Abstract
Objective
To determine whether polymorphisms of the surfactant protein B gene may be associated with increased mortality in patients with the acute respiratory distress syndrome.
Design
A prospective cohort study.
Setting
Four adult intensive care units at a tertiary academic medical center.
Patients
Two hundred fourteen white patients who had met criteria for acute respiratory distress syndrome.
Interventions
None.
Measurements and Main Results
Patients were genotyped for a variable nuclear tandem repeat polymorphism in intron 4 of the surfactant protein B gene and the surfactant protein B gene +1580 polymorphism. For the variable nuclear tandem repeat surfactant protein B gene polymorphism, patients were found to have either a homozygous wild-type genotype or a variant genotype consisting of either a heterozygous insertion or deletion polymorphism. Logistic regression was performed to analyze the relationship of the polymorphisms to mortality in patients with acute respiratory distress syndrome. In multivariate analysis, the presence of variable nuclear tandem repeat surfactant protein B gene polymorphism was associated with a 3.51 greater odds of death at 60 days in patients with acute respiratory distress syndrome as compared to those patients with the wild-type genotype (95% confidence interval 1.39-8.88, p = 0.008). There was no association found between the +1580 variant and outcome (p = 0.15).
Conclusions
In this study, the variable nuclear tandem repeat surfactant protein B gene polymorphism in intron 4 is associated with an increased 60 day mortality in acute respiratory distress syndrome after adjusting for age, severity of illness, and other potential confounders. Additional studies in other populations are needed to confirm this finding.
Keywords: acute respiratory distress syndrome, surfactant, pulmonary, surfactant protein SFTP-B, SFTP-B +1580, genetic polymorphism
The acute respiratory distress syndrome (ARDS) is defined as non-hydrostatic pulmonary edema clinically characterized by bilateral infiltrates on chest x-ray and hypoxemia in the absence of left atrial hypertension (1). Although survival of patients with ARDS has improved due to the use of ventilation using low tidal volumes, mortality remains high in the range of 30% to 40% (2). Older age, sepsis, chronic liver disease, multi-organ dysfunction, multiple blood transfusions, and ventilation with large tidal volumes are associated with increased mortality in ARDS (2-7). Genetic factors, including polymorphisms in the angiotensin converting enzyme, interleukin-1, and tumor necrosis factor genes have been associated with altered mortality in ARDS (8, 9).
Pulmonary surfactant is a combination of proteins and phospholipids normally present in alveolar fluid. It serves to prevent alveolar atelectasis, allow for normal ventilation and perfusion matching, and is immunologically active. It is recognized that pulmonary surfactant functions abnormally in patients with ARDS (10, 11). Surfactant protein B (SFTP-B) is one of the vital hydrophobic surfactant proteins that normally serves to reduce alveolar surface tension and thus allow for normal alveolar physiologic function (11, 12). Reduced levels of SFTP-B have been found in the lungs in animal models of hypoxemic respiratory failure and in patients with ARDS (10, 13, 14).
The surfactant protein B gene (SFTP-B) is located on chromosome 2 and is approximately 9500 base-pairs in length. The variable nuclear tandem repeat (VNTR) SFTP-B polymorphism is defined by the presence of or deletion of a 20 base-pair conserved sequence with a variable number of CA repeats in intron 4 of the SFTP-B gene as previously described (15). Wildtype carries no insertion or deletion. The SFTP-B +1580 constitutes a single polymorphism in exon 4 of the SFTP-B gene and is characterized by a cytosine for thymine variation at position 1580 which causes amino acid 131 to change from threonine to isoleucine (16). Both of these polymorphisms have been associated with ARDS in previous studies (16, 17). A relationship between the presence of these polymorphisms and outcome in ARDS has not been described. We hypothesized that these polymorphisms as described above may be associated with increased mortality in patients with ARDS.
Some of the results of this study have previously been reported in the form of an abstract (18).
MATERIALS AND METHODS
Study Population
The study population consisted of ARDS patients who were prospectively enrolled into the Molecular Epidemiology of ARDS study. The details of the study have been previously described (7, 17). In the parent study, critically ill patients admitted to the medical, cardiac, surgical, and neurosurgical intensive care units at the Massachusetts General Hospital in Boston, MA with study-defined sepsis, trauma, aspiration or multiple transfusion were eligible for the study (Table 1). Patients were excluded from the study if they fulfilled any of the following criteria: age <18, absolute neutrophil count <500 cells/μL (unless because of sepsis), treatment with immunosuppressants except corticosteroids or immunostimulants such as granulocyte colony-stimulating factor in the preceding 21 days, presence of a do-not-intubate or comfort-measures-only status, a history of human immunodeficiency virus, a history of solid organ or bone marrow transplantation, or a history of interstitial lung disease which may mimic ARDS. The study was approved by the Human Subjects Committees of the Massachusetts General Hospital and the Harvard School of Public Health. Informed consent was obtained from all patients or their appropriate surrogate.
Table 1.
Clinical risk factors for acute respiratory distress syndrome used as screening criteria for study inclusion41
| Risk Factor | Description |
|---|---|
| Sepsis | Known or strongly suspected source of systemic infection (e.g., urinary tract, pulmonary, intraabdominal infections, or soft tissue infection) and at least two of the following findings: 1) temperature >38°C (100.4°F) or <36°C (96.8°F); 2) heart rate >90 beats/min; 3) respiratory rate >20 breaths/min or PaCO 2 <32 mm Hg; 4) WBC >12,000 cells/μL, <4000 cells/μL, or >10% bands |
| Septic shock | Fulfill requirements for sepsis and any one of the following: 1) sepsis-induced hypotension as defined by systolic BP of <90 mm Hg or reduction of ≥40 mm Hg from baseline for at least 30 min, unresponsive to 500 mL fluid resuscitation; 2) need for vasopressors to maintain systolic BP of ≥90 mm Hg within baseline |
| Trauma | Multiple fractures and/or pulmonary contusions |
| Multiple transfusions | ≥8 units packed RBCs transfused within 24 hrs |
| Aspiration | Witnessed or documented with aspiration of gastric contents from airways or endotracheal tube |
BP, blood pressure; RBC, red blood cell; WBC, white blood cell.
Enrolled subjects were followed prospectively for development of ARDS defined as respiratory failure requiring mechanical ventilation and fulfillment of the American-European Consensus Conference criteria for ARDS as previously described (1, 7, 17). Only patients who developed ARDS are analyzed in this study.
Baseline demographic data were collected on each patient and included such information as past history of ARDS, diabetes, alcohol use, chronic liver disease, or smoking. Clinical information such as PaO2:FiO2 ratio, type of mechanical ventilation, treatment with steroids, and number and type of blood transfusions were recorded. Patients were followed for all-cause 60 day mortality.
DNA Extraction and Genotyping
Blood samples were collected from each enrolled patient in a 10 mL ethylenediaminetetraacetic acid containing tube. DNA was then extracted from blood using PureGene kits (Gentra Systems, Minneapolis, MN) per manufacturer’s protocol.
Extracted DNA was then used as a template for amplification of the VNTR SFTP-B gene using polymerase chain reaction as previously described (15). The following primers were used in polymerase chain reaction: 5′-CTGGTCATCGACTACTTCCA-3′ and 5′-TGTGTGTGAGAGTGAGGGTGTAAG-3′. The polymorphism consisted of either an insertion or deletion of a 20 base pair conserved sequence along with a variable number of dinucleotide CA repeats. Patients with a 606 base-pair band were considered to have the wild-type genotype. Patients with one or more smaller or larger bands were considered to have either an insertion or deletion variant, respectively. For quality control, a random 5% of the samples were repeated to assess the reproducibility of results. Two authors independently reviewed all agarose gels. There was 100% concordance of randomly repeated samples and 100% agreement in independent gel interpretation between two individuals. The rate of successful genotyping was 99.5%.
The allelic discrimination of the SFTP-B +1580 gene polymorphism was assessed with the ABI PRISM 7900 Sequence Detection System (Applied Biosystems, Foster City, CA), using the fluorogenic 5′ nuclease assay with Taqman minor groove binder probes. The wildtype Taqman minor groove binder probes were 6-carboxyfluorescein-labeled and the mutant probes were factor V Leiden probe-labeled. The primers and probes for the +1580 gene (rs 1130866) polymorphism assays were ordered from Applied Biosystems. Genotyping was performed by laboratory personnel blinded to subject status, and a random 10% of the samples were repeated to validate genotyping procedures. Two authors independently reviewed all genotyping results. The rate of genotyping success was 99.9%.
Statistical Analysis
All patients with ARDS were classified by presence of the C or T allele at the +1580 site of SFTP-B and by presence or absence of one or more VNTR variant (insertion or deletion) SFTP-B polymorphisms. Patients with heterozygous insertion/wildtype, heterozygous deletion/wildtype, and homozygous insertion polymorphisms were grouped together as variant SFTP-B as in previously published studies (17, 19). Compliance and Lung Injury Score were calculated as previously described (20). Dichotomous baseline characteristics were compared using the Fisher’s exact test and continuous variables were compared using the Student’s t-test if normally distributed or the Wilcoxon ranked sum test if not normally distributed.
A logistic regression model with 60-day mortality in ARDS as the dependent covariate was created. Independent covariates were selected by those found to be significantly related to outcome on univariate analysis or those covariates which had been reported in the literature as being related to outcome in patients with ARDS such as older age, chronic liver disease, red blood cell transfusion, sepsis, and treatment with corticosteroids (2-7, 21, 22).
Administration of any steroids at any time during the first 29 days of the study was included in the analysis as a dichotomous covariate. Age, Acute Physiology and Chronic Health Evaluation III score, and number of units of red blood cells transfused were kept as continuous covariates in the model. Acute Physiology and Chronic Health Evaluation III score was recalculated without the age component. Interaction between variant SFTP-B and the other covariates was tested with interaction terms. Potential confounders were evaluated by adding each covariate individually to a regression model of SFTP-B and ARDS mortality at 60 days. If the odds ratio (OR) of variant SFTP-B changed by >10%, the added covariate was considered to be a confounder. Conformation to the Hardy-Weinberg equilibrium was calculated using the chi-square goodness of fit test as has been done in previous studies (17).
Linkage disequilibrium between the SFTP-B +1580, VNTR SFTP-B polymorphism and their frequencies was estimated using the expectation maximization algorithm (23). Haplotypes were coded as an additive fashion. ARDS mortality was regressed on haplotype counts by logistic regression, using the most common haplotype as reference haplotype.
All analyses were performed using SAS statistical software (SAS Institute, Cary, NC) version 9.1. A p value ≤0.05 was considered significant.
RESULTS
Baseline Characteristics
Between September 1999 and October 2002, 238 of 1040 patients in the Molecular Epidemiology of ARDS Study developed ARDS. These patients were genotyped for the VNTR SFTP-B polymorphism and +1580 polymorphism. As 90% of the ARDS patients were white and significant racial differences have been noted in the distribution of SFTP-B polymorphisms, all subsequent analyses were restricted to the 214 white patients (24). Missing data on baseline characteristics occurred in only two patients. A total of 115 of 214 (54%) subjects survived to day 60. Clinical differences between survivors and nonsurvivors in ARDS are detailed in Table 2.
Table 2.
Baseline clinical factors for survivors and nonsurvivors with ARDS
| Survivors | Nonsurvivors | p | |
|---|---|---|---|
| Female gender | 50 (43%) | 51 (52%) | 0.27 |
| Chronic liver disease | 4 (4%) | 9 (9%) | 0.15 |
| Sepsis without shock | 44 (38%) | 24 (24%) | 0.039 |
| Septic shock | 53 (46%) | 63 (64%) | 0.013 |
| Direct pulmonary injury | 80 (70%) | 65 (66%) | 0.56 |
| Steroids given during ARDS | 39 (34%) | 36 (36%) | 0.77 |
| Age | 58 (18, 90) | 72 (22, 97) | <0.0001 |
| APACHE III | 51 (3, 99) | 66 (16, 131) | <0.0001 |
| Units of RBCs transfused | 1.0 (0, 31) | 2.0 (0, 63) | <0.009 |
For dichotomous variables, the number of patients is listed with percent of total in parenthesis. For the continuous covariates age, APACHE III score, and number of units of RBCs transfused, the median is listed with the range in parentheses.
ARDS, acute respiratory distress syndrome; APACHE, Acute Physiology and Chronic Health Evaluation; RBC, red blood cell.
Presence of VNTR SFTP-B Polymorphism and Risk of Death 60 Days
Thirty-nine (18%) patients had a variant form of SFTP-B (8 (4%) patients heterozygous insertion/wildtype, 24 (11%) patients heterozygous deletion/wildtype, seven (3%) patients homozygous insertion), 175 (82%) patients had the homozygous wildtype VNTR SFTP-B gene. There was no significant departure from Hardy Weinberg equilibrium (p = 0.39).
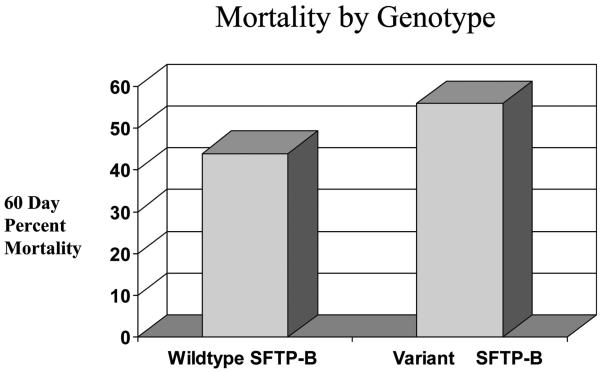
For those patients with one more or more VNTR SFTP-B variants, 17 of 39 (44%) patients survived compared with 98 of 175 (56%) patients homozygous for the wildtype allele who survived. This difference was not statistically significant on crude analysis (OR 1.65 [0.82, 3.32]; p = 0.16) (Fig. 1). Frequency of the VNTR SFTP-B genotypes in those who survived or died at day 60 are listed in Table 3.
Figure 1.
Distribution of survivors and nonsurvivors based on surfactant protein B gene (SFTP-B) genotype. For patients with wildtype SFTP-B, 98 patients (56%) survived and 77 (44%) died. For patients with variable nuclear tandem repeat (VNTR) SFTP-B polymorphism, 17 patients (44%) survived and 22 (56%) died.
Table 3.
Frequency and χ2 analysis of VNTR SFTP-B and +1580 genotypes in those who survived and died at day 60
| Wildtype VNTR SFTP-B | Variant VNTR SFTP-B | p | +1580 TT | +1580 CT | +1580 CC | p | |
|---|---|---|---|---|---|---|---|
| Survived at day 60 | 98 (85.2) | 17 (14.8) | 34 (29.6) | 57 (49.6) | 24 (20.9) | ||
| Died at day 60 | 77 (77.8) | 22 (22.2) | 0.16 | 21 (21.2) | 53 (53.5) | 25 (25.3) | 0.44 |
Percent of each genotype is listed in parentheses.
VNTR, variable nuclear tandem repeat; SFTP-B, surfactant protein B gene.
Significant predictors for 60-day mortality in ARDS on multivariate analysis are displayed in Table 4. After adjusting for these significant predictors on multivariate analysis, ARDS patients with one or more variant VNTR SFTP-B allele had significantly increased odds of death at 60 days (OR 3.51 [1.39, 8.88]; p value 0.008). There were no significant interactions between the variant SFTP-B polymorphism and other covariates in the model. Presence of variant SFTP-B was not related to Lung Injury Score (OR 0.86 [0.71, 1.06]; p = 0.16), nor was 60-day mortality related to Lung Injury Score (OR 0.87 [0.64, 1.2]; p = 0.39), or respiratory system compliance (OR 1.07 [0.90, 1.3]; p = 0.42). There was no significant change in the relationship of the VNTR SFTP-B polymorphism to mortality when the analysis was stratified either by direct lung injury, pneumonia, or gender.
Table 4.
Logistic regression model with covariates significantly related to mortality in white patients with acute respiratory distress syndrome at 60 days
| OR (95% CI) | p | |
|---|---|---|
| SFTP-B variant | 3.51 (1.39-8.88) | 0.008 |
| Age | 1.07 (1.04-1.09) | <0.0001 |
| Chronic liver disease | 4.94 (1.09-22.3) | 0.038 |
| APACHE III score | 1.04 (1.02-1.06) | 0.0002 |
| Red cell transfusion (per unit transfused) | 1.11 (1.03-1.18) | 0.0036 |
OR, odds ratio; CI, confidence interval; SFTP-B, surfactant protein B gene; APACHE, Acute Physiology and Chronic Health Evaluation.
The covariates in the multivariate model for ARDS mortality were examined further to determine which covariate affected the crude estimate most significantly. On adding age to a crude model of VNTR SFTP-B and mortality at 60 days, variant VNTR SFTP-B becomes significantly related to mortality (OR 2.25 [1.02, 4.95]; p = 0.044). Age is therefore a negative confounder of the relationship of the VNTR SFTP-B and mortality. It served to drive the multivariate OR away from the null and accounting for it yields a significant relationship between the presence of variant VNTR SFTP-B and mortality at 60 days. Systematic addition of the other covariates changed the estimate for variant VNTR SFTP-B allele and ARDS by <10% with no qualitative change in statistical significance.
To better examine the effect of age on the association between the variant VNTR SFTP-B and ARDS mortality, the analysis was stratified by the median age. On stratifying the final regression model along median age of 65, variant SFTP-B was related to mortality at 60 days among patients older than 65 with an OR of 3.0 (0.94, 9.5); p = 0.063. Among the younger subset, variant SFTP-B was related to mortality with an OR of 1.95 (0.56, 6.8); p = 0.29.
Presence of +1580 Polymorphism and Risk of Death 60 Days
There was no significant departure in allele distribution of SFTP-B +1580 from Hardy Weinberg equilibrium (p = 0.68). There was no significant relationship of the SFTP-B +1580 polymorphism to outcome in ARDS in this cohort on univariate or multivariate analysis. On crude analysis, SFTP-B +1580 was not related to mortality at 60 days with an OR of 1.27 (0.86, 1.87); p = 0.22. SFTP-B +1580 was not related to mortality at 60 days on multivariate analysis with an OR of 1.40 (0.88, 2.23); p = 0.15. No association between the variant +1580 and ARDS mortality was found after stratifying by pneumonia (p = 0.58) or direct pulmonary injury (p = 0.57). There was no significant difference in the SFTP-B +1580 genotypes in those who survived or died at day 60 (p = 0.44) as shown in Table 3.
Haplotype Analysis
Linkage disequilibrium analysis showed that all of the Lewontin’s D’ values between polymorphisms at +1580 and the VNTR SFTP-B were 1.0, indicating high linkage disequilibrium among these polymorphisms. A total of four haplotypes were deduced. However, global tests did not find any associations between haplotypes and ARDS mortality (p = 0.97, df = 3).
DISCUSSION
There are four major known surfactant proteins: SFTP-A, SFTP-B, SFTP-C, and SFTP-D and a minor group of proteins derived from serum (12). SFTP-A and SFTP-D belong to the collectin superfamily of proteins and are active immunologically in stimulating bacterial phagocytosis and macrophage chemotaxis (25-27). SFTP-B and SFTP-C are hydrophobic proteins crucial to survival. They reduce the hydrophilic molecular attractant forces in surfactant, serve to spread the fluid, and thus lower alveolar surface tension (12). These functions are important for maintaining normal physiologic lung function.
Abnormal function of surfactant in ARDS may contribute to atelectasis, shunt, and ventilation-perfusion mismatch. It may also predispose to infection and contribute to ventilator induced lung injury (28). Studies have shown alterations in the concentrations of surfactant proteins and changes in the surfactant function in ARDS. In bronchoalveolar lavage (BAL) specimens from ARDS patients, surface lipid-protein complexes are not surface active like surfactant from healthy controls and surface tension is increased (10, 11). Phospholipid concentrations from patients with respiratory failure have been shown to be similar to those of infants with respiratory distress syndrome (29).
Several studies in animals have suggested that decreased levels of SFTP-B or abnormalities in the SFTP-B gene are related to hypoxemic respiratory failure. In two mouse models of pneumocystis carinii pneumonia, levels of SFTP-B from BAL samples and SFTP-B gene expression were both reduced (13). In a bleomycin model of lung injury in rats, mature SFTP-B levels were decreased by 90% at 7 days postinjury. Lost surface activity of BAL phospholipids of the injured rats was restored by administration of exogenous SFTP-B (14). Transgenic mice lacking the SFTP-B gene die shortly after birth because of respiratory distress (30).
Similarly, humans with congenital absence of SFTP-B also develop a rapidly fatal respiratory distress syndrome (31). In infants, polymorphisms in SFTP-B have been associated with respiratory distress syndrome (29, 32, 33). Polymorphisms in SFTP-B have also been associated with ARDS (16, 17). A single nucleotide polymorphism (SFTP-B +1580) was found to be associated with the development of ARDS, septic shock, and the need for mechanical ventilation in adult patients with community-acquired pneumonia (16).
This study prospectively follows a cohort of patients with ARDS and shows an association between presence of the relatively common VNTR SFTP-B (found in 18% of the population in this study) and increased mortality in multivariate analysis. Specifically, the presence of the variant VNTR SFTP-B polymorphism is associated with a significantly increased risk of death at 60 days (OR 3.51 [95% confidence interval 1.38-8.88]; p = 0.008) after adjustment for the potential confounders. This risk was higher in older subjects who have a higher mortality in ARDS as a group. Currently, the functional significance of the VNTR SFTP-B polymorphism is not known. The variant VNTR SFTP-B gene could be related to mortality in ARDS directly—perhaps through the production of a faulty surfactant protein. Studies examining the levels and function of the SFTP-B protein in BAL fluid in human ARDS patients and SFTP-B mRNA levels along with genetics would help to answer this question. Given that the variant VNTR SFTP-B is present in an intron, it may not have a direct pathophysiologic role, but may instead be linked to another causally related polymorphism. In this case, this SFTP-B polymorphism may only serve as an epidemiologic marker of increased risk of mortality in those patients with ARDS. Alternatively, the polymorphism may play a role in protein splicing that has been hypothesized to be part of the role of introns. Further study will help to clarify the role of SFTP-B in ARDS and perhaps provide further basis for treatment of the disease.
Why the variant SFTP-B might have a greater relationship to mortality in patients who are older is unclear. Genes have been linked to multiple diseases differentially based on age. Age of onset of Alzheimer’s disease has been linked to apolipoprotein E polymorphisms and age of onset of diabetes has been linked to differences in human leukocyte antigen class II haplotypes (34-36). There have also been associations of polymorphisms based on age in ARDS. There is a stronger association of the -308GA tumor necrosis factor-α with mortality in younger patients (37). The interleukin-10 -1082GG genotype is associated with ARDS, but only in the presence of a significant interaction between the -1082GG genotype and age (38).
There is a suggestion that levels of SFTP-A may change with age. SFTP-A levels in bronchial lavage were found in one study to decrease with age in healthy volunteers (although there was no significant change in BAL levels of SFTP-A) (39). In another study, the ratio of SFTP-A1 to SFTP-A in BAL fluid was found to decrease with age, although the total SFTP-A content in BAL fluid was unchanged (40). On review of the literature, there has been no report of any relationship of levels of SFTP-B to age. The role of aging in the function and production of surfactant is also unknown. An age of illness threshold to the effects of SFTP-B and a mechanism by which this might occur needs to be further explored.
We found no relationship in this cohort between the SFTP-B +1580 polymorphism and mortality in ARDS, either when examined in the total population or when examined in the subgroups of patients with pneumonia or direct lung injury. A prior association between SFTP-B +1580 and the development of ARDS and need for mechanical ventilation was described in a group of patients with community acquired pneumonia (16). No association was found between this polymorphism and mortality but the low mortality rate (6.5%) in that study limited the power to detect an association.
We recognize several limitations in this study. First, there was no measurement of a phenotypic expression of the genes such as concentration of SFTP-B in BAL fluid. Second, this study examines the relationship of SFTP-B to mortality only in white patients because of the predominance of the group in the cohort and reported racial differences in SFTP-B. Its results may therefore have limited applicability to other racial groups. The evident relationship between SFTP-B and increased mortality in older patients needs to be further explored.
CONCLUSION
Surfactant plays an important role in lung function and has been shown to function abnormally in patients with ARDS. In this cohort, a VNTR in intron 4 of the SFTP-B is associated with increased 60 day mortality in ARDS after adjusting for age, severity of illness and other potential confounders. Additional studies in other populations are needed to confirm this finding.
ACKNOWLEDGMENTS
We thank Thomas McCabe, Kelly McCoy, Chris Schwartzenburg, and Julia Shin for patient recruitment and data collection; Marcia Chertok and Janna Frelich for data management; and Andrea Shafer and Li Su for laboratory support.
Supported, in part, by NIH grant R01 HL060710 (to DCC), NIH training grant T32 HL007874 (to PFC), and K23 HL 67197 from NHLBI (to MNG).
Footnotes
See also p. 2678.
The authors have not disclosed any potential conflicts of interest.
REFERENCES
- 1.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 2.Acute Respiratory Distress Syndrome Network (ARDSNet) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. New Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 3.Zilberberg MD, Epstein SK. Acute lung injury in the medical ICU. Am J Respir Crit Care Med. 1998;157:1159–1164. doi: 10.1164/ajrccm.157.4.9704088. [DOI] [PubMed] [Google Scholar]
- 4.Monchi M, Bellenfant F, Cariou A, et al. Early predictive factors of survival in the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1998;158:1076–1081. doi: 10.1164/ajrccm.158.4.9802009. [DOI] [PubMed] [Google Scholar]
- 5.Luhr OR, Antonsen K, Karlsson M, et al. Incidence and mortality after acute respiratory failure and acute respiratory distress syndrome in Sweden, Denmark, and Iceland. Am J Respir Crit Care Med. 1999;159:1849–1861. doi: 10.1164/ajrccm.159.6.9808136. [DOI] [PubMed] [Google Scholar]
- 6.Ware L, Mathay M. The acute respiratory distress syndrome. New Engl J Med. 2000;244:575–579. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 7.Gong MN, Thompson BT, Williams P, et al. Clinical predictors of and mortality in acute respiratory distress syndrome: Potential role of red cell transfusion. Crit Care Med. 2005;33:1191–1198. doi: 10.1097/01.ccm.0000165566.82925.14. [DOI] [PubMed] [Google Scholar]
- 8.Marshall RP, Webb S, Bellingan GJ, et al. Angiotensin converting enzyme insertions/deletion polymorphism is associated with susceptibility and outcome in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2002;166:646–650. doi: 10.1164/rccm.2108086. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe E, Hirasawa H, Oda S, et al. Extremely high interleukin-6 blood levels and outcome in the critically ill are associated with tumor necrosis factor and interleukin-1 related gene polymorphisms. Crit Care Med. 2005;33:89–97. doi: 10.1097/01.ccm.0000150025.79100.7d. [DOI] [PubMed] [Google Scholar]
- 10.Gregory TJ, Longmore WJ, Moxley MA, et al. Surfactant chemical composition and biophysical activity in acute respiratory distress syndrome. J Clin Invest. 1991;88:1976–1981. doi: 10.1172/JCI115523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hallman M, Spragg R, Harell JH, et al. Evidence of lung surfactant abnormality in respiratory failure. Study of bronchoalveolar lavage phospholipids, surface activity, phospholipase activity, and plasma myoinositol. J Clin Invest. 1982;70:673–683. doi: 10.1172/JCI110662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pantelidis P, Veeraraghavan S, du Bois RM. Surfactant gene polymorphisms and interstitial lung diseases. Respir Res. 2002;3:14. doi: 10.1186/rr163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beers MF, Atochina EN, Preston AM, et al. Inhibition of lung surfactant protein B expression during pneumocystis carinii pneumonia in mice. J Lab Clin Med. 1999;133:406–407. doi: 10.1016/s0022-2143(99)90019-7. [DOI] [PubMed] [Google Scholar]
- 14.Savani RC, Godinez RI, Godinez MH, et al. Respiratory distress after intratracheal bleomycin: Selective deficiency of surfactant proteins B and C. Am J Physiol Lung Cell Mol Physiol. 2001;281:L685–L696. doi: 10.1152/ajplung.2001.281.3.L685. [DOI] [PubMed] [Google Scholar]
- 15.Floros J, Veletza SV, Kotikalapudi P, et al. Dinucleotide repeats in human surfactant protein-B gene and respiratory distress syndrome. Biochem J. 1995;305:583–590. doi: 10.1042/bj3050583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quasney MW, Waterer GW, Dahmer MK, et al. Association between surfactant protein B +1580 polymorphism and the risk of respiratory failure in adults with community-acquired pneumonia. Crit Care Med. 2004;32:1115–1119. doi: 10.1097/01.ccm.0000124872.55243.5a. [DOI] [PubMed] [Google Scholar]
- 17.Gong MN, Zhou MD, Li-Lian X, et al. Polymorphism in the surfactant protein-B gene, gender, and the risk of direct pulmonary injury and ARDS. Chest. 2004;125:203–211. doi: 10.1378/chest.125.1.203. [DOI] [PubMed] [Google Scholar]
- 18.Currier PF, Gong MN, Pothier LJ, et al. Surfactant protein-B polymorphism is associated with increased mortality at 28 days in ARDS patients. Am J Respir Crit Care Med. 2005:A243. [Google Scholar]
- 19.Max M, Pison U, Floros J. Frequency of AP-B and SP-A1 gene polymorphisms in the acute respiratory distress syndrome (ARDS) Appl Cardiopulm Pathophysiol. 1996;6:111–118. [Google Scholar]
- 20.Murray JF, Matthay MA, Luce JM, et al. An expanded definition of the adult respiratory distress syndrome. Ann Rev Respir Dis. 1988;138:720–723. doi: 10.1164/ajrccm/138.3.720. [DOI] [PubMed] [Google Scholar]
- 21.Lee HS, Lee JM, Kim MS, et al. Low-dose steroid therapy at an early phase of postoperative acute respiratory distress syndrome. Ann Thorac Surg. 2005;79:405–410. doi: 10.1016/j.athoracsur.2004.07.079. [DOI] [PubMed] [Google Scholar]
- 22.Meduri GU, Headley MD, Golden E, et al. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome. JAMA. 1998;280:159–165. doi: 10.1001/jama.280.2.159. [DOI] [PubMed] [Google Scholar]
- 23.Zhai R, Zhou W, Gong MN, et al. Inhibitor κB-α haplotype GTC is associated with susceptibility to acute respiratory distress syndrome in Caucasians. Crit Care Med. 2007;35:893–898. doi: 10.1097/01.CCM.0000256845.92640.38. [DOI] [PubMed] [Google Scholar]
- 24.Veletza SV, Rogan PK, TenHave T, et al. Racial differences in allelic distribution at the human pulmonary surfactant protein B gene locus (SFTP-B) Exp Lung Res. 1996;22:489–494. doi: 10.3109/01902149609046037. [DOI] [PubMed] [Google Scholar]
- 25.Lawson PR, Reid KB. The roles of surfactant proteins A and D in innate immunity. Immunol Rev. 2000;173:66–78. doi: 10.1034/j.1600-065x.2000.917308.x. [DOI] [PubMed] [Google Scholar]
- 26.O’Neill S, Lesperance E, Klass D. Human lung lavage surfactant enhances staphylococcal phagocytosis by alveolar macrophages. Am Rev Respir Dis. 1984;130:1177–1179. doi: 10.1164/arrd.1984.130.6.1177. [DOI] [PubMed] [Google Scholar]
- 27.Hoffman R, Claypool W, Katyal S, et al. Augmentation of rat alveolar macrophage migration by surfactant protein. Am Rev Respir Dis. 1987;135:1358–1362. doi: 10.1164/arrd.1987.135.6.1358. [DOI] [PubMed] [Google Scholar]
- 28.Haitsma JJ, Papadakos PJ, Lachmann B. Surfactant therapy for acute lung injury/acute respiratory distress syndrome. Curr Opin Crit Care. 2004;10:18–22. doi: 10.1097/00075198-200402000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Klein JM, Thompson MW, Snyder JM, et al. Transient surfactant protein B deficiency in a term infant with severe respiratory failure. J Pediatr. 1998;132:198–200. doi: 10.1016/s0022-3476(98)70439-1. [DOI] [PubMed] [Google Scholar]
- 30.Clark JC, Wert SE, Bachurski CJ, et al. Targeted disruption of the surfactant protein B gene disrupts surfactant homeostasis causing respiratory failure in newborn mice. Proc Natl Acad Sci USA. 1995;92:7794–7798. doi: 10.1073/pnas.92.17.7794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nogee LM, Garnier G, Dietz HC, et al. A mutation in the surfactant protein B gene responsible for fatal neonatal respiratory disease in multiple kindreds. J Clin Invest. 1994;93:1860–1863. doi: 10.1172/JCI117173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tredano M, van Elburg RM, Kaspers AG, et al. Compound SFTPB 1549C→GAA (121ins2) and 457delC heterozygosity in severe congenital lung disease and surfactant protein B (SFTP-B) deficiency. Hum Mutat. 1999;14:502–509. doi: 10.1002/(SICI)1098-1004(199912)14:6<502::AID-HUMU9>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 33.Makri V, Hospes B, Stoll-Becker S, et al. Polymorphisms of surfactant protein B encoding gene: Modifiers of the course of neonatal respiratory distress syndrome? Eur J Pediatr. 2002;161:604–608. doi: 10.1007/s00431-002-1046-1. [DOI] [PubMed] [Google Scholar]
- 34.Harwood DG, Barker WW, Ownby RL, et al. Apolipoprotein E polymorphism and age of onset for Alzheimer’s disease in a bi-ethnic sample. Int Psychogeriatr. 2004;16:317–326. doi: 10.1017/s104161020400033x. [DOI] [PubMed] [Google Scholar]
- 35.Wijsman EM, Daw EW, Yu X, et al. APOE and other loci affect age-at-onset in Alzheimer’s disease families with PS2 mutation. Am J Med Genet B Neuropsychiatr Genet. 2005;132:14–20. doi: 10.1002/ajmg.b.30087. [DOI] [PubMed] [Google Scholar]
- 36.Murao S, Makino H, Kaino Y, et al. Differences in the contribution of HLA-DR and -DQ haplotypes to susceptibility to adult- and childhood-onset type 1 diabetes in Japanese patients. Diabetes. 2004;53:2684–2690. doi: 10.2337/diabetes.53.10.2684. [DOI] [PubMed] [Google Scholar]
- 37.Gong MN, Zhou W, Williams PL, et al. -308GA and TNFB polymorphisms in acute respiratory distress syndrome. Eur Respir J. 2005;26:382–389. doi: 10.1183/09031936.05.00000505. [DOI] [PubMed] [Google Scholar]
- 38.Gong MN, Thompson BT, Williams PL, et al. Interleukin-10 polymorphism in position-1082 and acute respiratory distress syndrome. Eur Respir J. 2006;27:674–681. doi: 10.1183/09031936.06.00046405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Betsuyaku T, Kuroki Y, Nagai K, et al. Effects of ageing and smoking on SP-A and SP-D levels in bronchoalveolar lavage fluid. Eur Respir J. 2004;24:964–970. doi: 10.1183/09031936.04.00064004. [DOI] [PubMed] [Google Scholar]
- 40.Tagaram HRS, Wang G, Umstead TM, et al. Characterization of a human surfactant protein A1 (SP-A1) gene-specific antibody; SP-A1 content variation among individuals of varying age and pulmonary health. Am J Physiol Lung Cell Mol Physiol. 2006;292:L1052–L1063. doi: 10.1152/ajplung.00249.2006. [DOI] [PubMed] [Google Scholar]
- 41.Bone RC, Balk RA, Cerra FB, et al. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]