Abstract
Gene cassettes of class 1 integrons in Escherichia coli isolates from urine specimens collected in Korea during the last 2 decades were characterized. intI1 was detected in 54% of the isolates, yet gene cassette regions were amplified in only 43% of the isolates. intI2 was detected in 29 (5%) isolates, and no intI3 was detected in this study. Twenty-one different genes, including genes encoding resistance to antibiotics, an alcohol dehydrogenase gene (adhE), and unknown genes, were detected. The genes most commonly found in class 1 integrons were those for aminoglycoside and trimethoprim resistance. The occurrence of aminoglycoside resistance genes in class 1 integrons decreased, and the presence of dfr genes increased rapidly, during the last 2 decades. Single-gene cassettes were predominant during the 1980s, while multigene cassettes predominated from the 1990s on. The aadA1, aadA2, and blaP1-aadA2 gene cassettes were frequently found in isolates from the 1980s but were not detected in isolates recovered since 2000. dfrA12-aadA2 and dfrA17-aadA5 were the most prevalent gene cassettes among isolates recovered from the 1990s on. In conclusion, class 1 integrons would appear to be responsible for resistance to antibiotics commonly used to treat urinary tract infections, and selection of a specific gene cassette was found to occur over the course of time.
The pathogens causing urinary tract infections are almost always predictable: Enterobacteriaceae, especially Escherichia coli, are the primary etiologic agents among both outpatients and inpatients. The prevalence of E. coli in urinary tract infections is currently estimated at 50 to 60% in Korea. Multiresistance to various antibiotics has been commonly found in urinary tract isolates of Enterobacteriaceae in Korea, where more than 50% of the isolates are resistant to three or more antibiotics (6, 7), while only 7.1% of urinary tract isolates in the United States are multiresistant to antibiotics (13). Antibiotic resistance genes have frequently been found to be encoded by determinants carried on mobile genetic elements, such as plasmids, transposons, and integrons, which are then responsible for the horizontal transfer of antibiotic resistance genes (11). The dissemination of antibiotic resistance genes has led to the rapid emergence of antibiotic resistance among bacterial populations.
Integrons are elements that participate in a powerful site-specific recombination system and play a major role in spreading antibiotic resistance genes in a clinical setting. Many antibiotic resistance genes found in gram-negative bacteria are part of a gene cassette inserted into an integron (12). Four classes of integrons have been identified according to their respective integrase (intI) genes (1, 10, 12). Class 1 integrons make up most of the integrons found in clinical isolates and are strongly associated with multiple-antibiotic resistance (9). To date, more than 50 different class 1 integrons and 60 different gene cassettes have been described, including gene cassettes conferring resistance to aminoglycosides, penicillins, cephalosporins, carbapenems, trimethoprim, chloramphenicol, rifampin, erythromycin, and quaternary ammonium compounds (4). Several studies have already investigated the prevalence of integrons and characterized gene cassettes in gram-negative bacteria (3, 9, 14, 15). However, these studies have only evaluated clinical isolates recovered from restricted areas during short periods. Accordingly, the aim of the present study was to determine the incidence of integrons, to characterize the antibiotic resistance genes inserted into class 1 integrons, and to determine the changes in gene cassettes in class 1 integrons among E. coli isolates from urine specimens collected in Korea during the last 2 decades.
MATERIALS AND METHODS
Bacterial strains.
A total of 621 E. coli strains were isolated at Kyungpook National University Hospital, Daegu, Korea, during three time periods: 1980 to 1985 (n = 243), 1996 to 1997 (n = 177), and 2001 to 2002 (n = 201). Isolates were obtained from urine specimens from patients with significant bacteriuria (≥105 CFU/ml) and were identified by use of a standard biochemical test.
Template DNA preparation.
The organisms were inoculated into 2 ml of Trypticase soy broth (Difco, Detroit, Mich.) and were incubated for 20 h at 37°C with shaking. The bacteria were then harvested by centrifugation at 10,000 × g for 5 min. After the supernatant was removed, the pellet was resuspended in 500 μl of sterilized deionizing water. Next, the cells were lysed by boiling for 10 min, and any cell debris was removed by centrifugation for 5 min at 11,500 × g. The supernatant was then used as the source of the template for PCR amplification.
PCR amplification of integrase genes.
To determine whether the E. coli isolates carried integrons, the conserved regions of the int genes were amplified with the degenerate primer pair hep35-hep36, and the PCR products were further restricted by using either RsaI or HinfI to determine the class of integrons, as described by White et al. (15).
Amplification and sequencing of gene cassette regions.
The gene cassette regions for class 1 and class 2 integrons were amplified with primer pairs hep58-hep59 and hep74-hep51, respectively, as described by White et al. (15, 16). To determine whether different isolates carried identical gene cassette, the cassette genes of every isolate were characterized. That is, each cassette gene PCR amplification product with a distinctive size (number of base pairs) was sequenced. PCR products of the same size were compared by restriction fragment length polymorphism to discover whether they had the same content. At least two different restriction endonucleases were chosen for each restriction fragment length polymorphism assay. To analyze the sequences of the gene cassette regions of the integrons, the PCR products were ligated with a pGEM T-easy vector (Promega, Madison, Wis.) and introduced into E. coli DH5α cells. Sequencing reactions were performed using a double-stranded plasmid preparation by dideoxy chain termination with T7 and Sp6 primers.
Southern hybridization.
Plasmid DNA was extracted by the method of Birnboim and Doly (2). After agarose gel electrophoresis of the plasmid, the denatured DNAs were transferred to a positively charged nylon membrane (Boehringer Mannheim, Mannheim, Germany) by using a capillary method. For the hybridization assays, a digoxigenin DNA labeling and detection kit (Roche, Mannheim, Germany) was used according to the manufacturer's instructions. Hybridization procedures were performed under high-stringency conditions. The probes were labeled with digoxigenin-11-dUTP by random labeling methods, and the probe for detecting the integrons used the purified PCR products of intI1.
RESULTS
Integron carriage in E. coli urinary tract isolates.
The incidence of class 1 and class 2 integrons among E. coli isolates from urine specimens collected in Korea over the last 2 decades is shown in Table 1. Among the 621 E. coli isolates tested, intI1 and intI2 were detected in 333 (54%) and 29 (5%) isolates, respectively. No intI3 was detected. Ten isolates carried two different class 1 integrons, and eight isolates carried both class 1 and class 2 integrons. The overall incidence of integrons increased slightly, from 54% in isolates from the 1980s to 58% in isolates recovered since 2000.
TABLE 1.
Incidence of integrons in E. coli isolates
| Yr isolated | No. of isolates tested | No. of isolates carrying:
|
Total no. (%) of isolates carrying intI1 and/or intI2 | ||
|---|---|---|---|---|---|
| intI1 | intI2 | intI1 and intI2 | |||
| 1980-1985 | 243 | 121a | 5 | 5 | 131 (53.9) |
| 1996-1997 | 177 | 99b | 4 | 1 | 104 (57.6) |
| 2001-2002 | 201 | 98c | 4 | 2 | 104 (58.2) |
One isolate carried two kinds of gene cassettes.
Six isolates carried two kinds of gene cassettes.
Three isolates carried two kinds of gene cassettes.
Characterization of gene cassettes.
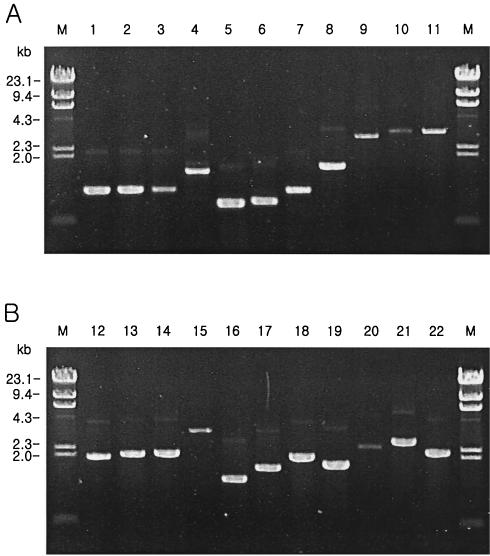
To characterize the gene cassettes in the integrons, the gene cassette regions were amplified and sequenced. Among the 334 isolates amplified, intI1 gene cassette regions were amplified in 276 isolates. The sizes of the gene cassette regions ranged from 0.7 to 3.5 kb (Fig. 1). No gene cassette regions were amplified for the remaining 58 isolates (9 isolates from 1980 to 1985, 17 isolates from 1996 to 1997, and 32 isolates from 2001 to 2002), possibly due to the lack of a 3′ conserved segment or modification of the primer binding sites (15). Twenty-one different genes, including genes encoding resistance to aminoglycosides (aadA1, aadA2, aadA2-1, aadA5, aacA4, and aadB), β-lactams (blaP1 and oxa2), chloramphenicol (catB, catB4, catB8, cmlA, and cmlA5), or trimethoprim (dfrA1, dfrA5, dfrA7, dfrA12, and dfrA17), a gene encoding an alcohol dehydrogenase enzyme (adhE), and three unknown genes, were detected (Table 2). The genes most commonly found among class 1 integrons were aminoglycoside and trimethoprim resistance genes. Six isolates carried an incomplete adhE gene; this gene encodes the alcohol dehydrogenase E protein, which was first found in class 1 integrons, indicating that class 1 integrons are associated with the transfer of a gene encoding a metabolic enzyme. All the class 2 integrons carried the same gene cassettes as those found in Tn7, namely, dfrA1, sat1, and aadA1.
FIG. 1.
PCR products of 21 different gene cassettes in class 1 (lanes 1 to 21) and class 2 (lane 22) integrons. Lanes: 1, aadA2-1; 2, aacA4; 3, dfrA1-unknown open reading frame; 4, aadB-oxa2; 5, blaP1-aadA1; 6, aadA1; 7, aadA2; 8, blaP1-aadA2; 9, dfrA12-aadA2; 10, dfrA17-aadA5; 11, dfrA1-aadA2; 12, adhEΔ; 13, aacA4-catB4-dfrA1-unknown gene; 14, dfrA5; 15, dfrA7; 16, aadB-aadA1; 17, aadB-cmlA5; 18, aacA4-aadA2; 19, aadB-cmlA; 20, unknown open reading frame-aacA4-catB8; 21, aacA4-catB; 22, dfrA1-sat-aadA; M, lambda DNA fragments digested by HindIII.
TABLE 2.
Characterization and incidence of gene cassettes in class 1 integrons of E. coli isolated during the last 2 decades
| Gene cassette | No. of gene cassettes
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1980 | 1981 | 1982 | 1983 | 1984 | 1985 | 1996 | 1997 | 2001 | 2002 | Total | |
| aadA1 | 13 | 10 | 6 | 6 | 2 | 2 | 3 | 51 | |||
| aadA2 | 4 | 3 | 2 | 1 | 2 | 12 | |||||
| aadA2-1 | 1 | 1 | |||||||||
| aacA4 | 1 | 1 | |||||||||
| dfrA5 | 2 | 4 | 1 | 7 | |||||||
| dfrA7 | 1 | 1 | |||||||||
| adhEΔ | 1 | 1 | 4 | 6 | |||||||
| aadB-aadA1 | 2 | 1 | 3 | ||||||||
| aacA4-aadA2 | 2 | 2 | |||||||||
| aadB-cmlA | 2 | 1 | 3 | ||||||||
| aadB-cmlA5 | 1 | 6 | 7 | ||||||||
| aacA4-calB | 1 | 1 | 2 | ||||||||
| blaP1-aadA1 | 5 | 1 | 1 | 8 | |||||||
| blaP1-aadA2 | 1 | 7 | 5 | 6 | 7 | 26 | |||||
| aadB-oxa2 | 1 | 2 | 3 | ||||||||
| dfrA1-unknown ORFa | 1 | 1 | |||||||||
| dfrA1-aadA2 | 4 | 4 | 1 | 1 | 10 | ||||||
| dfrA12-aadA2 | 5 | 7 | 6 | 14 | 8 | 12 | 10 | 62 | |||
| dfrA17-aadA5 | 16 | 11 | 16 | 14 | 57 | ||||||
| Unknown ORF aacA4-catB8 | 1 | 1 | |||||||||
| aacA4-catB4-dfrA1-unknown gene | 1 | 1 | 1 | 3 | |||||||
| Total | 24 | 14 | 10 | 19 | 20 | 17 | 44 | 41 | 37 | 32 | 267 |
ORF, open reading frame.
Changes of gene cassettes in class 1 integrons.
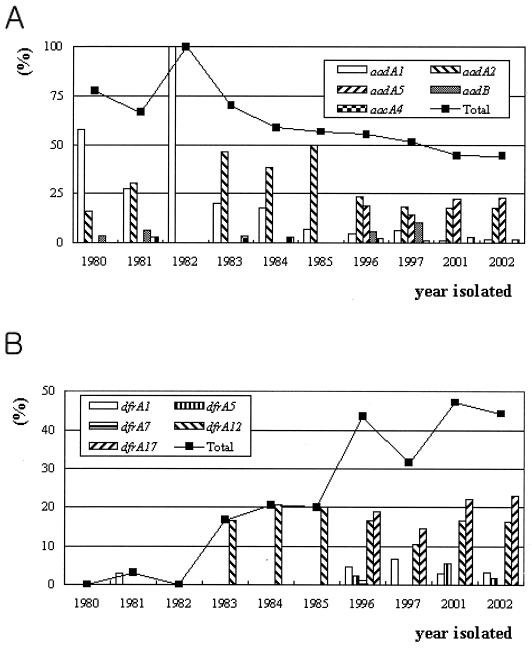
The prevalences of aminoglycoside and trimethoprim resistance genes associated with class 1 integrons in E. coli urinary tract isolates were found to change over time. The incidence of aadA1 among the integrons was above 50% in 1980 but thereafter decreased rapidly up to the present. The incidence of aadA2 increased beginning in 1980, reached 50% in 1985, and then decreased to below 25% from the 1990s on (Fig. 2). These changes may have been caused by the emergence of the dfrA12-aadA2 gene cassette in 1983 and the disappearance of the blaP1-aadA2 gene cassette in the 1990s. Only two dfr (trimethoprim resistance gene) types, dfrA1 and dfrA12, were detected in the 1980s, while three different dfr types, dfrA5, dfrA7, and dfrA17, were newly detected in the 1990s. Among the dfr genes, dfrA12 and dfrA17 were commonly detected in class 1 integrons from isolates recovered in the 1990s and 2001 to 2002.
FIG. 2.
Changes in prevalence of gene cassettes encoding aminoglycoside resistance (A) or trimethoprim resistance (B) among class 1 integrons during the last 2 decades. Numbers of aminoglycoside or trimethoprim resistance genes detected are expressed as percentages of the total number of cassette genes isolated.
Genetic localization of class 1 integrons.
Twenty isolates were randomly selected, and class 1 integrons were localized by Southern hybridization. The plasmid patterns of the original isolates were very different from each other, yet the intI1 gene was hybridized at plasmids of similar sizes (70 to 90 kb) in most of the isolates (data not shown). The plasmids of one isolate did not hybridize with the intI1 gene. This strain may have harbored the integron in a chromosome.
DISCUSSION
The present study characterized class 1 integrons and cassette genes conferring resistance to several classes of antibiotics in E. coli isolates collected in cases of urinary tract infection during the last 2 decades. Among 621 E. coli isolates, 339 (54.6%) included a class 1 integron, as detected by PCR with primers specific for intI. Other reports have also revealed the prevalence of class 1 integrons in gram-negative clinical isolates—54% in Taiwan (14), 59% in France (3), and 49% in Australia (15)—indicating that class 1 integrons are widespread among gram-negative clinical isolates.
Three significant gene cassette changes were detected in class 1 integrons during the last 2 decades in Korea. First, cassette genes encoding resistance to aminoglycosides were found to be predominant in the class 1 integrons of E. coli isolates from the 1980s, but their prevalence was slightly decreased by early 2000 (Fig. 2A). Second, only one cassette gene encoding resistance to trimethoprim was detected until 1982, but since the introduction of the dfrA12 and dfrA17 cassette genes to class 1 integrons in 1983 and the late 1980s and in the early 1990s, respectively, the prevalence of dfr cassettes has increased rapidly up to the present (Fig. 2B). Finally, the gene cassettes of 51.3% (58 of 113) of the class 1 integrons isolated during the 1980s were composed of a single cassette gene, while most of the gene cassettes isolated during the 1990s and since 2000 were composed of two or more cassette genes. The first and second changes may have been caused by antibiotic selective pressure. Aminoglycoside antibiotics were widely used for treating urinary tract infections in Korea during the 1980s. In the 1990s, however, quinolone and trimethoprim-sulfamethoxazole were preferred for treating E. coli infections in Korea. Such specific selection pressure may have supported the acquisition and maintenance of a trimethoprim resistance cassette by class 1 integrons and may also explain the increase in the number of different types of cassettes encoding dihydrofolate reductase. Furthermore, all the dfr cassettes, except for one, were located directly behind the 5′ conserved segment, which is closest to the promoter, thereby providing high-level expression and conditional resistance. In the present study, the increase in the prevalence of multiple-gene cassettes in class 1 integrons during the last 2 decades supports the suggestion that class 1 integrons facilitate their bacterial hosts' acquisition of resistance to a broad spectrum of antibiotic agents (12).
Based on the present results, the gene cassettes most commonly detected among class 1 integrons of E. coli isolates from the 1990s and 2000 to 2001 were dfrA17-aadA5 and dfrA12-aadA2. Although the dfrA12-aadA2 gene cassette was first detected in E. coli isolates from 1983, it is not known exactly when dfrA17-aadA5 was introduced to class 1 integrons. Lee et al. have reported that dfrA17 and dfrA12 were commonly detected among dfr genes in E. coli urinary tract isolates recovered from the same hospital during 1994 to 1996 (8). Therefore, dfrA17 may have been introduced to class 1 integrons in the late 1980s or early 1990s. Furthermore, the aadA5 cassette gene was detected only with the dfrA17 cassette gene, indicating that the dfrA17 and aadA5 cassette genes were introduced to class 1 integrons almost simultaneously, during 1986 to 1993, in Korea. The same combinations of cassette genes in class 1 integrons, such as aadA, blaP1-aadA2, dfrA12-aadA2, and dfrA17-aadA5, have also been detected in other areas of the world. For example, the dfrA12-aadA2 gene cassette pattern has been reported for E. coli urinary tract isolates recovered from Taiwan, Turkey, and Finland (3, 5), while the dfrA17-aadA5 gene cassette was recently found in E. coli urinary tract isolates from Australia and Taiwan (3, 16).
These results suggest that international travel and the import and export of animals may contribute to the dissemination of class 1 integrons or their host strains to different areas in the world. In addition, some researchers have proposed that gene cassettes become stably integrated over a long period (3, 9). Moreover, transfer of the entire integron, via a plasmid or transposon, is more frequent than single-gene mobilization or integration within the integron (9). In the present study, most integrons were located in plasmids and could be transferred to other strains. Yet the prevalence of each particular combination gene cassette differed according to the geographic area. In Taiwan, dfrA12-orfF-aadA2 was the gene cassette most commonly detected in E. coli strains isolated in 1993 and 1994, whereas in Australia, aadA1 was the gene cassette most frequently identified in E. coli strains isolated from 1998 to 1999 (3, 15). The results from Taiwan and Australia were similar to those for E. coli isolates from the 1990s and 1980s, respectively, in the present study. The differences in prevalence among different countries may have been caused by the different antibiotic therapy regimens used for bacterial infections in each country.
A new gene cassette, an incomplete adhE gene encoding the alcohol dehydrogenase E protein, was found in six isolates. It could have been picked up through an aberrant recombination event during integron transfer and may provide no selective advantage for the organism.
In conclusion, class 1 integrons were found to be widely disseminated among E. coli urinary tract isolates in Korea; the gene cassettes were continuously changed based on antibiotic selective pressure; and their sizes were increased by the introduction of new cassette genes. The location of integrons in plasmids may contribute to the horizontal dissemination of antibiotic resistance gene cassettes. Furthermore, antibiotic resistance genes and gene cassettes associated with metabolites were both introduced into class 1 integrons. Accordingly, the study of integrons and their associated gene cassettes can provide important information on the mechanisms of acquisition of multiple antibiotic resistance genes in clinical isolates.
Acknowledgments
This study was supported by a grant from the Korea Health 21 R&D Project, Ministry of Health and Welfare, Republic of Korea (02-PJ1-PG3-20201-0005).
REFERENCES
- 1.Arakawa, Y., M. Murakami, K. Suzuki, H. Ito, R. Wacharotayakun, S. Ohsuka, N. Kato, and M. Ohta. 1995. A novel integron-like element carrying the metallo β-lactamase gene blaIMP. Antimicrob. Agents Chemother. 39:1612-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birnboim, H. C., and J. Doly. 1979. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 24:1513-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang, C. Y., L. L. Chang, Y. H. Chang, T. M. Lee, and S. F. Chang. 2000. Characterization of drug resistance gene cassettes associated with class 1 integrons in clinical isolates of Escherichia coli from Taiwan, ROC. J. Med. Microbiol. 49:1097-1102. [DOI] [PubMed] [Google Scholar]
- 4.Fluit, A. C., and F. J. Schmitz. 1999. Class 1 integrons, gene cassettes, mobility, and epidemiology. Eur. J. Clin. Microbiol. Infect. Dis. 18:761-770. [DOI] [PubMed] [Google Scholar]
- 5.Heikkila, E., M. Skurnik, L. Sundstrom, and P. Huovinen. 1993. A novel dihydrofolate reductase cassette inserted in an integron borne on a Tn21-like element. Antimicrob. Agents Chemother. 37:1297-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim, S. W., J. Y. Lee, W. J. Park, Y. H. Cho, and M. S. Yun. 2000. Antibiotic sensitivity to the causative organism of acute simple urinary tract infection for recent 3 years. Korean J. Infect. Dis. 32:380-387. [Google Scholar]
- 7.Ko, H. S. 1999. A study of the changes of antibiotic sensitivity to the causative organisms of urinary tract infection for recent 5 years. Korean J. Urol. Assoc. 40:809-816. [Google Scholar]
- 8.Lee, J. C., J. Y. Oh, J. W. Cho, J. C. Park, J. M. Kim, S. Y. Seol, and D. T. Cho. 2001. The prevalence of trimethoprim-resistance-conferring dihydrofolate reductase genes in urinary isolates of Escherichia coli in Korea. J. Antimicrob. Chemother. 47:599-604. [DOI] [PubMed] [Google Scholar]
- 9.Martinez-Freijo, P., A. C. Fluit, F. J. Schmitz. V. S. C. Grek, J. Verhoef, and M. E. Jones. Class I integrons in gram-negative isolates from different European hospitals and association with decreased susceptibility to multiple antibiotic compounds. J. Antimicrob. Chemother. 42:689-696. [DOI] [PubMed]
- 10.Mazel, D., B. Dychinco, V. A. Webb, and J. Davies. 1988. A distinctive class of integron in the Vibrio cholerae genome. Science 280:605-608. [DOI] [PubMed] [Google Scholar]
- 11.Ploy, M. C., T. Lambert, J. P. Couty, and F. Denis. 2000. Integrons: an antibiotic resistance gene capture and expression system. Clin. Chem. Lab. Med. 38:483-487. [DOI] [PubMed] [Google Scholar]
- 12.Recchia, G. D., and R. M. Hall. 1995. Gene cassettes: a new class of mobile element. Microbiology 141:3015-3027. [DOI] [PubMed] [Google Scholar]
- 13.Sahm, D. F., C. Thornsberry, D. C. Mayfield, M. E. Jones, and J. A. Karlowsky. 2001. Multidrug-resistant urinary tract isolates of Escherichia coli: prevalence and patient demographics in the United States in 2000. Antimicrob. Agents Chemother. 45:1402-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sallen, B., A. Rajoharison, S. Desvarenne, and C. Mabilat. 1995. Molecular epidemiology of integron-associated antibiotic resistance genes in clinical isolates of Enterobacteriaceae. Microb. Drug Resist. 1:195-202. [DOI] [PubMed] [Google Scholar]
- 15.White, P. A., C. J. McIver, and W. D. Rawlinson. 2001. Integrons and gene cassettes in the Enterobacteriaceae. Antimicrob. Agents Chemother. 45:2658-2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White, P. A., C. J. Mclver, Y. M. Deng, and W. D. Rawlinson. 2000. Characterization of the two gene cassettes aadA5 and dfrA17. FEMS Microbiol. Lett. 182:265-269. [DOI] [PubMed] [Google Scholar]