Abstract
Purpose
Although the role of Toll-like receptors (TLRs) in bacterial infection and sepsis is well characterized, recent studies have also shown that TLR4 and TLR2 can play an important role in contributing to acute inflammatory processes and organ dysfunction in settings in which LPS or other bacterial products are not present. This review presents not only insights into pathophysiologic mechanisms that contribute to organ dysfunction and outcome in critical illness, but also direct therapeutic approaches to ameliorating such TLR-mediated responses that may potentially be of clinical benefit in critically ill patients.
Method
Literature review of the role of TLR4 and TLR2 in sterile inflammation relevant to critical care medicine using PubMed search, including original papers in English from 1990 to 2010.
Conclusion
There is increasing evidence that TLR4 and TLR2 are not only receptors for bacterial products, but also can be activated through other mechanisms relevant to the pathophysiology of critical illnesses. There is evidence that TLR4 and TLR2 are involved in ischemia-reperfusion injury and trauma where Gram-negative or Gram-positive bacteria are not detectible in the circulation or local organ sites, such as the lungs. In these settings TLRs can transduce other proinflammatory signals and thereby contribute to cellular activation leading to acute lung injury and other organ system dysfunction. The consequences of TLR4 and TLR2 activation through reactive oxygen species (ROS), heat shock proteins, and other non-LPS dependent mechanisms may be different from those associated with binding of the membrane component of bacteria to TLR4 or TLR2 and may produce different signatures of gene activation and release of proinflammatory mediators.
Keywords: TLR4, TLR2, Sterile inflammation, Ischemia-reperfusion, Trauma, Critical care medicine
Introduction
Toll-like receptors (TLRs) were initially characterized by their interactions with bacterial ligands and involvement in cellular activation associated with infection and sepsis. Toll-like receptors 2 (TLR2) and 4 (TLR4) were originally described as recognizing pathogen-associated molecular patterns (PAMPs) derived from bacteria and other microorganisms. However, recent studies have shown that both TLR2 and TLR4 can recognize non-microbial ligands, including danger-associated molecular patterns (DAMPs) and other products of inflamed tissue. Engagement of TLR2 and TLR4 initiates signaling through intracellular pathways that lead to activation of transcription factors, such as nuclear factor-κB (NF-κB) and the interferon regulatory factor 3 (IRF3), that result in transcription of genes, including proinflammatory cytokines and other immunoregulatory molecules. Recent studies in animals have shown that activation of TLR2 and TLR4 by interaction with non-microbial mediators can play an important role in contributing to organ dysfunction in settings associated with critical illness, such as hemorrhage and ischemia/reperfusion injury, in which LPS or other bacterial products are not present.
In addition to recent studies that have provided greater detail concerning the signaling pathways activated by TLR2 and TLR4, there is increasing understanding of the nature of the molecular interactions occurring between TLR2 and TLR4 with accessory molecules as well as with their ligands and antagonists. New approaches to inhibit TLR2 and TLR4, including antibodies and small molecules, provide therapeutic approaches that may have clinical utility in critical care medicine.
In this article, we review the potential roles of TLR2 and TLR4 in contributing to non-septic acute organ dysfunction and also speculate on the potential utility of inhibiting TLR2- and TLR4-associated cellular activation in improving outcome from critical illnesses in which microbial products do not appear to play a pathogenic role. In order to review the possible role of TLR2 and TLR4 in pathophysiologic processes relevant to critical care medicine, we searched the PubMed database by successively entering the terms Toll-like receptor, TLR, TLR2, or TLR4 with the following words: sterile inflammation, ischemia-reperfusion, trauma, hemorrhage, multiorgan failure, heat shock protein, HMGB1, reactive oxygen species, hyaluronic acid and critical care.
TLR 2 and 4 signaling pathways
Engagement of ligands with the TLR2/TLR1 or TLR2/ TLR6 heterodimer or the TLR4 homodimer induces activation of intracellular signaling pathways through recruitment of the Toll-like/interleukin 1 receptor (TIR) adapters MyD88 and Mal, resulting in activation of the IκBα kinase (IKK) complex with subsequent degradation of IκBα, the inhibitor of NF-κB, in the 26S proteasome [1–3]. Decreased cytoplasmic concentrations of IκBα permit NF-κB to translocate from the cytosol to the nucleus and activate κB-dependent genes, which include proinflammatory cytokines and other mediators of inflammatory and immune responses [1, 4].
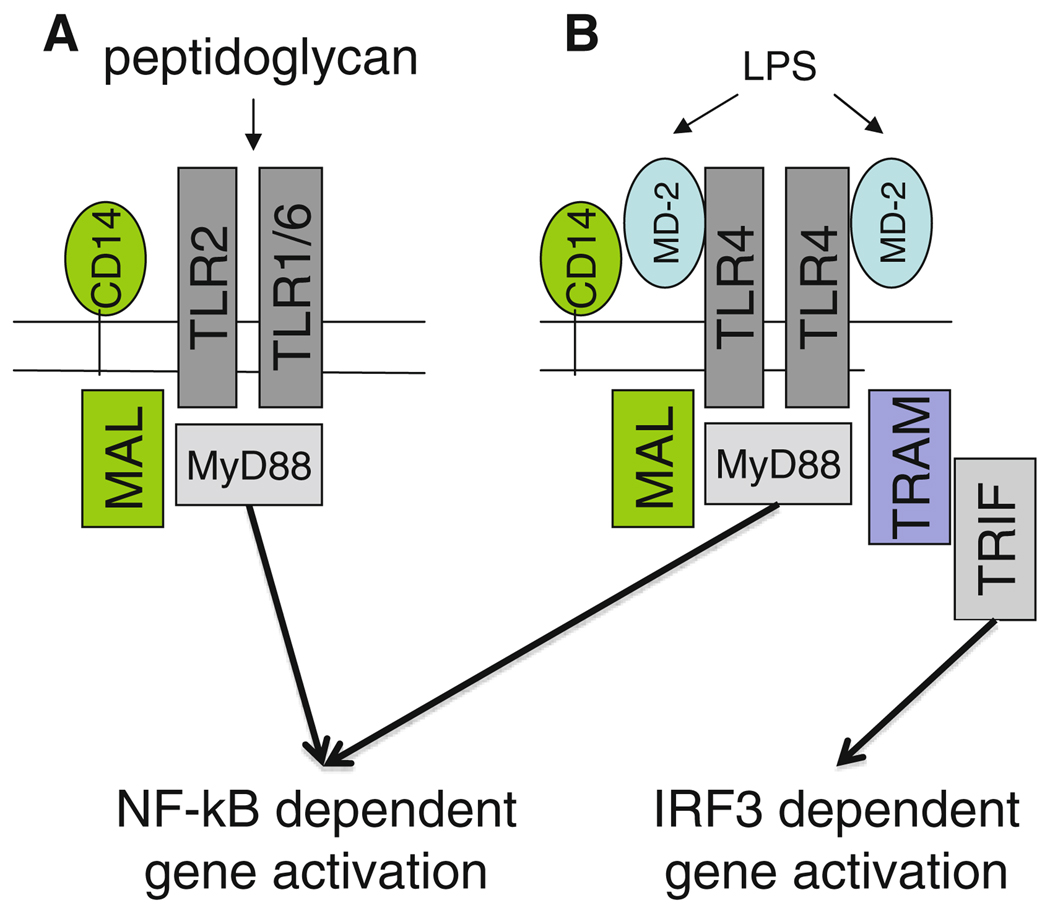
Engagement of TLR2 or TLR4 by their ligands results in cellular activation through a common pathway that involves the TIR adapters myeloid differentiation primary response gene (88) (MyD88) and MyD88 adapter-like (Mal) [1]. In addition, TLR4-induced signaling can occur through an alternate pathway that utilizes Toll/IL-1 receptor domain-containing adaptor inducing IFN-β (TRIF) and TRIF-related adaptor molecule (TRAM), which are additional TIR-associated scaffolding proteins, and this results in the dimerization and activation of interferon regulatory factor 3 (IRF3), thereby leading to the transcription of IRF3-dependent genes, such as interferon γ [1, 5] (Fig. 1).
Fig. 1.
Septic activation of TLR2 or TLR4
The serum factor CD 14 facilitates TLR2 and TLR4 activation through transferring TLR-specific ligands, such as peptidoglycan or lipoteichoic acid, to TLR2 or LPS to TLR4 (Fig. 1). TLR4 alone does not confer responsiveness of cells to LPS. TLR4 activation requires an additional protein, MD-2, which is an accessory molecule that complexes with TLR4 [6]. Mice lacking MD-2 do not respond to LPS and are protected from endotoxemia-associated mortality [7].
Participation of TLR2 and TLR4 in ischemia-reperfusion injury
Under physiological conditions, reactive oxygen species (ROS) participate in intracellular signaling pathways, transcriptional regulation, and other cellular events involved in maintaining cellular and tissue homeostasis [8]. However, in the setting of ischemia-reperfusion and other processes related to critical illness, there is increased production of ROS through mitochondrial electron transport mechanisms, activation of the purine/xanthine oxidase system, and NADPH oxidase activation [9–11].
There is increasing evidence showing that TLR4 can transduce proinflammatory signals produced by ROS. In vivo studies demonstrated decreased organ dysfunction following hemorrhage, myocardial infarction, or kidney ischemia-reperfusion in transgenic mice lacking TLR4 or the TLR4-related scaffolding protein MyD88 (Table 1) [12–14]. In these models of sterile inflammation, increased circulating levels of LPS are not present, and there is no evidence that LPS is responsible for TLR4 activation. For example, TLR4 was shown to be a key receptor that played a central role in neutrophil activation, increases in pulmonary concentrations of TNF-α and the development of acute lung injury after hemorrhage, a situation in which circulating concentrations of xanthine oxidase and production of ROS are increased, even though there is no detectible LPS in the circulation [12]. Similarly, TLR4 has been implicated in mesenteric endothelial dysfunction following hemorrhage and resuscitation, a situation in which there is no increase in circulating endotoxin [15]. Infarction size after coronary ligation was decreased in C3H/HeJ mice, which express non-functional TLR4, as compared to control C3H/HeN mice, showing that TLR4 is required to transduce cellular activation signals initiated by myocardial ischemia [13].
Table 1.
Activation of TLR2 or TLR4 by non-microbial ligands
| Disease related | Stimuli/ligand | TLR | Co-factors | Pattern of signaling and gene activation |
References |
|---|---|---|---|---|---|
| Ischemia reperfusion |
Xanthine oxidase or NADPH oxidase dependent superoxide |
TLR2 TLR4 |
MyD88 dependent | Enhanced expression of TNFα, MIP-2, IL-6, IL-1β, MCP1, IL-12, KC |
[12–19, 28–30] |
| Hemolysis, trauma, hematomas, rhabdomyolisis, hemorrhage |
Heme | TLR4 | MD-2 independent | Enhanced expression of TNFα, KC No expression of IL-6, IL-12 and IP-10 |
[38] |
| Trauma | Small fragmented Hyaluronic acid |
TLR2 TLR4 |
MD-2, MyD88 and CD44 dependent CD14 independent |
Enhanced expression of TNFα, MIP-2, IL-6, CXCL-2 TLR4 dependent activation of MMP13 and TGFβ (not activated by LPS) |
[39, 41, 45] |
| Trauma | HMGB1 | TLR2 TLR4 |
Appears to be dependent on binding of cofactors, such as IL-1β, DNA, and LPS, and interaction with the receptors for the co- factors, such as IL-1R, TLR9, or TLR4 MyD88, TIRAP, IRAK-1, IRAK-2, dependent and partially dependent of IRAK-4 |
Enhanced activation of TNF-α, IL-1α, IL-1β IL-1Ra, IL-6, IL-8, MIP-1α, MIP-1β MIP-2 No expression of IL-10 or IL-12 |
[48, 54] |
| Ischemia-reperfusio, trauma, exercise, stress |
Heat shock proteins (Hsp) |
TLR2 TLR4 |
MD-2, MyD88 and TRAF6 dependent (Hsp 60) Not characterized for each Hsp |
Gene activation pattern different for each Hsp |
[55, 56, 65, 67, 68] |
Similar to the situation with TLR4, the absence of TLR2 is associated with smaller infarct size, reduced endothelial dysfunction and diminished leukocyte infiltration after cardiac ischemia-reperfusion resulting from ligation of the left anterior descending coronary artery [16]. TLR2, TLR4 and the associated intracellular protein MyD88 contribute to renal dysfunction following ischemia reperfusion injury resulting from interruption of renal blood flow [14, 17–19].
ROSs, such as superoxide, participate in initiating and enhancing acute inflammatory responses [20–22]. In vivo studies have demonstrated that enhanced superoxide generation in the extracellular milieu is proinflammatory. For example, excessive production of extracellular superoxide through xanthine oxidase or by inhibition of dismutation of superoxide to hydrogen peroxide resulting from the absence of extracellular superoxide dismutase (EC-SOD) leads to more severe hemorrhage-induced organ dysfunction [23, 24]. In contrast, enhanced removal of extracellular superoxide in mice overexpressing EC-SOD or through the administration of gene therapy resulting in increased expression of EC-SOD is associated with beneficial effects on organ dysfunction after hemorrhage and other ischemic insults [25, 26].
Xanthine oxidase and NADPH oxidase appear to be important sources of extracellular superoxide in the setting of ischemia–reperfusion injury. We and others have demonstrated that inhibition of superoxide production by xanthine oxidase, through allopurinol treatment, or from NADPH oxidase, as a result of blocking translocation to the membrane of the NADPH phox 47 subunit, protected mice from proinflammatory processes initiated by hemorrhage or ischemia-reperfusion injury [9–11, 27]. We have also shown that extracellular superoxide derived from xanthine oxidase is able to activate neutrophils and induce neutrophil-mediated proinflammatory responses through a TLR4-dependent mechanism [28]. The catalytic ability of xanthine oxidase to produce superoxide is required to activate TLR4-associated signaling pathways and proin-flammatory responses [28]. This suggests that superoxide rather than xanthine oxidase or NADPH oxidase is a major mediator of the proinflammatory response. In addition, our studies show that xanthine oxidase can bind to TLR4 and that superoxide must be delivered by xanthine oxidase in close proximity to TLR4 in order to activate neutrophils [28]. Similarly, the NADPH subunit NOX4 (gp 47phox) can bind to TLR4 [29], and the NADPH subunit NOX2 (gp91phox) can associate with TLR2 [30].
The precise mechanism by which extracellular super-oxide induces cell activation through TLR4 is currently not well understood. While superoxide may directly affect TLR4 dimerization, an essential step required for initiation of intracellular signaling pathways, it is also possible that superoxide may affect TLR4-related signaling through indirect mechanisms. For example, increased activation of inducible nitric oxide synthase (iNOS) and resultant production of nitric oxide (NO) accompany septic shock and other critical illnesses [31]. Because superoxide reacts with NO, forming peroxynitrite, it is possible that interactions between TLR4 and peroxynitrite may induce cellular activation without requiring any direct association of superoxide with TLR4.
ROSs other than superoxide also appear to modulate inflammatory responses through TLR4-dependent mechanisms. For example, hydrogen peroxide, which is produced by dismutation of superoxide, has been shown to increase membrane lipid raft localization of TLR4 during hemorrhagic shock [32]. Of note, other TLRs may also be implicated in ROS-dependent inflammation as shown by recent studies in which hyperoxia or intestinal ischemia induced ALI were demonstrated to be TLR3 dependent [33, 34].
Participation of TLR2 and TLR4 in trauma
Tissue injury, as well as systemic or regional hypoper-fusion as a result of hemorrhage associated with accidental trauma, induce the release of endogenous ligands for TLR4 by dying or necrotic cells. Activation of TLR4 through interaction with these endogenous, non-microbial mediators contributes to inflammation and the development of ALI and other organ dysfunction.
Heme activates TLR4 by mechanisms distinct from those utilized by LPS
Heme, a central component of hemoglobin, is composed of an atom of iron linked to four ligand groups of porphyrin. Pathophysiologic conditions associated with hemolysis or extensive tissue damage produce increased amounts of free heme in the bloodstream. For example, large amounts of free heme and heme-associated proteins are found after rhabdomyolysis due to trauma, ischemia/ reperfusion injury, hemoglobinopathies, resolution of hematomas, hemorrhage or muscle injury [35, 36]. Free heme is associated with increased generation of ROS [37]. Exposure of macrophages to heme resulted in increased production of the proinflammatory cytokine, tumor necrosis factor-alpha (TNF-α), through a mechanism dependent on MyD88, TLR4 and CD14 [38]. The activation of TLR4 by heme requires iron and the vinyl groups of the porphyrin ring [38].
Activation of TLR4-dependent signaling pathways by heme appears to depend on an interaction distinct from the one between TLR4/MD2 and LPS because anti-TLR4/ MD2 antibodies or a lipid A antagonist inhibit LPS-induced TNF-α secretion, but not that induced by exposure of macrophages to heme (Table 1) [38]. Conversely, protoporphyrin IX antagonized heme-induced activation of TLR4 without affecting that produced by LPS [38].
Fragmented hyaluronic acid, which is increased in the setting of severe trauma and acute lung injury, activates TLR2 and TLR4
Hyaluronic acid (HA), a major extracellular matrix glycosaminoglycan, has been shown to be present in smaller fragments at the sites of inflammation and tissue damage [39, 40]. In vivo, high molecular weight HA (2–6 × 106 Da) can be depolymerized and converted to low molecular weight fragments (0.2 × 106 Da) via enzymatic degradation by hyaluronidase β-glucuronidase and hexosaminidase. Degradation of HA and release of small molecular weight HA fragments from the extracellular matrix has been shown to occur after traumatic injury [41]. The importance of HA fragments in contributing to inflammation under in vivo conditions is indicated by the fact that not only is lower molecular weight HA associated with active inflammatory processes, but also that a decrease in CD44-dependent clearance of HA leads to enhanced lung inflammation and injury (Table 1) [42–44]. Small molecular weight HA fragments were found to require TLR2 and TLR4 associated with MD-2, as well as MyD88, to stimulate mouse macrophages to produce inflammatory chemokines and cytokines [39, 45].
Small HA fragments appear to activate TLR4-associated signaling pathways in a manner that is different from that induced by LPS. In particular, small HA fragments require MD-2, but not CD14, to produce activation of TLR4-associated signaling events (Table 1). Such HA fragment associated properties are potentiated by the accessory molecule CD44, as small HA fragments induce physical association between TLR4 and CD44 on the cell membrane [41]. The patterns of gene activation, as determined by gene arrays, are different in monocytes stimulated by HA fragments as compared to those found after exposure to LPS (Table 1) [41]. TNFα, MIP-2, regulated upon activation, normal T cell expressed and secreted (RANTES) and monocyte chimoattractant protein 1 (MCP-1) induced by HA are expressed in a similar manner as with LPS, but other NF-κB-dependent molecules such as granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-1α are expressed differently in HA-stimulated cells as compared to those stimulated with LPS [41]. These findings demonstrate that sterile activation of TLR4 may not induce the same pattern of gene expression as does activation of TLR4 in sepsis. How these differing patterns of cellular activation occur have not been delineated, but one can speculate that association of TLR4 with CD44 is involved in modulating different spectrums of gene expression.
TLR4 participates in late inflammatory responses in which HMGB1 plays a contributory role
High mobility group box-1 protein (HMGB1), originally described as a nuclear nonhistone DNA-binding protein, has been shown to act as an extracellular participant in inflammation, particularly when bound to proinflammatory mediators such as IL-1β, LPS or DNA [46–49]. Experiments in mice found that HMGB1 levels in serum are increased at late time points after endotoxin exposure [46, 50]. We have recently shown that circulating levels of HMGB1 are elevated within 6 h after accidental trauma in humans [51]. The proinflammatory effects of HMGB1 were demonstrated in mice by the ability of HMGB1 to produce acute lung injury after direct intratracheal injection [52]. Administration of anti-HMGB1 antibodies protects mice from LPS-induced lethality even if the therapy is delayed several hours and is given after the appearance of the early proinflammatory cytokine response [46]. Administration of anti-HMGB1 antibodies also decreased the severity of LPS-induced acute lung injury, even though pulmonary concentrations of proinflammatory cytokines, such as IL-1β or TNF-α, remain elevated [52]. Similarly, in septic mice with peritonitis, mortality can be reduced if anti-HMGB1 antibodies are given as long as 24 h after the initiation of infection due to cecal ligation and perforation [53].
Transient transfection in immortalized human embryonic kidney 293 cells demonstrated that HMGB1 induced cellular activation and NF-κB-dependent transcription through TLR2 or TLR4 [54]. Co-immunoprecipitation studies showed interaction between HMGB1 and TLR2 as well as between HMGB1 and TLR4. Such interactions between HMGB1 and TLR2 and TLR4 provide an explanation for the ability of HMGB1 to induce cellular activation and generate inflammatory responses that are similar to those initiated by LPS [54]. However, we and others have demonstrated that HMGB1 itself has limited or no proinflammatory activity, and only develops the ability to induce cytokine production by macrophages and other cell populations after binding to DNA or through association with proinflammatory mediators, such as IL-1β (Table 1) [48]. Such results suggest that the reported associations between HMGB1 and TLR2 or TLR4 may be due to co-factors bound to HMGB1, and not necessarily to direct interactions of HMGB1 with TLR2 or TLR4. Future studies will be necessary to resolve this issue.
Heat shock proteins (HSP) induce proinflammatory cytokine release through TLR2 and TLR4
Members of the heat shock protein family, including Hsp60, Hsp70, Hsp72, Hsp90 and gp96, are capable of inducing production of proinflammatory cytokines via CD14/TLR2 and CD14/TLR4 receptor complex-mediated signal transduction pathways [55]. Hsp60 induces the expression of TNFα via TLR2 and increases TNFα expression through interacting with TLR4 and MD-2, MyD88 and TNF receptor associated factor 6 (TRAF6) (Table 1) [56]. The primary function of the HSPs appears to be as molecular chaperones in which they recognize and bind to nascent polypeptide chains and partially folded protein intermediates, thereby preventing aggregation and misfolding of such polypeptides [57–59].
HSPs are expressed constitutively and at increased levels under pathophysiologic conditions. For example, HSPs leak into the extracellular compartment after necrotic cell death [60]. In addition, HSPs can be released extracellularly independently of necrotic cell death in response to a number of conditions, including ischemia-reperfusion injury [55, 61], trauma [62] and intense exercise [63]. However, the mechanism by which HSPs produce cellular activation through TLR2- and TLR4-dependent mechanisms is not well delineated, and there is continuing controversy concerning the significance of interactions between HSPs and TLRs [64–66].
Recent studies have used recombinant HSPs, which contain no LPS, to investigate the mechanism by which they may produce cellular activation. Those studies demonstrate that exposure of cells to HSPs results in a specific pattern of proinflammatory cytokine expression, different from that induced by LPS, through a TLR4-dependent mechanism. For example, a recent study found that Hsp72 is released by cells during hepatic ischemia-reperfusion injury [67], and stimulation of hepatocytes with purified human recombinant HSP72 did not induce production of TNF-α or IL-6, but did result in dose-dependent increases in MIP-2 release. Production of MIP-2 was significantly decreased in hepatocytes obtained from TLR4 knockout mice [67]. A second study showed that Hsp70 plays a role in ischemia reperfusion injury by TLR4-dependent mechanisms and that recombinant Hsp70 induced NF-κB activation as well as the expression of TNF-α, IL1β and IL-6 and depression of myocardial contractility in a TLR4-dependent manner [68].
Perspectives
Increasing data demonstrate an important role for TLR2 and TLR4 in contributing to organ dysfunction in experimental settings related to critical illness, including sepsis, trauma, hyperoxia and ischemia reperfusion injury. However, fundamental questions remain to be addressed in order to develop specific interventions directed at modulating such TLR2- or TLR4-associated signaling. Indeed, given the growing number of putative non-microbial ligands that appear capable of inducing cellular activation through TLR2 and TLR4, major issues relate to the nature of the complex formed between such ligands with TLR2 or TLR4 and the most effective way to interrupt proinflammatory signaling induced by these interactions.
Inflammation initiated by interaction of heme with TLR4 is MD-2 independent and that produced by small fragments of hyaluronic acid is CD 14 independent (see Table 1). Such findings demonstrate that TLR4, and presumably TLR2, can be activated through mechanisms other than those utilized by PAMPs and imply that pharmacological interventions specifically aimed at these pathways of cellular interaction may reduce pathologic inflammatory processes that contribute to critical illness without compromising the beneficial roles of TLR2 and TLR4 in innate immunity against bacterial infection. Therapeutic strategies that may be effective in modulating non-septic TLR2- or TLR4-induced organ dysfunction include receptor agonists, receptor antagonists and signal transduction inhibitors.
The association of ischemia-reperfusion or trauma with TLR4-induced organ dysfunction suggests that down-regulation of TLR4-associated cellular activation may be useful in improving outcome in these clinical settings. Ligand-specific interventions that target proximal events induced by TLR2 or TLR4 engagement may have specific utility in non-septic critical illness in which TLR2 or TLR4 plays a role. For example, even before the description of TLRs, antagonists of lipid A were under development as treatment for gram-negative sepsis and endotoxemia. The lipid A analog E5564 (eritoran) inhibits TLR4 activation and is currently being investigated in clinical trials for sepsis [69]. The use of E5564 in a model of myocardial ischemia-reperfusion in mice reduced infarct size as well as the production of proinflammatory cytokines whose transcription is dependent on NF-jB [70]. E5564 is also able to antagonize the interaction of the protein ligand fibronectin EDA with TLR4 [71]. These studies suggest that E5564 may be useful for clinical conditions other than sepsis and endotoxemia in which TLR4 plays a central role in cellular activation.
Similar to lipid A analogs for TLR4, lipoteic acid analogs, such as Lactobacillus plantarum lipoteichoic acid, inhibit signal transduction induced by engagement of TLR2. Lactobacillus plantarum lipoteichoic acid also diminished the production of TNF-α after exposure of cells to the TLR2 ligand staphylococcus aureus lipoteic acid [72]. Similarly, a series of novel synthetic phospholipids that are TLR2 antagonists have been synthesized, but there are no data on the use of these compounds in experimental situations relevant to critical illness [73].
Disrupting the association of ligand with its receptor using specific peptides, small molecules or antibodies is a classical way to diminish signaling and cellular activation. We have recently demonstrated that heparin, which releases xanthine oxidase from association with the cell membrane and TLR4, decreases superoxide-dependent activation of TLR4-associated intracellular signaling events. This finding implies that heparin may be useful in diminishing inflammation and organ dysfunction in pathophysiologic conditions, such as hemorrhage or intestinal ischemia, in which increased circulating and cell-associated concentrations of xanthine oxidase are present. The ability of heparin to decrease xanthine oxidase-induced TLR4 activation provides a potential mechanism for a beneficial effect of heparin in sepsis that is not related to its anticoagulant actions [28]. In addition, since the binding of xanthine oxidase to the cell surface is mediated through interaction with heparan sulfate chains, peptides that can block the binding site of xanthine oxidase to glycosaminoglycans may also specifically diminish interactions between TLR4 and xanthine oxidase. A potential benefit of such peptides would be in blocking the proinflammatory effects of xanthine oxidase-generated superoxide without compromising the role of TLR4 in the host defense against bacterial infection. Similarly, specific therapies able to inhibit interactions of NADPH oxidase subunits with the TIR domain of TLR4 could result in a decrease of TLR4-dependent inflammation in the setting of ischemia reperfusion. Ligands, such as heme, that can activate TLR4-induced cellular activation without the presence of MD-2 might be disengaged from TLR4 by small interfering molecules such as protoporphyrin IX, thereby reducing their proinflammatory effects but without inhibiting beneficial innate immune responses to infection [38].
Antibodies to the extracellular domain of TLR2 have been successful in reducing mortality in mice exposed to TLR2 ligands [74]. An antibody directed to the extracellular domain of TLR2 (T2.5) abrogates the binding of lipoteichoic acid to TLR2. Such findings suggest that anti-TLR2 antibodies may be useful in reducing TLR2-dependent non-septic inflammation and organ dysfunction.
Small molecule approaches to modulate intracellular pathways initiated by TLR2 or TLR4 engagement are currently under investigation. For example, the cyclohexene derivative TAK-242 is a small synthetic molecular inhibitor of TLR4, but not TLR2, signaling that binds Cys747 in the intracellular signaling domain of TLR4 [75]. TAK-242 inhibits the TLR4-induced TRIF signaling pathway as well as the MyD88-dependent pathway [75]. A recently developed splice variant of TRAM [TRAM adapter with gold domain (TAG)] specifically inhibits the MyD88-independent signaling pathway upon LPS treatment by disrupting the interaction of TRIF with TRAM [76].
With the recent delineation of the crystal structures of the TLR2/TLR6 heterodimer and of the TLR4 homodimer associated with MD-2, it is now possible to explore how non-microbial ligands bind to TLR2 and TLR4. The availability of structural information concerning the interaction of TLR4/MD-2 and TLR2/TLR6 with microbial and non-microbial ligands will provide insights into the mechanisms by which TLR2 and TLR4 are activated and may suggest novel approaches for inhibiting excessive signaling through these receptors. Crystallography and associated functional studies may provide important insights into how cellular activation induced by DAMPS and other non-microbial TLR2 or TLR4 ligands can be modulated without compromising host defense mechanisms important in response to nosocomial and other infections.
A number of ligands, other than those discussed in this review, may contribute to non-septic inflammation through TLR2- or TLR4-associated pathways in critically ill patients. For example, TLR2 and TLR4 appear to be involved in ethanol- and acetaminophen-induced liver injury, ventilator-induced lung injury, aspiration-associated lung injury as well as in life-threatening asthma exacerbation [77–84].
Conclusions
The data discussed in this review show that TLR2 and TLR4 are not only receptors for bacterial products, but also can be activated through additional mechanisms relevant to the pathophysiology of critical illness. The consequences of activation of TLR2 and TLR4 by ROS and other mechanisms not related to infection may be different from those associated with binding of microbial products and may produce different signatures of gene activation and release of proinflammatory mediators. Future investigation will be necessary to characterize more completely the nature of the interactions among TLR2, TLR4 and other TLRs with non-microbial mediators of inflammation as well as their pathophysiologic significance in critically ill patients.
The ability of ROS, hyaluronic acid fragments, HMGB1 and other non-microbial mediators to contribute to acute inflammatory processes and organ dysfunction through pathways involving TLR2 or TLR4 may have important therapeutic implications for patients in intensive care units. In particular, therapeutic approaches directly aimed at diminishing formation of complexes between TLR2 or TLR4 and specific ligands or modulating intracellular signaling events initiated by the engagement of TLR2 or TLR4 may be beneficial not only in sepsis, but also in settings, such as after severe trauma and blood loss, where infection or endotoxemia does not appear to occur or to play a major role. Future experiments and clinical trials will be necessary to further explore the therapeutic implications of these hypotheses.
Contributor Information
Emmanuel Lorne, Pole Anesthésie Réanimation, Centre Hospitalier Universitaire d’Amiens, Université Jules Verne de Picardie, Place Victor Pauchet, 80054 Amiens Cedex, France; INSERM, ERI-12, Amiens, France.
Hervé Dupont, Pole Anesthésie Réanimation, Centre Hospitalier Universitaire d’Amiens, Université Jules Verne de Picardie, Place Victor Pauchet, 80054 Amiens Cedex, France; INSERM, ERI-12, Amiens, France.
Edward Abraham, Department of Medicine, University of Alabama, Birmingham, AL 35205, USA; Center for Free Radical Biology, University of Alabama, Birmingham, AL 35205, USA.
References
- 1.Gay NJ, Gangloff M. Structure and function of Toll receptors and their ligands. Annu Rev Biochem. 2007;76:141–165. doi: 10.1146/annurev.biochem.76.060305.151318. [DOI] [PubMed] [Google Scholar]
- 2.Vogel SN, Fitzgerald KA, Fenton MJ. TLRs: differential adapter utilization by toll-like receptors mediates TLR-specific patterns of gene expression. Mol Interv. 2003;3:466–477. doi: 10.1124/mi.3.8.466. [DOI] [PubMed] [Google Scholar]
- 3.McGettrick AF, O’Neill LA. The expanding family of MyD88-like adaptors in Toll-like receptor signal transduction. Mol Immunol. 2004;41:577–582. doi: 10.1016/j.molimm.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Baeuerle PA, Baltimore D. I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science. 1988;242:540–546. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- 5.Fitzgerald KA, Rowe DC, Barnes BJ, Caffrey DR, Visintin A, Latz E, Monks B, Pitha PM, Golenbock DT. LPS-TLR4 signaling to IRF-3/7 and NF-kappa B involves the toll adapters TRAM and TRIF. J Exp Med. 2003;198:1043–1055. doi: 10.1084/jem.20031023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, Kimoto M. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189:1777–1782. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagai Y, Akashi S, Nagafuku M, Ogata M, Iwakura Y, Akira S, Kitamura T, Kosugi A, Kimoto M, Miyake K. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat Immunol. 2002;3:667–672. doi: 10.1038/ni809. [DOI] [PubMed] [Google Scholar]
- 8.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 9.Shenkar R, Abraham E. Mechanisms of lung neutrophil activation after hemorrhage or endotoxemia: roles of reactive oxygen intermediates, NF-kappa B, and cyclic AMP response element binding protein. J Immunol. 1999;163:954–962. [PubMed] [Google Scholar]
- 10.Tan LR, Waxman K, Clark L, Eloi L, Chhieng N, Miller B, Young A. Superoxide dismutase and allopurinol improve survival in an animal model of hemorrhagic shock. Am Surg. 1993;59:797–800. [PubMed] [Google Scholar]
- 11.Shiotani S, Shimada M, Taketomi A, Soejima Y, Yoshizumi T, Hashimoto K, Shimokawa H, Maehara Y. Rho-kinase as a novel gene therapeutic target in treatment of cold ischemia/ reperfusion-induced acute lethal liver injury: effect on hepatocellular NADPH oxidase system. Gene Ther. 2007;14:1425–1433. doi: 10.1038/sj.gt.3303000. [DOI] [PubMed] [Google Scholar]
- 12.Barsness KA, Arcaroli J, Harken AH, Abraham E, Banerjee A, Reznikov L, McIntyre RC. Hemorrhage-induced acute lung injury is TLR-4 dependent. Am J Physiol Regul Integr Comp Physiol. 2004;287:R592–R599. doi: 10.1152/ajpregu.00412.2003. [DOI] [PubMed] [Google Scholar]
- 13.Oyama J, Blais C, Jr, Liu X, Pu M, Kobzik L, Kelly RA, Bourcier T. Reduced myocardial ischemia-reperfusion injury in toll-like receptor 4-deficient mice. Circulation. 2004;109:784–789. doi: 10.1161/01.CIR.0000112575.66565.84. [DOI] [PubMed] [Google Scholar]
- 14.Wu H, Chen G, Wyburn KR, Yin J, Bertolino P, Eris JM, Alexander SI, Sharland AF, Chadban SJ. TLR4 activation mediates kidney ischemia/ reperfusion injury. J Clin Invest. 2007;117:2847–2859. doi: 10.1172/JCI31008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benhamou Y, Favre J, Musette P, Renet S, Thuillez C, Richard V, Tamion F. Toll-like receptors 4 contribute to endothelial injury and inflammation in hemorrhagic shock in mice. Crit Care Med. 2009;37:1724–1728. doi: 10.1097/CCM.0b013e31819da805. [DOI] [PubMed] [Google Scholar]
- 16.Favre J, Musette P, Douin-Echinard V, Laude K, Henry JP, Arnal JF, Thuillez C, Richard V. Toll-like receptors 2-deficient mice are protected against postischemic coronary endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2007;27:1064–1071. doi: 10.1161/ATVBAHA.107.140723. [DOI] [PubMed] [Google Scholar]
- 17.Leemans JC, Stokman G, Claessen N, Rouschop KM, Teske GJ, Kirschning CJ, Akira S, van der Poll T, Weening JJ, Florquin S. Renal-associated TLR2 mediates ischemia/reperfusion injury in the kidney. J Clin Invest. 2005;115:2894–2903. doi: 10.1172/JCI22832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pulskens WP, Teske GJ, Butter LM, Roelofs JJ, van der Poll T, Florquin S, Leemans JC. Toll-like receptor-4 coordinates the innate immune response of the kidney to renal ischemia/ reperfusion injury. PLoS One. 2008;3:e3596. doi: 10.1371/journal.pone.0003596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shigeoka AA, Holscher TD, King AJ, Hall FW, Kiosses WB, Tobias PS, Mackman N, McKay DB. TLR2 is constitutively expressed within the kidney and participates in ischemic renal injury through both MyD88-dependent and -independent pathways. J Immunol. 2007;178:6252–6258. doi: 10.4049/jimmunol.178.10.6252. [DOI] [PubMed] [Google Scholar]
- 20.Abraham E, Singer M. Mechanisms of sepsis-induced organ dysfunction. Crit Care Med. 2007;35:2408–2416. doi: 10.1097/01.ccm.0000282072.56245.91. [DOI] [PubMed] [Google Scholar]
- 21.Matthay MA, Zimmerman GA, Esmon C, Bhattacharya J, Coller B, Doerschuk CM, Floros J, Gimbrone MA, Jr, Hoffman E, Hubmayr RD, Leppert M, Matalon S, Munford R, Parsons P, Slutsky AS, Tracey KJ, Ward P, Gail DB, Harabin AL. Future research directions in acute lung injury: summary of a national heart, lung, and blood institute working group. Am J Respir Crit Care Med. 2003;167:1027–1035. doi: 10.1164/rccm.200208-966WS. [DOI] [PubMed] [Google Scholar]
- 22.Torres M, Forman HJ. Redox signaling and the MAP kinase pathways. Biofactors. 2003;17:287–296. doi: 10.1002/biof.5520170128. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz MD, Repine JE, Abraham E. Xanthine oxidase-derived oxygen radicals increase lung cytokine expression in mice subjected to hemorrhagic shock. Am J Respir Cell Mol Biol. 1995;12:434–440. doi: 10.1165/ajrcmb.12.4.7695923. [DOI] [PubMed] [Google Scholar]
- 24.Bowler RP, Arcaroli J, Abraham E, Patel M, Chang LY, Crapo JD. Evidence for extracellular superoxide dismutase as a mediator of hemorrhage-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2003;284:L680–L687. doi: 10.1152/ajplung.00191.2002. [DOI] [PubMed] [Google Scholar]
- 25.Li Q, Bolli R, Qiu Y, Tang XL, Guo Y, French BA. Gene therapy with extracellular superoxide dismutase protects conscious rabbits against myocardial infarction. Circulation. 2001;103:1893–1898. doi: 10.1161/01.cir.103.14.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bowler RP, Arcaroli J, Crapo JD, Ross A, Slot JW, Abraham E. Extracellular superoxide dismutase attenuates lung injury after hemorrhage. Am J Respir Crit Care Med. 2001;164:290–294. doi: 10.1164/ajrccm.164.2.2011054. [DOI] [PubMed] [Google Scholar]
- 27.Shenkar R, Abraham E. Hemorrhage induces rapid in vivo activation of CREB and NF-kappa B in murine intraparenchymal lung mononuclear cells. Am J Respir Cell Mol Biol. 1997;16:145–152. doi: 10.1165/ajrcmb.16.2.9032121. [DOI] [PubMed] [Google Scholar]
- 28.Lorne E, Zmijewski JW, Zhao X, Liu G, Tsuruta Y, Park YJ, Dupont H, Abraham E. Role of extracellular superoxide in neutrophil activation: interactions between xanthine oxidase and TLR4 induce proinflammatory cytokine production. Am J Physiol Cell Physiol. 2008;294:C985–C993. doi: 10.1152/ajpcell.00454.2007. [DOI] [PubMed] [Google Scholar]
- 29.Park HS, Jung HY, Park EY, Kim J, Lee WJ, Bae YS. Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J Immunol. 2004;173:3589–3593. doi: 10.4049/jimmunol.173.6.3589. [DOI] [PubMed] [Google Scholar]
- 30.Yang CS, Shin DM, Kim KH, Lee ZW, Lee CH, Park SG, Bae YS, Jo EK. NADPH oxidase 2 interaction with TLR2 is required for efficient innate immune responses to mycobacteria via cathelicidin expression. J Immunol. 2009;182:3696–3705. doi: 10.4049/jimmunol.0802217. [DOI] [PubMed] [Google Scholar]
- 31.Cobb JP. Nitric oxide synthase inhibition as therapy for sepsis: a decade of promise. Surg Infect (Larchmt) 2001;2:93–100. doi: 10.1089/109629601750469410. discussion 100–101. [DOI] [PubMed] [Google Scholar]
- 32.Powers KA, Szaszi K, Khadaroo RG, Tawadros PS, Marshall JC, Kapus A, Rotstein OD. Oxidative stress generated by hemorrhagic shock recruits Toll-like receptor 4 to the plasma membrane in macrophages. J Exp Med. 2006;203:1951–1961. doi: 10.1084/jem.20060943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murray LA, Knight DA, McAlonan L, Argentieri R, Joshi A, Shaheen F, Cunningham M, Alexopolou L, Flavell RA, Sarisky RT, Hogaboam CM. Deleterious role of TLR3 during hyperoxia-induced acute lung injury. Am J Respir Crit Care Med. 2008;178:1227–1237. doi: 10.1164/rccm.200807-1020OC. [DOI] [PubMed] [Google Scholar]
- 34.Cavassani KA, Ishii M, Wen H, Schaller MA, Lincoln PM, Lukacs NW, Hogaboam CM, Kunkel SL. TLR3 is an endogenous sensor of tissue necrosis during acute inflammatory events. J Exp Med. 2008;205:2609–2621. doi: 10.1084/jem.20081370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Letarte PB, Lieberman K, Nagatani K, Haworth RA, Odell GB, Duff TA. Hemin: levels in experimental subarachnoid hematoma and effects on dissociated vascular smooth-muscle cells. J Neurosurg. 1993;79:252–255. doi: 10.3171/jns.1993.79.2.0252. [DOI] [PubMed] [Google Scholar]
- 36.Nath KA, Vercellotti GM, Grande JP, Miyoshi H, Paya CV, Manivel JC, Haggard JJ, Croatt AJ, Payne WD, Alam J. Heme protein-induced chronic renal inflammation: suppressive effect of induced heme oxygenase-1. Kidney Int. 2001;59:106–117. doi: 10.1046/j.1523-1755.2001.00471.x. [DOI] [PubMed] [Google Scholar]
- 37.Jeney V, Balla J, Yachie A, Varga Z, Vercellotti GM, Eaton JW, Balla G. Pro-oxidant and cytotoxic effects of circulating heme. Blood. 2002;100:879–887. doi: 10.1182/blood.v100.3.879. [DOI] [PubMed] [Google Scholar]
- 38.Figueiredo RT, Fernandez PL, Mourao- Sa DS, Porto BN, Dutra FF, Alves LS, Oliveira MF, Oliveira PL, Graca-Souza AV, Bozza MT. Characterization of heme as activator of Toll-like receptor 4. J Biol Chem. 2007;282:20221–20229. doi: 10.1074/jbc.M610737200. [DOI] [PubMed] [Google Scholar]
- 39.Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, Miyake K, Freudenberg M, Galanos C, Simon JC. Oligosaccharides of Hyaluronan activate dendritic cells via Toll-like receptor 4. J Exp Med. 2002;195:99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Agren UM, Tammi RH, Tammi MI. Reactive oxygen species contribute to epidermal hyaluronan catabolism in human skin organ culture. Free Radic Biol Med. 1997;23:996–1001. doi: 10.1016/s0891-5849(97)00098-1. [DOI] [PubMed] [Google Scholar]
- 41.Taylor KR, Yamasaki K, Radek KA, Di Nardo A, Goodarzi H, Golenbock D, Beutler B, Gallo RL. Recognition of hyaluronan released in sterile injury involves a unique receptor complex dependent on Toll-like receptor 4, CD44, and MD-2. J Biol Chem. 2007;282:18265–18275. doi: 10.1074/jbc.M606352200. [DOI] [PubMed] [Google Scholar]
- 42.Teriete P, Banerji S, Noble M, Blundell CD, Wright AJ, Pickford AR, Lowe E, Mahoney DJ, Tammi MI, Kahmann JD, Campbell ID, Day AJ, Jackson DG. Structure of the regulatory hyaluronan binding domain in the inflammatory leukocyte homing receptor CD44. Mol Cell. 2004;13:483–496. doi: 10.1016/s1097-2765(04)00080-2. [DOI] [PubMed] [Google Scholar]
- 43.Wang Q, Teder P, Judd NP, Noble PW, Doerschuk CM. CD44 deficiency leads to enhanced neutrophil migration and lung injury in Escherichia coli pneumonia in mice. Am J Pathol. 2002;161:2219–2228. doi: 10.1016/S0002-9440(10)64498-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Teder P, Vandivier RW, Jiang D, Liang J, Cohn L, Pure E, Henson PM, Noble PW. Resolution of lung inflammation by CD44. Science. 2002;296:155–158. doi: 10.1126/science.1069659. [DOI] [PubMed] [Google Scholar]
- 45.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 46.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 47.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 48.Sha Y, Zmijewski J, Xu Z, Abraham E. HMGB1 develops enhanced proinflammatory activity by binding to cytokines. J Immunol. 2008;180:2531–2537. doi: 10.4049/jimmunol.180.4.2531. [DOI] [PubMed] [Google Scholar]
- 49.Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, Parroche P, Drabic S, Golenbock D, Sirois C, Hua J, An LL, Audoly L, La Rosa G, Bierhaus A, Naworth P, Marshak-Rothstein A, Crow MK, Fitzgerald KA, Latz E, Kiener PA, Coyle AJ. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–496. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 50.Ulloa L, Batliwalla FM, Andersson U, Gregersen PK, Tracey KJ. High mobility group box chromosomal protein 1 as a nuclear protein, cytokine, and potential therapeutic target in arthritis. Arthritis Rheum. 2003;48:876–881. doi: 10.1002/art.10854. [DOI] [PubMed] [Google Scholar]
- 51.Peltz ED, Moore EE, Eckels PC, Damle SS, Tsuruta Y, Johnson JL, Sauaia A, Silliman CC, Banerjee A, Abraham E. HMGB1 is markedly elevated within 6 hours of mechanical trauma in humans. Shock. 2009;32:17–22. doi: 10.1097/shk.0b013e3181997173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. HMG-1 as a mediator of acute lung inflammation. J Immunol. 2000;165:2950–2954. doi: 10.4049/jimmunol.165.6.2950. [DOI] [PubMed] [Google Scholar]
- 53.Yang H, Ochani M, Li J, Qiang X, Tanovic M, Harris HE, Susarla SM, Ulloa L, Wang H, DiRaimo R, Czura CJ, Wang H, Roth J, Warren HS, Fink MP, Fenton MJ, Andersson U, Tracey KJ. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci USA. 2004;101:296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park JS, Gamboni-Robertson F, He Q, Svetkauskaite D, Kim JY, Strassheim D, Sohn JW, Yamada S, Maruyama I, Banerjee A, Ishizaka A, Abraham E. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol. 2006;290:C917–C924. doi: 10.1152/ajpcell.00401.2005. [DOI] [PubMed] [Google Scholar]
- 55.Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- 56.Vabulas RM, Ahmad-Nejad P, da Costa C, Miethke T, Kirschning CJ, Hacker H, Wagner H. Endocytosed HSP60s use toll-like receptor 2 (TLR2) and TLR4 to activate the toll/ interleukin-1 receptor signaling pathway in innate immune cells. J Biol Chem. 2001;276:31332–31339. doi: 10.1074/jbc.M103217200. [DOI] [PubMed] [Google Scholar]
- 57.Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- 58.Fink AL. Chaperone-mediated protein folding. Physiol Rev. 1999;79:425–449. doi: 10.1152/physrev.1999.79.2.425. [DOI] [PubMed] [Google Scholar]
- 59.Qian SB, McDonough H, Boellmann F, Cyr DM, Patterson C. CHIP-mediated stress recovery by sequential ubiquitination of substrates and Hsp70. Nature. 2006;440:551–555. doi: 10.1038/nature04600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol. 2000;12:1539–1546. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- 61.Asea A. Mechanisms of HSP72 release. J Biosci. 2007;32:579–584. doi: 10.1007/s12038-007-0057-5. [DOI] [PubMed] [Google Scholar]
- 62.Pittet JF, Lee H, Morabito D, Howard MB, Welch WJ, Mackersie RC. Serum levels of Hsp 72 measured early after trauma correlate with survival. J Trauma. 2002;52:611–617. doi: 10.1097/00005373-200204000-00001. discussion 617. [DOI] [PubMed] [Google Scholar]
- 63.Febbraio MA, Ott P, Nielsen HB, Steensberg A, Keller C, Krustrup P, Secher NH, Pedersen BK. Exercise induces hepatosplanchnic release of heat shock protein 72 in humans. J Physiol. 2002;544:957–962. doi: 10.1113/jphysiol.2002.025148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsan MF, Gao B. Cytokine function of heat shock proteins. Am J Physiol Cell Physiol. 2004;286:C739–C744. doi: 10.1152/ajpcell.00364.2003. [DOI] [PubMed] [Google Scholar]
- 65.Osterloh A, Veit A, Gessner A, Fleischer B, Breloer M. Hsp60-mediated T cell stimulation is independent of TLR4 and IL-12. Int Immunol. 2008;20:433–443. doi: 10.1093/intimm/dxn003. [DOI] [PubMed] [Google Scholar]
- 66.Tsan MF, Gao B. Heat shock proteins and immune system. J Leukoc Biol. 2009;85:905–910. doi: 10.1189/jlb.0109005. [DOI] [PubMed] [Google Scholar]
- 67.Galloway E, Shin T, Huber N, Eismann T, Kuboki S, Schuster R, Blanchard J, Wong HR, Lentsch AB. Activation of hepatocytes by extracellular heat shock protein 72. Am J Physiol Cell Physiol. 2008;295:C514–C520. doi: 10.1152/ajpcell.00032.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zou N, Ao L, Cleveland JC, Jr, Yang X, Su X, Cai GY, Banerjee A, Fullerton DA, Meng X. Critical role of extracellular heat shock cognate protein 70 in the myocardial inflammatory response and cardiac dysfunction after global ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2008;294:H2805–H2813. doi: 10.1152/ajpheart.00299.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mullarkey M, Rose JR, Bristol J, Kawata T, Kimura A, Kobayashi S, Przetak M, Chow J, Gusovsky F, Christ WJ, Rossignol DP. Inhibition of endotoxin response by e5564, a novel Toll-like receptor 4-directed endotoxin antagonist. J Pharmacol Exp Ther. 2003;304:1093–1102. doi: 10.1124/jpet.102.044487. [DOI] [PubMed] [Google Scholar]
- 70.Shimamoto A, Chong AJ, Yada M, Shomura S, Takayama H, Fleisig AJ, Agnew ML, Hampton CR, Rothnie CL, Spring DJ, Pohlman TH, Shimpo H, Verrier ED. Inhibition of Toll-like receptor 4 with eritoran attenuates myocardial ischemia-reperfusion injury. Circulation. 2006;114:I270–I274. doi: 10.1161/CIRCULATIONAHA.105.000901. [DOI] [PubMed] [Google Scholar]
- 71.Okamura Y, Watari M, Jerud ES, Young DW, Ishizaka ST, Rose J, Chow JC, Strauss JF., III The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem. 2001;276:10229–10233. doi: 10.1074/jbc.M100099200. [DOI] [PubMed] [Google Scholar]
- 72.Kim HG, Lee SY, Kim NR, Ko MY, Lee JM, Yi TH, Chung SK, Chung DK. Inhibitory effects of Lactobacillus plantarum lipoteichoic acid (LTA) on Staphylococcus aureus LTA-induced tumor necrosis factor-alpha production. J Microbiol Biotechnol. 2008;18:1191–1196. [PubMed] [Google Scholar]
- 73.Spyvee MR, Zhang H, Hawkins LD, Chow JC. Toll-like receptor 2 antagonists. Part 1: preliminary SAR investigation of novel synthetic phospholipids. Bioorg Med Chem Lett. 2005;15:5494–5498. doi: 10.1016/j.bmcl.2005.08.080. [DOI] [PubMed] [Google Scholar]
- 74.Meng G, Rutz M, Schiemann M, Metzger J, Grabiec A, Schwandner R, Luppa PB, Ebel F, Busch DH, Bauer S, Wagner H, Kirschning CJ. Antagonistic antibody prevents toll-like receptor 2-driven lethal shock-like syndromes. J Clin Invest. 2004;113:1473–1481. doi: 10.1172/JCI20762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Takashima K, Matsunaga N, Yoshimatsu M, Hazeki K, Kaisho T, Uekata M, Hazeki O, Akira S, Iizawa Y, Ii M. Analysis of binding site for the novel small-molecule TLR4 signal transduction inhibitor TAK-242 and its therapeutic effect on mouse sepsis model. Br J Pharmacol. 2009;157:1250–1262. doi: 10.1111/j.1476-5381.2009.00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Palsson-McDermott EM, Doyle SL, McGettrick AF, Hardy M, Husebye H, Banahan K, Gong M, Golenbock D, Espevik T, O’Neill LA. TAG, a splice variant of the adaptor TRAM, negatively regulates the adaptor MyD88-independent TLR4 pathway. Nat Immunol. 2009;10:579–586. doi: 10.1038/ni.1727. [DOI] [PubMed] [Google Scholar]
- 77.Imai Y, Kuba K, Neely GG, Yaghubian-Malhami R, Perkmann T, van Loo G, Ermolaeva M, Veldhuizen R, Leung YH, Wang H, Liu H, Sun Y, Pasparakis M, Kopf M, Mech C, Bavari S, Peiris JS, Slutsky AS, Akira S, Hultqvist M, Holmdahl R, Nicholls J, Jiang C, Binder CJ, Penninger JM. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133:235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vaneker M, Joosten LA, Heunks LM, Snijdelaar DG, Halbertsma FJ, van Egmond J, Netea MG, van der Hoeven JG, Scheffer GJ. Low-tidal-volume mechanical ventilation induces a toll-like receptor 4-dependent inflammatory response in healthy mice. Anesthesiology. 2008;109:465–472. doi: 10.1097/ALN.0b013e318182aef1. [DOI] [PubMed] [Google Scholar]
- 79.Blanco AM, Perez-Arago A, Fernandez-Lizarbe S, Guerri C. Ethanol mimics ligand-mediated activation and endocytosis of IL-1RI/ TLR4 receptors via lipid rafts caveolae in astroglial cells. J Neurochem. 2008;106:625–639. doi: 10.1111/j.1471-4159.2008.05425.x. [DOI] [PubMed] [Google Scholar]
- 80.Fernandez-Lizarbe S, Pascual M, Gascon MS, Blanco A, Guerri C. Lipid rafts regulate ethanol-induced activation of TLR4 signaling in murine macrophages. Mol Immunol. 2008;45:2007–2016. doi: 10.1016/j.molimm.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 81.Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34:101–108. doi: 10.1053/jhep.2001.25350. [DOI] [PubMed] [Google Scholar]
- 82.Yohe HC, O’Hara KA, Hunt JA, Kitzmiller TJ, Wood SG, Bement JL, Bement WJ, Szakacs JG, Wrighton SA, Jacobs JM, Kostrubsky V, Sinclair PR, Sinclair JF. Involvement of Toll-like receptor 4 in acetaminophen hepatotoxicity. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1269–G1279. doi: 10.1152/ajpgi.00239.2005. [DOI] [PubMed] [Google Scholar]
- 83.Genuneit J, Cantelmo JL, Weinmayr G, Wong GW, Cooper PJ, Riikjarv MA, Gotua M, Kabesch M, von Mutius E, Forastiere F, Crane J, Nystad W, El-Sharif N, Batlles-Garrido J, Garcia-Marcos L, Garcia-Hernandez G, Morales-Suarez-Varela M, Nilsson L, Braback L, Saraclar Y, Weiland SK, Cookson WO, Strachan D, Moffatt MF. A multi-centre study of candidate genes for wheeze and allergy: the International Study of Asthma and Allergies in Childhood Phase 2. Clin Exp Allergy. 2009;39:1875–1888. doi: 10.1111/j.1365-2222.2009.03364.x. [DOI] [PubMed] [Google Scholar]
- 84.Smit LA, Siroux V, Bouzigon E, Oryszczyn MP, Lathrop M, Demenais F, Kauffmann F. CD14 and toll-like receptor gene polymorphisms, country living, and asthma in adults. Am J Respir Crit Care Med. 2009;179:363–368. doi: 10.1164/rccm.200810-1533OC. [DOI] [PubMed] [Google Scholar]