Abstract
It is claimed that apoA-I expression is repressed in mice by cholic acid (CA) and its taurine conjugate, taurocholic acid (TCA) via farnesoid X receptor (FXR) activation. We measured apoA-I expression in mice, hamsters, and rats treated with highly potent and selective synthetic FXR agonists or with TCA. All of the synthetic agonists bound to FXR with high affinity in a scintillation proximity assay. However, TCA did not compete with the radioligand up to the highest concentration used (100 μM). The C-site regulatory region of apoA-I, through which FXR has been reported to regulate its expression, is completely conserved across the species investigated. In both male and female human apoA-I-transgenic mice, we reproduced the previously reported strong inhibition of human apoA-I expression upon treatment with the typical supraphysiological dose of TCA used in such studies. However, in contrast to some previous reports, TCA did not repress murine apoA-I expression in the same mice. Also, more-potent and -selective FXR agonists did not affect human or murine apoA-I expression in this model. In LDL receptor-deficient mice and Golden Syrian hamsters, selective FXR agonists did not affect apoA-I expression, whereas in Wistar rats, some even increased apoA-I expression. In conclusion, selective FXR agonists do not repress apoA-I expression in rodents. Repression of human apoA-I expression by TCA in transgenic mice is probably mediated through FXR-independent mechanisms.
Keywords: small heterodimer partner, apolipoprotein, cholesterol, bile
Increased plasma LDL-cholesterol (LDL-C) and reduced HDL-cholesterol (HDL-C) are known risk factors for cardiovascular disease, the most important cause of morbidity and mortality in the Western world (1). Apolipoprotein A-I (apoA-I), a key constituent of HDL, representing ∼64% of its protein mass (2), is synthesized primarily in the liver and intestine (3). Rubin et al. (4) showed, using human apoA-I-transgenic mice, that apoA-I overexpression caused an increase in plasma HDL-C and protected against diet-induced atherosclerosis. Conversely, apoA-I knockout mice exhibited decreased HDL-C and an increased susceptibility to atherogenesis (5). Similarly, Plump, Scott, and Breslow (6) demonstrated that expression of human apoA-I suppressed atherosclerosis in apoE-deficient mice. The anti-atherogenic effect of elevated plasma apoA-I is thought to arise from its central role in reverse cholesterol transport, the main pathway by which excess cholesterol is removed from peripheral tissues and transported to the liver, where it is partially converted into bile acids and then excreted in bile (7). The regulation of plasma apoA-I concentrations has been widely studied in rodents and primates. In particular, many nuclear hormone receptors have been shown to directly or indirectly regulate apoA-I expression. These include peroxisome proliferator-activated receptor α (PPARα) (in humans) (8), retinoid-X receptor-alpha (RXR-α) (9), hepatic nuclear factor 4-alpha (HNF-4α) (10), liver receptor homolog 1 (LRH-1) (11), apolipoprotein A-I-regulatory protein 1 (ARP-1)/Coup-TFII (12) and farnesoid X receptor (FXR) (13).
FXR is mainly expressed in liver, small intestine, kidneys, and adrenal glands (14), where it heterodimerizes with RXR-α (15) and binds to specific FXR response elements (FXREs) on genomic DNA composed of an inverted repeat of two AGGTCA half sites separated by one nucleotide (IR-1). In 1999, certain bile acids, e.g., chenodeoxycholic acid (CDCA), were identified as endogenous ligands that could bind to and activate FXR and so regulate bile acid synthesis and profile (16). It is well known that FXR agonist treatment affects the expression of its target genes, for example, increasing hepatic expression of small heterodimer partner (SHP) (17) and, consequently, decreasing the expression of CYP7A1 (17, 18) and sterol-12α hydroxylase (CYP8B1) (19), effects that can be used to monitor FXR activation in vivo. We have also shown that the affinity of synthetic agonists to bind to FXR in vitro correlates well with their plasma lipid-lowering activity in mice in vivo (unpublished observations).
FXR is reported to be involved in regulating lipoprotein and glucose metabolism, as shown by the elevated serum cholesterol and triglyceride levels and perturbed glucose homeostasis in FXR-deficient mice (20) and the normalization seen following treatment of high-fat diet-fed FXR-sufficient animals with FXR agonists (21). Interestingly, Leiss and von Bergmann (22) showed that treatment of gallstone patients with CDCA lowered their serum HDL-C by 46%, implying that agonism of FXR might decrease apoA-I expression. However, this report seems to be the exception; several other studies, in some cases more detailed and more apoA-I focused, showed that treatment of gallstone or cerebrotendinous xanthomatosis patients with CDCA had no effect on hepatic apoA-I mRNA and plasma apoA-I and no effect on HDL-C (23–31). Also, it was recently reported that a specific FXR agonist strongly decreased plasma triglycerides and LDL-C in rhesus monkeys without affecting plasma HDL-C (32). In studies in mice, the very weak, endogenous FXR agonist CA is used in preference to CDCA probably because the latter is rapidly inactivated by conversion to muricholic acid. Using supraphysiological doses of CA multiple authors have demonstrated a decrease in apoA-I expression and HDL-C in C57BL/6 mice. Subsequent reports claimed that these reductions in HDL-C (and apoA-I expression in mice) were a consequence of repression of apoA-I transcription by FXR activation. Claudel et al (13) proposed that FXR binds as a monomer to the C site in the apoA-I promoter and so represses its expression. On the other hand, Delerive et al. (11) claimed that the C site contains a recognition sequence for LRH-1 and that downregulation of apoA-I expression by FXR is mediated by SHP inhibiting LRH-1-regulated transcription. The in vivo studies in mice conducted to explore the mechanism by which FXR might decrease apoA-I expression were, however, flawed, because supraphysiological doses of either cholic acid (CA) or taurocholic acid (TCA) were used. Unfortunately, these reports led to the belief that FXR agonists downregulate apoA-I expression, an effect that would be a major disadvantage of FXR agonist therapy (33).
In this study, we compared the effects on apoA-I transcription and cholesterol metabolism of several structurally diverse, potent, and selective synthetic FXR agonists with those of the bile acid TCA in human apoA-I transgenic mice, and of synthetic FXR agonists in LDL receptor deficient (LDLr−/−) mice, hamsters, and rats. We showed that the C site regulatory region of apoA-I, through which FXR is reported to repress apoA-I expression, is completely conserved across the species investigated and in humans. We would, therefore, expect that any regulatory activity of the compounds would correlate inversely with their potency against FXR. However, what we showed was that the endogenous apoA-I mRNA expression in the livers was not- or only weakly decreased in both male and female mice, unchanged in hamsters and even increased in rats treated with FXR agonists. In contrast, TCA strongly decreased human apoA-I expression in the transgenic mice. These data indicate that FXR agonists do not inhibit apoA-I expression in the species investigated. The strong inhibition of human apoA-I expression by TCA in transgenic mice is probably mediated via an FXR-independent mechanism.
MATERIALS AND METHODS
Compounds used in animal studies
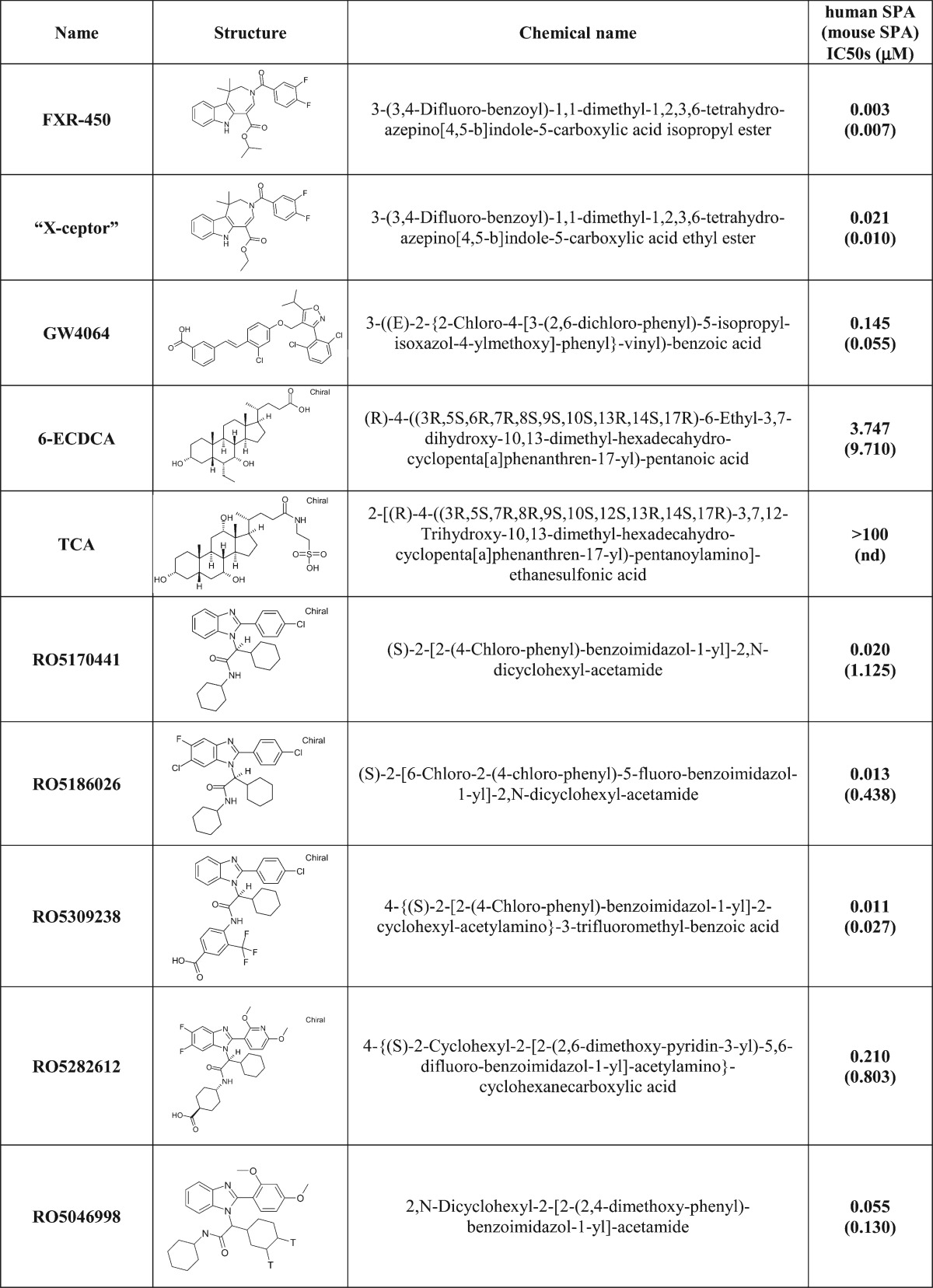
Compounds used included FXR-450 (3-(3,4-difluoro-benzoyl)-1,1-dimethyl-1,2,3,6-tetrahydro-azepino[4,5-b]indole-5-carboxylic acid isopropyl ester), the X-Ceptor compound (3-(3,4-difluoro-benzoyl)-1,1-dimethyl-1,2,3,6-tetrahydro-azepino[4,5-b]indole-5-carboxylic acid ethyl ester), GW4064 (3-((E)-2-{2-chloro-4-[3-(2,6-dichloro-phenyl)-5-isopropyl-isoxazol-4-ylmethoxy]phenyl}-vinyl)-benzoic acid), 6-ECDCA ((4R)-4-((3R,5S,6R,7R,8S,9S,10S,13R,14S,17R)-6-ethyl-3,7-dihydroxy-10,13-dimethyl-hexadecahydro-cyclopenta[a]phenanthren-17-yl)-pentanoic acid), CDCA ((R)-4-((3R,5S,6R,7R,8S,9S,10S,13R,14S,17R)-3,7-dihydroxy-10,13-dimethyl-hexadecahydro-cyclopenta[a]phenanthren-17-yl)-pentanoic acid), TCA (2-[(R)-4-((3R,5S,7R,8R,9S,10S,12S,13R,14S,17R)-3,7,12-trihydroxy-10,13-dimethyl-hexadecahydro-cyclopenta[a]phenanthren-17-yl)-pentanoylamino]ethanesulfonic acid). Benzimidazole derivatives RO5186026 ((S)-2-[6-chloro-2-(4-chloro-phenyl)-5-fluoro-benzoimidazol-1-yl]2,N-dicyclohexyl-acetamide), RO5170441 ((S)-2-[2-(4-chloro-phenyl)-benzoimidazol-1-yl]2,N-dicyclohexyl-acetamide), RO5282612 (4-(S)-2-cyclohexyl-2-[2-(2,6-dimethoxy-pyridin-3-yl)-5,6-difluoro-benzoimidazol-1-yl]acetylamino-cyclohexanecarboxylic acid) and RO5309238 (4-{(S)-2-[2-(4-chloro-phenyl)-benzoimidazol-1-yl]2-cyclohexyl-acetylamino}-3-trifluoromethyl-benzoic acid) were synthesized by Roche (patent WO2008/000643 and US2009/0163552 and Table 1).
TABLE 1.
Chemical name, structure and IC50s in a human and mouse scintillation proximity binding assay
Scintillation proximity binding assay
Binding of test substances to the human FXR ligand binding domain (LBD) was measured in a radioligand displacement scintillation proximity assay (SPA). The assay buffer contained 50 mM Hepes, pH 7.4, 10 mM NaCl, 5 mM MgCl2, and 0.01% Chaps. GST-FXR LBD fusion protein was bound to glutathione yttrium silicate SPA beads (Amersham) by shaking in a small volume of buffer at room temperature, and then diluted so that 40 nM protein and 10 μg of beads were added to each well of a 96-well plate in a volume of 100 μl. [3H]RO5046998 (55 Ci/mmol), 40 nM, was added to each well in a volume of 50 μl, and the reaction was incubated at room temperature for 30 min in the presence of test compounds in 50 μl buffer. RO5046998 is 2,N-dicyclohexyl-2-[2-(2,4-dimethoxy-phenyl)-benzoimidazol-1-yl]acetamide, one of the most potent FXR agonists identified from an early high-throughput screening assay (Table 1). The amount of radioligand remaining bound was determined using Optiplates (Perkin-Elmer) and a Packard TopCount. Dose-response curves were generated within a range of concentrations from 10−9 M to 10−4 M, and the IC50 for the test compounds was calculated using a customized XLfit template.
Animals
Preliminary time course studies with some of the selective FXR agonists in mice indicated that a dosing period of ≥5 days was sufficient for any changes in plasma lipids to stabilize. Male and female, human apoA-I-transgenic mice [C57BL/6-Tg(apoA1)1RubJ; Jackson Laboratory, 11–12 and 9–12-week–old, respectively] were assigned to treatment groups (n = 6/grp) based on age (females only) and plasma human apoA-I and HDL-C levels and fed a standard rodent diet (Kliba #3433). Mice were fed 10 g/d of powdered diet mixed with water (1:1, w/w). Compounds were administered for 5 days either by oral gavage daily (microsuspension in gelatin 7.5%) or, in the case of TCA, by food admix [0.5% TCA in the wet diet (i.e., ∼1% of dry weight)]. In most cases, all of the diet was eaten by the next day but any diet remaining was weighed and then discarded.
Male LDLr knockout mice were fed a high-fat diet (Kliba #2157 + 0.02% cholesterol) for 17 days prior to assignment to treatment groups (n = 6/grp) by their plasma HDL-C, LDL-C, and triglyceride levels. They were then treated with FXR agonists daily for 5 days by oral gavage (microsuspension in gelatin 7.5%).
Male Golden Syrian hamsters (8–9 weeks old; Charles River Laboratories, Germany) were fed a high-fat diet (Kliba #2453 + 0.05% cholesterol) for 17 days prior to the study. They were then assigned to treatment groups (n = 6/grp) according to their plasma HDL-C, LDL-C, and triglyceride levels. Hamsters were treated with FXR agonists daily for 11 days by oral gavage (microsuspension in gelatin 7.5%).
Male Wistar rats (7–8 weeks old; RCC, Switzerland) were fed normal rodent diet (Kliba #3436) and assigned to treatments (n = 5/grp) according to their plasma HDL-C, and triglycerides and body weight. Animals were dosed daily for 14 days by oral gavage.
Terminal blood samples were collected after a 4 h fast and 2 h postdosing either by retro-orbital sampling or, in the female apoA-I-transgenic mice, after decapitation. Plasma was then prepared. All remaining animals were then euthanized by decapitation under anesthesia, and livers were immediately removed, placed in RNAlater, kept at 4°C for 24 h, and then frozen. Study protocols were approved by the Veterinarian Central Office of Kanton Basel-Stadt, Switzerland to F. Hoffmann-La Roche, AG Basel, Switzerland.
Lipid analysis
Total cholesterol, HDL-C (direct method), LDL-C, triglyceride, and human apoA-I levels were determined using standard methods on an automated analyzer (Hitachi 912; Roche Diagnostics AG).
Lipoprotein profiles by fast-protein liquid chromatography
An equal volume of plasma was pooled from all the animals in each group and the plasma lipoproteins were separated by size-exclusion chromatography [Superose-6 gel fast-protein liquid chromatography (FPLC); AKTA system, Pharmacia]. Total cholesterol in the fractions was quantified using a fluorometric assay (34). Lipoprotein distribution was calculated assuming a Gaussian distribution for each peak, using a nonlinear, least-squares curve-fitting procedure to calculate the area under the curve.
Gene expression
Frozen liver (30 mg) was homogenized in 1ml Trizol (Invitrogen) in a Fastprep (Qbiogene) machine. After chloroform extraction, RNA was extracted from lysates using a PureLink micro-to-midi kit (Invitrogen). RNA was reverse transcribed using the Transcriptor cDNA first-strand synthesis kit (Roche Applied Science). Quantitative PCR reactions were performed in an LC480 (Roche Applied Science). In the case of human, mouse, and rat, specific primers and probes were ordered from Applied Biosystems and reactions were performed using LC480 Probes Master (Roche Applied Science) following procedures given by the manufacturer. Catalogue numbers for probes and primers are mm99999915_g1 (mouse GAPDH), mm00442278_m1 (mouse SHP), mm00437569_m1 (mouse apoA-I), hs00163641_m1 (human apoA-I), Rn01775763_g1 (rat GAPDH), Rn00562483_g1 (rat apoA-I), and Rn00579921_s1 (rat CYP8B1).
Hamster primers were derived from published sequences and ordered from Microsynth AG, and qPCR reactions were performed using LC480 SYBR Green I Master (Roche Applied Science). Sequences were GAPDH forward, 5′-AGGTTGTCTCCTGCGACTTCA-3′, GAPDH reverse, 5′-GCATCAAAGGTGGAAGAGTGG-3′; SHP forward, 5′-AGGGAGGCCTTGGATGTC-3′, SHP reverse, 5′-AGAAGGACGGCAGGTTCC-3′; apoA-I forward, 5′-TGGCTGTGCTCTTCCTGACC-3′, and apoA-I reverse, 5′-CTCTGCCGCTGTCTTTCACC-3′.
Statistics
Significant differences between control and treated groups were assessed by one-way ANOVA followed by Dunnett's procedure for posthoc comparisons using JMP6 software. Significance was defined as p value less than 0.05.
RESULTS
Potency and selectivity of FXR ligands
Mice, hamsters, and rats were treated with various FXR agonists that differed in structure and affinity for the human and murine FXR LBD as shown in Table 1 and described in the literature (35–37). Whereas all of the fully synthetic and selective FXR agonists bound to the human and murine FXR LBD with IC50s of ≤0.21 μM and ≤1.1 μM, respectively, 6-ECDCA was 18- and 9-fold less potent, respectively and TCA was inactive against human FXR up to the highest concentration used (100 μM). For comparison, the IC50 of CDCA, the most-potent physiological FXR agonist, and its taurine conjugate were 125 μM and 46 μM, respectively, in the human SPA binding assay. Selectivity of the compounds for FXR was determined versus peroxisome proliferator-activated receptor (PPAR)-α, -β, -δ, liver x receptor (LXR)-α and -β, and retinoid X receptor (RXR)-α for all the compounds used in this study except TCA. None of the compounds tested was able to activate PPAR-, LXR-, or RXR-mediated reporter-gene transcription (data not shown). Selectivity over the human GPBAR1 receptor was also measured. We confirmed a previous report (37) that 6-ECDCA and TCA are agonists of human GPBAR1 (EC50: 1 μM and 23 μM, respectively). None of the other FXR agonists were active against human GPBAR1.
Regulation of apoA-I expression
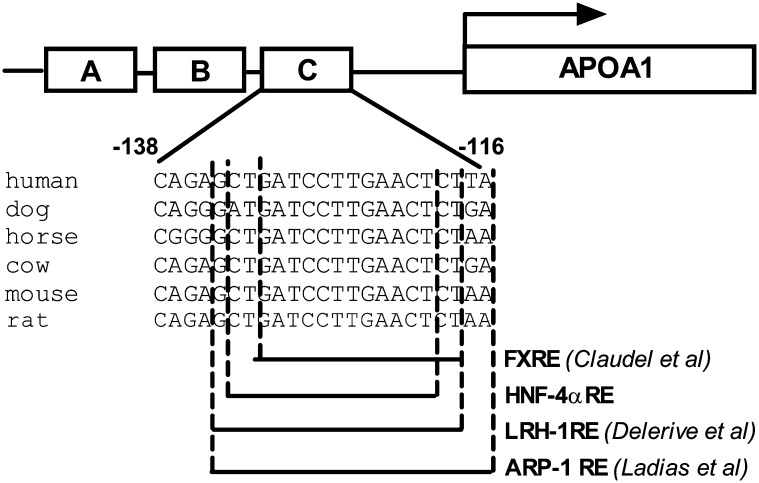
As discussed, the C site of the apoA-I promoter region has been identified as a key element in the regulation of apoA-I transcription. We took a comprehensive comparative bioinformatics approach to identifying conserved noncoding DNA sequences in the promoter region of the apoA-I gene across several species with the assumption that conserved stretches would harbor all authentic transcriptional regulatory elements. BLAST (38) comparisons were performed to provide anchor points for more-sensitive analysis of orthologous genomic regions. The conserved genomic regions were then analyzed using homology-based gene predictions [based on Genewise (39) and polymerase II promoter predictions (Roche, unpublished algorithm)]. The C site was the only region that was well conserved between horses, cows, dogs, humans, rats, and mice (Fig. 1). The fact that the comparative genomic approach did not identify any other well-conserved regions in the apoA-I promoter region reinforced the importance of site C in the regulation of apoA-I expression. It is also interesting to note that our comparative genomic software identified this region (−134 GCTGATCCTTGAACT−120) of the C site as an HNF-4α binding site based on similarity to the matrix M00638 from TransFac (40). The same sequence is included in the region (−132 TGATCCTTGAACTCTAAGTTCCAC−109) in the C site identified by Chan, Nakabayashi, and Wong (41) as an HNF-4α binding site. It is reported that the portion of the C site (−132 TGATCCTTGAACT−120) that we and Chan, Nakabayashi, and Wong (41), Delerive et al. (11), and Claudel et al. (13) identified as an HNF-4α, LRH-1, and FXR binding site, respectively, also binds ARP-1 (12), a transcription factor that is known to downregulate apoA-I expression.
Fig. 1.
Nucleotide sequence conservation of the C site of the apoA-I promoter region. Alignment of the conserved regulatory element from the human, mouse, rat, dog, horse, and cow C site in the apoA-I promoter region. FXRE was identified by Claudel et al. (13), LRH1-RE, by Delerive et al. (11). The HNF-4α-responsive element was identified using TransFac (40).
Effect of synthetic FXR agonists and TCA in human apoA-I-transgenic mice
Male and female human apoA-I-transgenic mice were treated with either vehicle or one of either four or three structurally diverse FXR agonists, respectively, or TCA for 5 days. Food intake and body weight gain were unaffected by treatment. Hepatic human and murine apoA-I and murine SHP mRNA levels as well as plasma human apoA-I , cholesterol, HDL-C and lipoprotein profiles were measured (Figs. 2, 3).
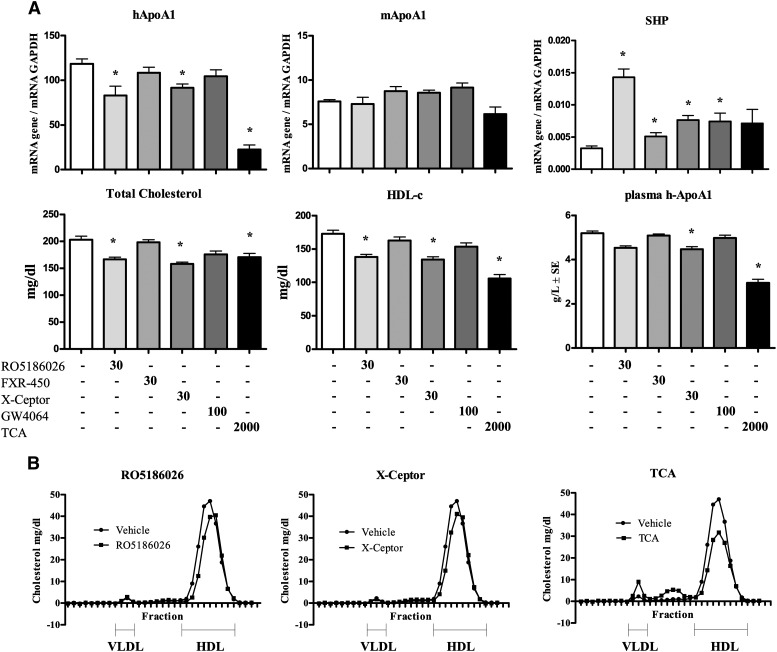
Fig. 2.
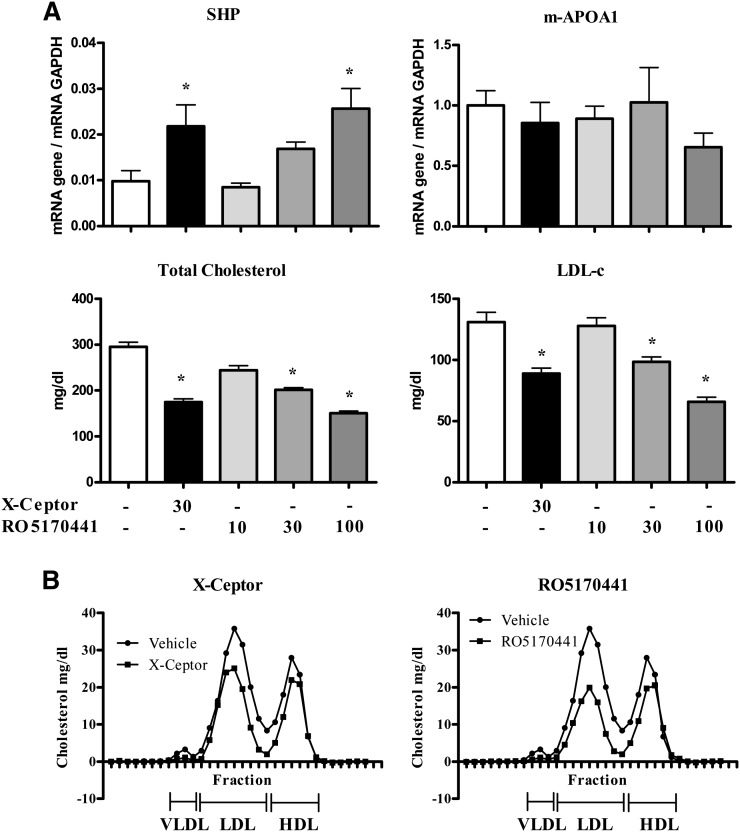
Hepatic gene expression and plasma lipid parameters in male human apoA-I-transgenic mice treated daily for 5 days with either vehicle, RO5186026 (30 mg/kg), FXR-450 (30 mg/kg), the X-Ceptor compound (30 mg/kg), GW4064 (100 mg/kg), or TCA (2,000 mg/kg). A: Human and murine apoA-I and SHP expression was measured by quantitative PCR and normalized to GAPDH (N = 6/grp). Plasma total cholesterol, HDL-C, and human apoA-I levels were measured as described in Materials and Methods (N = 6/grp). Significant differences between the experimental groups (* P < 0.05) were determined by ANOVA followed by a Dunnett's T-test. Values are means ± SD. B: Pooled plasma lipoprotein FPLC profiles of mice treated with either vehicle, RO5186026, the X-Ceptor compound, or TCA.
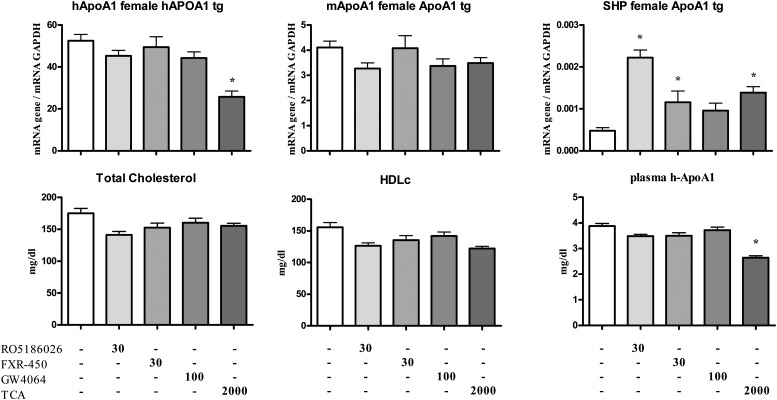
Fig. 3.
Hepatic gene expression and plasma lipid parameters in female human apoA-I-transgenic mice treated daily for 5 days with either vehicle, RO5186026 (30 mg/kg), FXR-450 (30 mg/kg), GW4064 (100 mg/kg), or TCA (2,000 mg/kg). Human and murine apoA-I and SHP expression was measured by quantitative PCR and normalized to GAPDH (N = 6/grp). Plasma total cholesterol, HDL-C, and human apoA-I levels were measured as described in Materials and Methods (N = 6/grp). Significant differences between the experimental groups (* P < 0.05) were determined by ANOVA followed by a Dunnett's T-test. Values are means ±SE.
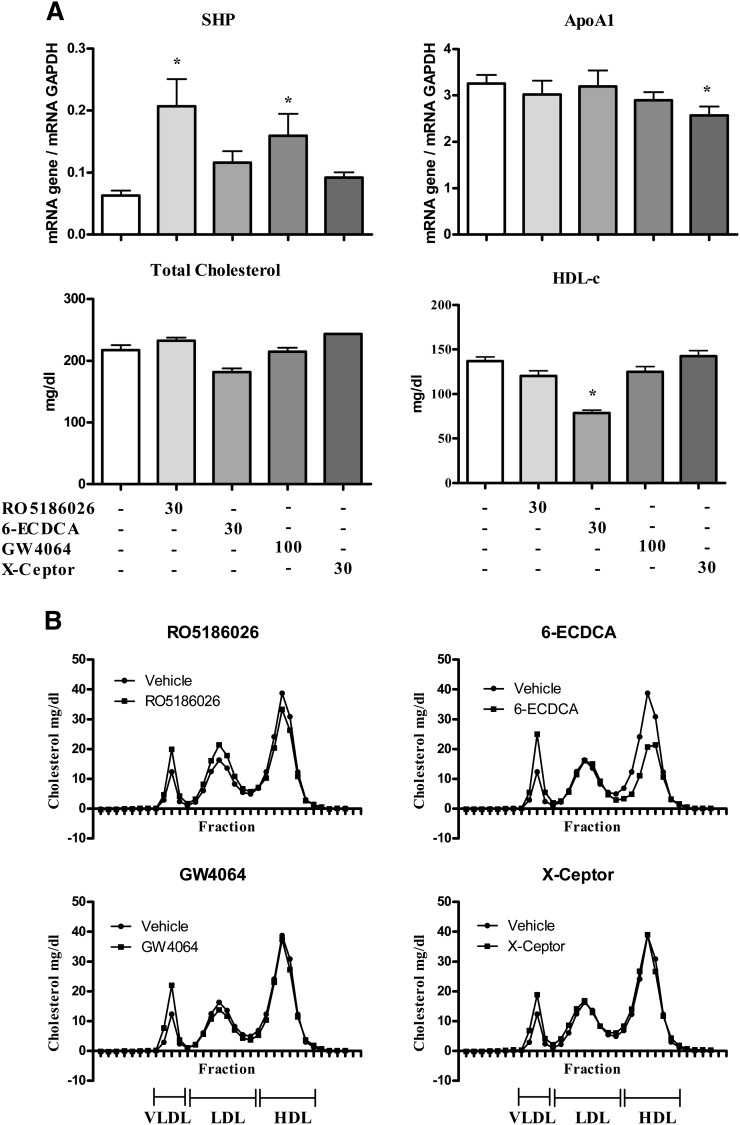
TCA increased SHP expression by ∼3-fold in female mice but the trend for an increase in males was not statistically significant. RO5186026 was the most potent FXR activator increasing SHP expression by ∼4- to 5-fold in both sexes (Figs. 2A and 3). FXR-450, the X-Ceptor compound and GW4064 were moderately active in the male mice, increasing SHP expression by ∼2-fold. In female mice FXR-450 increased SHP expression by 2.2-fold, whereas the trend for an increase caused by GW4064 was not statistically significant.
As reported in studies on female transgenic mice, TCA strongly decreased human apoA-I expression in both male and female mice (81% and 51%, respectively). Of the selective FXR agonists, RO5186026 and the X-Ceptor compound were weakly active in males, decreasing human apoA-I mRNA levels by 30% and 23%, respectively, whereas none of the selective agonists affected human apoA-I expression significantly in females. Surprisingly, considering the identical sequence of site C in humans and mice and previously literature reports, none of the compounds affected the expression of murine apoA-I mRNA in either sex [or in male LDLr−/− mice (see below)].
As might be expected from the human apoA-I gene expression results, TCA strongly decreased human apoA-I in the plasma of both male and female mice (41% and 34%, respectively). Of the synthetic FXR agonists, only the X-ceptor compound caused a small (14%) decrease in plasma human apoA-I in male mice.
Although TCA had no effect on plasma total and HDL-C in female mice, these parameters were decreased by 16% and 39%, respectively, in the males, an effect that appeared to be associated with an increase in VLDL-C and LDL-C (Fig. 2B). Also in male mice, RO5186026 and the X-Ceptor compound caused small decreases in total- (−18% and −22%, respectively) and HDL-C (−20% and −22%, respectively; see also Fig. 2B), whreas GW4064 and FXR-450 had no effect on these plasma lipid parameters. In female mice, none of the selective FXR agonists affected plasma total or HDL-C significantly.
Effect of synthetic FXR agonists in LDLr−/− mice
We determined the effect of FXR agonists in male LDLr −/− mice, a model that has been shown to respond to LDL-C modifying drugs including FXR agonists (42, 43). LDLr −/− mice were treated for 5 days with the X-Ceptor compound or various doses of RO5170441, a potent FXR agonist (Fig. 4). Food intake and body weight gain were unaffected by treatment. RO5170441 caused a dose-dependent decrease in plasma total cholesterol (−24% and −41% at 30 mg/kg and 100 mg/kg, respectively) and LDL-C (−27% and −49% at 30 mg/kg and 100 mg/kg, respectively) and a weak decrease in HDL-C, as shown by FPLC profiles (Fig. 4B). The X-Ceptor compound (30 mg/kg) produced similar effects (Fig. 4A), with a strong reduction in plasma total cholesterol (−34%) and LDL-C (−37%) and a weak decrease in HDL-C (Fig. 4B). The X-Ceptor compound caused a 2.5-fold increase in hepatic SHP expression, comparable to its effect in male human apoA-I-transgenic mice. RO5170441 dose-dependently increased the expression of SHP up to 2.6-fold at 100 mg/kg. Even though both compounds activated FXR-mediated transcription of SHP, yet again there was no change in the expression of murine apoA-I (Fig. 4).
Fig. 4.
Hepatic gene expression and plasma lipid parameters in male LDLr−/− mice were treated daily for 5 days with either vehicle, the X-Ceptor compound (30 mg/kg), or RO5170441 (10, 30, or 100 mg/kg). A: Murine apoA-I and SHP expression was measured by quantitative PCR and normalized to GAPDH (N = 6/grp). Plasma total cholesterol and LDL-C levels in mice treated with FXR agonists were measured as described in Materials and Methods (N = 6/grp). Significant differences between the experimental groups (*P < 0.05) were determined by ANOVA, followed by a Dunnett's T-test. Values are means ±SD. B: Pooled plasma lipoprotein FPLC profiles of LDLr−/− mice treated with either vehicle, the X-ceptor compound, or RO5170441 (100 mg/kg/d).
Effect of synthetic FXR agonists in hamsters
We investigated the potential regulation of apoA-I by FXR agonists in male hamsters. Animals were treated for 12 days with either RO5186026, 6-ECDCA, GW4064, or the X-Ceptor compound (Fig. 5). Food intake and body weight gain were unaffected by treatment. RO5186026 was the most-potent FXR agonist activating SHP expression by 3.2-fold, followed by GW4064 (2.5-fold, Fig. 5A). The X-Ceptor compound and 6-ECDCA did not modulate the expression of SHP. However, although the more-potent FXR agonists had no effect on apoA-I expression, the X-ceptor compound caused a small decrease (−21%, Fig. 5A). None of the treatments affected total plasma cholesterol levels in the hamsters (Fig. 5A). Interestingly, 6-ECDCA exhibited a slightly different profile from those of the other FXR agonists by decreasing HDL-C (Figs. 5A, B) while potentially increasing VLDL-C.
Fig. 5.
Hepatic gene expression and plasma lipid parameters in male Golden Syrian hamsters treated daily for 12 days with either vehicle, RO5186026 (30 mg/kg), 6-ECDCA (30 mg/kg), GW4064 (100 mg/kg), or the X-ceptor compound (30 mg/kg). A: ApoA-I and SHP expression was measured by quantitative PCR and normalized to GAPDH (N = 6/grp). Plasma total cholesterol and HDL-C levels were measured as described in Materials and Methods (N = 6/grp). Significant differences (* P < 0.05) were determined by ANOVA, followed by Dunnett's T-test). Values are means ±SD. B: Pooled plasma lipoprotein FPLC profiles of vehicle or compound-treated hamsters.
Effect of synthetic FXR agonists in rats
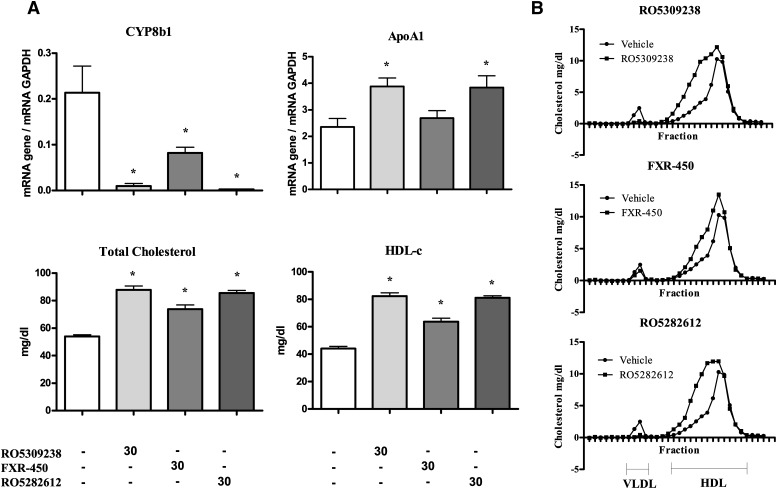
Rats were treated for 14 days with either FXR-450, RO5282612, or RO5309238. FXR activation by all three compounds was assessed indirectly by measuring the inhibition of CYP8B1 expression (Fig. 6A). FXR-450 was the least-potent compound, repressing CYP8B1 expression by 62%, whereas RO5309238 and RO5282612 dramatically reduced the expression of CYP8B1, by 95% and 99%, respectively. Food intake and body weight gain were unaffected by treatment. Interestingly, the most-potent compounds, RO5309238 and RO5282612, caused significant (63%) increases in apoA-I expression The changes in apoA-I expression were mirrored by increases in plasma total cholesterol and HDL-C levels. FPLC profile analysis revealed that the increase in total cholesterol was mainly caused by an increase in large HDL-C, as shown by the displacement of the HDL peak to the left on treatment (Fig. 6B). A decrease in VLDL was also detected with the more-potent FXR agonists.
Fig. 6.
Hepatic gene expression and plasma lipid parameters in male Wistar rats treated daily for 14 days with either vehicle, RO5309238 (30 mg/kg), FXR-450 (30 mg/kg), or RO5282612 (30 mg/kg). A: ApoA-I and CYP8B1 expression was measured by quantitative PCR and normalized to GAPDH (N = 5/grp). Plasma total cholesterol and HDL-C levels were measured as described in Materials and Methods (N = 5/grp). Significant differences between the experimental groups (* P < 0.05) were determined by ANOVA, followed by a Dunnett's T-test). Values are means ±SD. B: Pooled plasma lipoprotein FPLC profiles of vehicle and compound-treated rats.
DISCUSSION
Low levels of plasma HDL (cholesterol) and of apoA-I, its major apolipoprotein, are associated with an increased risk of coronary heart disease. It has long been reported that feeding CA or its taurine conjugate to mice reduces hepatic apoA-I mRNA and plasma apoA-I and HDL-C. For example, Srivastava, Srivastava, and Averna (44) showed that feeding wild-type or human apoA-I-transgenic mice (gender not stated) with 1% CA led to an ∼18% and 36% to 54% decrease in hepatic murine apoA-I or human apoA-I mRNA, respectively, and a 30% and ∼60% decrease in plasma murine apoA-I or human apoA-I protein, respectively. Subsequently, the decrease in apoA-I expression was claimed to be mediated by CA acting as an FXR agonist. Claudel et al. (13) reported a marked decrease in human and murine apoA-I mRNA and human plasma apoA-I (∼80%) in female human apoA-I-transgenic mice fed 0.5% TCA in the diet. They proposed the unusual mechanism of FXR binding as a monomer to the C site in the apoA-I promoter leading to repression of its expression. On the other hand, Delerive et al. (11) reported multiple in vitro studies that supported their hypothesis that the C site contains a recognition sequence for LRH-1 and that downregulation of apoA-I expression by FXR agonists is mediated, via inhibition of LRH-1-regulated transcription, by SHP. This hypothesis was, however, not supported by a study they conducted in mice in which hepatic overexpression of LRH-1 had no effect on apoA-I expression. Also, contrary to what might be expected of this hypothesis, lipoprotein profiles are reported to be unchanged in SHP−/− mice (45), apoA-I was even slightly lower or unchanged in FXR−/− mice (20, 46), and HDL-C was unchanged in hepatic LRH-1−/− mice (47).
It is interesting to note that the FXR agonist used in vivo in these studies was CA or its taurine conjugate. As previously reported by others (16, 48), we demonstrated that CA does not bind to FXR itself. It is, however, likely that the livers of mice fed CA will contain increased amounts of its bacterial metabolite, deoxycholic acid (49), a weak FXR agonist (16, 50) (IC50 of TDCA = 9 μM in our human FXR SPA). Feeding CA could, therefore, indirectly increase FXR agonist activity in vivo. Alternatively, CA could downregulate the expression of apoA-I via mechanisms other than through FXR, e.g., via ARP-1, a transcription factor that also binds to an overlapping section of the C site, as demonstrated by Ladias and Karathanasis (12) and Srivastava, Srivastava, and Averna (44). Treatment of healthy volunteers or gallstone or cerebrotendinous xanthomatosis patients with deoxycholic acid (DCA) or CDCA, the most potent of the bile acid FXR agonists, had no effect on hepatic apoA-I mRNA expression and plasma apoA-I and either no or minimal effect on HDL-C (23–29, 51). The only exception to these almost exclusively negative findings that we are aware of was a reported 46% decrease in HDL-C with a high dose CDCA (1 g/d) (22). The reason for this discrepancy is not known, although it may relate to the high dose of CDCA used and/or the method of HDL-C determination.
The generation of potent and selective FXR agonists that are active in vivo has made it possible to fully evaluate this hypothesis. Interestingly, Evans et al. (52) recently reported that the potent and selective FXR agonist FXR-450 did not change hepatic apoA-I mRNA levels in LDLr−/− and human apoA-I-transgenic mice and in rats, thus casting further doubt on the role of FXR in the regulation of apoA-I expression. We extended this finding by comparing the effect of feeding 1% (dry w/w) TCA with that of pharmacological doses of FXR-450 and other potent and selective FXR agonists on hepatic apoA-I expression in vivo.
Potency and selectivity of the FXR agonists in vitro
The two bile acid derivatives 6-ECDCA and TCA were shown to have either low or no affinity, respectively, for FXR in the SPA. However, as reported elsewhere, 6-ECDCA and TCA exhibit polypharmacology, being active against GPBAR1 as well as FXR (53). On the other hand, FXR-450, GW4064, the X-ceptor compound, and the benzimidazoles RO5186026, RO5170441, RO5282612, and RO5309238 were highly active against FXR in vitro and in vivo but inactive against other nuclear hormone receptors and GPBAR1.
Comparative genomics of the apoA-I promoter C site
The C site of the apoA-I promoter was identified previously in in vitro studies as a key element in the regulation of apoA-I transcription by FXR. We showed that the C site is the only region in the promoter region of apoA-I that was almost completely conserved between mice, rats, horses, dogs, cows, and humans. If FXR is involved in regulating the expression of apoA-I via the C site, as proposed by Delerive et al. (11) and Claudel et al. (13), the homology of this promoter sequence would suggest that the regulation of gene expression should be similar in all these species and, of course, in both sexes. However, this was not the case. Even within the same strain of apoA-I-transgenic mouse, we showed that TCA, a bile acid that did not bind to human FXR in our SPA, caused a substantial decrease of human apoA-I expression while having no effect on murine apoA-I expression. On the other hand, compounds that were as or more potent and more-selective FXR agonists in vivo had little or mostly no effect on human apoA-I, mouse apoA-I, or hamster apoA-I expression, whereas in rats, they even increased rat apoA-I expression. Comparative genomic analysis of the C site of the apoA-I promoter led us to characterize it as an HNF-4α binding site. This is in agreement with a previous report by Chan, Nakabayashi, and Wong (41). The control of HNF-4α-induced upregulation of gene expression, including that of apoA-I, is complex (2). Repression of HNF-4α-induced transcription has been ascribed to increased expression of the nuclear hormone SHP, potentially via FXR activation, or of ARP-1 (2, 54). As described above and by Srivastava and Srivastava (2), ARP-1 has been shown to bind to the same sequence in site C (and in site A) as HNF-4α, and so the two could directly compete to repress or induce expression, respectively. For the reasons given above, it is unlikely that TCA regulates apoA-I expression through SHP-mediated downmodulation of HNF-4α activity, inasmuch as this would be FXR dependent. However, it is possible that TCA induces an increase in the proportion of ARP-1 to HNF-4α protein, their relative activities, or their interaction, which leads to a reduction in apoA-I expression. Interestingly, in hepatic HNF-4α, conditional knockout mice, even though plasma triglycerides, TC, LDL-C, and HDL-C were dramatically decreased, this was not accompanied by a decrease in hepatic apoA-I mRNA or plasma protein (55). This suggests that hepatic HNF-4α might not be involved in the regulation of apoA-I expression by TCA in vivo.
Dose of CA versus bile acid pool size and synthetic rate
The dose of CA or TCA required to decrease the expression of apoA-I in mice is very high, typically 0.5%–1% of the diet. For example, the 1% dietary TCA used in the present study equated to a dose of ∼2,000 mg/kg/d, ∼50 mg per mouse per day, or ∼400 μmol/100g body weight/d. This is four to six times the normal bile acid pool size (62.5–100 μmol/100g body weight) given every day and ∼36 to 48 times the normal bile acid synthetic rate (8.3–11 μmol/d/100 g body weight) in untreated mice (56, 57). Because the doses of CA or TCA used in these studies are so much higher than the synthetic rate of bile acid that is normally sufficient to maintain bile acid pool size, it is quite possible that any changes in the regulation of gene expression that are induced (e.g., of apoA-I ) could be attributable to nonphysiological mechanisms.
Sex and strain effects
Claudel et al. (13) used female human apoA-I-transgenic mice on a C57BL/6 background and noted a decrease in both human and murine apoA-I expression on feeding TCA, whereas we used male and female transgenic mice of the same strain fed TCA and, although we could replicate the effect they had seen on human apoA-I expression, we saw no effect on murine apoA-I expression in either sex. Paigen et al. (58) reported that female C57BL mice fed an atherogenic diet containing 0.5% CA also responded with a large downregulation of HDL-C, whereas males did not (50% vs. ∼0% decrease). Interestingly, testosterone treatment of female C57BL mice reduced the CA-induced decrease in HDL-C. Fuchs et al. (59) also reported no change in apoA-I mRNA or protein in male C57BL mice fed a high-cholesterol diet containing 0.5% CA, whereas Cyp7A1, a marker of FXR activation, was downregulated (−47%), as expected. Lastly, Masson et al. (60) did manage to induce a decrease in hepatic apoA-I mRNA and in plasma apoA-I in male 129/C57BL fed 1% CA. These reports suggest that female C57BL mice may be more sensitive than males to the downregulation of murine apoA-I by CA. An alternative explanation is that the effect in both sexes is just inconsistent. We have no explanation for the inconsistency of what is likely, for the reasons provided, to be a nonphysiological response. Curiously, downregulation of the human apoA-I transgene in mice by TCA appears to occur consistently in both sexes. However, it should be noted that the human gene was 16-fold more highly expressed than the endogenous mouse gene, so it may be more sensitive to regulation, as was proposed by Srivastava and Srivastava (2). Others have also shown that the regulation of apoA-I by CA containing high-fat diets is dependent on the strain of mouse, with female C57BL/6 mice showing big decreases in hepatic apoA-I mRNA or plasma apoA-I, as expected, whereas these parameters were either increased or unchanged in female C3H or BALB/c mice (61, 62). The literature, therefore, only supports an inconsistent suppressive effect of supraphysiological doses of CA and not of selective FXR agonists on murine apoA-I expression in vivo, in C57BL/6 mice. Suppression of human apoA-I expression in transgenic mice by CA is substantial and may be more consistent, but is not mediated via FXR.
Because hamsters, rats, and mice of different sexes and strains carrying the same regulatory C site on the apoA-I gene do not respond consistently to FXR agonist treatment, we contend that this adds to the substantial literature evidence against earlier hypotheses that FXR agonists suppress apoA-I expression.
In summary, we report here that the C site in the apoA-I promoter, through which FXR is believed to regulate apoA-I expression, is almost completely conserved across several mammalian species, including humans, mice, and rats. Treatment of human apoA-I-transgenic mice with the very weak FXR agonist TCA substantially repressed human apoA-I expression, but, surprisingly, not murine apoA-I expression. Treatment with structurally diverse compounds that were as potent or more potent and more-selective FXR agonists than TCA had little or mostly no effect on human apoA-I mRNA or human plasma protein levels and no effect on murine apoA-I mRNA. Also, selective FXR agonists did not change apoA-I expression in LDLR−/− mice or in hamsters and even increased apoA-I expression in rats. Hence, we saw no correlation between the increased transcription of FXR target genes and the repression of apoA-I expression in vivo. These data support the hypothesis that the strong inhibition of human apoA-I expression by supraphysiological doses of TCA in transgenic mice is driven by mechanisms other than via FXR. Inhibition of apoA-I expression is not, therefore, a property associated with activation of FXR by specific and potent agonists and should not hinder their development as therapeutic agents.
Acknowledgments
The authors wish to thank Franz Schuler for his strong support in this work, as well as Astride Schnoebelen, Anita van der Klooster, Marie-Thérèse Traendlin, and Heinz Meyer for their expert technical support with the GPBAR1 binding and FXR scintillation proximity binding assays and animal pharmacology.
Footnotes
Abbreviations:
- ARP-1
- apolipoprotein A-I regulatory protein 1
- CA
- cholic acid
- CDCA
- chenodeoxycholic acid
- CYP8B1
- sterol-12α hydroxylase
- DCA
- deoxycholic acid
- FPLC
- fast-protein liquid chromatography
- FXR
- farnesoid X receptor
- FXRE
- FXR response element
- HDL-C
- HDL-cholesterol
- HNF-4α
- hepatic nuclear factor 4-alpha
- LBD
- ligand binding domain
- LDL-C
- LDL-cholesterol
- LRH-1
- liver receptor homolog 1
- RXR-α
- retinoid-X receptor alpha
- SHP
- small heterodimer partner
- SPA
- scintillation proximity assay
- TCA
- taurocholic acid
REFERENCES
- 1.Hokanson J. E., Austin M. A. 1996. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. J. Cardiovasc. Risk. 3: 213–219. [PubMed] [Google Scholar]
- 2.Srivastava R. A., Srivastava N. 2000. High density lipoprotein, apolipoprotein A-I, and coronary artery disease. Mol. Cell. Biochem. 209: 131–144. [DOI] [PubMed] [Google Scholar]
- 3.Elshourbagy N. A., Boguski M. S., Liao W. S., Jefferson L. S., Gordon J. I., Taylor J. M. 1985. Expression of rat apolipoprotein A-IV and A-I genes: mRNA induction during development and in response to glucocorticoids and insulin. Proc. Natl. Acad. Sci. USA. 82: 8242–8246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubin E. M., Krauss R. M., Spangler E. A., Verstuyft J. G., Clift S. M. 1991. Inhibition of early atherogenesis in transgenic mice by human apolipoprotein AI. Nature. 353: 265–267. [DOI] [PubMed] [Google Scholar]
- 5.Williamson R., Lee D., Hagaman J., Maeda N. 1992. Marked reduction of high density lipoprotein cholesterol in mice genetically modified to lack apolipoprotein A-I. Proc. Natl. Acad. Sci. USA. 89: 7134–7138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plump A. S., Scott C. J., Breslow J. L. 1994. Human apolipoprotein A-I gene expression increases high density lipoprotein and suppresses atherosclerosis in the apolipoprotein E-deficient mouse. Proc. Natl. Acad. Sci. USA. 91: 9607–9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmitz G., Kaminski W. E., Orso E. 2000. ABC transporters in cellular lipid trafficking. Curr. Opin. Lipidol. 11: 493–501. [DOI] [PubMed] [Google Scholar]
- 8.Staels B., Auwerx J. 1998. Regulation of apo A-I gene expression by fibrates. Atherosclerosis. 137: S19–S23. [DOI] [PubMed] [Google Scholar]
- 9.Kockx M., Princen H. M., Kooistra T. 1998. Fibrate-modulated expression of fibrinogen, plasminogen activator inhibitor-1 and apolipoprotein A-I in cultured cynomolgus monkey hepatocytes–role of the peroxisome proliferator-activated receptor-alpha. Thromb. Haemost. 80: 942–948. [PubMed] [Google Scholar]
- 10.Hertz R., Sheena V., Kalderon B., Berman I., Bar-Tana J. 2001. Suppression of hepatocyte nuclear factor-4alpha by acyl-CoA thioesters of hypolipidemic peroxisome proliferators. Biochem. Pharmacol. 61: 1057–1062. [DOI] [PubMed] [Google Scholar]
- 11.Delerive P., Galardi C. M., Bisi J. E., Nicodeme E., Goodwin B. 2004. Identification of liver receptor homolog-1 as a novel regulator of apolipoprotein AI gene transcription. Mol. Endocrinol. 18: 2378–2387. [DOI] [PubMed] [Google Scholar]
- 12.Ladias J. A., Karathanasis S. K. 1991. Regulation of the apolipoprotein AI gene by ARP-1, a novel member of the steroid receptor superfamily. Science. 251: 561–565. [DOI] [PubMed] [Google Scholar]
- 13.Claudel T., Sturm E., Duez H., Torra I. P., Sirvent A., Kosykh V., Fruchart J. C., Dallongeville J., Hum D. W., Kuipers F., et al. 2002. Bile acid-activated nuclear receptor FXR suppresses apolipoprotein A-I transcription via a negative FXR response element. J. Clin. Invest. 109: 961–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forman B. M., Goode E., Chen J., Oro A. E., Bradley D. J., Perlmann T., Noonan D. J., Burka L. T., McMorris T., Lamph W. W., et al. 1995. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell. 81: 687–693. [DOI] [PubMed] [Google Scholar]
- 15.Seol W., Choi H. S., Moore D. D. 1995. Isolation of proteins that interact specifically with the retinoid X receptor: two novel orphan receptors. Mol. Endocrinol. 9: 72–85. [DOI] [PubMed] [Google Scholar]
- 16.Parks D. J., Blanchard S. G., Bledsoe R. K., Chandra G., Consler T. G., Kliewer S. A., Stimmel J. B., Willson T. M., Zavacki A. M., Moore D. D., et al. 1999. Bile acids: natural ligands for an orphan nuclear receptor. Science. 284: 1365–1368. [DOI] [PubMed] [Google Scholar]
- 17.Lu T. T., Makishima M., Repa J. J., Schoonjans K., Kerr T. A., Auwerx J., Mangelsdorf D. J. 2000. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol. Cell. 6: 507–515. [DOI] [PubMed] [Google Scholar]
- 18.Goodwin B., Jones S. A., Price R. R., Watson M. A., McKee D. D., Moore L. B., Galardi C., Wilson J. G., Lewis M. C., Roth M. E., et al. 2000. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol. Cell. 6: 517–526. [DOI] [PubMed] [Google Scholar]
- 19.del Castillo-Olivares A., Gil G. 2001. Suppression of sterol 12alpha-hydroxylase transcription by the short heterodimer partner: insights into the repression mechanism. Nucleic Acids Res. 29: 4035–4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sinal C. J., Tohkin M., Miyata M., Ward J. M., Lambert G., Gonzalez F. J. 2000. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 102: 731–744. [DOI] [PubMed] [Google Scholar]
- 21.Fiorucci S., Mencarelli A., Palladino G., Cipriani S. 2009. Bile-acid-activated receptors: targeting TGR5 and farnesoid-X-receptor in lipid and glucose disorders. Trends Pharmacol. Sci. 30: 570–580. [DOI] [PubMed] [Google Scholar]
- 22.Leiss O., von Bergmann K. 1982. Different effects of chenodeoxycholic acid and ursodeoxycholic acid on serum lipoprotein concentrations in patients with radiolucent gallstones. Scand. J. Gastroenterol. 17: 587–592. [DOI] [PubMed] [Google Scholar]
- 23.Albers J. J., Grundy S. M., Cleary P. A., Small D. M., Lachin J. M., Schoenfield L. J. 1982. National Cooperative Gallstone Study: the effect of chenodeoxycholic acid on lipoproteins and apolipoproteins. Gastroenterology. 82: 638–646. [PubMed] [Google Scholar]
- 24.Bateson M. C., Iqbal J. 1979. Chenodeoxycholic acid, postprandial serum-triglycerides, and H.D.L. cholesterol. Lancet. 1: 930. [DOI] [PubMed] [Google Scholar]
- 25.Einarsson C., Hillebrant C. G., Axelson M. 2001. Effects of treatment with deoxycholic acid and chenodeoxycholic acid on the hepatic synthesis of cholesterol and bile acids in healthy subjects. Hepatology. 33: 1189–1193. [DOI] [PubMed] [Google Scholar]
- 26.Hillebrant C., Nyberg B., Angelin B., Axelson M., Bjorkhem I., Rudling M., Einarsson C. 1999. Deoxycholic acid treatment in patients with cholesterol gallstones: failure to detect a suppression of cholesterol 7alpha-hydroxylase activity. J. Intern. Med. 246: 399–407. [DOI] [PubMed] [Google Scholar]
- 27.Kuriyama M., Tokimura Y., Fujiyama J., Utatsu Y., Osame M. 1994. Treatment of cerebrotendinous xanthomatosis: effects of chenodeoxycholic acid, pravastatin, and combined use. J. Neurol. Sci. 125: 22–28. [DOI] [PubMed] [Google Scholar]
- 28.Nilsson L. M., Abrahamsson A., Sahlin S., Gustafsson U., Angelin B., Parini P., Einarsson C. 2009. Bile acids and lipoprotein metabolism: effects of cholestyramine and chenodeoxycholic acid on human hepatic mRNA expression. Biochem. Biophys. Res. Commun. 357: 707–711.. [DOI] [PubMed] [Google Scholar]
- 29.Perez-Aguilar F., Breto M., Alegre B., Berenguer J. 1985. Increase in serum total cholesterol and low-density lipoprotein cholesterol by high-dose chenodeoxycholic acid in patients with radiolucent gallstones significantly reversed during preventive low dose after gallstone dissolution. Digestion. 31: 225–233. [DOI] [PubMed] [Google Scholar]
- 30.Schoenfield L. J., Lachin J. M. 1981. Chenodiol (chenodeoxycholic acid) for dissolution of gallstones: the National Cooperative Gallstone Study. A controlled trial of efficacy and safety. Ann. Intern. Med. 95: 257–282. [DOI] [PubMed] [Google Scholar]
- 31.Wang D. Q., Tazuma S., Cohen D. E., Carey M. C. 2003. Feeding natural hydrophilic bile acids inhibits intestinal cholesterol absorption: studies in the gallstone-susceptible mouse. Am. J. Physiol. Gastrointest. Liver Physiol. 285: G494–502. [DOI] [PubMed] [Google Scholar]
- 32.Lundquist J. T., Harnish D. C., Kim C. Y., Mehlmann J. F., Unwalla R. J., Phipps K. M., Crawley M. L., Commons T., Green D. M., Xu W., et al. 2010. Improvement of physiochemical properties of the tetrahydroazepinoindole series of farnesoid X receptor (FXR) agonists: beneficial modulation of lipids in primates. J. Med. Chem. 53: 1774–1787. [DOI] [PubMed] [Google Scholar]
- 33.Fiorucci S., Mencarelli A., Distrutti E., Palladino G., Cipriani S. 2010. Targetting farnesoid-X-receptor: from medicinal chemistry to disease treatment. Curr. Med. Chem. 17: 139–159. [DOI] [PubMed] [Google Scholar]
- 34.Heider J. G., Boyett R. L. 1978. The picomole determination of free and total cholesterol in cells in culture. J. Lipid Res. 19: 514–518. [PubMed] [Google Scholar]
- 35.Flatt B., Martin R., Wang T. L., Mahaney P., Murphy B., Gu X. H., Foster P., Li J., Pircher P., Petrowski M., et al. 2009. Discovery of XL335 (WAY-362450), a highly potent, selective, and orally active agonist of the farnesoid X receptor (FXR). J. Med. Chem. 52: 904–907. [DOI] [PubMed] [Google Scholar]
- 36.Maloney P. R., Parks D. J., Haffner C. D., Fivush A. M., Chandra G., Plunket K. D., Creech K. L., Moore L. B., Wilson J. G., Lewis M. C., et al. 2000. Identification of a chemical tool for the orphan nuclear receptor FXR. J. Med. Chem. 43: 2971–2974. [DOI] [PubMed] [Google Scholar]
- 37.Pellicciari R., Fiorucci S., Camaioni E., Clerici C., Costantino G., Maloney P. R., Morelli A., Parks D. J., Willson T. M. 2002. 6Alpha-ethyl-chenodeoxycholic acid (6-ECDCA), a potent and selective FXR agonist endowed with anticholestatic activity. J. Med. Chem. 45: 3569–3572. [DOI] [PubMed] [Google Scholar]
- 38.Altschul S. F., Madden T. L., Schaffer A. A., Zhang J., Zhang Z., Miller W., Lipman D. J. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25: 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Birney E., Clamp M., Durbin R. 2004. GeneWise and Genomewise. Genome Res. 14: 988–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matys V., Kel-Margoulis O. V., Fricke E., Liebich I., Land S., Barre-Dirrie A., Reuter I., Chekmenev D., Krull M., Hornischer K., et al. 2006. TRANSFAC and its module TRANSCompel: transcriptional gene regulation in eukaryotes. Nucleic Acids Res. 34: D108–D110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chan J., Nakabayashi H., Wong N. C. 1993. HNF-4 increases activity of the rat apo A1 gene. Nucleic Acids Res. 21: 1205–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Repa J. J., Turley S. D., Quan G., Dietschy J. M. 2005. Delineation of molecular changes in intrahepatic cholesterol metabolism resulting from diminished cholesterol absorption. J. Lipid Res. 46: 779–789. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y. X., Martin-McNulty B., Huw L. Y., da Cunha V., Post J., Hinchman J., Vergona R., Sullivan M. E., Dole W., Kauser K. 2002. Anti-atherosclerotic effect of simvastatin depends on the presence of apolipoprotein E. Atherosclerosis. 162: 23–31. [DOI] [PubMed] [Google Scholar]
- 44.Srivastava R. A., Srivastava N., Averna M. 2000. Dietary cholic acid lowers plasma levels of mouse and human apolipoprotein A-I primarily via a transcriptional mechanism. Eur. J. Biochem. 267: 4272–4280. [DOI] [PubMed] [Google Scholar]
- 45.Kerr T. A., Saeki S., Schneider M., Schaefer K., Berdy S., Redder T., Shan B., Russell D. W., Schwarz M. 2002. Loss of nuclear receptor SHP impairs but does not eliminate negative feedback regulation of bile acid synthesis. Dev. Cell. 2: 713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lambert G., Amar M. J., Guo G., Brewer H. B., Jr, Gonzalez F. J., Sinal C. J. 2003. The farnesoid X-receptor is an essential regulator of cholesterol homeostasis. J. Biol. Chem. 278: 2563–2570. [DOI] [PubMed] [Google Scholar]
- 47.Mataki C., Magnier B. C., Houten S. M., Annicotte J. S., Argmann C., Thomas C., Overmars H., Kulik W., Metzger D, Auwerx J., et al. 2007. Compromised intestinal lipid absorption in mice with a liver-specific deficiency of liver receptor homolog 1. Mol. Cell Biol. 27: 8330–8339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crestani M., Karam W. G., Chiang J. Y. 1994. Effects of bile acids and steroid/thyroid hormones on the expression of cholesterol 7 alpha-hydroxylase mRNA and the CYP7 gene in HepG2 cells. Biochem. Biophys. Res. Commun. 198: 546–553. [DOI] [PubMed] [Google Scholar]
- 49.Li-Hawkins J., Gafvels M., Olin M., Lund E. G., Andersson U., Schuster G., Bjorkhem I., Russell D. W., Eggertsen G. 2002. Cholic acid mediates negative feedback regulation of bile acid synthesis in mice. J. Clin. Invest. 110: 1191–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang H., Chen J., Hollister K., Sowers L. C., Forman B. M. 1999. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol. Cell. 3: 543–553. [DOI] [PubMed] [Google Scholar]
- 51.Wang Y., Jones P. J., Woollett L. A., Buckley D. D., Yao L., Granholm N. A., Tolley E. A., Heubi J. E. 2006. Effects of chenodeoxycholic acid and deoxycholic acid on cholesterol absorption and metabolism in humans. Transl. Res. 148: 37–45. [DOI] [PubMed] [Google Scholar]
- 52.Evans M. J., Mahaney P. E., Borges-Marcucci L., Lai K., Wang S., Krueger J. A., Gardell S. J., Huard C., Martinez R., Vlasuk G. P., et al. 2008. A synthetic farnesoid X receptor (FXR) agonist promotes cholesterol lowering in models of dyslipidemia. Am. J. Physiol. Gastrointest. Liver Physiol. 296: G543–G552.. [DOI] [PubMed] [Google Scholar]
- 53.Pellicciari R., Gioiello A., Macchiarulo A., Thomas C., Rosatelli E., Natalini B., Sardella R., Pruzanski M., Roda A., Pastorini E., et al. 2009. Discovery of 6alpha-ethyl-23(S)-methylcholic acid (S-EMCA, INT-777) as a potent and selective agonist for the TGR5 receptor, a novel target for diabesity. J. Med. Chem. 52: 7958–7961. [DOI] [PubMed] [Google Scholar]
- 54.Russell D. W. 2009. Fifty years of advances in bile acid synthesis and metabolism. J. Lipid Res. 50 (Suppl.):120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hayhurst G. P., Lee Y. H., Lambert G., Ward J. M., Gonzalez F. J. 2001. Hepatocyte nuclear factor 4alpha (nuclear receptor 2A1) is essential for maintenance of hepatic gene expression and lipid homeostasis. Mol. Cell. Biol. 21: 1393–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mardones P., Quinones V., Amigo L., Moreno M., Miquel J. F., Schwarz M., Miettinen H. E., Trigatti B., Krieger M., VanPatten S., et al. 2001. Hepatic cholesterol and bile acid metabolism and intestinal cholesterol absorption in scavenger receptor class B type I-deficient mice. J. Lipid Res. 42: 170–180. [PubMed] [Google Scholar]
- 57.Schwarz M., Russell D. W., Dietschy J. M., Turley S. D. 1998. Marked reduction in bile acid synthesis in cholesterol 7alpha-hydroxylase-deficient mice does not lead to diminished tissue cholesterol turnover or to hypercholesterolemia. J. Lipid Res. 39: 1833–1843. [PubMed] [Google Scholar]
- 58.Paigen B., Holmes P. A., Mitchell D., Albee D. 1987. Comparison of atherosclerotic lesions and HDL-lipid levels in male, female, and testosterone-treated female mice from strains C57BL/6, BALB/c, and C3H. Atherosclerosis. 64: 215–221. [DOI] [PubMed] [Google Scholar]
- 59.Fuchs M., Ivandic B., Muller O., Schalla C., Scheibner J., Bartsch P., Stange E. F. 2001. Biliary cholesterol hypersecretion in gallstone-susceptible mice is associated with hepatic up-regulation of the high-density lipoprotein receptor SRBI. Hepatology. 33: 1451–1459. [DOI] [PubMed] [Google Scholar]
- 60.Masson D., Lagrost L., Athias A., Gambert P., Brimer-Cline C., Lan L., Schuetz J. D., E, Schuetz G., Assem M. 2005. Expression of the pregnane X receptor in mice antagonizes the cholic acid-mediated changes in plasma lipoprotein profile. Arterioscler. Thromb. Vasc. Biol. 25: 2164–2169. [DOI] [PubMed] [Google Scholar]
- 61.Dueland S., France D., Wang S. L., Trawick J. D., Davis R. A. 1997. Cholesterol 7alpha-hydroxylase influences the expression of hepatic apoA-I in two inbred mouse strains displaying different susceptibilities to atherosclerosis and in hepatoma cells. J. Lipid Res. 38: 1445–1453. [PubMed] [Google Scholar]
- 62.Ishida B. Y., Blanche P. J., Nichols A. V., Yashar M., Paigen B. 1991. Effects of atherogenic diet consumption on lipoproteins in mouse strains C57BL/6 and C3H. J. Lipid Res. 32: 559–568. [PubMed] [Google Scholar]