk Factors Collaboration group has reported a significant and curvilinear association between plasma Lp(a) levels and CHD, defined as first MI or CHD death, in 126,634 participants in 36 different prospective studies (11). In this study, there was no association between plasma Lp(a) levels and nonvascular death (11). In addition, a meta-analysis of 31 published prospective studies in which baseline Lp(a) levels were measured with somewhat different methodologies indicated an odds ratio of 1.45 (CI = 1.32–1.58) for CHD in subjects in the upper tertile of Lp(a) levels, relative to subjects in the lower tertile (10). Our results in men in the FOS are consistent with these reports from large populations: men with elevated plasma levels of Lp(a) had a greater than 2-fold increased risk of CHD, relative to men with low levels of Lp(a). The lower limit for the upper tertile of Lp(a) protein levels in men was approximately 40 nmol/l and for the upper tertile of Lp(a) levels (Wako) was approximately 20 mg/dl. The increased risk of CHD associated with high Lp(a) levels remained statistically significant after adjustment for other lipoprotein levels and other established risk factors for CHD, supporting the independent role of Lp(a) in CHD development. In men, the association between plasma Lp(a) concentrations and CHD remained significant after isoform size was entered into the hazard prediction model. In addition, when the predominant apo(a) isoform size was the primary variable, models adjusting for plasma Lp(a) levels abolished the predictive value of the isoform size. Similar models using both isoform sizes provided comparable results. This finding emphasizes the fact that plasma Lp(a) levels are more informative than is apo(a) isoform size in risk prediction in the Framingham cohort.
Previous studies have examined the role of apo(a) isoform size on CHD prediction (24–30), and some of these studies have attempted to separate the prediction relative to isoform size from that of plasma levels of Lp(a). In a small case-control study, Kraft et al. (26) have reported that both Lp(a) levels and isoform size were associated with CHD, but when both variables were entered in the multivariate model, the strength of the association was higher for apo(a) isoform size. Similarly, Rifai et al. (29) have found both Lp(a) levels and apo(a) isoform size to be significant predictors of CHD in a small case-control study, and when both variables were entered in the same multivariate model, only apo(a) isoform size remained significantly associated with CHD. In three separate Danish populations, Kamstrup et al. (28) have reported an increased risk of CHD (HR = 1.3 to 1.4) in subjects in the lowest quartile of number of apo(a) kringle-42 repeats versus the highest quartile. In one of the three populations, after further adjustment for Lp(a) levels, the association between the number of kringle-42 repeats was attenuated but remained significant (28). It has been suggested that the stronger association between apo(a) isoform and CHD could be explained by a greater atherogenicity of Lp(a) particles with lower number of repeats (28, 29). However, in two Asian Indian populations, Lp(a) levels were associated with CHD risk independently of apo(a) isoform size (30). In our prospective study, the multivariate analysis including Lp(a) and apo(a) isoform size in the same model clearly indicates that the majority of the increased risk of CHD by Lp(a) in men is attributable to the concentration of the lipoprotein particle, and that isoform size contributes to a smaller degree. The contribution of isoform size to risk prediction could be explained by isoform size being one, but not the only, determinant of plasma Lp(a) levels (8). Genetic variations at the apo(a) locus other than kringle-42 repeats have been shown to be strongly associated with plasma Lp(a) levels (9, 31, 32). Genetic studies support the causative relationship between plasma Lp(a) levels and CHD. The PROCARDIS Consortium Study has shown a significant CHD risk prediction associated with two SNPs in the apo(a) gene locus, rs3798220 and rs10455872 (9). These variants were both associated with plasma Lp(a) levels, and the association between these gene variants and CHD was abolished when plasma Lp(a) levels were entered into the model. In our population, even though the rs3798220 SNP was significantly associated with Lp(a) levels, it was not a significant predictor of CHD. Our finding is probably due to the fact that this SNP is only one of the several genetic determinants of Lp(a) levels, and further supports the concept that Lp(a) concentrations are directly associated with CHD risk.
Our comparison of different Lp(a) assays indicates that the two immuno-based assays had similar CHD prediction value. However, it should be pointed out that the Lp(a) concentrations as measured by the Wako assay were overestimated up to 130% in subjects with very large predominant apo(a) isoform sizes, and underestimated up to 37% in subjects with small isoform sizes. On the other hand, the cholesterol content of Lp(a) was not significantly associated with CHD in multivariate analysis. This may suggest that either the cholesterol content of the particle, which is influenced by the metabolism of the particle, similar to the cholesterol content in LDL, does not entirely account for the atherogenicity of Lp(a), or that the methodology for Lp(a) cholesterol assessment is not accurate. Although our and other works confirm the causative link between elevated Lp(a) and CHD, the mechanism responsible for the atherogenicity of Lp(a) is still not clear. Lp(a) has been shown to serve as a scavenger of oxidized phospholipids (14, 33), and this functional property may be responsible for pro-atherogenic and pro-inflammatory effects of Lp(a). Also, due to the high degree of homology between apo(a) and plasminogen (3), it has been hypothesized that Lp(a) interferes with the fibrinolytic process and thus may be pro-thrombotic (34). Both of these putative functions of Lp(a) are attributed to the apo(a) component.
In women, we did not find a significant association between CHD and Lp(a) levels. This is in contrast to other large prospective and case-control studies indicating a similar association between high Lp(a) levels and CHD risk in men and women (9, 11). Our power calculation analysis indicated that both a higher event rate and a greater number of participants would have been needed to detect a significant association between Lp(a) levels and CHD risk in women in the FOS.
Our study supports a significant and independent role of Lp(a) on cardiovascular disease in men.
Footnotes
Abbreviations:
- BMI
- body mass index
- CHD
- coronary heart disease
- CV
- coefficient of variation
- ECG
- electrocardiogram
- FOS
- Framingham Offspring Study
- HR
- hazard ratio
- Lp(a)
- Lipoprotein(a)
- SNP
- single-nucleotide polymorphism
- TC
- total cholesterol
- TG
- triglyceride
This work was supported by grants HL-60935, HL-74753, HL-083813, and HL-030086 from the National Institutes of Health and by the U.S. Department of Agriculture, under agreement No. 58-1950-7-707; by NHLBI N01-HC-25195 and HL-60935 from the National Institutes of Health (L.A.C., C.C.W.), Bethesda, MD. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the US Department of Agriculture. The authors report no conflict of interest.
REFERENCES
- 1.Berg K. 1963. A new serum type system in man: the Lp system. Acta Pathol. Microbiol. Scand. 59: 369–382. [DOI] [PubMed] [Google Scholar]
- 2.Utermann G., Weber W. 1983. Protein composition of Lp(a) lipoprotein from human plasma. FEBS Lett. 154: 357–361. [DOI] [PubMed] [Google Scholar]
- 3.McLean J. W., Tomlinson J. E., Kuang W-J., Eaton D. L., Chen E. Y., Fless G. M., Scanu A. M., Lawn R. M. 1987. cDNA sequence of human apolipoprotein(a) is homologous to plasminogen. Nature. 330: 132–137. [DOI] [PubMed] [Google Scholar]
- 4.Marcovina S. M., Hobbs H. H., Albers J. J. 1996. Relation between number of apolipoprotein(a) kringle 4 repeats and mobility of isoforms in agarose gel: basis for a standardized isoform nomenclature. Clin. Chem. 42: 436–439. [PubMed] [Google Scholar]
- 5.Utermann G., Menzel H. J., Kraft H. G., Duba H. C., Kemmler H. G., Seitz C. 1987. Lp(a) glycoprotein phenotypes. Inheritance and relation to Lp(a)-lipoprotein concentrations in plasma. J. Clin. Invest. 80: 458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rader D. J., Cain W., Ikewaki K., Talley G., Zech L. A., Usher D., Brewer H. B. 1994. The inverse association of plasma lipoprotein(a) concentrations with apolipoprotein(a) isoform size is not due to differences in Lp(a) catabolism but to differences in production rate. J. Clin. Invest. 93: 2758–2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jenner J. L., Seman L. J., Millar J. S., Lamon-Fava S., Welty F. K., Dolnikowski G. G., Marcovina S. M., Lichtenstein A. H., Barrett P. H., deLuca C., et al. 2005. The metabolism of apolipoproteins (a) and B-100 within plasma Lipoprotein(a) in human beings. Metabolism. 54: 361–369. [DOI] [PubMed] [Google Scholar]
- 8.Boerwinkle E., Leffert C. G., Lin J., Lackner C., Chiesa G., Hobbs H. H. 1992. Apolipoprotein(a) gene accounts for greater than 90% of the variation in plasma lipoprotein(a) concentrations. J. Clin. Invest. 90: 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarke R., Peden J. F., Hopewell J. C., Kyriakou T., Goel A., Heath S., Parish S., Barlera S., Franzosi M. G., Rust S., for the PROCARDIS Consortium. 2009. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N. Engl. J. Med. 361: 2518–2528. [DOI] [PubMed] [Google Scholar]
- 10.Bennet A., Di Angelantonio E., Erqou S., Eirikdottir G., Sigurdsson G., Woodward M., Rumley A., Lowe G. D., Danesh J., Gudnason V. 2008. Lipoprotein(a) levels and risk of future coronary heart disease. Arch. Intern. Med. 168: 598–608. [DOI] [PubMed] [Google Scholar]
- 11.The Emerging Risk Factors Collaboration. 2009. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. J. Am. Med. Assoc. 302: 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ignatescu M., Kostner K., Zorn G., Kneussl M., Maurer G., Lang I. M., Huber K. 1998. Plasma Lp(a) levels are increased in patients with chronic thromboembolic pulmonary hypertension. Thromb. Haemost. 80: 231–232. [PubMed] [Google Scholar]
- 13.Kang C., Dominguez M., Loyau S., Miyata T., Durlach V., Angles-Cano E. 2002. Lp(a) particles mold fibrin-binding properties of apo(a) in size-dependent manner: a study with different-length recombinant apo(a), native Lp(a), and monoclonal antibody. Arterioscler. Thromb. Vasc. Biol. 22: 1232–1238. [DOI] [PubMed] [Google Scholar]
- 14.Tsimikas S., Tsironis L. D., Tselepis A. D. 2007. New insights into the role of lipoprotein(a)-associated lipoprotein-associated phospholipase A2 in atherosclerosis and cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 27: 2094–2099. [DOI] [PubMed] [Google Scholar]
- 15.Feinleib M., Kannel W. B., McNamara P. M., Garrison R. J., Castelli W. P. 1975. The Framingham Offspring Study: design and preliminary data. Prev. Med. 4: 518–525. [DOI] [PubMed] [Google Scholar]
- 16.Friedewald W. T., Levy R. I., Fredrickson D. S. 1972. Estimation of the concentration of low density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 18: 499–502. [PubMed] [Google Scholar]
- 17.Marcovina S. M., Albers J. J., Gabel B., Koschinsky M. L., Gaur V. P. 1995. Effect of the number of apolipoprotein(a) kringle 4 domains on immunochemical measurements of lipoprotein(a). Clin. Chem. 41: 246–255. [PubMed] [Google Scholar]
- 18.Dati F, Tate JR, Marcovina S, International Federation of Clinical Chemistry and Laboratory Medicine, IFCC Working Group for Lipoprotein(a) Assay Standardization. 2004. First WHO/IFCC international reference reagent for lipoprotein(a) for immunoassay-Lp(a) SRM 2B. Clin. Chem. Lab. Med. 42: 670–676. [DOI] [PubMed] [Google Scholar]
- 19.Seman L. J., Jenner J. L., McNamara J. R., Schaefer E. J. 1994. Quantification of lipoprotein(a) in plasma by assaying cholesterol in lectin-bound plasma fraction. Clin. Chem. 40: 400–403. [PubMed] [Google Scholar]
- 20.Gabriel S. B., Schaffner S. F., Nguyen H., Moore J. M., Roy J., Blumenstiel B., Higgins J., DeFelice M., Lochmenr A., Faggart M., et al. 2002. The structure of haplotype blocks in the human genome. Science. 296: 2225–2229. [DOI] [PubMed] [Google Scholar]
- 21.Schaefer E. J., Lamon-Fava S., Jenner J. L., McNamara J. R., Ordovas J. M., Davis C. E., Abolafia J. M., Lippel K., Levy R. I. 1994. Lipoprotein(a) levels and risk of coronary heart disease in men. The Lipid Research Clinics Coronary Primary Prevention Trial. J. Am. Med. Assoc. 271: 999–1003. [DOI] [PubMed] [Google Scholar]
- 22.Bostom A. G., Cupples L. A., Jenner J. L., Ordovas J. M., Seman L. J., Wilson P. W., Schaefer E. J., Castelli W. P. 1996. Elevated plasma lipoprotein(a) and coronary heart disease in men aged 55 years and younger. A prospective study. J. Am. Med. Assoc. 276: 544–548. [DOI] [PubMed] [Google Scholar]
- 23.Bostom A. G., Gagnon D. R., Cupples L. A., Wilson P. W., Jenner J. L., Ordovas J. M., Schaefer E. J., Castelli W. P. 1994. A prospective investigation of elevated lipoprotein(a) detected by electrophoresis and cardiovascular disease in women. The Framingham Heart Study. Circulation. 90: 1688–1695. [DOI] [PubMed] [Google Scholar]
- 24.Klausen I. C., Sjol A., Hansen P. S., Gerdes L. U., Moller L., Lemming L., Schroll M., Faegerman O. 1997. Apolipoprotein(a) isoforms and coronary heart disease in men: a nested case-control study. Atherosclerosis. 132: 77–84. [DOI] [PubMed] [Google Scholar]
- 25.Lundstam U., Herlitz J., Karlsson T., Linden T., Wiklund O. 2002. Serum lipids, lipoprotein(a), and apolipoprotein(a) isoforms as prognostic markers in patients with coronary heart disease. J. Intern. Med. 251: 111–118. [DOI] [PubMed] [Google Scholar]
- 26.Kraft H. G., Lingenhel A., Kochl S., Hoppichler F., Kronenberg F., Abe A., Muhlberger V., Schonitzer D., Utermann G. 1996. Apolipoprotein(a) kringle IV repeat number predicts risk for coronary heart disease. Arterioscler. Thromb. Vasc. Biol. 16: 713–719. [DOI] [PubMed] [Google Scholar]
- 27.Katsouras C. S., Karabina S. A., Tambaki A. P., Goudevenos J. A., Michalis L. K., Tsironis L. D., Stroumbis C. S., Elisaf M. S., Sideris D. A., Tselepis A. D. 2001. Serum lipoprotein(a) concentrations and apolipoprotein(a) isoforms: association with the severity of clinical presentation in patients with coronary heart disease. J. Cardiovasc. Risk. 8: 311–317. [DOI] [PubMed] [Google Scholar]
- 28.Kamstrup P. R., Tybjaerg-Hansen A., Steffensen R., Nordestgaard B. G. 2009. Genetically elevated lipoprotein(a) and increased risk of myocardial infaction. J. Am. Med. Assoc. 301: 2331–2339. [DOI] [PubMed] [Google Scholar]
- 29.Rifai N., Ma J., Sacks F. M., Ridker P. M., Hernandez W. J. L., Stampfer M. J., Marcovina S. 2004. Apolipoprotein(a) size and lipoprotein(a) concentration and future risk of angina pectoris with evidence of severe coronary atherosclerosis in men: The Physicians’ Health Study. Clin. Chem. 50: 1364–1371. [DOI] [PubMed] [Google Scholar]
- 30.Geethanjali F. S., Luthra K., Lingenhel A., Kanagasaba-Pathy A. S., Jacob J., Srivastava L. M., Vasisht S., Kraft H. G., Utermann G. 2003. Analysis of the apo(a) size polymorphism in Asian Indian populations: association with Lp(a) concentration and coronary heart disease. Atherosclerosis. 169: 121–130. [DOI] [PubMed] [Google Scholar]
- 31.Wade D. P., Clarke J. G., Lindahl G. E., Liu A. C., Zysow B. R., Meer K., Schwartz K., Lawn R. M. 1993. 5′ Control regions of the apolipoprotein(a) gene and members of the related plasminogen gene family. Proc. Natl. Acad. Sci. USA. 90: 1369–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luke M. M., Kane J. P., Liu D., Rowland C. M., Shiffman D., Cassano J., Catanese J. J., Pullinger C. R., Leong D. U., Arellano A. R., et al. 2007. A polymorphism in the protease-like domain of apolipoprotein(a) is associated with severe coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 27: 2030–2036. [DOI] [PubMed] [Google Scholar]
- 33.Kiechl S., Willeit J., Mayr M., Viehweider B., Oberhollenzer M., Kronenberg F., Wiedermann C. J., Oberthaler S., Xu Q., Witztum J. L., et al. 2007. Oxidized phospholipids, lipoprotein(a), lipoprotein-associated phospholipase A2 activity, and 10-year cardiovascular outcomes. Prospective results from the Bruneck Study. Arterioscler. Thromb. Vasc. Biol. 27: 1788–1795. [DOI] [PubMed] [Google Scholar]
- 34.Boffa M. B., Marcovina S. M., Koschinsky M. L. 2004. Lipoprotein(a) as a risk factor for atherosclerosis and thrombosis: mechanistic insights from animal models. Clin. Biochem. 37: 333–343. [DOI] [PubMed] [Google Scholar]