Abstract
Phytosterols (plant sterols and stanols) can lower intestinal cholesterol absorption, but the complex dynamics of the lipid digestion process in the presence of phytosterol esters (PEs) are not fully understood. We performed a clinical experiment in intubated healthy subjects to study the time course of changes in the distribution of all lipid moieties present in duodenal phases during 4 h of digestion of meals with 3.2 g PE (PE meal) or without (control meal) PE. In vitro experiments under simulated gastrointestinal conditions were also performed. The addition of PE did not alter triglyceride (TG) hydrolysis in the duodenum or subsequent chylomicron TG occurrence in the circulation. In contrast, cholesterol accumulation in the duodenum aqueous phase was markedly reduced in the presence of PE (−32%, P < 0.10). In vitro experiments confirmed that PE reduces cholesterol transfer into the aqueous phase. The addition of PE resulted in a markedly reduced presence of meal-derived hepta-deuterated cholesterol in the circulation, i.e., in chylomicrons (−43%, PE meal vs. control; P < 0.0001) and plasma (−54%, PE meal vs. control; P < 0.0001). The present data show that addition of PE to a meal does not alter TG hydrolysis but displaces cholesterol from the intestinal aqueous phase and lowers chylomicron cholesterol occurrence in humans.
Keywords: micelles, sitosterol, campesterol, sitostanol, hepta-deuterated cholesterol, isotope enrichment
Phytosterols or plant sterols are present in most plant-based food products and have structural similarity to cholesterol, differing only in aliphatic side chain length and saturation. Sitosterol, campesterol, and stigmasterol have unsaturated moieties in the sterol core (compared with stanols) and are the major plant sterols in human diets. They are mainly provided by vegetable oils, nuts, unrefined grains, and, to a much lower extent, fruits and vegetables (1, 2). Other important dietary sources of plant sterols or stanols are phytosterol-enriched food products (e.g., margarines, which are typically enriched with the fatty acid esters of plant stanols or sterols) and a variety of enriched low-fat food products (e.g., yogurt, milk preparations, and beverages). The consumption of these esterified plant sterol-enriched foods results in LDL cholesterol-lowering effects in various groups and other approved health claims (3, 4). Although the total and LDL cholesterol-lowering effects of plant sterols have been known since the 1950s and repeatedly demonstrated in more recent trials (1), the detailed mechanistic events behind this metabolic phenomenon as occurring in the intestinal lumen are not yet fully understood. In vitro work has suggested that free plant sterols can be dispersed in mixed micelles, thus displacing free cholesterol moieties and resulting in lower cholesterol availability for uptake by the enterocyte apical brush border membrane. Reduced intestinal absorption of cholesterol in the presence of esterified plant sterols has been reported in humans (2–4). Because esterified plant sterols are highly hydrophobic moieties, their physicochemical behavior in the gut should be different from that of amphipathic free plant sterols and cholesterol. As such, esterified sterols are expected to be concentrated in the core of the emulsified fat droplets (5), whereas free sterols should be distributed between the fat droplets’ surface, the vesicles and mixed micelles in the aqueous phase, and insoluble material in the small intestine content (6). In addition, whereas emulsified dietary triglycerides (TGs) are hydrolyzed by gastric and pancreatic lipases, esterified plant sterols or cholesterol can be hydrolyzed by specific pancreatic lipases, such as cholesterol esterase (7). Two studies have described the distribution of various plant stanols and cholesterol in the human distal jejunum content for 1 h after constant infusion of low-fat formula (3). Although these studies provided the first description of intestinal processing of sterols in the presence of plant stanols in humans, the complex dynamics of the overall lipid digestion process and their concomitant efficacy on cholesterol intestinal uptake were not investigated. In addition to luminal transfer of cholesterol into mixed micelles, other molecular mechanisms have been proposed at the intestinal level: mucosal cholesterol efflux involving intestinal LXR-mediated targets (e.g., ABC transporters) (8), cholesterol internalization (NPC1L1) (9), and synthesis (HMG-CoA reductase) and esterification (ACAT) (7). However, the relevance of these molecular mechanisms has not yet been demonstrated in vivo.
The present study was performed in intubated healthy subjects with a unique sophisticated design to determine i) the time course of changes in the distribution of all lipid moieties present in the oily, aqueous, and insoluble phases coexisting in the duodenum during digestion of meals with or without phytosterol esters (PEs) over 4 h, and ii) the relationship between lipid and sterol processing in the duodenum and the resulting incorporation of (deuterated) cholesterol obtained from the meal in intestinally derived chylomicrons for 8 h, as well as the relative rate of cholesterol absorption. Experiments under simulated in vitro gastrointestinal conditions were performed to acquire insight into micellar distribution and competition for both cholesterol and phytosterol. The data obtained provide for the first time a full comprehensive description of the effects of phytosterols on the lipid digestion process and postprandial secretion of cholesterol in humans.
MATERIALS AND METHODS
Study population
Twelve healthy male subjects were included in the study. Inclusion criteria were: 18–45 years of age, body mass index of 18–25 kg/m2, and good general health with no medical conditions affecting the gastrointestinal tract or liver and no use of medication known to affect cholesterol or bile acid metabolism. Fasting plasma total cholesterol, LDL cholesterol, TGs, and glucose concentrations were below 5, 3.1, 1.5, and 6.3 mmol/l, respectively. Subjects with high alcohol intake (>140 g/w), unconventional diets, or high physical activity (>4.5 h/w) were excluded.
The study protocol was reviewed and approved by the local medical ethics committee, and every subject approved and signed an informed consent.
Experimental protocol
In a randomized, single-blind crossover design, 12 healthy volunteers were given two different meals in 500 ml of liquid: the control meal did not contain phytosterol ester (PE), whereas the other meal did contain PE (PE meal). A wash-out period of 1 month between test meals was used. The procedure started at approximately 7:00 AM, when the volunteers were intubated after an overnight fast. A single lumen radiopaque nasoduodenal tube with one sampling point (outer diameter 4.7 mm, 108 cm; ref 8888-264846, Kendall Argyle, Sherwood Medical, Tullamore, Ireland) was placed in the small intestine at the ligament of Treitz (30 cm from the pylorus) under X-ray monitoring as previously described (5). After the tube was fitted, the subjects sat upright until the end of the protocol to limit variation in gastric emptying rates.
An antecubital vein was then catheterized with an intravenous cannula (5066 20G; Optiva Medex, UK). Volunteers consumed the 500 ml liquid meal containing deuterium-enriched cholesterol warmed to 37°C within 15 min. After 240 min, the nasoduodenal tube was removed. To follow the deuterated cholesterol in the plasma (10), the subject ingested a solid meal of commercial foods (lasagna, wheat bread, egg, yogurt, sugar, and water, providing 1820 kJ and 22 g fat) at 300 min. The protocol ended after 480 min.
Meal composition
The two sequences tested were as follows: a liquid 500 ml control meal [3,595 kJ, 41 g fat, 31 g protein, 92 g carbohydrate of Fortisip (Nutricia, UK) + 26.7 g Becel regular spread (no PE, 9.3 g fat; Unilever, The Netherlands)] and a liquid 500 ml PE meal [Fortisip (Nutricia, UK) + 26.7 g Becel pro.activ with PE (3.2 g of PE, which is equivalent to 2.0 g of phytosterols, and 9.3 g of fat; Unilever, The Netherlands)]. Phytosterols were β-sitosterol (65.1%), campesterol (15.2%), and β-sitostanol (11.9%). The remaining portion (7.8%) consisted of other phytosterols.
Hepta-deuterated cholesterol (25,26,26,26,27,27,27 2H7 [2H7] cholesterol) (D7C) with >99% total enrichment was purchased from CDN Isotopes (CIL Cluzeau Info Labo, France). A single dose of 80 mg D7C was dissolved in 1 ml sunflower oil (10) and added to the 500 ml liquid meal. Natural cholesterol (40 mg) was added in the form of raw egg yolk (2.1 g). The liquid meal was homogenized using an Ultraturrax (5 min, position 1) at 37–40°C to ensure dispersion of all ingredients.
Duodenum content and blood sampling
The duodenum content samples (5–10 ml) were taken by aspiration from the nasoduodenal tube using a syringe at 0, 20, 40, 60, 90, 120, 150, 180, and 240 min after the two meal sequences. The duration corresponds to the full liquid meal digestion process (11). Immediately after collection, a protease-lipase-microbial inhibitor cocktail was added, and samples were stored in the refrigerator as previously described (5). Blood samples were collected in tubes containing EDTA at 0, 60, 120, 180, 240, 390, and 480 min. The first five time points allowed the determination of excursion of TGs into the plasma after digestion of the meal sequences, as previously performed after a test meal in healthy subjects (12). The two subsequent samplings were performed for accurate follow-up of delayed chylomicron and plasma D7-cholesterol enrichment as previously described (13). Blood samples were centrifuged within 1 h of collection at 1,700 g for 10 min. Except for the aliquot used for chylomicron isolation, the plasma samples were stored at −80°C until analysis.
Analytical determinations
Duodenum samples.
A careful sequential separation process based on previous studies in intubated volunteers (5, 11, 14) was used. Aliquots of 4 ml of duodenum content were added to 7 ml ultrapure water and centrifuged for 50 min at 50,500 g (4°C) using a Sorvall WX100 Ultracentrifuge (Thermo Scientific, France) and a SW Ti 40 rotor (Beckmann, France) in conditions similar to those previously published (6), taking care to avoid full breakage of emulsion droplets (5, 14). The floating creamy layer (subsequently known as the oil phase) was collected first, followed by the clear infranatant (aqueous phase). Finally the precipitated material (pellet) at the bottom of the tube was suspended in 1 ml 0.9% NaCl. All fractions prepared from fresh duodenal samples were stored at −80°C until analysis.
Extraction, separation and quantification of lipid species.
The following lipid species in the three collected fractions were separated and quantified by GC during a 60 min run: FFAs, monoglycerides (MGs), diglycerides (DGs), TGs, free cholesterol, free phytosterols, cholesteryl esters (CEs), and PEs. Briefly, the 0.7 ml aqueous phase, 0.5 ml pellet phase, or the 0.3 ml oil phase was extracted twice with hexane and once with diethyl ether (v/v). The extracts were evaporated and silylated with N,O-bis-trimethylsilyl-trifluoroacetamide (Macherey Nagel; Hoerdt, France) using pyridine as the solvent (Sigma, France) before analysis by GC. An Autosystem XL Perkin Elmer apparatus equipped with a Chrompack, CP-Sil5 10 m column (CP7730, Varian; Les Ulis, France) was used. A Programmed-temperature On-Column injector with a temperature gradient from 80°C to 360°C at 10°/min and a detector temperature of 370°C was employed. Calibrations were made using dedicated mixtures of standard moieties (Sigma, France). Peaks corresponding to known lipid species (i.e., FFAs, MGs, DGs, TGs, PEs, and CEs) were integrated and pooled. Cholesterol and phytosterol peaks were separately integrated. PE represents the sum of the main PE moieties present (sitosterol, campesterol and sitostanol). The characterization of individual compounds within each lipid species was confirmed by TLC. A GC method comparison was also performed with the techniques used by the Unilever laboratory in Vlaardigen, The Netherlands. Phospholipids (PLs), namely phosphatidylcholine, levels were determined from 10 µl aliquots using a commercial kit (PA150, Biomérieux, France). Total bile salts (BS) were assayed from 20 µl aliquots with an enzymatic method using α-hydroxysteroid dehydrogenase (H1506-50UN, Sigma, France) and β-nicotinamide adenine dinucleotide (N7004-5G, Sigma, France). Lipid moiety concentrations of each phase were calculated and expressed as mmol/l duodenum content.
[2H7] cholesterol (D7C) determination.
[2H7] cholesterol (D7C) concentrations in the three phases were determined by GC-MS using an HP 6890 series II gas chromatograph fitted with an HP 7673 automatic sampler and interfaced to an HP 5973 A mass spectrometer, as previously reported (10, 13). Single-ion monitoring was performed on the following fragments: D7C, ion m/z = 336; epicoprostanol, ion m/z = 370; and endogenous free cholesterol, ion m/z = 329. D7C was expressed as μmol/l duodenum content.
Determination of D7C in chylomicrons and plasma.
Chylomicrons were isolated from 5 ml fresh plasma by ultracentrifugation as previously described (10, 12). The floating creamy layer was carefully collected and stored at −80°C until analyzed. D7C concentration was determined from 0.2 ml chylomicron fraction as described above. Total lipids (mostly TGs) were extracted by the Bligh and Dyer method (15) from 0.6 ml chylomicron fraction and measured gravimetrically after solvent evaporation.
In vitro uptake of phytosterol and cholesterol in the aqueous micellar phase.
In vitro digestion experiments performed with a semi-solid meal were used to mimic human gastrointestinal conditions. The semi-solid meal was prepared by mixing 130 g milk with 30 g white bread and 5 g spread (with or without PE), which contained 75 mg cholesterol (12.3% carbohydrate, 4.1% proteins, 2.5% fat, and 0.05% cholesterol). The gastric emptying profile was modeled according to Minekus et al. (16). After gastric digestion for 30 min, 35% of the semi-solid meal matrix was transferred from the gastric phase (pH 2.2) to the intestinal phase (pH 6.5). After 60 min, another 20% was transferred, and the last 45% was transferred into the intestinal phase after 90 min. The simulated gastric fluid contained 0.4% pepsin (Sigma Aldrich 77160), 0.4% Rhizopus oryzae lipase, 0.87% NaCl, and 0.04% KCL in 1 M HCl. The simulated intestinal fluid contained 4.0% bile extract (Sigma Aldrich B8631), 15% pancreatin (Sigma Aldrich P1625, 3× USP), 0.1% cholesterol esterase (Sigma Aldrich 26745), 0.87% NaCl, and 0.04% KCl in water. After the addition of intestinal enzymes, samples were collected after 30, 60, 90, and 150 min, and the concentrations of FFA, phytosterol, and cholesterol were measured in the aqueous phase and expressed as percent of total initial values. Experiments and assays were performed in duplicate.
Calculations and statistical methods.
The data are presented as mean ± SEM of 12 subjects. The area under the curve (AUC) for lipid species (mmol/l) versus time (AUC60, AUC240, AUC480 for 60, 240, or 480 min, respectively) was calculated using the trapezoidal method. For each dependent variable, the normality of distribution was validated using the Kolmogorov-Smirnov test. The dependent variables at each time point and AUCs were statistically compared between meal groups using a univariate general linear model. Because subjects were their own controls, each model was adjusted for the subject effect. The longitudinal meal group effect on dependent variables (free cholesterol, free phytosterols, FFA, MGs, DGs, TGs, PLs, BSs, PEs, CEs, D7C) over time was analyzed using a mixed linear model with a one-order autoregressive covariance structure. Bonferroni's adjustments were applied when multiple two-by-two comparisons were made. All statistical tests were performed using the SAS software package. All results with a P value <0.05 were considered statistically significant.
RESULTS
Lipid composition and changes in the aqueous, oil, and pellet phases of duodenal contents
The three coexisting phases (aqueous, oil, and pellet) of duodenum samples were isolated to analyze the concentrations of specific lipid moieties. Table 1 (for PE, free cholesterol, free phytosterols, CEs, D7Cs) and supplementary Table I (for TGs, DGs, MGs, FFAs, PLs, BS) show the time course of concentration changes of all lipid parameters measured in the aqueous phase of the duodenal contents after the liquid meals with or without PE.
TABLE 1.
Mean concentrations (μmol/l ± SEM) of sterols (free phytosterol, PE, free cholesterol, CE, and D7C) in the different digestion phases (aqueous, oil, pellet) along the digestion process time
| Meals | Time (min) | Aqueous phase |
Oil phase |
Pellet |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C meal | P | PE | C | CE | D7C | P | PE | C | CE | D7C | P | PE | C | CE | D7C | |
| 20 | nd | nd | 582 ± 132 | nd | 18.1 ± 3.2 | nd | nd | 132 ± 3 3 | 38.9 ± 13.8 | 11.7±5.1 | nd | nd | 134 ±4 0 | 15.9 ± 14.3 | 3.3± 0.5 | |
| 40 | nd | nd | 472 ± 84 | nd | 18.7 ± 3.2 | nd | nd | 137 ± 36 | 53.7 ±16.3 | 14.9 ±4.5 | nd | nd | 126 ± 58 | 11.1± 8.0 | 3.5 ± 0.6 | |
| 60 | nd | nd | 260 ± 72 | nd | 15.3 ± 2.8 | nd | nd | 89 ± 14 | 49.5 ±18.5 | 13.1 ±4.4 | nd | nd | 75 ± 17 | 12.9 ± 10.5 | 4.7 ± 1.4 | |
| 90 | nd | nd | 133 ± 49 | nd | 12.5 ± 3.6 | nd | nd | 73 ± 16 | 65.7 ±21.9 | 14.9 ±3.7 | nd | nd | 61± 13 | 37.7 ± 18.0 | 5.6 ± 1.1 | |
| 120 | nd | nd | 55 ± 13 | nd | 8.2 ± 2.3 | nd | nd | 50 ± 6 | 62.8 ±15.3 | 12.2±1.7 | nd | nd | 68 ± 19 | 24.3 ± 12.9 | 7.3 ± 2.0 | |
| 150 | nd | nd | 33 ± 9 | nd | 5.4 ± 1.1 | nd | nd | 55 ± 10 | 92.0 ±22.2 | 19.0 ±5.0 | nd | nd | 64 ± 13 | 25.0 ± 6.4 | 8.7 ± 1.9 | |
| 180 | nd | nd | 63 ± 32 | nd | 9.5 ± 4.0 | nd | nd | 72 ± 20 | 95.0 ±26.0 | 18.4 ±4.9 | nd | nd | 49 ± 14 | 13.0 ± 4.5 | 8.4 ±.4 | |
| 240 | nd | nd | 27 ±13 | nd | 4.8 ± 1.7 | nd | nd | 113 ± 42 | 104.9 ±34.7 | 19.5 ± 3.6 | nd | nd | 63 ± 21 | 29.0 ± 9.6 | 10.9 ± 2.2 | |
| PE meal | 20 | 138 ± 34 | nd | 450 ±120 | nd | 23.9 ± 4.3 | 76 ± 22 | 686 ± 22 | 126 ± 22 | 88.8 ±18.8 | 18.7 ± 4.3 | 18 ±4 | 2 ±1 | 112 ± 46 | 3.6 ± 1.5 | 3.6 ± 0.7 |
| 40 | 123 ± 18 | nd | 331 ±50 | nd | 19.0 ± 3.2 | 84 ± 17 | 970 ± 17 | 133 ± 27 | 87.6 ±23.7 | 14.4 ± 4.2 | 22 ±7 | 5 ±3 | 148 ± 46 | 6.8 ± 3.3 | 4.4 ± 0.6 | |
| 60 | 106 ± 25 | nd | 206 ±49 | nd | 19.1 ± 3.9 | 46 ± 9 | 535 ±9 | 72 ± 16 | 56.5 ±8.8 | 9.1 ±2.2 | 32 ±12 | 7 ±4 | 63 ± 22 | 4.2 ± 1.8 | 3.2 ±0.5 | |
| 90 | 49 ± 15 | nd | 92 ±30 | nd | 9.3 ± 2.5 | 27 ±6 | 306 ±6 | 51 ± 12 | 57.9 ±14.4 | 7.7 ±2.8 | 26 ±10 | 43 ±35 | 32 ±11 | 22.7 ±17.2 | 4.8 ± 1.9 | |
| 120 | 38 ± 10 | nd | 45 ±17 | nd | 6.5 ± 2.0 | 46 ±13 | 705±13 | 53 ± 11 | 70.2 ±11.7 | 14.5 ±5.4 | 29 ±16 | 25 ±12 | 73 ±25 | 12.2 ±3.5 | 5.0 ± 1.3 | |
| 150 | 30 ± 6 | nd | 37 ± 10 | nd | 4.6 ± 1.0 | 35 ±10 | 540 ±10 | 41 ± 11 | 65.4 ±10.7 | 11.1 ±3.7 | 32 ±10 | 58 ±23 | 72 ±24 | 23.3 ±7.7 | 5.7 ± 1.1 | |
| 180 | 29 ± 7 | nd | 32 ± 11 | nd | 5.2 ± 0.7 | 70 ±24 | 904 ±24 | 54 ± 8 | 104.2 ±18.5 | 26.4 ±6.2 | 36 ±11 | 176 ±57 | 66 ±24 | 62.1 ±17.7 | 11.4 ± 2.4 | |
| 240 | 24 ± 11 | nd | 17 ± 5 | nd | 7.9 ± 2.7 | 62 ±11 | 964 ±11 | 107 ± 32 | 79.2 ±12.4 | 29.4 ±5.6 | 75 ±32 | 117 ±57 | 72 ±32 | 30.3 ±11.7 | 9.8 ± 2.7 | |
C, free cholesterol; P, free phytosterol; nd, not detected.
Aqueous phase.
FFAs and, to a lesser extent, MGs are noticeably present in the aqueous phase up to 20 mmol/l and 4 mmol/l, respectively, after the PE and control meals. The highest concentrations were recorded during the first hour of digestion, and concentrations gradually decreased in the following 3 h. No significant differences between the meals were found for FFA or MGs at the different time points. As expected, small amounts of DG were detected in the duodenum aqueous phase, whereas TG levels were not measurable. The PL, provided by both meals and bile, and BS, occurred in the aqueous phase, and comparable changes were observed over time with concentrations up to 7 mmol/l for PL and 9 mmol/l for BS. Free phytosterols reached comparable concentrations (∼0.1 mmol/l) during the first hour of digestion after the meal with PE and then decreased. As expected, no PE was detected in aqueous phases. Free cholesterol concentrations were high during the first hour of digestion and then decreased; higher concentrations were detected after the control meal than the meal with PE. CEs were not present. D7C displayed kinetics similar to those of cholesterol. The cholesterol/(PL + FFA + MG) ratios in the aqueous phase at the time points were calculated, and noticeable differences related to the presence or absence of PE in the test meals were not detected.
Oil phase.
MG, FFA, DG (up to 2 mmol/l), and TG (up to 4 mmol/l) accumulated in the oil phase (see supplementary Table I). No time dependency or meal effects were observed over 4 h for the moieties in this phase. PL also accumulated in the oil phase, but only small amounts of BS were detected. As expected, PE accumulated in the oil phase (up to 1 mmol/l) after the meal with PE, whereas free phytosterol and cholesterol did so only minimally (up to 0.1 mmol/l). Some CE was found in the oil phase (concentrations up to 0.1 mmol/l) (Table 1).
Pellet.
FFA and MG accumulated in the pellet (up to 5 mmol/l and 0.8 mmol/l, respectively) and, as expected, very low concentrations of DG and TG were quantified, especially after 2 h (see supplementary Table I). PL noticeably accumulated (up to 3.8 mmol/l at 150 min for control meal), whereas BS concentrations were comparable at all time points. Phytosterol, PE, and CE did not accumulate in the pellet, but a small increase in PE was detected after 150 min (Table 1). Cholesterol accumulated up to 0.1 mmol/l, as did D7C. No noticeable differences in the lipid moieties were observed between the two meals in the pellet phase.
Apparent lipolysis rate
This process can be quantified using the FFA aqueous phase (±pellet)/TGoil AUC ratio at 240 min as shown in Fig. 1. The FFA aqueous phase/TGoil AUC ratios were 2.3 and 2.8 for the control meal and the PE meal, respectively. The FFA (aqueous phase + pellet)/TGoil AUC ratios were 4.21 and 4.04 for the control meal and the PE meal, respectively. Indeed, no significant differences for these two parameters were detected after meals with or without PE.
Fig. 1.
Apparent lipolysis rate expressed as AUC 240 min ratios of released FFAs in the aqueous phase (± pellet) to intact TGs in the oil phase. Results shown are the mean (± SEM) of ratios after a meal with PEs (gray) or without PE as control (white).
In vitro aqueous/micellar distribution of lipid species.
In the in vitro digestion model of meals with cholesterol and bile, cholesterol was detected in the aqueous (micellar) phase gradually over time and reached comparable final concentrations (t = 150 min, end duodenal phase) for the control meal (0.52 mmol/l) and PE meal (0.48 mmol/l). The FFA release in both conditions was not different, indicating a similar rate and extent of lipolysis. The cholesterol concentration in the aqueous (micellar) phase during digestion was somewhat lower (about −20% percent of initial concentration) in the PE meal, compared with the control meal after 60 min (Fig. 2). As expected, the percentage of phytosterol increased with time with the PE meal but was not detectable with the control meal. Phytosterol incorporation in the aqueous phase was lower compared with cholesterol. The absolute concentration of both sterols (cholesterol plus phytosterol) was higher compared with that of cholesterol in the control meal.
Fig. 2.
Incorporation of FFAs, free cholesterol, and free phytosterols in the aqueous (micellar) phase formed during in vitro digestion of a control and a phytosterol esters-containing meal. For experimental details, see the Materials and Methods section. Time represents the time of incubation under duodenal conditions. Y-axis represents the aqueous phase concentration for each lipid species relative to the initial concentration.
Distribution of sterols in the human duodenum phases
The distribution of sterols in the three phases of the human duodenum contents has been determined for the overall digestion process by calculating the AUC240. As shown in Fig. 3, cholesterol concentration in the aqueous phase was lower in the presence of PE as compared with control (−27%, P < 0.1). No differences in the AUC240 of the pellet and oil phase cholesterol concentrations were found in the presence or absence of PE. CE preferentially accumulated in the oil phase without influence of PE. PE accumulated in the oil phase as well (Fig. 4A), while phytosterol accumulated in the aqueous and pellet phases. The apparent rate of hydrolysis of PE calculated by the PE/phytosterol AUC240 ratios was ∼12%. The cholesterol aqueous phase/pellet AUC240 ratio was lower after the meal with PE (−28%, P < 0.05) than the control meal (Fig. 4B). A similar reduction was observed for AUC60 (data not shown). The calculated D7C aqueous phase/pellet AUC240 was 26% lower (not significant) after the meal with PE compared with the control (data not shown). The phytosterol/cholesterol ratios in the aqueous, oil, and pellet phases were comparable (Fig. 4A).
Fig. 3.
Distribution expressed in AUC240 (mmol/min/l) of free cholesterol and CEs in the different duodenum phases (aqueous, oil, and pellet) after a meal with PEs (gray) or without PE as control (white). Results are the mean (± SEM) of AUC240 min.
Fig. 4.
Distribution (ratios) of sterol species in the different duodenum phases (aqueous, oil, and pellet) after a meal with PE (gray) or without PE as control (white). A: Phytosterol esters/phytosterols and phytosterols/cholesterol ratios. B: Cholesterol aqueous phase/pellet ratio. Results are the mean (± SEM) of AUC240 min ratios.
Chylomicron D7C and lipids
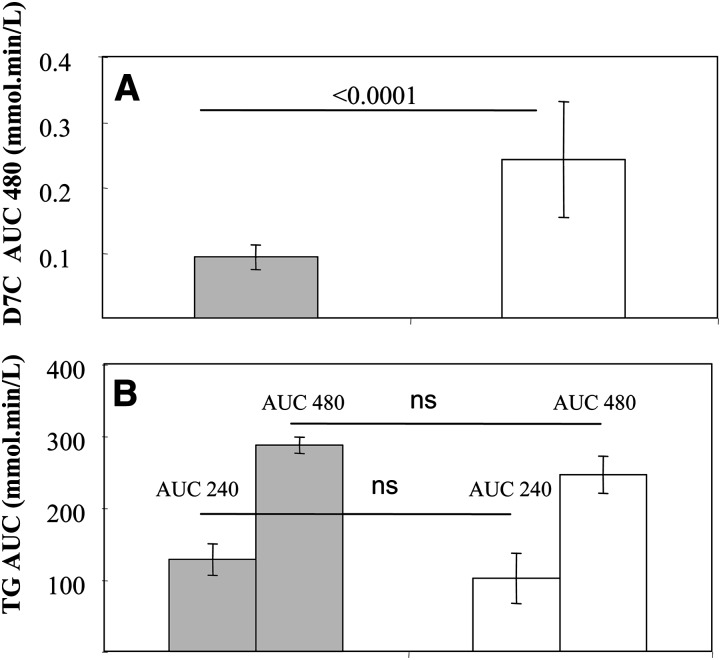
The presence of D7C, which was added to the initial meals in chylomicrons was followed for 480 min, with a second meal without D7C or PE being consumed at 240 min (Fig. 5). As expected (10), D7C slightly increased in chylomicrons at 180 min, decreased at 240 min, increased at 360 min, and then decreased at 480 min with the control meal. After the PE meal, smaller chylomicron D7C increases were observed at 180 min, and much smaller increases were observed at 360 and 480 min, as compared with the control meal (P < 0.05). Chylomicron D7C AUCs over 480 min had a significant reduction (43%, P < 0.0001) observed after the meal with PE (Fig. 6A). No change for total chylomicron lipids (mostly TG) was observed at 240 or 480 min (Fig. 6B). Moreover, the time course of chylomicron lipid occurrence was not different after the two meals (data not shown).
Fig. 5.
Kinetics of occurrence of absorbed meal D7C in chylomicrons after a meal with PE (solid line) or without PE as control (dashed line). For experimental details, see the Materials and Methods section. Results are means (± SEM). * Significantly different values, P < 005.
Fig. 6.
A: D7C AUCs 480 min in chylomicrons after a meal with PE (gray) and without PE as control (white); as shown, mean value (± SEM) after the meal with PE significantly different from the one after the control meal, P < 0.0001. B: TG AUCs for 240 min and 480 min, means (± SEM); as shown, no significant differences (ns) between the meal with PE (gray) and without PE (white) values were found. All data shown are means (± SEM).
Plasma D7C
The occurrence of D7C in the plasma was measured for 480 min (Fig. 7). The tracer/tracee (isotopic enrichment) ratio of plasma cholesterol (Fig. 7A) shows a significant reduction (−58%, P < 0.0001) in plasma after the meal with PE, compared with the control meal. Significantly lower plasma D7C levels (AUC 480) were detected after the meal with PE, compared with the control meal (−54%, P < 0.0001) (Fig. 7B).
Fig. 7.
A: Tracer (D7C)/tracee ratios (isotopic enrichement, °/°°) of plasma cholesterol. B: D7C AUCs 480 min in plasma after a meal with PE (gray) or without PE as control (white); as shown, values after the meal with PE significantly different from those after the control meal, P < 0.0001. All data shown are means (± SEM).
DISCUSSION
This study showed that the addition of PE does not alter fat digestion in the duodenum and subsequent chylomicron TG secretion into the circulation of healthy subjects. The addition of PE noticeably increased free plant sterol concentrations in the duodenum phases and reduced cholesterol availability in the aqueous phase, resulting in a markedly reduced meal-derived cholesterol in the circulation, i.e., chylomicrons and plasma. Reduced cholesterol uptake in the aqueous phase during in vitro experiments confirmed our in vivo findings.
We have determined the lipid distribution patterns by separating the three main phases of the duodenum contents during fat digestion (5, 6, 17). Based on previous observations in humans (5), we found herein that the oil phase contains high concentrations of TGs and lower levels of DGs, PLs, CEs (present in the initial meal), and PEs when the components are present in the meal, whereas MGs and (protonated) FFAs accumulated in the lipid phase. Following 4 h of digestion, the volume of the lipid phase greatly declined due to TG and sterol ester hydrolysis by gastric lipase, the pancreatic colipase-lipase system, and cholesterol esterase (18–20). This study shows that adding PE to a meal does not noticeably alter lipid concentrations in the oil phase.
As expected, the aqueous phase contained very low concentrations of hydrophobic lipid moieties (TG, DG, CE, PE) but high concentrations of amphipathic moieties such as MG, FFA, PL, and BS. Due to the extensive absorption of the amphipathic moieties from the duodenum mucosa, their concentrations greatly decreased over 4 h of digestion. These results are in agreement with previous studies showing no change in bile acid patterns after chronic plant stanol intake in colectomized patients (21). The addition of PE resulted in elevated free phytosterol concentrations in the aqueous phase, probably resulting from PE hydrolysis by gut-aspecific lipases, as shown by others (3).
The components of the pellet phase after the control meal contained high concentrations of FFA, PL, MG, and cholesterol, and lower levels of hydrophobic lipids and BS, as expected, based on previous studies (5). This finding illustrates that the aggregated pellet material is essentially a lamellar crystal phase of excess amphipathic lipids, including cholesterol (22). The presence of PE in the meal did not alter this pattern, but free phytosterols accumulated in the pellet.
We calculated the apparent rates of dietary TG hydrolysis as previously described (5) from the concentrations of the lipid moieties TG and FFA. Comparable results (∼80% lipolysis) were observed after the two meal sequences over 4 h, indicating that added PE did not interfere with the hydrolysis of dietary fat in the small intestine of healthy humans, in line with data obtained in ileostomized patients (23). In the present study, the results from an in vitro model confirmed that added PE does not interfere with fat hydrolysis (3).
PE mainly accumulated in the oil phase, as expected, given its hydrophobicity, which is in line with previous observations of phytostanols (24). Thus, PE has poor solubility in mixed micelles or small vesicles and cannot accumulate in the aqueous phase to directly interfere with free cholesterol moieties. In fact, PE is hydrolyzed in the duodenum. The apparent hydrolysis rate was ∼12% during fat digestion in the duodenum in the present study. Higher rates (40–88%) have previously been reported using a design based on constant lipid infusion and the collection of jejunal samples (3, 24) or measuring ileal output in ileostomized subjects (23).
The behavior of free cholesterol resembles that of free phytosterol, which is likely, given their close physicochemical properties (25). Cholesterol increased to comparable levels in the three duodenum phases. However, the cholesterol concentration was noticeably lower (−27%) in the aqueous phase after the PE meal than the control meal. The cholesterol aqueous/pellet ratio was 28% lower after the meal with PE than after the control meal. A 34% reduction in free cholesterol concentration in the jejunum aqueous phase has been reported when phytostanols were added (3). This indicates that the addition of PE greatly decreased the capacity to incorporate cholesterol in the dispersed structures (vesicles and mixed micelles) present in the aqueous phase. These in vivo observations confirm in vitro results in which free sitostanol or sitosterol was not only interchangeable with free cholesterol during mixed micelle formation but also effectively competed with cholesterol already solubilized in mixed micelles (25, 26). Indeed, the free phytosterols generated from PE hydrolysis reached concentrations similar to free cholesterol in the aqueous phase, and to a lesser degree, in the pellet. In accordance with in vitro data, the stoichiometry of phytosterol and cholesterol in the duodenum after consumption of 3,250 mg PE is compatible with a marked competition between the two moieties for incorporation into vesicles and micelles present in the aqueous phase. In addition to bile acids, PLs are also important in this process, given that phytosterol and cholesterol solubility in the aqueous phase is highly dependent on the presence of PL (18, 27). When present in excess during fat digestion, liquid crystals/lamellar structures made of amphipaths and lipolytic products, namely PL and cholesterol (and phytosterol when present), readily precipitate, as observed in this and other studies (5, 11, 24). This finding is further supported by the coprecipitation mechanism identified in vitro (28). Due to the coexistence of vesicular structures and mixed micelles in the intestinal aqueous phase (6), it was not possible to determine the lipid composition of the mixed micelles per se. Thus, given that cholesterol absorption by enterocytes is lower from vesicles than mixed micelles (29), the true micellar cholesterol availability cannot be precisely determined from the present data. Nevertheless, our results clearly show that cholesterol available for mucosal uptake from the aqueous phase is markedly reduced (∼30%) when PE is added. One could have speculated that a reduced cholesterol mucosal uptake might result in increased cholesterol in the aqueous phase. However, the opposite was found in the presence of PE, which indicates that unabsorbed cholesterol is distributed into the other phases, especially the insoluble pellet.
The presence of PE noticeably increased CE concentration in the oil phase of the duodenum content, which is in agreement with previous studies showing such a phenomenon in the jejunum with added plant stanols (3, 24). De novo esterification of free plant stanols and cholesterol during infusion in the human duodenum has been recently observed (24), although the underlying mechanisms remain to be elucidated. The fact that cholesterol can be esterified and accumulates in the oil phase suggests an additional mechanism by which PE can reduce cholesterol accumulation in the aqueous phase and thus reduce cholesterol availability for mucosal uptake.
Reduced cholesterol incorporation into micelles and vesicles (expressed in %) present in the aqueous phase during digestion of the PE meal in the in vitro experiments confirms the hypothesized competition for micellar space and uptake during digestion. However, the data do not indicate a strict 1:1 stoichiometric displacement; a considerable amount of cholesterol remains in the aqueous phase even when a comparable number of micelles were formed according to FFA concentrations.
Because chylomicron cholesterol output depends on the cholesterol absorption rate of the small intestine, we hypothesized a reduction in the extent of cholesterol absorption when PE is added to meals. This was studied by assaying ingested intestinally derived D7-cholesterol (D7C) in plasma (27, 30). The assays were performed 8 h after meal intake, a duration allowing most ingested dietary cholesterol to occur in the plasma and reach a steady-state equilibrium (10). In the present study, we found that the addition of PE to the meal significantly lowered the presence of meal-derived D7C (−54%) compared with the control meal. This result supports previous studies in which decreased cholesterol absorption was observed in ileostomized patients [−32% with plant stanol or sterol esters (2) or −40% with plant stanol esters (22, 31)], healthy subjects [−34–37% with sitostanol and lecithins (27)], and hypercholesterolemic patients [−18% with plant sterols (32)]. Here, we clearly showed that the reduction in D7-chylomicron cholesterol after PE intake is significant and large (43%), highlighting the direct relationship between duodenum processing and intestinal resecretion of cholesterol. This effect is specific, given that chylomicron lipids (mostly TG) were not affected by the presence of PE in the meal. It appears that the reduction in cholesterol output in chylomicrons or plasma is somewhat greater than the cholesterol decrease observed in the duodenum aqueous phase. This could be because the duodenal sampling was at a single location, whereas the chylomicron response integrates the whole small intestine. In addition, the greater reduction in chylomicrons suggests that additional mechanisms modulate key transporters controlling cholesterol absorption, as reported by others (33, 34), and the enzyme for intracellular sterol esterification (35).
In conclusion, this study shows that adding PEs to a meal does not alter fat hydrolysis in the duodenum nor chylomicron release, but specifically reduces free cholesterol availability for intestinal absorption and subsequent cholesterol resecretion in chylomicrons in healthy subjects.
Acknowledgments
The authors thank Jack Seijen ten Hoorn for providing the in vitro data on micellar incorporation, and Matthieu Maillot for his expertise in statistical analysis.
Footnotes
Abbreviations:
- BS
- bile salts
- CE
- cholesteryl ester
- D7C
- hepta-deuterated cholesterol
- DG
- diglyceride
- FFA
- free fatty acid
- MG
- monoglyceride
- PE
- phytosterol ester
- PL
- phospholipid
- TG
- triglyceride
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of one table.
REFERENCES
- 1.Demonty I., Ras R. T., van der Knaap H. C., Duchateau G. S., Meijer L., Zock P. L., Geleijnse J. M., Trautwein E. A. 2009. Continuous dose-response relationship of the LDL-cholesterol-lowering effect of phytosterol intake. J. Nutr. 139: 271–284. [DOI] [PubMed] [Google Scholar]
- 2.Normen L., Dutta P., Lia A., Andersson H. 2000. Soy sterol esters and beta-sitostanol ester as inhibitors of cholesterol absorption in human small bowel. Am. J. Clin. Nutr. 71: 908–913. [DOI] [PubMed] [Google Scholar]
- 3.Nissinen M., Gylling H., Vuoristo M., Miettinen T. A. 2002. Micellar distribution of cholesterol and phytosterols after duodenal plant stanol ester infusion. Am. J. Physiol. Gastrointest. Liver Physiol. 282: G1009–G1015. [DOI] [PubMed] [Google Scholar]
- 4.Richelle M., Enslen M., Hager C., Groux M., Tavazzi I., Godin J. P., Berger A., Metairon S., Quaile S., Piguet-Welsch C., et al. 2004. Both free and esterified plant sterols reduce cholesterol absorption and the bioavailability of beta-carotene and alpha-tocopherol in normocholesterolemic humans. Am. J. Clin. Nutr. 80: 171–177. [DOI] [PubMed] [Google Scholar]
- 5.Armand M., Borel P., Pasquier B., Dubois C., Senft M., Andre M., Peyrot J., Salducci J., Lairon D. 1996. Physicochemical characteristics of emulsions during fat digestion in human stomach and duodenum. Am. J. Physiol. 271: G172–G183. [DOI] [PubMed] [Google Scholar]
- 6.Hernell O., Staggers J. E., Carey M. C. 1990. Physical-chemical behavior of dietary and biliary lipids during intestinal digestion and absorption. II. Phase analysis and aggregation states of luminal lipids during duodenal fat digestion in healthy adult human beings. Biochemistry. 29: 2041–2056. [DOI] [PubMed] [Google Scholar]
- 7.Tavani D. M., Nes W. R., Billheimer J. T. 1982. The sterol substrate specificity of acyl CoA:cholesterol acyltransferase from rat liver. J. Lipid Res. 23: 774–781. [PubMed] [Google Scholar]
- 8.Plat J., Nichols J. A., Mensink R. P. 2005. Plant sterols and stanols: effects on mixed micellar composition and LXR (target gene) activation. J. Lipid Res. 46: 2468–2476. [DOI] [PubMed] [Google Scholar]
- 9.Field F. J., Born E., Mathur S. N. 2004. Stanol esters decrease plasma cholesterol independently of intestinal ABC sterol transporters and Niemann-Pick C1-like 1 protein gene expression. J. Lipid Res. 45: 2252–2259. [DOI] [PubMed] [Google Scholar]
- 10.Beaumier-Gallon G., Dubois C., Senft M., Vergnes M. F., Pauli A. M., Portugal H., Lairon D. 2001. Dietary cholesterol is secreted in intestinally derived chylomicrons during several subsequent postprandial phases in healthy humans. Am. J. Clin. Nutr. 73: 870–877. [DOI] [PubMed] [Google Scholar]
- 11.Armand M., Borel P., Dubois C., Senft M., Peyrot J., Salducci J., Lafont H., Lairon D. 1994. Characterization of emulsions and lipolysis of dietary lipids in the human stomach. Am. J. Physiol. 266: G372–G381. [DOI] [PubMed] [Google Scholar]
- 12.Dubois C., Beaumier G., Juhel C., Armand M., Portugal H., Pauli A. M., Borel P., Latge C., Lairon D. 1998. Effects of graded amounts (0–50 g) of dietary fat on postprandial lipemia and lipoproteins in normolipidemic adults. Am. J. Clin. Nutr. 67: 31–38. [DOI] [PubMed] [Google Scholar]
- 13.Beaumier-Gallon G., Lanfranchi J., Vergnes M. F., Lairon D., Pastor J., Pauli A. M., Portugal H. 1998. Method for simultaneous measurements of traces of heptadeuterated cholesterol and cholesterol by gas chromatography-mass spectrometry: application in humans. J. Chromatogr. B Biomed. Sci. Appl. 718: 23–32. [DOI] [PubMed] [Google Scholar]
- 14.Armand M., Pasquier B., Andre M., Borel P., Senft M., Peyrot J., Salducci J., Portugal H., Jaussan V., Lairon D. 1999. Digestion and absorption of 2 fat emulsions with different droplet sizes in the human digestive tract. Am. J. Clin. Nutr. 70: 1096–1106. [DOI] [PubMed] [Google Scholar]
- 15.Bligh E. G., Dyer W. J. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 16.Minekus M., Marteau P., Havenaar R., Huis in't Veld J. H. J. 1995. A multicompartmental dynamic computer-controlled model simulating the stomach and small intestine. Altern. Lab. Anim. 23: 197–209. [Google Scholar]
- 17.Hofmann A. F., Borgstroem B. 1964. The intraluminal phase of fat digestion in man: the lipid content of the micellar and oil phases of intestinal content obtained during fat digestion and absorption. J. Clin. Invest. 43: 247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carey M. C., Small D. M., Bliss C. M. 1983. Lipid digestion and absorption. Annu. Rev. Physiol. 45: 651–677. [DOI] [PubMed] [Google Scholar]
- 19.Lowe M. E. 2002. The triglyceride lipases of the pancreas. J. Lipid Res. 43: 2007–2016. [DOI] [PubMed] [Google Scholar]
- 20.Lairon D. 2008. Macronutrient intake and modulation on chylomicron production and clearance. Atheroscler. Suppl. 9: 45–48. [DOI] [PubMed] [Google Scholar]
- 21.Miettinen T. A., Vuoristo M., Nissinen M., Jarvinen H. J., Gylling H. 2000. Serum, biliary, and fecal cholesterol and plant sterols in colectomized patients before and during consumption of stanol ester margarine. Am. J. Clin. Nutr. 71: 1095–1102. [DOI] [PubMed] [Google Scholar]
- 22.Staggers J. E., Hernell O., Stafford R. J., Carey M. C. 1990. Physical-chemical behavior of dietary and biliary lipids during intestinal digestion and absorption. I. Phase behavior and aggregation states of model lipid systems patterned after aqueous duodenal contents of healthy adult human beings. Biochemistry. 29: 2028–2040. [DOI] [PubMed] [Google Scholar]
- 23.Normen L., Ellegard L., Janssen H. G., Steenbergen H., Trautwein E., Andersson H. 2006. Phytosterol and phytostanol esters are effectively hydrolysed in the gut and do not affect fat digestion in ileostomy subjects. Eur. J. Nutr. 45: 165–170. [DOI] [PubMed] [Google Scholar]
- 24.Nissinen M. J., Vuoristo M., Gylling H., Miettinen T. A. 2007. Respective hydrolysis and esterification of esterified and free plant stanols occur rapidly in human intestine after their duodenal infusion in triacyl- or diacylglycerol. Lipids. 42: 603–612. [DOI] [PubMed] [Google Scholar]
- 25.Ikeda I., Tanabe Y., Sugano M. 1989. Effects of sitosterol and sitostanol on micellar solubility of cholesterol. J. Nutr. Sci. Vitaminol. (Tokyo). 35: 361–369. [DOI] [PubMed] [Google Scholar]
- 26.Mel'nikov S. M., Seijen ten Hoorn J. W., Eijkelenboom A. P. 2004. Effect of phytosterols and phytostanols on the solubilization of cholesterol by dietary mixed micelles: an in vitro study. Chem. Phys. Lipids. 127: 121–141. [DOI] [PubMed] [Google Scholar]
- 27.Ostlund R. E., Jr, Spilburg C. A., Stenson W. F. 1999. Sitostanol administered in lecithin micelles potently reduces cholesterol absorption in humans. Am. J. Clin. Nutr. 70: 826–831. [DOI] [PubMed] [Google Scholar]
- 28.Mel'nikov S. M., Seijen ten Hoorn J. W., Bertrand B. 2004. Can cholesterol absorption be reduced by phytosterols and phytostanols via a cocrystallization mechanism? Chem. Phys. Lipids. 127: 15–33. [DOI] [PubMed] [Google Scholar]
- 29.Haikal Z., Play B., Landrier J. F., Giraud A., Ghiringhelli O., Lairon D., Jourdheuil-Rahmani D. 2008. NPC1L1 and SR-BI are involved in intestinal cholesterol absorption from small-size lipid donors. Lipids. 43: 401–408. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y., Vanstone C. A., Parsons W. D., Jones P. J. 2004. Validation of a single-isotope-labeled cholesterol tracer approach for measuring human cholesterol absorption. Lipids. 39: 87–91. [DOI] [PubMed] [Google Scholar]
- 31.Nissinen M. J., Gylling H., Miettinen T. A. 2006. Effects of plant stanol esters supplied in a fat free milieu by pastilles on cholesterol metabolism in colectomized human subjects. Nutr. Metab. Cardiovasc. Dis. 16: 426–435. [DOI] [PubMed] [Google Scholar]
- 32.Varady K. A., Houweling A. H., Jones P. J. 2007. Effect of plant sterols and exercise training on cholesterol absorption and synthesis in previously sedentary hypercholesterolemic subjects. Transl. Res. 149: 22–30. [DOI] [PubMed] [Google Scholar]
- 33.Jones P. J., AbuMweis S. S. 2009. Phytosterols as functional food ingredients: linkages to cardiovascular disease and cancer. Curr. Opin. Clin. Nutr. Metab. Care. 12: 147–151. [DOI] [PubMed] [Google Scholar]
- 34.Calpe-Berdiel L., Escola-Gil J. C., Blanco-Vaca F. 2009. New insights into the molecular actions of plant sterols and stanols in cholesterol metabolism. Atherosclerosis. 203: 18–31. [DOI] [PubMed] [Google Scholar]
- 35.Liu J., Chang C. C., Westover E. J., Covey D. F., Chang T. Y. 2005. Investigating the allosterism of acyl-CoA:cholesterol acyltransferase (ACAT) by using various sterols: in vitro and intact cell studies. Biochem. J. 391: 389–397. [DOI] [PMC free article] [PubMed] [Google Scholar]